Abstract
Nonalcoholic fatty liver disease (NAFLD) is the liver disease associated with obesity, diabetes and the metabolic syndrome. Although steatosis is a key histological feature, liver biopsies of patients with NAFLD can show a wide range of findings, including portal and lobular inflammation, ballooning and apoptotic hepatocellular injury, Mallory-Denk bodies, megamitochondria and fibrosis. Nonalcoholic steatohepatitis (NASH) is a progressive subtype of NAFLD first defined by analogy to alcoholic hepatitis. The characteristic finding in NASH is ballooning hepatocellular injury, often accompanied by Mallory-Denk bodies and some degree of fibrosis. Young children may have an alternate pattern of progressive NAFLD characterized by a zone 1 distribution of steatosis, inflammation and fibrosis. Several grading and staging systems exist for use in natural history studies and clinical trials, but all require adequate biopsies to minimize errors due to under sampling. Some information about the short term natural history is available from paired biopsy studies. These studies have demonstrated that while NASH generally shows fibrosis progression over time, some patients will show regression of disease.
Keywords: Steatohepatitis, Liver Biopsy, Steatosis, Histology, Scoring, Staging
Introduction
Non-alcoholic fatty liver disease (NAFLD) and its more severe subtype, non-alcoholic steatohepatitis (NASH), are steatotic liver diseases that develop in the absence of significant alcohol use. NAFLD is most often associated with obesity, specifically central obesity, insulin resistance, and other insulin resistance syndromes, type II diabetes, and hyperlipidemia. Because of these associations, NAFLD is now recognized as the hepatic manifestation of the metabolic syndrome. It demonstrates a spectrum of liver disease characterized by the accumulation of lipid, mainly in the form of macrovesicular steatosis. The histological manifestations range from mild steatosis in only 5% of hepatocytes to more severe forms with both lobular and/or portal inflammation, ballooning hepatocyte injury, and fibrosis in varying patterns of distribution to the end stage of cirrhosis.1 As with other chronic liver diseases, patients are at risk to develop hepatocellular or other primary liver carcinomas.2
There is a growing recognition of NAFLD as a serious disease. Even with the most conservative estimates NAFLD is very common in the US and probably is more common than hepatitis C and alcoholic liver disease and certainly significantly more prevalent in patients who are obese or diabetics. The prevalence of NASH is higher than previously estimated and is 5- to 6-fold higher than the estimated prevalence of chronic hepatitis C3,4. The prevalence of NAFLD has been estimated to range from 2.8% to 46% throughout the world depending on the study population and the diagnostic tool used to verify NAFLD (eg, serology, imaging, liver biopsy).4,5
Prevalence studies of NASH, the most clinically relevant subset of patients with NAFLD, depend on histologic evaluation. In an autopsy study from the late 1980s, the prevalence of steatohepatitis was significantly different between the markedly obese patients (18.5%) and the lean patients (2.7%).6 In Marchesini's study of well characterized NAFLD, 163 of 304 underwent liver biopsy and of those, 74% had NASH.7 More recently, Dr. Stephen Harrison and his team evaluated the prevalence of NAFLD and NASH in healthy US adults via ultrasound followed by liver biopsy. Of 328 patients who completed an ultrasound and questionnaire, 156 (48%) had at least 5% steatosis on ultrasound. And nearly 30% of patients with NAFLD had NASH on histologic review. Moreover, 9 (3%) of the NASH patients had significant fibrosis (more than stage 1). These patients were found to be more insulin resistant than those with mild fibrosis.4
Nonetheless, it remains challenging to estimate the true prevalence of a disease for which there is no serologic confirmatory tests and a liver biopsy is required for definite diagnosis.
Importance of liver biopsy in NAFLD
The presumptive diagnosis of NAFLD is often made in the setting of persistently elevated serum aminotransferases with a positive imaging study (often ultrasound), no history of significant alcohol use, and absence of other congenital or chronic liver diseases. However, 4 to 5% of patients with other chronic liver diseases may have NASH8 and autoantibodies may be present in significant titers in 20% of NAFLD patients.9,10 In order to correctly classify the liver disease and exclude other coincident liver diseases, a liver biopsy is required.1,11,12 While a liver biopsy can provide a wealth of information about the state of the liver, is it not practical to try to distinguish NAFLD from alcoholic liver disease (ALD) by histopathological examination only.13
A number of retrospective and prospective studies have suggested that simple steatosis without inflammation carries a benign prognosis while those with the lesions of NASH are more likely to progress to advanced fibrosis and cirrhosis.14,15 NASH has also been the focus of clinical trials1. It is therefore important to try to distinguish those with steatosis alone from those with NASH. Steatosis can be detected by imaging, using ultrasound, computed tomography or magnetic resonance imaging (MRI). Modern MRI methods can detect a fat fraction as low as 5%, making it useful for detecting clinically relevant steatosis.16 However, the distinction between steatosis and steatohepatitis cannot be made by current imaging methods nor can imaging detect the lobular arrangement of steatosis, which is useful to distinguish the pediatric pattern of NAFLD (described below).17 Non-invasive serological markers have had limited success at making the distinction between steatosis alone and NASH, although they may have value as screening tests to identify patients unlikely to have NASH.18,19 Use of liver enzyme tests alone can lead to overdiagnosis of NASH. Serum aminotransferases correlate poorly with histological activity. For example, in one early study NASH was incorrectly diagnosed by clinical tests alone prior to biopsy in 17% (4/24) of the cases studies by Sorbi et al.20 In this study, liver biopsy changed the diagnosis in 4 patients with suspected NASH; 3 had normal histology and the fourth had primary sclerosing cholangitis (later confirmed by imaging). Normal levels of aminotransferases are also no guarantee that significant disease is absent. Some fraction of patients with metabolic syndrome who have features of NAFLD or NASH including fibrosis and cirrhosis will have aminotransferases within the normal range; thus these patients might not be identified by serologic screening tools.20-22 A liver biopsy remains the only diagnostic tool that can distinguish steatosis from steatohepatitis, grade the severity of the liver disease and correctly classify cases with more than one diagnosis.
