Abstract
Background and Aim
Forkhead box protein P3 (FoxP3)+ regulatory T (Treg) cells play a fundamental role in maintaining the balance between the tissue-damaging and protective immune response to chronic hepatitis C (CHC) infection. Herein, we investigated the frequency of Treg cells in the colon and their potential relationship to the various CHC outcomes and hepatic histopathology.
Methods
Colonic biopsies were collected from three groups with CHC: treatment naïve (TN; n = 20), non-responders (NR; n = 20), sustained virologic response (SVR; n = 20), and a fourth healthy control group (n = 10). The plasma viral loads and cytokines levels were determined by quantitative real-time polymerase chain reaction, and ELISA, respectively. Liver biopsies were examined to assess inflammatory score and fibrosis stage. Colonic Treg frequency was estimated by immunohistochemistry using confocal microscopy.
Results
A significant increase in the frequency of colonic Treg was found in TN, and NR groups compared with the control and SVR group. The frequency of colonic Treg, plasma interleukin (IL)-10 and IL-4 levels were significantly positively correlated with viral load and negatively correlated with METAVIR inflammatory score, and fibrosis stages.
Conclusion
Colonic Treg cells are negatively correlated with liver inflammation and hepatitis C virus (HCV) viral load, which suggests a strong linkage between gut-derived Treg cell populations and HCV infection.
Keywords: colonic Treg, cytokines, HCV, liver histopathology, viral load
Introduction
Chronic hepatitis C (CHC) infection is a global worldwide health problem with an increasing burden year by year, particularly in areas with a high endemicity like Egypt.1 The World Health Organization estimates that approximately 200 million people worldwide are infected with HCV.2 In Egypt, it was estimated that 15% of Egyptians have serologic evidence of HCV infection.3
The ultimate outcome of HCV infection is determined by the host immune response. Patients with acute HCV infection who did not clear the virus developed chronicity.2,4,5 Persistent HCV-specific cytotoxic T-cell responses in the liver have been associated with the development of hepatic inflammation which may ultimately lead to liver cirrhosis.4,5 One of the potential mechanisms that might modulate hepatitis C virus (HCV)-specific immune responses is the inhibitory role of the regulatory T (Treg) cells. 6–8 Treg cells are a subtype of T cells that play a fundamental role in maintaining immune homeostasis to balance between the tissue-damaging and protective effects of the immune response. Treg cells are characterized by the expression of the Forkhead box protein P3 (FoxP3) transcription factor in the nucleus and is generally accepted as the single best marker for Treg cells.5,8–10 In cases of HCV infection, the role of Treg is still controversial and most of studies yielded conflicting reports.11–15 This conflict may be explained by the heterogeneity in the methods and sites of studying the frequency of Treg cells. In most reports, Treg cells were studied in the peripheral blood, which may not be a real mirror of the actual frequency of Treg cells in the microenvironment of the liver. To solve the problem, we herein focused on the potential immunologic link between the gut and the liver through a population of T cells that are capable of homing to both the liver and gut via portal recirculation as the two organs shared common embryological origins.12,13 There are strong evidences that Treg cells may play an important role in the induction of tolerance in the liver.11–13 Additionally, almost all mucosal lymphocytes that provide protection against gut pathogens or tolerance to commensal organisms entered the liver.11,12 In this study, we evaluated the role of colonic Treg cells in HCV infection by investigating the frequency of Treg cells in colonic tissue and examined its relationship to the various patterns of HCV treatment responses, viral persistence, and HCV pathogenesis.
Patients and methods
Patients’ recruitment
A hospital-based cross-sectional descriptive study was conducted to evaluate the role of colonic Treg cells in CHC patients. All participants were recruited from Assiut Liver Institute for Treatment of Hepatitis C Virus and Assiut University Hospitals outpatient clinics, Assiut, Egypt, between January 2012 and September 2012. Verbal and signed consent forms were obtained from participants according to an IRB protocol approved by Assiut University, College of Medicine Institutional Review Board.
Inclusion criteria for the patients were: positive for HCV antibodies by ELISA and HCV-RNA by real-time polymerase chain reaction (RT-PCR) without selection by race, age, or gender. Exclusion criteria were pregnancy, history of Schistosoma infection, inflammatory bowel diseases (IBDs) or suspected IBD, auto-immune diseases including rheumatoid arthritis, and any patients on systemic immunomodulators.
