Abstract
Periampullary adenocarcinoma, including pancreatic cancer, is a heterogeneous group of tumours with dismal prognosis, for which there is an urgent need to identify novel treatment strategies. The human epithelial growth factor receptors EGFR, HER2 and HER3 have been studied in several tumour types, and HER-targeting drugs have a beneficial effect on survival in selected types of cancer. However, these effects have not been evident in pancreatic cancer, and remain unexplored in other types of periampullary cancer. The prognostic impact of HER-expression in these cancers also remains unclear. The aim of this study was therefore to examine the expression and prognostic value of EGFR, HER2 and HER3 in periampullary cancer, with particular reference to histological subtype. To this end, protein expression of EGFR, HER2 and HER3, and HER2 gene amplification was assessed by immunohistochemistry and silver in situ hybridization, respectively, on tissue microarrays with tumours from 175 periampullary adenocarcinomas, with follow-up data on recurrence-free survival (RFS) and overall survival (OS) for up to 5 years. EGFR expression was similar in pancreatobiliary (PB) and intestinal (I) type tumours, but high HER2 and HER3 expression was significantly more common in I-type tumours. In PB-type cases receiving adjuvant gemcitabine, but not in untreated cases, high EGFR expression was significantly associated with a shorter OS and RFS, with a significant treatment interaction in relation to OS (pinteraction = 0.042). In I-type cases, high EGFR expression was associated with a shorter OS and RFS in univariable, but not in multivariable, analysis. High HER3 expression was associated with a prolonged RFS in univariable, but not in multivariable, analysis. Neither HER2 protein expression nor gene amplification was prognostic. The finding of a potential interaction between the expression of EGFR and response to adjuvant chemotherapy in PB-type tumours needs validation, and merits further study.
Introduction
Adenocarcinomas originating in the head of the pancreas, the distal bile duct, the ampulla of Vater and the duodenum are often grouped together as periampullary tumours, since they can be difficult to distinguish from each other clinically. Pancreatic cancer is the most common type of periampullary adenocarcinoma, accounting for 3% of all cancer in the USA [1] and the Nordic countries [2] and, due to very high lethality [3], 7% of all cancer-related deaths, making it the fourth most common cause of cancer-related death in the western world [1, 2]. After surgery, periampullary adenocarcinomas have traditionally been primarily categorized according to their anatomical origin, but recent research has shown that morphological subtype is a more rational basis for classification [4]. Pancreatobiliary (PB) type morphology dominates in periampullary tumours of pancreatic and distal bile duct origin, but is also seen in the ampulla of Vater [4, 5]. They have a significantly worse prognosis than tumours with an intestinal (I) type morphology, which are mainly found in the ampulla of Vater and in the duodenum [4–6].
Members of the HER (human epithelial growth factor receptor) family of tyrosine kinase receptors are essential for human development and growth, and they are overexpressed in several human cancers. They consist of four closely related transmembranous molecules, EGFR (HER1, ErbB-1), HER2 (Neu, ErbB-2), HER3 (ErbB-3) and HER4 (ErbB-4). Ligand-binding causes hetero- or homodimerization of receptors and intracellular transphosphorylation, which activates several intracellular signalling-cascades important for cell survival, proliferation and growth. Combinations of HERs give dimers that vary in stability, affinity for their ligands and activation of different signalling-cascades [7].
There are several drugs, targeting either the extracellular domains or the intracellular tyrosine kinase domains of the HERs, that give survival benefits in selected cases of breast, colon, gastric and lung cancer [7], and combinations of HER-active drugs have been shown to further improve survival compared with single HER-therapy [8].
Expression of EGFR is common in pancreatic cancer, and has been associated with metastatic potential [9], but several studies have not found any prognostic effect of EGFR expression on overall survival (OS) [9–11]. A meta-analysis did however find a survival disadvantage in pancreatic cancer expressing EGFR [12]. Addition of the EGFR tyrosine kinase inhibitor erlotinib to gemcitabine lead to an increased OS in patients with advanced pancreatic cancer [13], the improvement was however modest and erlotinib is therefore rarely used for treatment of pancreatic cancer in clinical practice. Other EGFR active drugs have not led to a prolonged OS, when added to standard chemotherapy [7]. EGFR expression has also been shown to be more common in PB-type than in I-type ampullary adenocarcinoma [14] and overexpression has been associated with shorter OS in I-type but not in PB-type tumours [15].
The reported rates of HER2 overexpression in pancreatic cancer, defined as 3+ in immunohistochemical staining or gene amplification by in situ hybridization (ISH), vary from 0%-11% [16–23]. Similarly to other tumour types, high expression of HER2 in pancreatic cancer has been associated with a shorter survival [24], but other studies have found the opposite [25]. Addition of the HER2 antibody trastuzumab to gemcitabine in metastatic pancreatic cancer overexpressing HER2 (2+ or 3+) gave no clear survival benefit compared with the expected survival upon gemcitabine alone [26].
In tumours of the ampulla, distal bile duct and gall bladder, the frequency of HER2 overexpression has been low, and comparable to pancreatic cancer in a few small studies [16, 17, 23], whereas larger studies have found overexpression in 6–13% of ampullary tumours [27, 28], and 23% and 17% of tumours of the bile duct and gall bladder [29].
In ampullary adenocarcinoma, HER2 gene amplification has been equally distributed over morphological types, and amplified cases have been wild-type for KRAS, NRAS and BRAF. There has also been an equal distribution of 2+ and 3+ immunohistochemistry among ISH amplified cases [27].
In one previous study on patients with resected pancreatic cancer, high HER3 expression was denoted in 41% of cases, and was found to be associated with a shorter OS [30], which is in line with studies on several other types of adenocarcinoma [31]. However, high HER3 expression has also been found to correlate with a prolonged survival in colorectal cancer [32, 33], breast cancer [34] and in gastric and oesophageal cancer [35]. Anti-HER3 antibodies have been shown to reduce growth in pancreatic cancer cell lines that are wild-type for KRAS [36]. To the best of our knowledge, the expression and prognostic impact of HER3 has not been studied in the full spectrum of periampullary adenocarcinoma.
Thus, the prognostic role of HERs in periampullary adenocarcinoma has mostly been studied in pancreatic cancer, often with conflicting results, and less in I-type adenocarcinomas. The efficacy of treatments targeted at EGFR, HER2 and HER3 are not well studied in periampullary cancer, in particular in I-type tumours. Therefore, the aim of this study was to analyse the expression and prognostic significance of EGFR, HER2 and HER3, with particular reference to morphological subtype, in a retrospective, consecutive cohort of 175 cases with periampullary cancer.