Pathology of NAFLD and NASH
The term NAFLD is typically used to refer to the whole constellation of nonalcoholic steatotic disease, while NASH represents a histologically specific pattern of liver disease. NASH may be identified in the presence of other chronic liver diseases8, while if only steatosis is present, it may not be related to the usual metabolic causes of NAFLD23. NASH is the most common form of histologically progressed NAFLD and is almost always associated with some degree of fibrosis. It is unclear whether patients with steatosis but without NASH can progress to cirrhosis, although children with the pediatric pattern of NAFLD (discussed below) can develop advanced fibrosis without the typical features of NASH.
By definition, NAFLD has steatosis which is more than 5%.1 The presence of less than 5% of steatosis is not regarded as clinically significant. This degree of steatosis is present in all cases of NASH with some exceptions, and these include cases with advanced fibrosis/cirrhosis, and rare cases of steatohepatitis which are due to drug exposure to amiodarone. Steatosis from patients with NASH is most often of the macrovesicular type with both large droplets of fat that push the nucleus aside and small droplets of fat that leave the nucleus in the center of the cell. However, in both alcoholic and nonalcoholic FLD, small patches of microvesicular steatosis may also be present (Figure 1). Diffuse microvesicular steatosis is not a feature of NAFLD but rather a manifestation of acute liver injury resulting from severe acute mitochondrial injury due to variety of etiologies, including alcohol, drugs (tetracycline, zidovudine, cocaine, valproic acid, aspirin (Reyes' syndrome)), acute fatty liver of pregnancy, and rare metabolic disorders like carnitine deficiency. In most cases, the steatosis is mild to moderate in degree but may vary from minimal to marked.18 The distribution of steatosis within the hepatic acinus is characteristic; steatosis is most intense around the central veins (predominantly in zones 2 and 3) and the periportal areas are often spared in early disease.24,25
Figure 1.

Patterns of steatosis in NAFLD. A. Zone 3 center steatosis with sparing of periportal areas (40×). B. Zone 1 periportal steatosis with sparing of central vein region (100×). C. A patch of microvesicular steatosis in a case of NASH (600×).
The inflammatory changes in steatohepatitis are nonspecific and variable but are usually mild in comparison to chronic hepatitis or chronic cholestatic liver disease. Some degree of lobular inflammation is common in NAFLD (Figure 2). It is typically mild mixed inflammation but is nearly always present in an adequate needle liver biopsy. Clusters of mononuclear cells including T cells and macrophages infiltrate into hepatocyte plates. Microgranulomas are a common finding. Scattered apoptotic bodies/acidophil bodies are common but are not a conspicuous finding. Neutrophils may surround the ballooned hepatocytes, especially cells that have Mallory-Deck Bodies (MDBs) in a pattern called satellitosis. Severe, confluent inflammation or bridging necrosis is not a feature of steatohepatitis and should make one consider other diseases such as autoimmune hepatitis or drug injury. Mild portal inflammation and mild ductular reaction are more common in NASH than in cases of steatosis alone18. Interface hepatitis when present, it is focal and mild, but as with bridging necrosis, significant interface hepatitis should make one think of other chronic inflammatory liver diseases. Although, portal inflammation is not a diagnostic criterion of NAFLD, it is reported to be associated with clinical and histological features of advanced disease26,27. As the amount of fibrosis increases in steatohepatitis, more inflammation is observed in portal areas or within fibrous bands, so that patients who are approaching cirrhosis will have more inflammation in their fibrous tracts more than patients who are early in their disease. However, when chronic portal inflammation is moderate or marked, one should consider excluding other chronic liver diseases. The presence of lymphoid aggregates, as in hepatitis C, numerous plasma cells, as in autoimmune hepatitis, and bile duct injury as in primary biliary cirrhosis is very unusual in NASH. Portal inflammation may persist or even increase after surgical or drug treatment for NASH, even as steatohepatitis resolves.28-30
Figure 2.

Inflammatory infiltrates in NAFLD. A. Lobular inflammation in NASH. A microgranuloma is seen to the right of the small lymphocytic infiltrate. (600×). B. Portal inflammation is mild and may focally involve the limiting plate. (400×)
The diagnostic feature of NASH is zone 3 hepatocellular injury. It takes the form of hepatocellular ballooning, with or without MDBs (Figure 3). The ballooned hepatocytes are generally larger than the surrounding hepatocytes and have distinctive rarified cytoplasm that is irregularly stranded and clumped. There is some variation in this histological appearance and cells with features that fall between classic ballooned hepatocytes and normal cells may be identified. Some investigators have used the loss of staining for keratin 8 and 18 as a useful marker of ballooning.31 Ballooning is not typically seen in young children, even those with fibrosis. The significance of ballooning was shown by the Cleveland Clinic study in which patients were subdivided in 4 types depending on histologic findings: types 1 and 2 had steatosis without or with inflammation, while types 3 and 4 there added ballooning and other features associated with the diagnosis of NASH.14 Cirrhosis developed more frequently in types 3 and 4. A follow-up study using the same patient cohort demonstrated that ballooning and MDBs were most closely associated with perivenular and perisinusoidal fibrosis in multivariable analysis of multiple histological features.32
Figure 3.
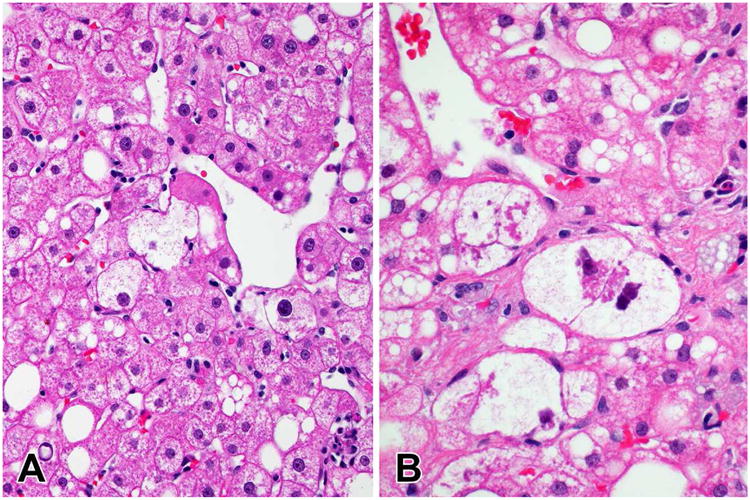
Ballooned hepatocytes and Mallory-Denk bodies in NASH. A. Ballooned hepatocytes near a central vein (400×). B. Ballooned hepatocytes with large Mallory-Denk body cytoplasmic inclusions (600×).