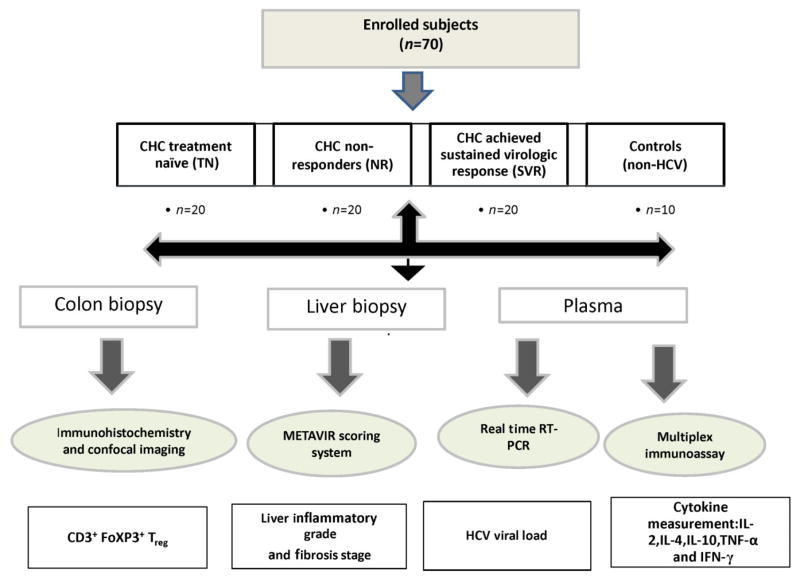
Patients with CHC were grouped into three groups; treatment naïve (TN, n = 20), patient non-responders (NR) to therapy (n = 20), and patients with sustained virologic response (SVR) to therapy (n = 20). The fourth group was healthy control subjects (n = 10) who were non-HCV infected, had a routine colonoscopic examination for colon cancer screening, and were negative. In case of naive group, colon biopsies and blood samples were taken at the same time of liver biopsy before the start of therapy. Meanwhile, in case of NR, colon biopsies and blood samples were taken within 6 months after the start of therapy so the gap between liver biopsies and colon biopsies was not more than 6 months. The average time gap between liver biopsies and both blood and colon biopsy in SVR was 1.5 years. Therapy to CHC is the standard of care that consists of pegylated interferon-α and ribavirin. Twenty milliliters of blood was drawn from each subject. Figure 1 is a flow chart describing the study design.
Figure 1.
Flow chart for study design for the role of colonic regulatory T (Treg) in hepatitis C virus (HCV) infection. Schematic presentation of the study design and the number of subjects enrolled in each group were plotted in Fig 1.
Colonic tissue sampling
After proper preparation, three rectal biopsies (from the descending colon 1–3 mm in size using biopsy forceps) were obtained from each patient and controls via flexible sigmoidscopy.
Serological and liver function tests
Liver function tests (serum alanine aminotransferase [ALT], aspartate aminotransferase [AST], and albumin) were performed using a chemical analyzer Hitachi 911 (Boehringer Ingelheim, Ingelheim, Germany). Serological markers as Hepatitis B Surface antigen (HBs-Ag), anti-HCV and anti-HIV were tested using commercially available micro particle enzyme immunoassay kits (AXSYM, Abbott Laboratories, Wiesbaden, Germany) as specified by the manufacturer.
Determination of HCV viral load by RT-PCR
HCV viral loads were determined in patients’ plasma using RT-PCR as described.16 Briefly, RNA was extracted from 140 μL of patient plasma with viral RNA Mini Kit (QIAGEN GmbH, Hilden, Germany, Cat No.52904), according to the manufacturer’s instruction. Quantitative measurement of HCV-RNA by real time was detected using PCR kit (artus® HCV RG RT-PCR supplied by QIAGEN GmbH, Cat No. 4518263) as specified by the manufacturer.
Assessment of liver inflammation and fibrosis
Liver biopsy specimens were obtained from all patients with CHC prior to treatment and used for assessment of liver inflammation and fibrosis. Hepatic histopathological findings were interpreted independently of clinical and biochemical data by a pathologist, according to the criteria described by METAVIR scoring system.17 Liver biopsy was not done for control group or after HCV therapy.
Examination of the frequency of Treg cells
The frequency of Treg and CD3 + T cells in colonic biopsies were evaluated using fluorescent immunohistochemistry as previously described.18 Two primary antibodies from Abcam (Cambridge, MA, USA), mouse monoclonal Anti-CD3 antibody [Clone PS1] (Cat No. ab699), and Rabbit monoclonal Anti-FoxP3 antibody [clone SP97] (Cat No ab99964), were incubated in a sequential manner with two secondary antibodies from Jackson Immunoresearch (West Groove, PA, USA), Alexa Fluor® 594 Donkey Anti-rabbit, and Alexa Fluor® 488-Donkey Anti-Mouse. The number of colonic Treg and CD3 + T cells were recorded by using Zeiss LASER scanning confocal microscopy (LSM710; Carl Zeiss GmbH, Jena, Germany) with high-power field (1HPF = 0.4 mm2). Ten HPFs were examined in each slide and averaged. Data were calculated as % frequency of Treg as the follows:
Liver biopsies were not available for immunohistochemistry staining of intrahepatic Treg.
Cytokine measurement
Selected panel of cytokines (interleukin [IL]-2, IL4, IL-10, γ-interferon [IFN-γ], and tumor necrosis factor-α [TNF-α]) concentrations in the plasma were determined by ELISA using Milliplex TM Multiplex kits (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol.
Statistical analysis
Data are provided as range (minimum, maximum); mean ± SD. Student’s t-test was used to examine the difference between two groups with a significance value at P = 0.05. The ANOVA test was used to examine the difference between groups of the enrolled subjects. Correlations between parameters measured were calculated using Spearman’s correlation coefficient. All statistical analysis was conducted by Graph Pad Prism 6 Software (San Diego, CA, US, A).
Results
Characterization of the enrolled subjects
Table 1 summarized the demographic and clinical characteristics of the enrolled subjects. The healthy control subjects were significantly older than TN group, NR, and SVR group (P = 0.0003, P = 0.0003, and P < 0.0001, respectively) and contained more females compared with TN, NR, and SVR groups (P = 0.002, P = 0.03, and P = 0.03, respectively).