Materials and Methods
Patients
The study cohort is a previously described retrospective consecutive series of pancreaticoduodenectomy specimens from all patients (n = 175) with periampullary adenocarcinoma, including pancreatic cancer, resected at the University hospitals of Lund and Malmö, Sweden, from January 1 2001 until December 31 2011 [37–41]. Data on survival were gathered from the Swedish National Civil Register. Follow-up started at the date of surgery and ended at death, at 5 years after surgery or at December 31 2013, whichever came first. Information on neoadjuvant and adjuvant treatment and recurrence was obtained from patient records. All haematoxylin & eosin stained slides from all cases were re-evaluated by one pathologist (JEL), blinded to the original report and outcome, as previously described, to get a uniform assessment of all histopathological parameters.
All EU and national regulations and requirements for handling human samples have been fully complied with during the conduct of this project; i.e. decision no. 1110/94/EC of the European Parliament and of the Council (OJL126 18,5,94), the Helsinki Declaration on ethical principles for medical research involving human subjects, and the EU Council Convention on human rights and Biomedicine. The study was approved of by the Ethics committee of Lund University (ref no 445/07), whereby the committee waived the need for consent other than by the option to opt out. All information from the patient records was anonymized and de-identified prior to analysis.
Tissue microarray construction
Tissue microarrays (TMAs) were constructed using a semi-automated arraying device (TMArrayer, Pathology Devices, Westminister, MD, USA). A standard set of three tissue cores (1 mm) were obtained from each of the 175 formalin fixated paraffin embedded primary tumours and from lymph node metastases from 105 of the cases, whereby one to three lymph node metastases were sampled in each case.
Immunohistochemistry, Silver In-Situ Hybridization and staining evaluation
All immunohistochemical staining and in situ hybridization (ISH) was performed on 4 μm TMA-sections. Immunohistochemistry for EGFR and HER3 was performed in an Autostainer Plus (Dako, Glostrup, Denmark) after automated pre-treatment with the PT-link system (Dako), using the monoclonal anti-EGFR antibody 31G7 (Zymed Laboratories Inc, San Francisco, CA, USA), diluted 1:25 and the monoclonal anti-HER3 antibody SP71 (Novus Biologicals LTD, Cambridge, UK), diluted 1:100, respectively. HER2 immunohistochemistry was performed on a BenchMark ULTRA instrument (Ventana Medical Systems, Inc. Tucson, AZ, USA). ULTRA Cell Conditioning (ULTRA CC1), pH9, was used for heat induced epitope retrieval (HIER). The monoclonal primary antibody PATHWAY anti-HER-2/neu (4B5), (Ventana Medical Systems, Inc.) was incubated for 20 minutes and the antibody-antigen complex was visualized with ultraView Universal DAB Detection kit (Ventana Medical Systems, Inc.)
HER2 ISH was also performed on the BenchMark ULTRA instrument (Ventana Medical Systems, Inc.). The peptide bonds were broken with ULTRA Cell Conditioning (ULTRA CC2), pH6, and ISH protease3. HER2 gene and Chromosome 17 (Chr17) were detected with INFORM HER2 Dual ISH DNA Probe Cocktail Assay (Ventana Medical Systems, Inc.). For visualization, ultraView SISH DNP Detection Kit and ultraView Red ISH DIG Detection Kit (Ventana Medical Systems, Inc.) were used, giving black and red chromogenic signals. As a final step, all slides were counterstained with haematoxylin.
The herein used anti-HER3 antibody has been validated by siRNA-mediated knockdown, immunocytochemistry and quantitative real-time PCR [35].
The immunohistochemical staining of EGFR, HER2 and HER3 was annotated by two independent observers (JEL and MH for EGFR, JEL and CFW for HER2/3) and consensus was reached in discordant cases.
EGFR, HER2 and HER3 protein expression was evaluated using the recommended protocol for HER2 testing in gastric and gastroesophageal junction cancer biopsies [42], taking complete, basolateral, or lateral membranous reactivity in a minimum of 5 clustered positive cancer cells into account, with the intensity recorded as 0, 1+, 2+ or 3+. Cytoplasmic staining was denoted as a separate category, but grouped with 1+ in the statistical analyses. Protein expression was grouped 0–2+ vs 3+, whereby 0–2+ was regarded as low expression and 3+ as high expression.
Assessment of ISH was performed according to the Ventana INFORM HER2 Dual ISH DNA Probe Cocktail Assay Interpretation Guide, and annotated by one pathologist (JEL). For each core 20 cancer cells were counted, and if the resulting HER2/Chr17 ratio fell within 1.5 and 2.5, another 20 cells were counted. A ratio above 2.0 was denoted as amplified. Assessment of HER2 ISH was only performed on cases that had either 2+ or 3+ immunohistochemical HER2 expression.
Statistical analysis
Chi square test was applied to analyse the relationship between the dichotomized expression of each biomarker and clinicopathological parameters. Two patients with PB-type adenocarcinomas who had received neoadjuvant chemotherapy were excluded from the correlation and survival analyses. Three additional patients were excluded from the survival analyses; two with I-type adenocarcinomas who died within one month from surgery due to complications and one with PB-type adenocarcinoma who emigrated 5 months after surgery.
Kaplan Meier estimates of 5-year RFS and OS and log rank test were applied to evaluate survival differences in strata according to high and low expression of each biomarker. For PB-type tumours, biomarker expression was also combined with given adjuvant treatment; gemcitabine vs none/other. To estimate the interaction between given adjuvant treatment and biomarker expression in relation to survival, the following interaction variable was constructed; gemcitabine-based adjuvant treatment (+/−) × biomarker (high/low). Hazard ratios (HR) and 95% confidence intervals (CI) for death and recurrence within 5 years were calculated by Cox regression proportional hazard’s modelling in unadjusted analysis and in a multivariable model adjusted for differentiation grade, T-stage, N-stage, perineural invasion, lymphatic invasion, vascular invasion, invasion in peripancreatic fat and adjuvant chemotherapy. A backward conditional method was used for variable selection in the adjusted model. The proportional hazards assumption was tested by examining log-log survival curves.