Mallory-Denk bodies are a common finding in both ALD and NASH although in NASH cases the MDBs are fewer in number and not as well formed as in typical cases of ALD. They may be seen in other diseases— particularly in severe cases of chronic cholestasis, in Wilson disease and as a reaction to certain drugs. In NASH, the MDBs are associated with the ballooned hepatocytes in zone 3. MDBs have been noted to be a marker of severity or a progressive disease in ASH and NASH. They result from active metabolic process and consist of aggregates of unfolded hyperphosphorylated keratins, heat shock proteins, ubiquitin and an early response gene product known as p6233. MDBs can be highlighted using immunostains for ubiquitin or p62. When immunostaining is performed, they may also be seen in non-ballooned hepatocytes.
Fibrosis in both ALD and NASH begins in the peri-central region or acinar zone 3, this fibrosis can become quite very complex in the perisinusoidal spaces and eventually will progress to bridging fibrosis and cirrhosis (Figure 4). This pattern of fibrosis is highly characteristic of steatohepatitis and is different from other chronic liver disease patterns where the central veins are involved later in the disease process. Periportal fibrosis develops after the perisinusoidal fibrosis and is manifested as trapping of hepatocytes around the edge of the portal area and extension of short strands of collagen into the surrounding parenchyma. Fibrotic bridges may eventually form single bands between the portal areas and central veins without hepatocyte trapping or island formation. Masson trichrome or other connective tissue stains are essential to highlight the fibrosis and particularly useful in early fibrosis of steatohepatitis. NASH cirrhosis may retain all of the lesions of active steatohepatitis34 but the steatosis may diminish below the 5% level. The active lesions of steatohepatitis may disappear in cirrhosis as well35,36 leading to a diagnosis of cryptogenic cirrhosis. It is likely that NASH is the underlying etiology of most cases of cryptogenic cirrhosis37-39.
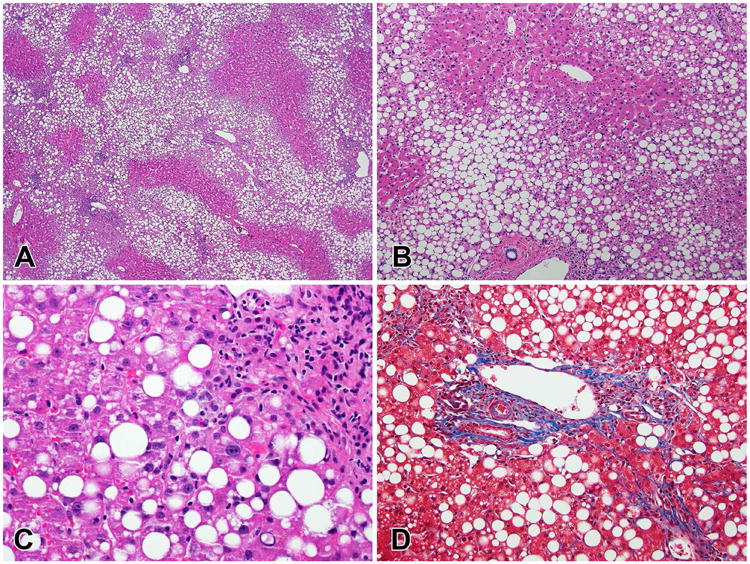
Figure 4.

Fibrosis progression in NASH. A. Early perisinusoidal fibrosis with delicate collagen strands between ballooned hepatocytes (600×). B. Advanced perisinusoidal fibrosis (200×). C. Bridging fibrosis with extensive networks of perisinusoidal fibrosis nearly encircling a regenerative nodule. (100×) D. Established cirrhosis (40×). (All Masson stains)
There are a variety of other findings in NAFLD and NASH that have less clinical and diagnostic significance. Megamitochondria or giant mitochondria have been described in ALD and NAFLD but they are more characteristic of alcohol-related steatohepatitis. These are small intracytoplasmic discrete, eosinophilic globules, 3 to 10 um diameter, sometimes are rounded and less often they can be needlelike shaped. They are bright red on Masson trichrome stain and negative with PAS stain, distinguishing them from alph-1-antitryspin globules. On ultrastructural examination, they are dysmorphic and contain crystalline inclusions.40 Lipogranulomas occur frequently in NASH, but are not helpful in the diagnosis. They may be associated with focal fibrosis and are frequently seen next to central veins or portal areas. This type of fibrosis, however, should not be confused with the perisinusoidal fibrosis of steatohepatitis, and there is no evidence to suggest that this localized change contributes to progressive fibrosis. Glycogenated nuclei are a common finding in NASH also, but may be seen in other metabolic diseases. The hepatocytes nuclei appear empty and the change is seen more often in periportal hepatocytes.
NAFLD and NASH in Children and Adolescents
With the increasing prevalence of obesity and diabetes in children and adolescents41 it is not surprising that NAFLD and NASH have been identified in this population. Both teenagers and younger children may show the same patterns of NAFLD and NASH as adults, with zone 3 centered ballooning injury, inflammation, steatosis and perisinusoidal fibrosis. The disease tends to be milder in children and biopsy surveys show only a few percent with cirrhosis. In a large study of children and adolescents enrolled by the NASH Clinical Research Network (NASH CRN) only 1 of 177 patients had cirrhosis on biopsy.42 Another multicenter study in North America found no cases of cirrhosis among 108 children with NAFLD and NASH.43 However, these same studies found 14% and 20% of their respective cohort had bridging fibrosis, confirming that advanced liver disease could be found in children.