Table 1.
Demographic and clinical data characteristics of the enrolled subjects
| Demographic characters | TN group (n = 20) | NR group (n = 20) | SVR group (n = 20) | Control group (n = 10) |
|---|---|---|---|---|
| Sex | ||||
| Male : female | 19:1 | 17:3 | 17:3 | 4:6 |
| Age | ||||
| Range (mean ± SD) | 19–54 (42 ± 10.4) | 21–54 (43.5 ± 8.6) | 20–51 (40.3 ± 7.8) | 42–64 (56 ± 6.8) |
| HCV viral load (106 IU/mL, [mean ± SD]) | 1.3 ± 1.2 | 0.9 ± 0.8 | NA | NA |
| Distribution of liver inflammatory grade | ||||
| Grade 1–2 | 12 | 11 | 14 | |
| Grade 3–4 | 8 | 9 | 6 | NA |
| Distribution of liver fibrosis stage | ||||
| Stage 1–2 | 14 | 13 | 18 | |
| Stage 3–4 | 6 | 7 | 2 | NA |
| ALT (IU/mL [mean ± SD]) | 50.9 ± 25.8 | 37 ± 22.5 | 32.5 ± 26.7 | 21 ± 7.4 |
| AST (IU/mL [mean ± SD]) | 36.5 ± 21.9 | 32.2 ± 23 | 25.9 ± 18.9 | 23 ± 11 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; NA, not applicable; NR, non-responders; SVR, sustained virologic response; TN, treatment naïve.
ALT levels were significantly higher in TN group (50.9 ± 25.8 U/mL) compared with healthy controls group (21 ± 7.4 U/mL; P = 0.005), SVR group (32.5 ± 26.7 U/mL; P = 0.006), and NR group (37 ± 22.5 U/mL; P = 0.04), while there was no significant difference in ALT levels among NR, SVR, and the healthy control groups. In addition, there was no significant difference between groups in AST levels (Table 1). There was no significant difference in HCV viral load between TN group (mean ± SD = 1.3 ± 1.2 × 106 IU/mL) compared with NR (0.9 ± 0.8 × 106 IU/mL). Also, there was no significant difference between HCV-infected groups in their METAVIR inflammatory grade and fibrosis stage prior to treatment.
Characterization of colonic Treg cells
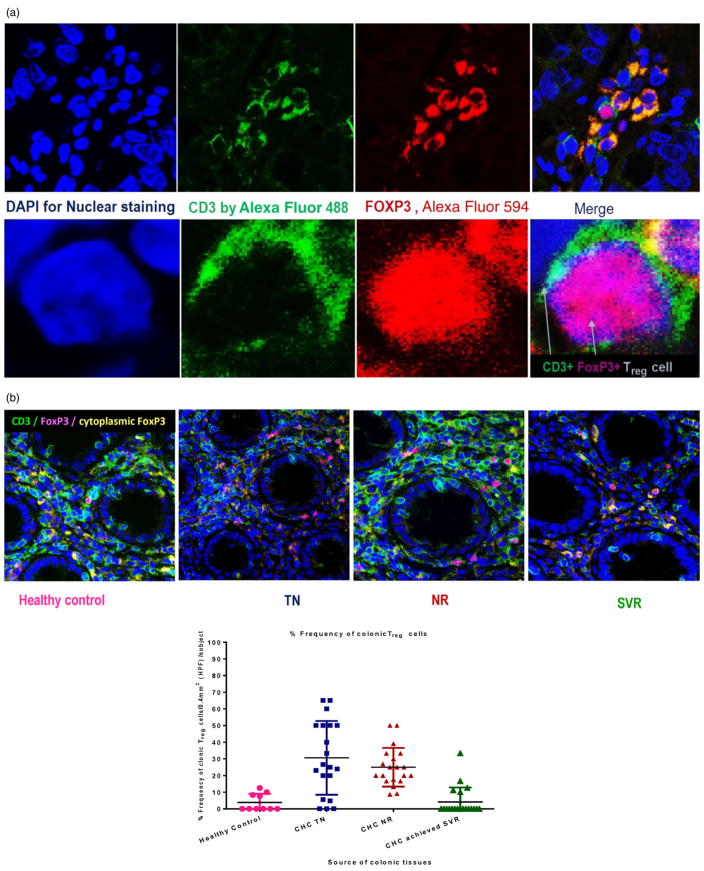
Colonic Treg cells were detected by indirect fluorescent immunohistochemical staining of CD3 and FoxP3. Treg cells were identified by the green surface CD3 (Alexa Fluor 488) and the red nuclear FoxP3 (Alexa Fluor 594) as demonstrated in Figure 2a.
Figure 2.