All tests were two sided. P-values <0.05 were considered significant, and no adjustments were made for the number of tests performed. All statistical analyses were performed using IBM SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Distribution of protein expression of EGFR, HER2 and HER3 and HER2 gene amplification
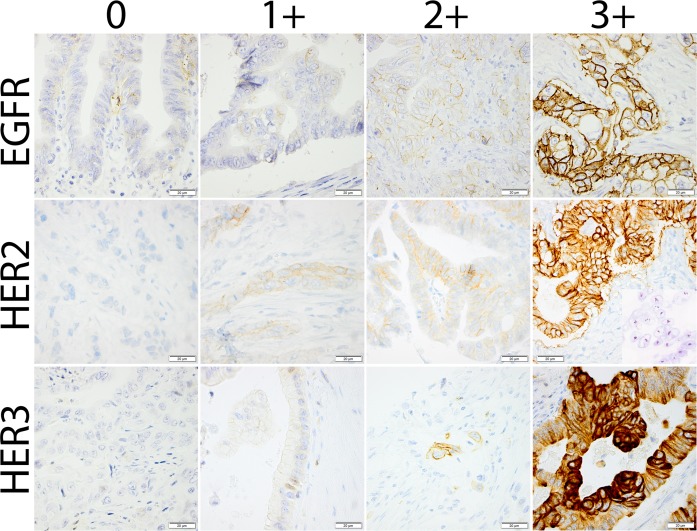
Examples of immunohistochemistry scores 0, 1+, 2+ and 3+ for EGFR, HER2 and HER3 expression and HER2 gene amplification by SISH are shown in Fig 1.
Fig 1. Sample immunohistochemical images.
Photomicrographs representing different categories of immunohistochemical staining for EGFR, HER2 and HER3, respectively. An image visualizing HER2 gene amplification by silver in situ hybridization is shown together with the HER2 3+ case.
The distribution of expression of EGFR, HER2 and HER3 in PB- and I-type primary tumours is shown in Table 1. For cases with 2+ or 3+ expression of HER2, the results of SISH for HER2 are also shown. The fraction of cases with 3+ expression differed significantly between PB-type and I-type tumours for HER3 (17% vs 51%, p<0.001), and for HER2 (0% vs 6%, p = 0.017), but not for EGFR. HER2 2+ expression was seen in 14% of both PB- and I-type tumours. SISH for HER2 failed in 46% of the cases, probably due to prolonged tissue fixation in formaldehyde. All assessable HER2 3+ cases showed amplification of HER2, as did one 2+ PB-type case of ampullary origin. In total, 7% (5/68) of ampullary adenocarcinoma and 8% (4/49) of I-type ampullary adenocarcinoma showed HER2 overexpression (either immunohistochemistry 3+ or SISH+). There were no cases with 3+ co-expression of all three HER family members.
Table 1. Expression of EGFR, HER2 and HER3, and amplification status for HER2 in pancreatobiliary and intestinal type periampullary adenocarcinoma.
| PB-type | I-type | All | ||
|---|---|---|---|---|
| n = 110 | n = 65 | n = 175 | ||
| Excluded due to neoadjuvant treatment | 2 | 0 | 2 | |
| EGFR IHC score | ||||
| 0 | 2 (2%) | 2 (3%) | 4 (2%) | |
| 1 | 18 (17%) | 16 (25%) | 34 (20%) | |
| 2 | 39 (36%) | 22 (35%) | 61 (36%) | |
| 3 | 48 (45%) | 23 (37%) | 71 (42%) | |
| Unassessable | 1 | 2 | 3 | |
| HER2 IHC score | ||||
| 0 | 48 (44%) | 27 (43%) | 75 (44%) | |
| 1 | 45 (42%) | 23 (37%) | 68 (40%) | |
| 2 | 15 (14%) | 9 (14%) | 24 (14%) | |
| 3 | 0 (0%) | 4 (6%) | 4 (2%) | |
| Unassessable | 0 | 2 | 2 | |
| HER2 SISH (for IHC 2/3+) | ||||
| Not amplified | 8 (53%) | 4 (31%) | 12 (43%) | |
| Amplified | 1 (7%) | 2 (15%) | 3 (11%) | |
| Failed SISH | 6 (40%) | 7 (54%) | 13 (46%) | |
| Not assessed | 93 | 52 | 145 | |
| HER3 IHC score | ||||
| 0 | 67 (63%) | 18 (29%) | 85 (50%) | |
| 1 | 18 (17%) | 12 (19%) | 30 (18%) | |
| 2 | 4 (4%) | 1 (2%) | 5 (3%) | |
| 3 | 18 (17%) | 32 (51%) | 50 (29%) | |
| Unassessable | 1 | 2 | 3 | |
IHC: immunohistochemistry. SISH: silver in situ hybridization. Due to rounding effects, percentages may not add up to 100.
Since there were no tumours with 3+ expression of HER2 in the PB-group, further analyses on associations and prognosis related to 3+ expression of HER2 in PB-type tumours could not be done.
In the full cohort there were 86 cases with evaluable stainings from both primary tumour and corresponding lymph node metastases. There were no significant differences in the proportion of 3+ expression of EGFR, HER2 or HER3 between primary tumours and corresponding metastases (data not shown).
Associations between EGFR, HER2 and HER3 protein expression and clinicopathological parameters
In PB-type adenocarcinomas there were no significant associations between 3+ expression of EGFR or HER3 and clinicopathological parameters (S1 Table).
In I-type adenocarcinomas, high EGFR expression was significantly associated with larger tumour size, but not with any other parameter. HER2 expression was not associated with any parameter. For HER3, there was an inverse association between high protein expression and tumour stage, perineural growth, blood vessel invasion, growth in peripancreatic fat and recurrence (S2 Table). When considering I-type adenocarcinomas of ampullary origin only, thus excluding duodenal origin, the associations remained significant for tumour stage (p<0.001), perineural growth (p = 0.002) and growth in peripancreatic fat (p<0.001) (data not shown).