The typical adult pattern of NASH is seen mainly in adolescents, while younger pre-adolescent children tend to have an alternate pattern of progressive liver disease. This pattern is characterized by steatosis that is most prominent in zone 1, forming a collar around the portal areas (Figure 5). The vacuoles of fat are largest in the hepatocytes nearest the portal areas and tend to decrease in diameter across the acinus to zone 3. Lobular and portal inflammation are present, but generally mild. Ballooned hepatocytes are difficult to identify, if present at all, and tend to be the same size as the surrounding non-ballooned hepatocytes. Mallory-Denk bodies are not seen in this pattern. The fibrosis pattern is also different, beginning around the portal areas, with hepatocyte trapping by collagen and extension of short septations into the surrounding parenchyma. When bridging fibrosis develops, the bridges connect the portal areas, leaving the central veins alone. Because of the zone 1 centric appearance of this pattern, the NASH CRN has termed this the zone 1 borderline pattern.23,42 Since this pattern of NAFLD usually lacks the characteristic features of NASH (namely typical balloon cells and Mallory-Denk bodies) there has been reluctance to use the diagnostic term steatohepatitis when describing this pattern. Table 1 contrasts the feature of classical NASH of adolescents and adults with the zone 1 borderline pattern.
Figure 5.
Zone 1 Pattern of NAFLD. A. Steatosis surrounds and bridges between portal areas (40×). B. Steatosis spares the central vein (top, center) but surrounds the portal area (100×). C. Portal inflammation infiltrates adjacent parenchyma (200×). D. Periportal fibrosis is present (Masson, 200×).
Table 1. Comparison of features of the pediatric zone 1 borderline pattern of NAFLD with typical steatohepatitis.
| Characteristic | ||
|---|---|---|
| Population | Mainly pre-adolescent children | Older adolescent children and adults |
| Steatosis | Zone 1 to pan-acinar distribution | Zone 3 to pan-acinar distribution |
| Inflammation | Portal inflammation equal to or greater than lobular inflammation in early disease | Lobular inflammation generally more prominent than portal inflammation in early disease |
| Ballooning | Indistinct or absent | Present, usually in zone 3 |
| Mallory-Denk Bodies | Absent | Present |
| Fibrosis | Periportal fibrosis to portal-portal bridging fibrosis | Perisinusoidal fibrosis in zone 3 to portal-central bridging fibrosis |
Grading, Staging and Scoring in NAFLD and NASH
As NAFLD and NASH became subjects of serious clinical study, it became necessary for pathologists to develop systematic approaches for assessing the severity of the various lesions described above. However, unlike chronic hepatitis, in which inflammation and fibrosis are the only histological features of importance, NAFLD and NASH have other characteristics that need to be tracked. Steatosis and ballooning injury are the main additions to inflammation and fibrosis in NAFLD scoring systems, but other features, including acidophil bodies, Mallory-Denk bodies and the zonal location of steatosis are also important. Fibrosis staging is straightforward in chronic hepatitis, proceeding from none to portal/periportal to bridging to cirrhosis in a linear fashion. While fibrosis in NAFLD and NASH proceeds generally from none to perisinusoidal to periportal and perisinusoidal to bridging to cirrhosis, the first manifestation of fibrosis may be periportal, particularly in children. The relative weight of perisinusoidal vs periportal fibrosis may vary and none of the current staging systems address this adequately. The risk is that efforts to simplify staging and grading for analysis may obscure key elements of pathophysiology. Pathologists and clinicians studying NAFLD and NASH should keep in mind that scoring systems are limited in their ability to capture the rich complexity of pathology in this disease.
There are three scoring systems currently in regular use: the Brunt system44, the NASH CRN system45 and the SAF system46. A comparison of grading and staging of individual histological features between these systems is shown in Table 2. The Brunt system was proposed primarily as a method for grading the severity of NASH and was not intended to be applied to cases that did not meet minimal criteria for steatohepatitis. The grading of steatohepatitis into mild, moderate and severe was based on an overall impression of the severity of steatosis, inflammation and ballooning, but most of the weight was given to ballooning. The NASH CRN system was developed specifically for use in clinical trials and natural history studies as a detailed method for tracking histological change. It was designed with both pediatric and adult patterns in mind and in order to cover the spectrum of changes from the mildest forms of steatosis alone to the most severe forms of steatohepatitis. A composite grade, the NAFLD Activity Score (NAS), was developed as a summary of the overall severity of injury. It is defined as the unweighted sum of the steatosis, lobular inflammation and ballooning scores and it varies from 0 to 8.45 A diagnostic categorization of the changes as NAFLD but not NASH, zone 1 or zone 3 borderline patterns or definite steatohepatitis is made separately from the NAS. The SAF system is the most recent addition and has not been widely used in clinical trials yet. In this system, steatosis (S), activity (A) and fibrosis (F) are separately assessed. Activity is defined as the sum of the lobular inflammation and ballooning scores and varies from 0-4. The SAF defines the presence or absence of NASH based directly on the scores, setting it apart from the other scoring systems (Table 3). Because it has no accommodation for the zone 1 pediatric pattern it should not be used to classify NAFLD and NASH in children. Cases with clear ballooning and inflammation but only minimal steatosis (<5%) would not be classified as NASH in this system, which may cause interpretation problems in cases of cirrhosis or in evaluating a therapy that affect steatosis without changing the other features.