Characterization of colonic regulatory T (Treg) by double immunofluorescence (IF) staining with anti CD3 and anti-Forkhead box protein P3 (FoxP3) antibody. Colonic Treg cells were detected by indirect fluorescent immunohistochemical staining of CD3 and FoxP3 using mouse monoclonal Anti-CD3 antibody [PS1] and rabbit monoclonal Anti-FOXP3 antibody [SP97]. Treg cells identified by the green surface CD3 (by Alexa Fluor® 488-Donkey Anti-Mouse secondary antibody) and the red nuclear FoxP3 (by Alexa Fluor® 594 Donkey Anti-Rabbit secondary antibody). FoxP3 also was expressed intracytoplasmic in some proliferation cells and appeared as orange color because of the merge between red FoxP3 and green CD3 (a). The frequency of colonic Treg cells in the study groups was plotted in panel b. Significant increase in the frequency of colonic Treg in patients with chronic hepatitis C (CHC) naïve to treatment was observed compared with healthy control and sustained virologic response (SVR) (P = 0.0009 and P < 0.0001, respectively). Additionally, the frequency of colonic Treg was significantly higher in non-responders (NR) compared with healthy controls, and SVR (P < 0.0001 and P < 0.001). No significant differences were observed in the frequency of colonic Treg in SVR group compared with healthy subjects or in the NR group compared with CHC naïve to treatment.
No significant differences were observed in the number of colonic CD3 + T cells among the different groups. However, there was a significant increase in the mean frequency of colonic Treg cells in TN group (mean ± SD; 30.62 ± 22.13%), compared with the healthy controls (3.90 ± 5.1%; P = 0.0009), and SVR group (4.18 ± 8.6%; P < 0.0001). Additionally, the frequency of colonic Treg was significantly higher in NR (25 ± 11.6%), compared with healthy controls, and SVR group (P < 0.0001 and P < 0.001, respectively). No significant differences in the frequency of colonic Treg in SVR group compared with healthy subjects, and in NR compared with TN group (P > 0.05) (Fig. 2b and Table 2).
Table 2.
Frequency of CD3+ T cells, colonic Treg cells and average values of plasma cytokines levels in the study groups
| TN group (n = 20) | NR group (n = 20) | SVR group (n = 20) | Control group (n = 10) | |
|---|---|---|---|---|
| CD3 T-cell number/HPF | ||||
| Range | 2–27 | 3–30 | 3–26 | 8–16 |
| (Mean ± SD) | 13 ± 8.5 | 14.4 ± 8.3 | 11.0 ± 5.8 | 12.2 (± 2.7) |
| Colonic Treg number | ||||
| Range | 0–13 | 1–10 | 0–2 | 0–2 |
| (Mean ± SD) | 3.5 ± 3.5 | 3.6 ± 2.6 | 0.3 ± 0.6 | 0.5 ± 0.7 |
| % Colonic Treg frequency (mean ± SD) | 30.62 ± 22.13 | 25 ± 11.6 | 4.18 ± 8.6 | 3.9 ± 5.1 |
| Plasma cytokine levels,† pg/mL (mean ± SD) | ||||
| IL-2 | 2.61 ± 1.160 | 5.18 ± 4.35 | 3.46 ± 0.67 | 5.78 ± 7.4 |
| 1L-4 | 185 ± 61.69 | 143 ± 116.15 | 47 ± 28 | 34.50 ± 62.61 |
| IL-10 | 18.98 ± 8.32 | 12.80 ± 9.29 | 7.75 ± 5.83 | 5.66 ± 5.746 |
| TNF-α | 23 ± 11 | 22.62 ± 9.38 | 15.21 ± 3.39 | 12.66 ± 4.82 |
| IFN-γ | 18.42 ± 26.37 | 17.17 ± 32.11 | 7.9115 ± 7.79 | 9.416 ± 2.23 |
The lower level of detection (LLD) for IL-2, TNF-α, and IFN-γ is (LLDs = 0.64 pg/mL) while the lower level of detection (LLD) for IL-4 and IL-10 is (LLDs = 3.2 pg/mL). Additionally, we did not consider analysis of the data if any of the cytokines levels in > 50% of the samples were below detection limit (BDL).
HPF, high-power field; IFN-γ, γ-interferon; IL, interleukin; NR, non-responders; SVR, sustained virologic response; TN, treatment naïve; TNF-α, tumor necrosis factor-α; Treg, regulatory T.
Correlation between colonic Treg frequency and viral load
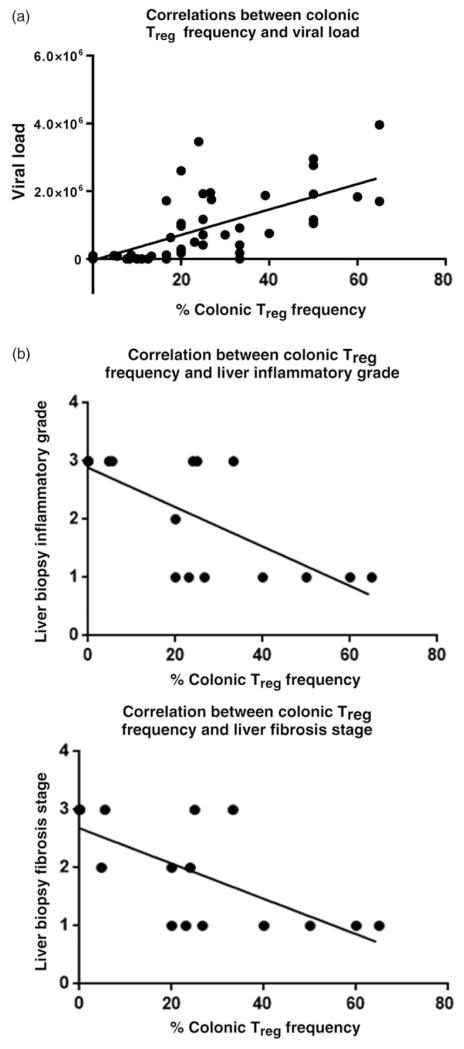
A significant positive correlation was detected between the frequency of colonic Treg and viral load (P < 0.0001, r = 0.73; 95% confidence interval [CI] = 0.6029–0.8264) in CHC-infected groups as shown in Figure 3a.