Impact of EGFR, HER2 and HER3 expression on 5-year recurrence-free and overall survival
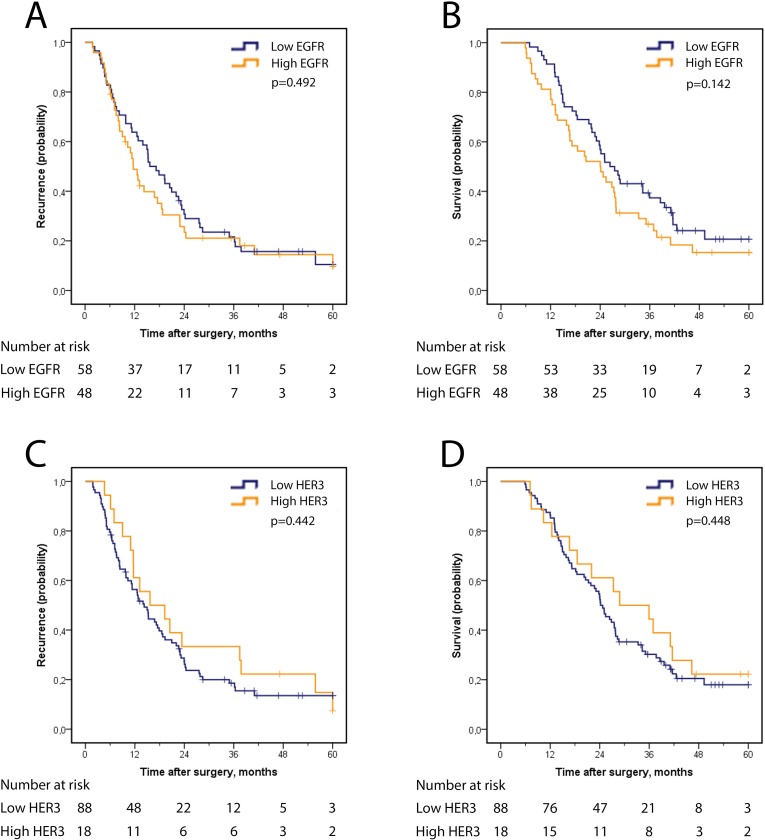
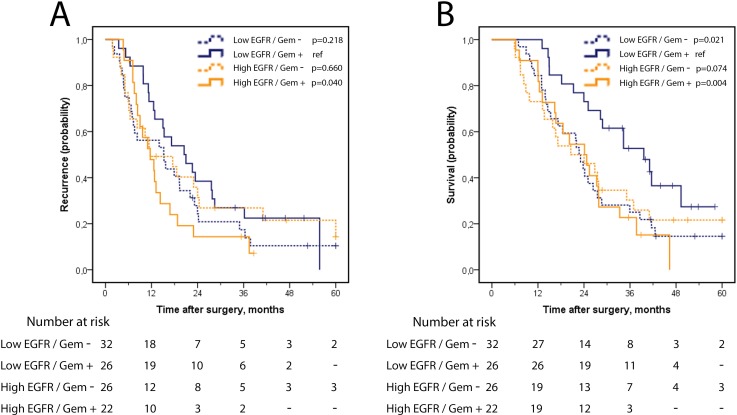
In the full group of PB-type cases, recurrence-free survival (RFS) and OS did not differ by expression of EGFR or HER3 (Fig 2A–2D). Analysis in strata according to adjuvant treatment, however, revealed a significantly reduced RFS and OS for patients that had received adjuvant gemcitabine and had tumours with high, as compared with low, EGFR expression (Fig 3A and 3B), whereas no survival difference was seen according to high or low EGFR expression among untreated PB-type cases (Fig 3). As further shown in Table 2, there was a significant treatment interaction between EGFR expression and adjuvant gemcitabine in relation to OS (pinteraction = 0.042), but not in relation to RFS. When considering only pancreatic tumour origin, a significantly shorter RFS (HR 2.47, 95% CI 1.02–6.00) and OS (HR 3.47, 95% CI 1.34–8.97) was seen in adjuvant gemcitabine treated cases with tumours displaying high EGFR expression, as compared with low EGFR expression, whereas no survival difference was seen according to high or low EGFR expression among cases not receiving adjuvant gemcitabine. There was however no significant interaction in relation to neither RFS, p(interaction) = 0.084 nor OS, p(interaction) = 0.160.
Fig 2. Recurrence-free and overall survival according to EGFR and HER3 expression in PB-type adenocarcinoma.
Kaplan Meier analysis of five-year recurrence-free survival in strata according to high and low expression of (A) EGFR and (C) HER3 and overall survival according to high and low expression of (B) EGFR and (D) HER3.
Fig 3. Recurrence-free and overall survival in strata according to EGFR expression and adjuvant gemcitabine in PB-type adenocarcinoma.
Kaplan Meier analysis of (A) five-year recurrence-free survival and (B) overall survival in combined strata according to EGFR expression (high/low) and adjuvant gemcitabine (yes/no).
Table 2. Cox proportional hazards analysis of the impact of EGFR expression on recurrence-free and overall survival in strata according to adjuvant gemcitabine in patients with PB-type adenocarcinoma.
| RFS | OS | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p(interaction) | HR (95% CI) | p(interaction) | ||
| Gem - | |||||
| Low EGFR (n = 32) | 1.00 | 1.00 | |||
| High EGFR (n = 26) | 0.84 (0.47–1.49) | 0.098 | 0.94 (0.53–1.68) | 0.042 | |
| Gem + | |||||
| Low EGFR (n = 26) | 1.00 | 1.00 | |||
| High EGFR (n = 22) | 1.93 (1.02–3.65) | 2.69 (1.34–5.42) | |||
Bold text indicates significant values
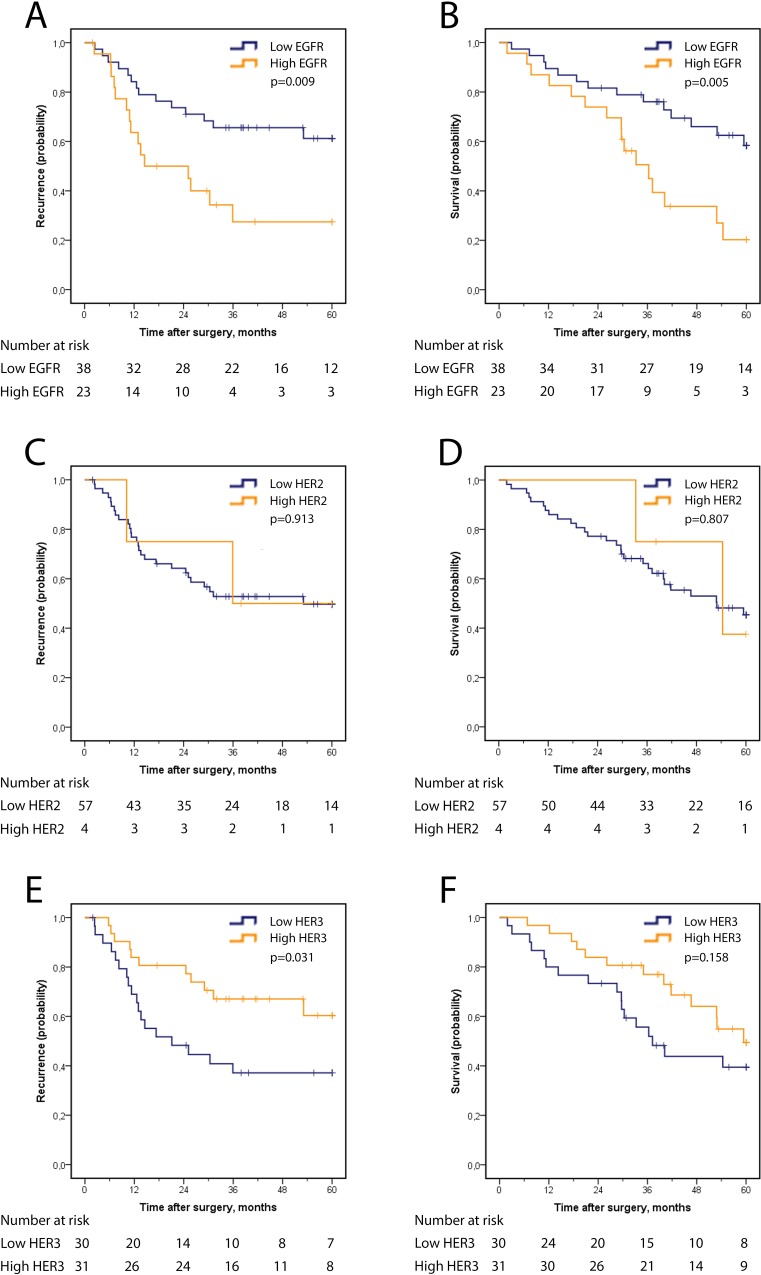
In I-type cases, Kaplan Meier analysis revealed a significantly shorter OS and RFS for cases with high EGFR expression (Fig 4A and 4B). Significance was retained in univariable Cox regression analysis for RFS (HR 2.58, 95% CI 1.23–5.38) and OS (HR 2.74, 95% CI 1.32–5.69), but not in multivariable analysis (Table 3). The prognostic value of EGFR expression did not differ according to adjuvant treatment in I-type tumours (data not shown), and there was no significant difference in RFS or OS between cases with high or low HER2 expression (Fig 4C and 4D and Table 3). There was a significant association between high HER3 expression and a longer RFS (p = 0.031), and significance was retained in univariable Cox regression analysis (HR 0.45, 95% CI 0.21–0.95), but not in multivariable analysis (Table 3). There was no significant association between HER3 expression and OS.
Fig 4. Recurrence-free and overall survival according to EGFR, HER2 and HER3 expression in I-type adenocarcinoma.
Kaplan Meier analysis of five-year recurrence-free survival in strata according to high and low expression of (A) EGFR, (C) HER2, (E) HER3 and overall survival according to high and low expression of (B) EGFR, (D) HER2, and (F) HER3.
Table 3. Cox proportional hazards analysis of the impact of EGFR, HER2 and HER3 expression on recurrence-free and overall survival in patients with I-type adenocarcinoma.
| RFS | OS | |||||