Table 2. Comparison of the essential elements of NAFLD/NASH grading and staging systems.
| Numerical Grade or Stage | Brunt System | NASH CRN System | SAF System |
|---|---|---|---|
| Fibrosis Stage | |||
|
| |||
| 0 | None | None | None |
| 1 | Zone 3 perisinusoidal fibrosis only | Perisinusoidal or periportal fibrosis; 3 substages defined | Perisinusoidal or periportal fibrosis; |
| 2 | Zone 3 perisinusoidal fibrosis and periportal fibrosis | Perisinusoidal and periportal fibrosis | Perisinusoidal and periportal fibrosis |
| 3 | Bridging fibrosis | Bridging fibrosis | Bridging fibrosis |
| 4 | Cirrhosis | Cirrhosis | Cirrhosis |
|
| |||
| Ballooning Grade | |||
|
| |||
| 0 | None | None | Only normal hepatocytes |
| 1 | Mild, zone 3 | Few | Few: Clusters of hepatocytes with rounded shape and reticulated cytoplasm |
| 2 | Prominent, zone 3 | Many | Many: Enlarged hepatocytes (≥2× normal) |
| 3 | Marked, zone 3 | ||
|
| |||
| Lobular Inflammation Grade | |||
|
| |||
| 0 | No foci | No foci | No foci |
| 1 | 1-2 foci per 20× field | <2 foci per 20× field | <2 foci per 20× field |
| 2 | 2-4 foci per 20× field | 2-4 foci per 20× field | >2 foci per 20× field |
| 3 | >4 foci per 20× field | >4 foci per 20× field | |
|
| |||
| Portal Inflammation Grade | |||
|
| |||
| 0 | None | None | |
| 1 | Mild | Mild | |
| 2 | Moderate | More than mild | |
| 3 | Severe | ||
|
| |||
| Steatosis Grade | |||
|
| |||
| 0 | None | <5% | <5% |
| 1 | ≤33% | 5% to 33% | 5% to 33% |
| 2 | 33% to 66% | 33% to 67% | 33% to 67% |
| 3 | >66% | >67% | >67% |
Table 3. The SAF Diagnostic Algorithm for Defining NASH.
| Steatosis (S) | Lobular Inflammation | Ballooning | Total Activity Score (A) | Diagnosis |
|---|---|---|---|---|
| 0 | 0, 1 or 2 | 0, 1 or 2 | 0 to 4 | Not NAFLD |
| 1, 2 or 3 | 0 | 0 | 0 | NAFL |
| 1 | 1 | NAFL | ||
| 2 | 2 | NAFL | ||
| 1 | 0 | 1 | NAFL | |
| 1 | 2 | NASH | ||
| 2 | 3 | NASH | ||
| 2 | 0 | 2 | NAFL | |
| 1 | 3 | NASH | ||
| 2 | 4 | NASH |
Sample and Observer Variability
Although, liver biopsy has remained as a core diagnostic tool and is regarded as the “gold standard” for evaluation of fatty liver diseases, there should be awareness of the sampling difficulties and problems of inter and intra-observer variation in grading and staging. The staging and grading systems described above were developed as semi-quantitative scoring systems for assessing the severity of injury. Interobserver variability studies have been performed for the NASH CRN and SAF system with adequate demonstration of reproducibility.45-47
In addition to issues of variation in interpretation, there are also potential pitfalls related to sampling.48,49 A needle biopsy of liver represents at best 1/50,000th of the entire liver and the severity of liver disease may not be evenly distributed throughout the liver. Bedossa and colleagues using computer modelling have shown that accurate grading and staging in chronic hepatitis requires a biopsy of at least 25mm, unfortunately an uncommon specimen in modern hepatological practice.48 Recent studies have shown that this is also an important factor in evaluating non-alcoholic fatty liver disease. In NAFLD, differences in findings, and thus in “grade” and “stage”, have been demonstrated in recent studies50,51. Several studies have shown that experienced liver pathologists can have good or excellent agreement on the lesions of interest in NAFLD: steatosis, ballooning, lobular inflammation and fibrosis45,47,52. It should be noted that studies that attempt to define sample variation generally do not account for the noise contributed by observer variation and so overestimate the potential to miss lesions based on sampling.
Histologically Based Natural History Studies
The natural history of NAFLD has been studied for decades—even before NASH was characterized as a finding in NAFLD. Prior to 1980, there was significant debate in the medical and pathological literature as to the significance of steatosis in non-alcoholic patients and its relationship to progressive fibrosis. Steatosis was a common finding, but evidence of chronic liver injury, namely advanced fibrosis and to a lesser extent inflammation, was often absent. Although alcohol-like steatohepatitic injury seemed to be present in certain specific cases such as following intestinal bypass,53 there was reluctance to extrapolate the association of steatosis and cirrhosis to the general population54,55.
The natural history of NAFLD has been explored in several types of studies. There have been large epidemiological studies with populations defined by liver enzyme elevation coupled with risk factors like obesity and diabetes. On a smaller scale there have been retrospective studies of patients biopsied in the past and shown to have NAFLD or NASH who have then been followed to clinically determined endpoints. The third type of study involves paired biopsies. These are short-term studies, with mean times between biopsies of 3 to 8 years. Information on disease progression can also be found in placebo arms of randomized clinical trials which use histology as an endpoint. Table 4 highlights a number of paired biopsy studies in NAFLD and NASH, while Table 5 shows studies with placebo groups that have paired biopsies.
Table 4. Longitudinal studies of NAFLD and NASH using Paired Biopsies.