Figure 3.
Correlation between colonic regulatory T (Treg) frequency and both viral load and liver histopathology in treatment-naïve (TN) chronic hepatitis C virus (HCV)-infected patients. A significant (P < 0.0001) positive correlation between frequency of colonic Treg cells and viral load (r = 0.73, with 95% confidence interval [CI] = 0.6029–0.8264) in TN patients was detected (a). On the other hand, a significant negative correlation was detected between the frequency of colonic Treg in TN with METAVIR necro-inflammatory grade (P = 0.0001, r = −0.7586, with 95% CI = −0.8993 to −0.4759) and fibrosis stage (P = 0.0002, r = −0.7385 with 95% CI = −0.8901 to −0.4396) (b).
Correlation between colonic Treg frequency and liver histopathology
A significant negative correlation between the frequency of colonic Treg with METAVIR necro-inflammation grade (P = 0.0001, r = −0.7586; 95% CI = −0.8993 to −0.4759), and fibrosis stage (P = 0.0002, r = −0.7385; 95% CI = −0.8901 to −0.4396) was detected in the TN group as shown in Figure 3b.
Additionally, there was a significant (P < 0.0001) positive correlation between ALT and both liver inflammation (r = 0.4983, with 95% CI = 0.2798–0.6678) and liver fibrosis (P < 0.0001) (r = 0.4983, with 95% CI = 0.2818–0.6689). However, there were no significant correlations between viral load and liver inflammation, fibrosis scores, or ALT levels (data not shown).
Cytokines profiles in the study groups and correlation with viral load, colonic Treg frequency, and liver pathology
Plasma IL-4, IL-10, and TNF-α were significantly elevated in TN group compared with SVR group and control group, and in NR group compared with SVR group and control group. However, there was no significant difference in the levels of plasma IL-2 and IFN-γ between the study groups as shown in Tables 2 and 3.
Table 3.
Statistical analysis of plasma cytokines levels in the study groups
| P-value Between-groups ANOVA | P-value TN versus NR | P-value TN versus SVR | P-value TN versus healthy | P-value NR versus SVR | P-value NR versus healthy | P-value SVR versus control | |
|---|---|---|---|---|---|---|---|
| IL-2 | 0.28 | 0.08 | 0.33 | 0.84 | > 0.99 | 0.09 | 0.38 |
| IL-4 | 0.0001* | 0.07 | < 0.0001* | 0.0002* | 0.0106* | 0.003* | 0.053 |
| IL-10 | 0.006* | 0.2918 | 0.003* | 0.009* | 0.04* | 0.01* | 0.93 |
| TNF-α | 0.0016* | 0.77 | 0.04* | 0.0069* | 0.0047* | 0.0009* | 0.0958 |
| IFN-γ | 0.51 | 0.99 | 0.60 | 0.77 | 0.6 | 0.55 | 0.47 |
Significant difference.
IFN-γ, γ-interferon; IL, interleukin; NR, non-responders; SVR, sustained virologic response; TN, treatment naïve; TNF-α, tumor necrosis factor-α.
Plasma levels of IL-4 and IL-10 were significantly positively correlated with both colonic Treg frequency and HCV viral load (and negatively correlated with METAVIR score of liver inflammation and fibrosis stage, as shown in Table 4). On the other hand, we did not find a significant correlation between plasma levels of IL-2, TNF-α, or IFN-γ with HCV viral load, colonic Treg frequency, and liver pathology.
Table 4.
Correlations between cytokines levels and HCV viral load, frequency of colonic Treg cells, and liver pathology
| Correlation with HCV viral load | Correlation with % Treg frequency | Correlation with liver biopsy inflammatory score | Correlation with liver biopsy fibrosis stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P-value | 95%CI | r | P-value | 95%CI | r | P-value | 95% CI | r | P-value | 95%CI | |
| IL-2 | 0.15 | 0.53 | −0.32 to 0.57 | −0.14 | 0.58 | −0.56 to 0.34 | 0.23 | 0.33 | −0.23 to 0.61 | 0.29 | 0.2 | −0.23 to 0.61 |
| IL-4 | 0.37 | 0.02* | 0.05 to 0.62 | 0.34 | 0.04* | 0.02 to 0.6 | −0.65 | 0.04* | −0.91 to −0.04 | −0.67 | 0.03* | −0.91 to −0.07 |
| IL-10 | 0.51 | < 0.0001* | 0.28 to 0.68 | 0.28 | 0.02* | 0.04 to 0.49 | −0.66 | 0.03* | −0.91 to −0.06 | −0.74 | 0.01* | −0.93 to −0.19 |
| TNF-α | 0.08 | 0.52 | −0.16 to 0.31 | 0.21 | 0.08 | −0.04 to 0.43 | 0.04 | 0.85 | −0.41 to 0.48 | −0.001 | 0.99 | −0.44 to 0.44 |
| IFN-γ | 0.09 | 0.48 | −0.16 to 0.33 | 0.11 | 0.39 | −0.14 to 0.35 | 0.10 | 0.67 | −0.36 to 0.52 | 0.14 | 0.55 | −0.32 to 0.55 |
Significant difference.