|---|---|---|---|---|---|---|
| n (events) | Univariable | Multivariable | n (events) | Univariable | Multivariable | |
| EGFR | ||||||
| Low | 38 (14) | 1.00 | 1.00 | 38 (14) | 1.00 | 1.00 |
| High | 23 (15) | 2.58 (1.23–5.38) | 1.80 (0.85–3.82) | 23 (16) | 2.74 (1.32–5.69) | 1.55 (0.68–3.53) |
| HER2 | ||||||
| Low | 57 (27) | 1.00 | 57 (28) | 1.00 | ||
| High | 4 (2) | 0.92 (0.22–3.89) | NI | 4 (2) | 0.84 (0.20–3.51) | NI |
| HER3 | ||||||
| Low | 30 (18) | 1.00 | 1.00 | 30 (17) | 1.00 | |
| High | 31 (11) | 0.45 (0.21–0.95) | 1.37 (0.52–3.62) | 31 (13) | 0.60 (0.29–1.23) | NI |
Multivariable analysis adjusted for tumour grade, N-stage (N0 vs N1), T-stage (T1-2 vs T3-4), perineural growth, lymphatic invasion, blood vessel invasion, invasion of perineural fat and adjuvant treatment. NI, not investigated. Bold text indicates significant values.
Discussion
In this study, we have evaluated the prognostic impact of EGFR, HER2 and HER3 in periampullary adenocarcinoma, by morphological type and adjuvant treatment. In intestinal-type adenocarcinoma, we found that high HER3 expression was a favourable prognostic factor and that high EGFR expression was an adverse prognostic factor, although none of these associations were independent of other prognostic factors. In addition, in pancreatobiliary-type tumours, EGFR expression was found to be an adverse prognostic factor only in cases that received adjuvant gemcitabine, and a positive effect of gemcitabine was only seen in cases with low EGFR expression, with a significant interaction between EGFR expression and adjuvant gemcitabine in relation to overall survival. HER2 expression was not prognostic, neither in intestinal-type, nor in pancreatobiliary-type tumours.
Our results regarding the expression of EGFR, HER2 and HER3 are comparable to previously published results, with the possible exception of EGFR expression in I-type adenocarcinomas, where we found 3+ expression in 38% of the cases, compared with 4% expression [14] and 19% 3+ expression [15] in other studies. In addition, we were not able to demonstrate the previously described association between HER2 and HER3 expression. Our results regarding the adverse prognostic effect of EGFR in the entire group of intestinal-type, but not in pancreatobiliary type, adenocarcinoma confirm the results of Xia et al [15], and further underscore the biological differences between pancreatobiliary and intestinal type periampullary adenocarcinomas. Our finding of a positive effect of gemcitabine only in cases with low EGFR expression has to our knowledge not been shown in these tumour types before. This finding is however compatible with the described increase in EGFR expression in colon cancer cell lines when resistance to chemotherapy was induced [43], and a better response to chemoradiotherapy in esophageal squamous cell carcinoma displaying low EGFR expression [44]. Our finding thus suggests that gemcitabine has a limited or no effect on survival in pancreatobiliary type tumours with high expression of EGFR. From a mechanistic viewpoint, inhibition of EGFR could theoretically seem like an attractive treatment option in these patients. However, immunohistochemical assessment of EGFR expression has not been a good predictor of response to the EGFR antibodies cetuximab or panitumumab in colorectal cancer, compared with EGFR copy number and KRAS mutation analysis [45–48]. Expression of EGFR also failed to predict response to the EGFR tyrosine kinase inhibitor erlotinib, when added to gemcitabine in the NCIC CTG PA.3 trial, in patients with locally advanced or metastatic pancreatic cancer [13]. Although the herein studied cohort contains both adjuvant treated and untreated patients, thus enabling identification of potential predictive biomarkers, firm conclusions on treatment prediction should not be drawn, given the retrospective character of the cohort. Another caveat is that several tests have been made in the present study, which increases the risk for type I errors, i.e. detecting a difference that is coincidental.