| Author (year) | N | Follow-up (years) | Fibrosis Progressed (N) | Fibrosis Regressed (N) | Factors |
|---|---|---|---|---|---|
| Lee (1989)61 | 13 | 3.5 | 5 | 0 | |
| Powell (1990)36 | 13 | 3.5 | 4 | 1 | |
| Bacon (1994)62 | 2 | 5.5 | 1 | 0 | |
| Teli (1995)15 | 12 | 1 | 0 | ||
| Ratziu (2000)63 | 14 | 5.2 | 2 | 4 | |
| Evans (2002)64 | 7 | 8.2 | 4 | 0 | |
| Harrison (2003)65 | 22 | 5.4 | 9 | 4 | AST |
| Fassio (2004)66 | 22 | 5.3 | 7 | 4 | Obesity, BMI |
| Adams (2005)67 | 103 | 3.2 | 38 | 30 | DM, BMI, Fibrosis stage |
| Hui (2005)68 | 17 | 6.1 | 9 | 0 | None |
| Ekstedt (2006)69 | 68 | 13.8 | 29 | 11 | None |
| Caldwell (2009)70 | 7 | 6 | 7 | ||
| Feldstein (2009)71 | 5 | 3.4 | 4 | 0 | |
| Sorrentino (2010)72 | 132 | 6.4 | 45 | 11 | Fibronectin, HTN, HOMA-IR |
| Wong (2010)73 | 52 | 3 | 14 | 13 | Increased BMI and Waist circumference |
| Hamaguchi (2010)74 | 39 | 2.4 | 11 | 12 | Hemoglobin A1c |
| Pais (2011)75 | 6 | 5 | 6 | ||
| Pais (2013)59 | 70 | 3.7 | 20 | 20 | Age, DM |
| McPherson (2015)58 | 108 | 6.6 | 45 | 20 | DM at follow-up |
| Total | 712 | 37% | 18% |
Abbreviations: AST – aspartate aminotransferase; BMI – body mass index; HTN – hypertension; DM – diabetes mellitus; HOMA-IR – Homeostatic model assessment of insulin resistance
Table 5. Changes in fibrosis stage among placebo treated patients in randomized trials.
| Author (year) | N | Treatment time (weeks) | Fibrosis Progressed (N) | Fibrosis Regressed (N) | Mean Fibrosis Change | Mean NAS Change |
|---|---|---|---|---|---|---|
| Harrison (2003)76 | 22 | 26 | 3 | 9 | -0.27 | -0.1 |
| Lindor (2004)77 | 57 | 104 | 12 | 12 | ||
| Belfort (2006)78 | 21 | 26 | 7 | -0.15 | -0.77 | |
| Dufour (2006)79 | 10 | 104 | 0.5 | |||
| Aithal (2008)80 | 30 | 52 | 6 | 6 | ||
| Ratziu (2008)81 | 31 | 52 | 6 | 5 | -0.18 | 0 |
| Abdelmalek (2009)82 | 28 | 52 | 0.4 | -1 | ||
| Haukeland (2009)83 | 24 | 26 | 4 | 0.08 | -0.42 | |
| Nelson (2009)84 | 6 | 52 | 0 | |||
| Leuschner (2010)85 | 91 | 78 | -0.08 | -1.03 | ||
| Promrat (2010)86 | 10 | 48 | -0.3 | -1.4 | ||
| Sanyal (2010)87 | 72 | 96 | 22 | -0.1 | -0.5 | |
| Lavine (2011)88 | 58 | 96 | 19 | -0.2 | -0.7 | |
| Van Wagner (2011)89 | 7 | 52 | 3 | 0 | 0.4 | -0.3 |
| Zein (2011)90 | 26 | 52 | 4 | 0.4 | -0.1 | |
| Wong (2013)91 | 20 | 24 | 5 | -0.1 | -0.3 | |
| Sanyal (2014)92 | 55 | 52 | 0 | -1 | ||
| Takeshita (2014)93 | 12 | 24 | 0 | -0.4 | ||
| Neuschwander-Tetri (2015)94 | 109 | 72 | 31 | 19 | 0.1 | -0.7 |
| Summary | 689 | 61/256 (24%) | 112/477 (23%) |
A number of general conclusions about fibrosis progression can be drawn from these studies. First, there is clear evidence of disease fluctuation in terms of fibrosis stage over the short term. While some proportion of the apparent progression and regression is related to sample and observer variation, the percent of the population that changes (20-30%) is greater than observational noise would contribute. It is interesting to note that placebo groups often show histological improvement both in fibrosis and disease activity—possibly due to good medical care while patients are enrolled in a clinical trial. Second, while the percent of progression and regression is similar in the short-term placebo treated groups, in the longer term paired biopsies there is a clear bias toward progression. This finding was emphasized by two recent meta-analyses.56,57 Singh et al. found a mean fibrosis progression rate of 0.07 fibrosis stages/year in patients with fatty liver alone and 0.14 stages/year in patients with NASH. They identified an association between rapid progression and hypertension as well as a low AST to ALT ratio at baseline, but not with other clinical or histological parameters. Argo and coworkers found that fibrosis progression was associated with age and inflammation on the initial biopsy, but not with baseline obesity, diabetes or hypertension. One of the most significant findings of the more recent meta-analysis as well as two of the most recent longitudinal studies58,59 has been the observation that patients with NAFLD who do not have features of NASH are still at some risk of fibrosis progression and may develop NASH on subsequent biopsies. Because disease activity and stage apparently fluctuate over time, baseline risk factors for disease progression may be difficult to identify. Indeed, while there is some suggestion that baseline obesity, diabetes and other features of the metabolic syndrome play a role in disease progression, the actual size of the risk remains unclear. Nevertheless, long-term follow-up studies emphasize the importance of fibrosis stage in prognosis60. Since almost all cases of NASH have some degree of fibrosis it may be difficult to define the contribution of the severity of NASH (separate from stage) to long term prognosis.
While natural history studies of NAFLD and NASH have shed some light on disease progression over the short term, there remain unanswered questions:
Is there a benign form of NAFLD or are all patients with NAFLD but not NASH at risk for progression?
Can we create a reliable predictive model of progression, either from baseline clinical and histological findings or from a combination of baseline data with some longitudinal data?
How much do we shift the natural history by providing patients with a good standard of care for obesity and diabetes, without offering a specific pharmacological intervention?
How are changes in fibrosis tied to changes in other histological and clinical parameters?
Longitudinal observational studies of NAFLD and NASH coupled with periodic biopsies have the potential to address these questions in ways that studies using only non-invasive methods cannot.
Key Points.
Nonalcoholic fatty liver disease (NAFLD) is a histologically complex disease with the potential to progress to cirrhosis
Nonalcoholic steatohepatitis is a specific progressive subtype of NAFLD, characterized by ballooning hepatocellular injury in zone 3, steatosis, inflammation and often fibrosis
NAFLD in pre-adolescent children has an acinar zone 1 pattern of steatosis, inflammation and fibrosis that usually lacks ballooning but may progress to advanced fibrosis
Liver biopsy remains the gold standard for confirmation of the diagnosis and definition of the severity of histological findings
Paired biopsy based natural history studies suggest that over the short term there may be fluctuation in disease severity and fibrosis
Summary.