CI, confidence interval; HCV, hepatitis C virus; IFN-γ, γ-interferon; IL, interleukin; NR, non-responders; SVR, sustained virologic response; TN, treatment naïve; TNF-α, tumor necrosis factor-α; Treg, regulatory T.
Discussion
Several studies have shown that HCV-specific CD8+ T cells derived from the peripheral blood or liver are impaired and display a reduced ability to proliferate or secrete anti-viral cytokines such as IFN-γ.14,15,19 The mechanisms contributing to CD8+ T-cell exhaustion in HCV are not completely understood; however, it may be partially explained by the intrinsic regulatory pathways such as signals mediated by the inhibitory receptor PD-1.14 and extrinsic regulatory pathway such as IL-10 or Treg cells.20 Most of prior studies focused to examine HCV-specific immune responses in the peripheral blood, and this may be due to the feasibility to perform these examinations; however, the results may be misrepresentative of the intrahepatic microenvironment.8 The difficulty of obtaining enough number of cells from liver biopsies without in vitro expansion, as well as the risk of obtaining repeated liver biopsies from patients represent a plausible hindrance.8 In order to solve these problems, we focused on the gut immune cells that are abundant and could be obtained with less risk by colonoscopy. Intrinsic gut-liver immune axis of the lymphocytes recirculate between the gut and liver through the portal circulation and share a common regulation and activation pathways. Most of the infiltrating T cells in the liver are primed cells suggesting that trafficking of memory T cells through the liver might contribute to immune surveillance.21 The existence of a population of long-lived memory T cells capable of homing both to the liver and the gut confirms the link between gut and liver diseases.12,13 Evidence, which support such finding, comes from observations that the gut adhesion molecules and chemokine (such as CCL25) are also detected on liver endothelium22 providing a mechanism for the recruitment of mucosal lymphocytes to the liver.12,23 Additionally, almost all mucosal lymphocytes that provide protection against gut pathogens circulate in the liver.11,12 Therefore, studying these gut immune cells may convey a third compartment, other than the peripheral circulation and the liver, that may be a useful image of the intrahepatic microenvironment.11,12 Based on these evidences, we examined the frequency of Treg cells in colonic tissue and investigated their association with the various outcomes of CHC therapy, viral persistence, plasma cytokines, and liver inflammation.
Our data indicated that the frequency of colonic Treg in CHC patients is higher than control. Our findings are in concordance with previous reports that demonstrated a higher number of FoxP3+Treg cells in the liver of HCV-infected patients compared with healthy control.24,25 However, lower frequency of peripheral Treg was reported in patients with HCV-mixed cryoglobulinemia vasculitis.26 No difference was observed in the frequency of Treg between TN and NR groups. On the contrary, we found a significant increase in the frequency of colonic Treg in TN compared with control and SVR groups. Moreover, the frequency of Treg was significantly higher in NR compared with controls and those with SVR. The difference in Treg frequency could not been explained simply by the age or gender differences between the groups as there was still a significant difference in Treg frequency between TN and SVR groups despite the lack of age or gender differences between them. Our observation that the frequency of Treg was comparable between control and SVR groups strongly supports a role of colonic Treg response in anti-HCV therapy. The increase in Treg frequency in NR and TN groups compared with the controls or SVR might be the result of the suppressive effect of HCV core protein with the induction of Treg in those HCV-infected patients.27,28
For further support of our finding, we examined the correlation between viral load and colonic Treg cells. A significant positive correlation was detected between the frequency of colonic Treg and viral load in CHC patients. Our findings contradict the previous report of no correlation between peripheral Treg and HCV viral load by Itose et al.25 However, they measured peripheral Treg and not colonic Treg, which might not be a real mirror of the microenvironment of Treg cells in HCV-infected patients.