Another possible limitation to the present study is the use of tissue microarrays, whereby the issue of representativity in relation to whole tissue sections may always be raised. One should however bear in mind that whole tissue sections also represent only a minor part of the tumour, and that the tissue microarray technique allows sampling from different regions in different tissue blocks, thus enabling detection of heterogeneous expression. With a few exceptions [49], the tissue microarray method accurately reflects the expression of different proteins, and is a well-validated platform for studies of biomarkers [50].
Our results on the incidence of HER2 overexpression (3+ and/or ISH amplification) are well in line with previous results, with a low frequency in pancreatobiliary-type tumours or pancreatic cancer, and a higher frequency in I-type adenocarcinomas, although the latter is lower than in gastric cancer [42]. The HER2-HER3 homodimer is a powerful activator of the PI3K/Akt pathway [51], causing aberrant proliferative and antiapoptotic intracellular signals [52]. Inhibition of HER2 activity, however, causes upregulation of HER3, and simultaneous blockage of HER2 and HER3 activity gives a more potent inhibition of HER2 dependent oncogenic features than blockage of one receptor alone [53]. In line with those findings there is one case report of second line therapy with the antibodies trastuzumab and pertuzumab, thus inhibiting both HER2 and its dimerization, for HER2 overexpressing metastatic ampullary cancer, describing stable disease, with some shrinkage of metastases, and a longer survival than expected upon standard chemotherapy [54]. Blockage of HER3 activity is thus of interest, and clinical trials with the HER3 antibody patritumab are ongoing [55, 56].
Our finding of a longer recurrence-free survival in I-type cases with high HER3 expression is unexpected, given the oncogenic features of HER3, and in contrast with previous reports on various non-gastrointestinal cancers [31], one on colon cancer [57], and two on gastric cancer [58, 59], but harmonize with a few studies on breast [34], colorectal [32, 33], and gastric and oesophageal cancer [35]. A possible explanation for our finding could be that high HER3 expression reflects a less proliferative tumour, which is in line with the described expression of HER3 in non-proliferating parts of colon epithelium and colon cancer [60]. Another explanation for the diverging results regarding the prognostic effect of HER3 could also be the use of different antibodies, and algorithms for assessing the expression. The antibody used in the present study is however well validated [35], and we have used the well-known protocol for assessing HER2-immunohistochemistry in biopsies of gastric cancer, to make the annotation easily reproducible.
In the current study, the least studied HER family member, HER4, was not included, but given the complex network of signalling pathways that combinations of HER dimers and ligands can activate, it is not unlikely that expression of HER4 may have prognostic or predictive implications in periampullary adenocarcinoma.
In summary, the results from the present study demonstrate that high EGFR expression is an unfavourable prognostic factor in in gemcitabine treated pancreatobiliary type adenocarcinoma. The finding of a potential interaction between EGFR expression and response to adjuvant gemcitabine in pancreatobiliary type tumours is novel and of potential clinical relevance, and therefore merits confirmation and further study, both in a mechanistic context as well as in additional patient cohorts. EGFR expression was also an unfavourable prognostic factor, although not independent from other factors, in intestinal type tumours. Expression of HER3 was found to differ between pancreatobiliary and intestinal type adenocarcinomas and to be a favourable prognostic factor, however not independent, in intestinal type adenocarcinoma. Overexpression of HER2 was observed in 8% of intestinal type ampullary adenocarcinoma, and was not associated with prognosis. It is feasible that further steps towards individualized therapy in periampullary adenocarcinoma will involve simultaneous targeting of several members of the HER family.
Supporting Information
M, median. IQR, interquartile range.
(DOCX)
M, median. IQR, interquartile range. Bold text indicates significant values.
(DOCX)
Acknowledgments
This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, the Gunnar Nilsson Cancer Foundation, the Mrs Berta Kamprad Foundation, the Swedish Government Grant for Clinical Research, Lund University Faculty of Medicine and University Hospital Research Grants.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, the Gunnar Nilsson Cancer Foundation, the Mrs Berta Kamprad Foundation, the Swedish Government Grant for Clinical Research, Lund University Faculty of Medicine and University Hospital Research Grants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. 10.3322/caac.21254 . [DOI] [PubMed] [Google Scholar]
- 2.Engholm G, Ferlay J, Christensen N, Kejs AMT, Johannesen TB, Khan S, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.1 (09.07.2015).: Association of the Nordic Cancer Registries. Danish Cancer Society; 2015. Available: http://www.ancr.nu. [Google Scholar]
- 3.Carpelan-Holmstrom M, Nordling S, Pukkala E, Sankila R, Luttges J, Kloppel G, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005;54(3):385–7. 10.1136/gut.2004.047191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170 10.1186/1471-2407-8-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westgaard A, Pomianowska E, Clausen OP, Gladhaug IP. Intestinal-type and pancreatobiliary-type adenocarcinomas: how does ampullary carcinoma differ from other periampullary malignancies? Ann Surg Oncol. 2013;20(2):430–9. 10.1245/s10434-012-2603-0 . [DOI] [PubMed] [Google Scholar]
- 6.Bronsert P, Kohler I, Werner M, Makowiec F, Kuesters S, Hoeppner J, et al. Intestinal-type of differentiation predicts favourable overall survival: confirmatory clinicopathological analysis of 198 periampullary adenocarcinomas of pancreatic, biliary, ampullary and duodenal origin. BMC Cancer. 2013;13:428 10.1186/1471-2407-13-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roskoski R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. 10.1016/j.phrs.2013.11.002 . [DOI] [PubMed] [Google Scholar]