NAFLD and its progressive subtype of NASH are histologically complex diseases in which the pattern of changes drives the diagnostic classification. A liver biopsy remains the best method for characterizing the complexity of injury. Disease progression to cirrhosis clearly occurs in patients with NASH, but some patients with steatosis alone may be at risk to develop NASH. The lesions of NAFLD and NASH can be graded and staged in a fashion similar to other liver diseases and these methods can be used to study histological change in patients enrolled in natural history studies and clinical trials.
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of internal medicine. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 4.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Seminars in liver disease. 2008;28(4):339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 6.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12(5):1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 7.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 8.Brunt EM, Ramrakhiani S, Cordes BG, et al. Concurrence of histologic features of steatohepatitis with other forms of chronic liver disease. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2003;16(1):49–56. doi: 10.1097/01.MP.0000042420.21088.C7. [DOI] [PubMed] [Google Scholar]
- 9.Vuppalanchi R, Gould RJ, Wilson LA, et al. Clinical significance of serum autoantibodies in patients with NAFLD: results from the nonalcoholic steatohepatitis clinical research network. Hepatology international. 2012;6:379–385. doi: 10.1007/s12072-011-9277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotler SJ, Kanji K, Keshavarzian A, Jensen DM, Jakate S. Prevalence and significance of autoantibodies in patients with non-alcoholic steatohepatitis. Journal of clinical gastroenterology. 2004;38(9):801–804. doi: 10.1097/01.mcg.0000139072.38580.a0. [DOI] [PubMed] [Google Scholar]
- 11.Adams LA, Angulo P. Role of liver biopsy and serum markers of liver fibrosis in non-alcoholic fatty liver disease. Clinics in liver disease. 2007;11(1):25–35. viii. doi: 10.1016/j.cld.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Brunt EM. Histopathology of non-alcoholic fatty liver disease. Clinics in liver disease. 2009;13(4):533–544. doi: 10.1016/j.cld.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Nakano M, Fukusato T. Histological study on comparison between NASH and ALD. Hepatology research : the official journal of the Japan Society of Hepatology. 2005;33(2):110–115. doi: 10.1016/j.hepres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 15.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22(6):1714–1719. [PubMed] [Google Scholar]
- 16.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58(6):1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassidy FH, Yokoo T, Aganovic L, et al. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics : a review publication of the Radiological Society of North America, Inc. 2009;29(1):231–260. doi: 10.1148/rg.291075123. [DOI] [PubMed] [Google Scholar]
- 18.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52(3):913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44(1):27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 20.Sorbi D, McGill DB, Thistle JL, Therneau TM, Henry J, Lindor KD. An assessment of the role of liver biopsies in asymptomatic patients with chronic liver test abnormalities. The American journal of gastroenterology. 2000;95(11):3206–3210. doi: 10.1111/j.1572-0241.2000.03293.x. [DOI] [PubMed] [Google Scholar]
- 21.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 22.Kleiner DE, Berk PD, Hsu JY, et al. Hepatic pathology among patients without known liver disease undergoing bariatric surgery: observations and a perspective from the longitudinal assessment of bariatric surgery (LABS) study. Seminars in liver disease. 2014;34(1):98–107. doi: 10.1055/s-0034-1371083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Seminars in liver disease. 2012;32(1):3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 24.Chalasani N, Wilson L, Kleiner DE, et al. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. Journal of hepatology. 2008;48(5):829–834. doi: 10.1016/j.jhep.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. Journal of hepatology. 2002;37(1):56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 26.Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(3):809–820. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gadd VL, Skoien R, Powell EE, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59(4):1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 28.Barker KB, Palekar NA, Bowers SP, Goldberg JE, Pulcini JP, Harrison SA. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. The American journal of gastroenterology. 2006;101(2):368–373. doi: 10.1111/j.1572-0241.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 29.Dixon JB, Bhathal PS, Hughes NR, O'Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39(6):1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 30.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38(4):1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 31.Guy CD, Suzuki A, Burchette JL, et al. Costaining for keratins 8/18 plus ubiquitin improves detection of hepatocyte injury in nonalcoholic fatty liver disease. Human pathology. 2012;43(6):790–800. doi: 10.1016/j.humpath.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gramlich T, Kleiner DE, McCullough AJ, Matteoni CA, Boparai N, Younossi ZM. Pathologic features associated with fibrosis in nonalcoholic fatty liver disease. Human pathology. 2004;35(2):196–199. doi: 10.1016/j.humpath.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Zatloukal K, French SW, Stumptner C, et al. From Mallory to Mallory-Denk bodies: what, how and why? Experimental cell research. 2007;313(10):2033–2049. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Charlton M, Kasparova P, Weston S, et al. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2001;7(7):608–614. doi: 10.1053/jlts.2001.25453. [DOI] [PubMed] [Google Scholar]
- 35.Abdelmalek M, Ludwig J, Lindor KD. Two cases from the spectrum of nonalcoholic steatohepatitis. Journal of clinical gastroenterology. 1995;20(2):127–130. doi: 10.1097/00004836-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29(3):664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 38.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32(4 Pt 1):689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 39.Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. The American journal of medicine. 2000;108(1):9–13. doi: 10.1016/s0002-9343(99)00315-0. [DOI] [PubMed] [Google Scholar]
- 40.Caldwell SH, Swerdlow RH, Khan EM, et al. Mitochondrial abnormalities in non-alcoholic steatohepatitis. Journal of hepatology. 1999;31(3):430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 41.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA : the journal of the American Medical Association. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 42.Patton HM, Lavine JE, Van Natta ML, et al. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135(6):1961–1971. e1962. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter-Kent C, Yerian LM, Brunt EM, et al. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology. 2009;50(4):1113–1120. doi: 10.1002/hep.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. The American journal of gastroenterology. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 45.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 46.Bedossa P, Poitou C, Veyrie N, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56(5):1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 47.Bedossa P, Consortium FP. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60(2):565–575. doi: 10.1002/hep.27173. [DOI] [PubMed] [Google Scholar]