T-cells also play a pivotal role in hepatic necro-inflammation and the subsequent fibrosis during their effort to limit viral replication.29,30 To achieve balance between liver damage and viral replication, Treg may suppress HCV-specific immune responses.31 In our study, a significant inverse correlation between the frequency of colonic Treg and the liver pathology was observed. The same finding is also noted by Bolacchi et al.7 and Sturm et al.,8 who reported that the Treg/CD8 ratio in liver is significantly lower in cirrhotic stage supporting the role of colonic Treg in controlling chronic inflammatory response and liver damage in CHC infection.7,30,32,33
Previous studies showed that plasma cytokines levels are altered during HCV infection compared with healthy control,34,35 and peripheral Treg frequency was positively correlated with cytokines levels such as IL-10, IL-4, and transforming growth factor-β.34,35 However, to our knowledge, no reports described the correlation between colonic Treg frequency and plasma cytokines profile in HCV infection. In our study, plasma IL-4, IL-10, and TNF-α were significantly elevated in TN compared with SVR and controls groups and in NR compared with SVR and controls groups. Plasma levels of IL-4 and IL-10 were also significantly positively correlated with both colonic Treg frequency and HCV viral load and negatively correlated with METAVIR score of liver inflammation and fibrosis stage. Because IL-4, IL-10, and TNF-α are surrogate markers of immune regulation by Treg, our findings further support the role of Treg and its cytokines in controlling chronic inflammatory response and liver damage in CHC infection.7,30,32,33
No significant difference in the levels of IL-2 and IFN-γ (Th1 cytokines) among the four groups was observed, and we did not find significant correlation between plasma Th1 cytokines levels with HCV viral load, colonic Treg frequency, and liver pathology. These data confirm the bias of the immune response toward Th22,34,36 in CHC and the role of Treg in this bias.36
We expected that the expansion of Treg cells during HCV infection is HCV specific24,37 because there were no reports of generalized immunosuppression in HCV infection.38 Our data suggested that the expansion of colonic Treg cells decreases the magnitude of HCV-specific T-cell responses in viremic patients and prevents clearing of the infection. Additionally, Treg cells may have a protective effect and limit the massive immunopathologic effect of the high viral load on the liver.
However, there is still an open question whether Treg cells are protective or harmful in CHC. We postulate that the effective host anti-HCV immune response may be associated with strong inflammatory reactions and liver damage. To minimize the damage to self, the activation of the immune system also triggers anti-inflammatory pathways through Treg responses. Both inflammatory and anti-inflammatory reactions are normal components of the immune response, which, together, fight infections while preventing immunopathology. In agreement with this scenario, the data presented here strongly support the hypothesis that Treg cells play an important role in the clinical presentations of CHC infection by suppressing HCV-specific immune response and suppression of hepatic inflammation.
To the best of our knowledge, our study is the first report to examine the frequency of gut Treg cells in CHC, and hence, our results may serve as a benchmark for future interventions. However, the small sample size and the lack of studying the Treg cells in the liver biopsy are limitations for our study.
Based on these observations, we recommend a further exploration of the liver-gut immune axis by studying both liver and gut histopathology longitudinally in HCV-infected patients. In conclusion, the gut Treg cells are highly associated with the hepatic pathologic damage and HCV pathogenesis that suggests a strong linkage between gut-derived Treg cell populations and hepatic insult.
Acknowledgments
We would like to thank the entire participants in this study, particularly the patients. We thank all members of the Department of Medical Microbiology and Immunology, Faculty of Medicine, Assiut University, Assiut, Egypt. We also thank the Flow Cytometry and Pathology Core Facility at the Digestive Health Center at Cincinnati Children’s Hospital Medical Center (CCHMC). This investigation was supported by Egyptian Government scholarship for Helal Hetta, the Grant office, Faculty of Medicine, Assiut University, Egypt, Merck Investigator Initiated Studies (IISP #: 38879 [Shata]); National Institute of Health Grant (K24DK070528 [Sherman]); and was supported in part by Public Health Service Grant (P30 DK078392 [The Gene Expression Microarray Core Cincinnati Children’s Hospital Medical Center]); National Institute of Health Grant (NIH P30 DK078392 [Core of the Digestive Disease Research Core Center in Cincinnati]).
Footnotes
This work was partially presented at The 64th Annual Liver Meeting of AASLD 2013 in Washington, DC, USA on November1-5, 2013. Abstract #2013.
Conflict of interest: There is no conflict of interest.
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Klenerman P, Thimme R. T cell responses in hepatitis C: the good, the bad and the unconventional. Gut. 2012;61:1226–34. doi: 10.1136/gutjnl-2011-300620. [DOI] [PubMed] [Google Scholar]
- 3.Gouda I, Nada O, Ezzat S, et al. Immunohistochemical detection of hepatitis C virus (genotype 4) in B-cell NHL in an Egyptian population: correlation with serum HCV-RNA. Appl Immunohistochem Mol Morphol. 2010;18:29–34. doi: 10.1097/PAI.0b013e3181ae9e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–52. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 5.Keynan Y, Card CM, McLaren PJ, Dawood MR, Kasper K, Fowke KR. The role of regulatory T cells in chronic and acute viral infections. Clin Infect Dis. 2008;46:1046–52. doi: 10.1086/529379. [DOI] [PubMed] [Google Scholar]