- 8.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. 10.1056/NEJMoa1413513 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y, et al. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int J Mol Med. 2003;11(3):305–9. . [PubMed] [Google Scholar]
- 10.Uegaki K, Nio Y, Inoue Y, Minari Y, Sato Y, Song MM, et al. Clinicopathological significance of epidermal growth factor and its receptor in human pancreatic cancer. Anticancer Res. 1997;17(5B):3841–7. . [PubMed] [Google Scholar]
- 11.Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23(1–2):74–9. 10.1159/000093497 . [DOI] [PubMed] [Google Scholar]
- 12.Luo G, Long J, Qiu L, Liu C, Xu J, Yu X. Role of epidermal growth factor receptor expression on patient survival in pancreatic cancer: a meta-analysis. Pancreatology. 2011;11(6):595–600. 10.1159/000334465 . [DOI] [PubMed] [Google Scholar]
- 13.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. 10.1200/JCO.2006.07.9525 . [DOI] [PubMed] [Google Scholar]
- 14.Mikhitarian K, Pollen M, Zhao Z, Shyr Y, Merchant NB, Parikh A, et al. Epidermal growth factor receptor signaling pathway is frequently altered in ampullary carcinoma at protein and genetic levels. Mod Pathol. 2014;27(5):665–74. 10.1038/modpathol.2013.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia M, Overman MJ, Rashid A, Chatterjee D, Wang H, Katz MH, et al. Expression and clinical significance of epidermal growth factor receptor and insulin-like growth factor receptor 1 in patients with ampullary adenocarcinoma. Hum Pathol. 2015;46(9):1315–22. 10.1016/j.humpath.2015.05.012 . [DOI] [PubMed] [Google Scholar]
- 16.Aloysius MM, Lobo DN, Rowlands BJ, Madhusudan S, Ilyas M, Zaitoun AM. HER-2/Neu overexpression is a rare event in peri-ampullary cancer: assessment using the HercepTest. Histopathology. 2009;55(2):236–7. 10.1111/j.1365-2559.2009.03351.x . [DOI] [PubMed] [Google Scholar]
- 17.Koeppen HK, Wright BD, Burt AD, Quirke P, McNicol AM, Dybdal NO, et al. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology. 2001;38(2):96–104. . [DOI] [PubMed] [Google Scholar]
- 18.Safran H, Steinhoff M, Mangray S, Rathore R, King TC, Chai L, et al. Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am J Clin Oncol. 2001;24(5):496–9. . [DOI] [PubMed] [Google Scholar]
- 19.Chou A, Waddell N, Cowley MJ, Gill AJ, Chang DK, Patch AM, et al. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 2013;5(8):78 10.1186/gm482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potti A, Ganti AK, Tendulkar K, Chitajallu S, Sholes K, Koch M, et al. HER-2/neu and CD117 (C-kit) overexpression in hepatocellular and pancreatic carcinoma. Anticancer Res. 2003;23(3B):2671–4. . [PubMed] [Google Scholar]
- 21.Stoecklein NH, Luebke AM, Erbersdobler A, Knoefel WT, Schraut W, Verde PE, et al. Copy number of chromosome 17 but not HER2 amplification predicts clinical outcome of patients with pancreatic ductal adenocarcinoma. J Clin Oncol. 2004;22(23):4737–45. 10.1200/JCO.2004.05.142 . [DOI] [PubMed] [Google Scholar]
- 22.Day JD, Digiuseppe JA, Yeo C, Lai-Goldman M, Anderson SM, Goodman SN, et al. Immunohistochemical evaluation of HER-2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol. 1996;27(2):119–24. . [DOI] [PubMed] [Google Scholar]
- 23.Ata A, Polat A, Serinsoz E, Sungur MA, Arican A. Prognostic value of increased HER2 expression in cancers of pancreas and biliary tree. Pathol Oncol Res. 2015;21(3):831–8. 10.1007/s12253-014-9847-x . [DOI] [PubMed] [Google Scholar]
- 24.Komoto M, Nakata B, Amano R, Yamada N, Yashiro M, Ohira M, et al. HER2 overexpression correlates with survival after curative resection of pancreatic cancer. Cancer Sci. 2009;100(7):1243–7. 10.1111/j.1349-7006.2009.01176.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koka V, Potti A, Koch M, Fraiman G, Mehdi S, Levitt R. Role of immunohistochemical identification of Her-2/neu and detection of variability in overexpression in pancreatic carcinoma. Anticancer Res. 2002;22(3):1593–7. . [PubMed] [Google Scholar]
- 26.Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C, et al. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22(5):706–12. . [DOI] [PubMed] [Google Scholar]
- 27.Hechtman JF, Liu W, Sadowska J, Zhen L, Borsu L, Arcila ME, et al. Sequencing of 279 cancer genes in ampullary carcinoma reveals trends relating to histologic subtypes and frequent amplification and overexpression of ERBB2 (HER2). Mod Pathol. 2015;28(8):1123–9. 10.1038/modpathol.2015.57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumhoer D, Zlobec I, Tornillo L, Dietmaier W, Wuensch PH, Hartmann A, et al. Immunophenotyping and oncogene amplifications in tumors of the papilla of Vater. Virchows Arch. 2008;453(6):579–88. 10.1007/s00428-008-0669-7 . [DOI] [PubMed] [Google Scholar]
- 29.Kawamoto T, Ishige K, Thomas M, Yamashita-Kashima Y, Shu S, Ishikura N, et al. Overexpression and gene amplification of EGFR, HER2, and HER3 in biliary tract carcinomas, and the possibility for therapy with the HER2-targeting antibody pertuzumab. J Gastroenterol. 2015;50(4):467–79. 10.1007/s00535-014-0984-5 . [DOI] [PubMed] [Google Scholar]
- 30.Hirakawa T, Nakata B, Amano R, Kimura K, Shimizu S, Ohira G, et al. HER3 overexpression as an independent indicator of poor prognosis for patients with curatively resected pancreatic cancer. Oncology. 2011;81(3–4):192–8. 10.1159/000333825 . [DOI] [PubMed] [Google Scholar]
- 31.Ocana A, Vera-Badillo F, Seruga B, Templeton A, Pandiella A, Amir E. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst. 2013;105(4):266–73. 10.1093/jnci/djs501 . [DOI] [PubMed] [Google Scholar]
- 32.Baiocchi G, Lopes A, Coudry RA, Rossi BM, Soares FA, Aguiar S, et al. ErbB family immunohistochemical expression in colorectal cancer patients with higher risk of recurrence after radical surgery. Int J Colorectal Dis. 2009;24(9):1059–68. 10.1007/s00384-009-0702-6 . [DOI] [PubMed] [Google Scholar]
- 33.Seo AN, Kwak Y, Kim WH, Kim DW, Kang SB, Choe G, et al. HER3 protein expression in relation to HER2 positivity in patients with primary colorectal cancer: clinical relevance and prognostic value. Virchows Arch. 2015;466(6):645–54. 10.1007/s00428-015-1747-2 . [DOI] [PubMed] [Google Scholar]