- 48.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38(6):1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Scheuer PJ. Liver biopsy size matters in chronic hepatitis: bigger is better. Hepatology. 2003;38(6):1356–1358. doi: 10.1016/j.hep.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein NS, Hastah F, Galan MV, Gordon SC. Fibrosis heterogeneity in nonalcoholic steatohepatitis and hepatitis C virus needle core biopsy specimens. American journal of clinical pathology. 2005;123(3):382–387. doi: 10.1309/EY72-F1EN-9XCB-1KXX. [DOI] [PubMed] [Google Scholar]
- 51.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 52.Younossi ZM, Gramlich T, Liu YC, et al. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1998;11(6):560–565. [PubMed] [Google Scholar]
- 53.Drenick EJ, Simmons F, Murphy JF. Effect on hepatic morphology of treatment of obesity by fasting, reducing diets and small-bowel bypass. The New England journal of medicine. 1970;282(15):829–834. doi: 10.1056/NEJM197004092821502. [DOI] [PubMed] [Google Scholar]
- 54.Popper H, Schaffner F. Editorial: Steatosis-Mallory's hyaline-cirrhosis: Can their relationships be resolved by an experiment of nature? Gastroenterology. 1974;67(1):185–188. [PubMed] [Google Scholar]
- 55.Thaler H. Editorial: Fatty liver-steatonecrosis-cirrhosis. Acta Hepatogastroenterol (Stuttg) 1975;22(5):271–273. [PubMed] [Google Scholar]
- 56.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. Journal of hepatology. 2009;51(2):371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015;13(4):643–654. e641–649. doi: 10.1016/j.cgh.2014.04.014. quiz e639-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. Journal of hepatology. 2015;62(5):1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 59.Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. Journal of hepatology. 2013;59(3):550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 60.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but no Other Histologic Features, Associates with Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.043. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Human pathology. 1989;20(6):594–598. doi: 10.1016/0046-8177(89)90249-9. [DOI] [PubMed] [Google Scholar]
- 62.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107(4):1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 63.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118(6):1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 64.Evans CD, Oien KA, MacSween RN, Mills PR. Non-alcoholic steatohepatitis: a common cause of progressive chronic liver injury? Journal of clinical pathology. 2002;55(9):689–692. doi: 10.1136/jcp.55.9.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. The American journal of gastroenterology. 2003;98(9):2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 66.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40(4):820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 67.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. Journal of hepatology. 2005;42(1):132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Hui AY, Wong VW, Chan HL, et al. Histological progression of non-alcoholic fatty liver disease in Chinese patients. Alimentary pharmacology & therapeutics. 2005;21(4):407–413. doi: 10.1111/j.1365-2036.2005.02334.x. [DOI] [PubMed] [Google Scholar]
- 69.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 70.Caldwell SH, Lee VD, Kleiner DE, et al. NASH and cryptogenic cirrhosis: a histological analysis. Annals of hepatology. 2009;8(4):346–352. [PMC free article] [PubMed] [Google Scholar]
- 71.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58(11):1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorrentino P, Terracciano L, D'Angelo S, Ferbo U, Bracigliano A, Vecchione R. Predicting fibrosis worsening in obese patients with NASH through parenchymal fibronectin, HOMA-IR, and hypertension. The American journal of gastroenterology. 2010;105(2):336–344. doi: 10.1038/ajg.2009.587. [DOI] [PubMed] [Google Scholar]
- 73.Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59(7):969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 74.Hamaguchi E, Takamura T, Sakurai M, et al. Histological course of nonalcoholic fatty liver disease in Japanese patients: tight glycemic control, rather than weight reduction, ameliorates liver fibrosis. Diabetes care. 2010;33(2):284–286. doi: 10.2337/dc09-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pais R, Pascale A, Fedchuck L, Charlotte F, Poynard T, Ratziu V. Progression from isolated steatosis to steatohepatitis and fibrosis in nonalcoholic fatty liver disease. Clinics and research in hepatology and gastroenterology. 2011;35(1):23–28. doi: 10.1016/j.gcb.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. The American journal of gastroenterology. 2003;98(11):2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 77.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39(3):770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 78.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. The New England journal of medicine. 2006;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 79.Dufour JF, Oneta CM, Gonvers JJ, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4(12):1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 80.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 81.Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135(1):100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 82.Abdelmalek MF, Sanderson SO, Angulo P, et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50(6):1818–1826. doi: 10.1002/hep.23239. [DOI] [PubMed] [Google Scholar]
- 83.Haukeland JW, Konopski Z, Eggesbo HB, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scandinavian journal of gastroenterology. 2009;44(7):853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 84.Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. Journal of clinical gastroenterology. 2009;43(10):990–994. doi: 10.1097/MCG.0b013e31819c392e. [DOI] [PubMed] [Google Scholar]
- 85.Leuschner UF, Lindenthal B, Herrmann G, et al. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52(2):472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 86.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England journal of medicine. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA : the journal of the American Medical Association. 2011;305(16):1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Wagner LB, Koppe SW, Brunt EM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Annals of hepatology. 2011;10(3):277–286. [PubMed] [Google Scholar]
- 90.Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54(5):1610–1619. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong VW, Wong GL, Chan AW, et al. Treatment of non-alcoholic steatohepatitis with Phyllanthus urinaria: a randomized trial. Journal of gastroenterology and hepatology. 2013;28(1):57–62. doi: 10.1111/j.1440-1746.2012.07286.x. [DOI] [PubMed] [Google Scholar]
- 92.Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M, Group EAS. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147(2):377–384. e371. doi: 10.1053/j.gastro.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 93.Takeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57(5):878–890. doi: 10.1007/s00125-013-3149-9. [DOI] [PubMed] [Google Scholar]
- 94.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