- 6.Hartling HJ, Gaardbo JC, Ronit A, et al. CD4(+) and CD8(+) regulatory T cells (Tregs) are elevated and display an active phenotype in patients with chronic HCV mono-infection and HIV/HCV co-infection. Scand J Immunol. 2012;76:294–305. doi: 10.1111/j.1365-3083.2012.02725.x. [DOI] [PubMed] [Google Scholar]
- 7.Bolacchi F, Sinistro A, Ciaprini C, et al. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:88–196. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sturm N, Thelu MA, Camous X, et al. Characterization and role of intra-hepatic regulatory T cells in chronic hepatitis C pathogenesis. J Hepatol. 2010;53:25–35. doi: 10.1016/j.jhep.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Magg T, Mannert J, Ellwart JW, Schmid I, Albert MH. Subcellular localization of FOXP3 in human regulatory and nonregulatory T cells. Eur J Immunol. 2012;42:1627–38. doi: 10.1002/eji.201141838. [DOI] [PubMed] [Google Scholar]
- 11.Adams DH, Eksteen B, Curbishley SM. Immunology of the gut and liver: a love/hate relationship. Gut. 2008;57:838–48. doi: 10.1136/gut.2007.122168. [DOI] [PubMed] [Google Scholar]
- 12.Hetta HF, Mehta MJ, Shata MTM. Gut immune response in the presence of hepatitis C virus infection. World J Immunol. 2014;4:52–62. [Google Scholar]
- 13.Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670–82. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- 14.Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–37. 37.e1–2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruener NH, Lechner F, Jung MC, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–8. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-sherif WT, Sayed SK, Afifi NA, EL-Amin HA. Occult hepatitis B infection among Egyptian chronic hepatitis C patients and its relation with liver enzymes and hepatitis B markers. Life Sci. 2012;9:467–74. [Google Scholar]
- 17.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 18.Shata MT, Abdel-Hameed EA, Hetta HF, Sherman KE. Immune activation in HIV/HCV-infected patients is associated with low-level expression of liver expressed antimicrobial peptide-2 (LEAP-2) J Clin Pathol. 2013;66:967–75. doi: 10.1136/jclinpath-2013-201581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spangenberg HC, Viazov S, Kersting N, et al. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42:828–37. doi: 10.1002/hep.20856. [DOI] [PubMed] [Google Scholar]
- 20.Thimme R, Opitz OG. Interleukin-10 and viral clearance: translation to viral hepatitis. Gastroenterology. 2007;132:2611–3. doi: 10.1053/j.gastro.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Ward SM, Jonsson JR, Sierro S, et al. Virus-specific CD8+ T lymphocytes within the normal human liver. Eur J Immunol. 2004;34:1526–31. doi: 10.1002/eji.200324275. [DOI] [PubMed] [Google Scholar]
- 22.Grant AJ, Lalor PF, Hubscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–72. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- 23.Eksteen B, Grant AJ, Miles A, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511–7. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rushbrook SM, Ward SM, Unitt E, et al. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–9. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itose I, Kanto T, Kakita N, et al. Enhanced ability of regulatory T cells in chronic hepatitis C patients with persistently normal alanine aminotransferase levels than those with active hepatitis. J Viral Hepat. 2009;16:844–52. doi: 10.1111/j.1365-2893.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- 26.Boyer O, Saadoun D, Abriol J, et al. CD4+CD25+ regulatory T-cell deficiency in patients with hepatitis C-mixed cryoglobulinemia vasculitis. Blood. 2004;103(Suppl 2):3428–30. doi: 10.1182/blood-2003-07-2598. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald AJ, Duffy M, Brady MT, et al. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J Infect Dis. 2002;185:720–7. doi: 10.1086/339340. [DOI] [PubMed] [Google Scholar]
- 28.Langhans B, Lechmann M, Ihlenfeldt H, et al. A hepatitis C virus (HCV) core protein derived peptide inhibits HCV specific lymphocyte proliferation. Eur J Med Res. 2000;5:115–20. [PubMed] [Google Scholar]
- 29.Claassen MA, de Knegt RJ, Tilanus HW, Janssen HL, Boonstra A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol. 2010;52:315–21. doi: 10.1016/j.jhep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Rehermann B. Interaction between the hepatitis C virus and the immune system. Semin Liver Dis. 2000;20:127–41. doi: 10.1055/s-2000-9946. [DOI] [PubMed] [Google Scholar]
- 31.Cabrera R, Tu Z, Xu Y, et al. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 32.Abrignani S. Immune responses throughout hepatitis C virus (HCV) infection: HCV from the immune system point of view. Springer Semin Immunopathol. 1997;19:47–55. doi: 10.1007/BF00945024. [DOI] [PubMed] [Google Scholar]
- 33.Rico MA, Quiroga JA, Subira D, et al. Features of the CD4+ T-cell response in liver and peripheral blood of hepatitis C virus-infected patients with persistently normal and abnormal alanine aminotransferase levels. J Hepatol. 2002;36:408–16. doi: 10.1016/s0168-8278(01)00281-1. [DOI] [PubMed] [Google Scholar]
- 34.Reiser M, Marousis CG, Nelson DR, et al. Serum interleukin 4 and interleukin 10 levels in patients with chronic hepatitis C virus infection. J Hepatol. 1997;26:471–8. doi: 10.1016/s0168-8278(97)80409-6. [DOI] [PubMed] [Google Scholar]
- 35.Sofian M, Aghakhani A, Farazi AA, et al. Serum profile of T helper 1 and T helper 2 cytokines in hepatitis C virus infected patients. Hepat Mon. 2012;12:e6156. doi: 10.5812/hepatmon.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolganiuc A, Szabo G. T cells with regulatory activity in hepatitis C virus infection: what we know and what we don’t. J Leukoc Biol. 2008;84:614–22. doi: 10.1189/jlb.1107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boettler T, Spangenberg HC, Neumann-Haefelin C, et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun J, Bodola F, Fan X, et al. Hepatitis C virus core and envelope proteins do not suppress the host’s ability to clear a hepatic viral infection. J Virol. 2001;75:11992–8. doi: 10.1128/JVI.75.24.11992-11998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]