- 34.Quinn CM, Ostrowski JL, Lane SA, Loney DP, Teasdale J, Benson FA. c-erbB-3 protein expression in human breast cancer: comparison with other tumour variables and survival. Histopathology. 1994;25(3):247–52. . [DOI] [PubMed] [Google Scholar]
- 35.Hedner C, Borg D, Nodin B, Karnevi E, Jirstrom K, Eberhard J. Expression and Prognostic Significance of Human Epidermal Growth Factor Receptors 1 and 3 in Gastric and Esophageal Adenocarcinoma. PLoS One. 2016;11(2):e0148101 10.1371/journal.pone.0148101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazrek Y, Dubreuil O, Garambois V, Gaborit N, Larbouret C, Le Clorennec C, et al. Anti-HER3 domain 1 and 3 antibodies reduce tumor growth by hindering HER2/HER3 dimerization and AKT-induced MDM2, XIAP, and FoxO1 phosphorylation. Neoplasia. 2013;15(3):335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elebro J, Jirstrom K. Use of a standardized diagnostic approach improves the prognostic information of histopathologic factors in pancreatic and periampullary adenocarcinoma. Diagn Pathol. 2014;9(1):80 10.1186/1746-1596-9-80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elebro J, Heby M, Gaber A, Nodin B, Jonsson L, Fristedt R, et al. Prognostic and treatment predictive significance of SATB1 and SATB2 expression in pancreatic and periampullary adenocarcinoma. J Transl Med. 2014;12(1):289 10.1186/s12967-014-0289-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elebro J, Ben Dror L, Heby M, Nodin B, Jirstrom K, Eberhard J. Prognostic effect of hENT1, dCK and HuR expression by morphological type in periampullary adenocarcinoma, including pancreatic cancer. Acta Oncol. 2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fristedt R, Elebro J, Gaber A, Jonsson L, Heby M, Yudina Y, et al. Reduced expression of the polymeric immunoglobulin receptor in pancreatic and periampullary adenocarcinoma signifies tumour progression and poor prognosis. PLoS One. 2014;9(11):e112728 10.1371/journal.pone.0112728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heby M, Elebro J, Nodin B, Jirstrom K, Eberhard J. Prognostic and predictive significance of podocalyxin-like protein expression in pancreatic and periampullary adenocarcinoma. BMC Clin Pathol. 2015;15:10 10.1186/s12907-015-0009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25(5):637–50. 10.1038/modpathol.2011.198 . [DOI] [PubMed] [Google Scholar]
- 43.Ekblad L, Johnsson A. Cetuximab sensitivity associated with oxaliplatin resistance in colorectal cancer. Anticancer Res. 2012;32(3):783–6. . [PubMed] [Google Scholar]
- 44.Hickey K, Grehan D, Reid IM, O'Briain S, Walsh TN, Hennessy TP. Expression of epidermal growth factor receptor and proliferating cell nuclear antigen predicts response of esophageal squamous cell carcinoma to chemoradiotherapy. Cancer. 1994;74(6):1693–8. . [DOI] [PubMed] [Google Scholar]
- 45.Cappuzzo F, Finocchiaro G, Rossi E, Janne PA, Carnaghi C, Calandri C, et al. EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients. Ann Oncol. 2008;19(4):717–23. 10.1093/annonc/mdm492 . [DOI] [PubMed] [Google Scholar]
- 46.Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6(5):279–86. 10.1016/S1470-2045(05)70102-9 . [DOI] [PubMed] [Google Scholar]
- 47.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23(9):1803–10. 10.1200/JCO.2005.08.037 . [DOI] [PubMed] [Google Scholar]
- 48.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–5. 10.1158/0008-5472.CAN-06-0191 . [DOI] [PubMed] [Google Scholar]
- 49.Linderoth J, Ehinger M, Akerman M, Cavallin-Stahl E, Enblad G, Erlanson M, et al. Tissue microarray is inappropriate for analysis of BCL6 expression in diffuse large B-cell lymphoma. Eur J Haematol. 2007;79(2):146–9. 10.1111/j.1600-0609.2007.00892.x . [DOI] [PubMed] [Google Scholar]
- 50.Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Kochli OR, et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol. 2001;159(6):2249–56. 10.1016/S0002-9440(10)63075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soltoff SP, Carraway KL 3rd, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14(6):3550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17(3):590–603. 10.1038/sj.leu.2402824 . [DOI] [PubMed] [Google Scholar]
- 53.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108(12):5021–6. 10.1073/pnas.1016140108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Hayer K, Farber J, Yeo CJ, Sama AR. HER-2-Positive Ampullary Adenocarcinoma: A Case Report. Case Reports in Pancreatic Cancer. 2015;1(1):7–10. 10.1089/crpc.2015.29004.koh [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LoRusso P, Janne PA, Oliveira M, Rizvi N, Malburg L, Keedy V, et al. Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2013;19(11):3078–87. 10.1158/1078-0432.CCR-12-3051 . [DOI] [PubMed] [Google Scholar]
- 56.Nishio M, Horiike A, Murakami H, Yamamoto N, Kaneda H, Nakagawa K, et al. Phase I study of the HER3-targeted antibody patritumab (U3-1287) combined with erlotinib in Japanese patients with non-small cell lung cancer. Lung Cancer. 2015;88(3):275–81. 10.1016/j.lungcan.2015.03.010 . [DOI] [PubMed] [Google Scholar]
- 57.Beji A, Horst D, Engel J, Kirchner T, Ullrich A. Toward the prognostic significance and therapeutic potential of HER3 receptor tyrosine kinase in human colon cancer. Clin Cancer Res. 2012;18(4):956–68. 10.1158/1078-0432.CCR-11-1186 . [DOI] [PubMed] [Google Scholar]
- 58.Hayashi M, Inokuchi M, Takagi Y, Yamada H, Kojima K, Kumagai J, et al. High expression of HER3 is associated with a decreased survival in gastric cancer. Clin Cancer Res. 2008;14(23):7843–9. 10.1158/1078-0432.CCR-08-1064 . [DOI] [PubMed] [Google Scholar]
- 59.Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL Jr., et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29(22):3030–6. 10.1200/JCO.2010.33.6313 . [DOI] [PubMed] [Google Scholar]
- 60.Jarde T, Kass L, Staples M, Lescesen H, Carne P, Oliva K, et al. ERBB3 Positively Correlates with Intestinal Stem Cell Markers but Marks a Distinct Non Proliferative Cell Population in Colorectal Cancer. PLoS One. 2015;10(9):e0138336 10.1371/journal.pone.0138336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
M, median. IQR, interquartile range.
(DOCX)
M, median. IQR, interquartile range. Bold text indicates significant values.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.