Abstract
Background
Ferritin is associated with various cardiometabolic risk factors such as dyslipidemia, hypertension, obesity, and insulin resistance in adults. We aimed to study the association between serum ferritin levels and dyslipidemia in adolescents, because dyslipidemia is considered an important modifiable cardiovascular risk factor in the young.
Methods
We analyzed 1,879 subjects (1,026 boys and 853 girls) from the 2009–2010 Korean National Health and Nutrition Examination Survey IV. Subjects were categorized into quartiles according to their lipid parameters, which were classified according to age and gender. Those in the highest quartile groups for total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglyceride concentrations were diagnosed as having dyslipidemia. Those in the lowest quartile for high-density lipoprotein cholesterol (HDL-C) values were diagnosed with abnormal levels.
Results
In boys, total cholesterol, LDL-C, and triglyceride concentrations were significantly correlated with serum ferritin levels. In both boys and girls, serum ferritin levels were negatively associated with HDL-C values, even after adjusting for all covariates. Furthermore, there was no significant correlation between serum ferritin levels and total cholesterol, LDL, and triglyceride concentrations in girls.
Conclusion
Serum ferritin levels were significantly associated with major dyslipidemia parameters, more prominently in boys than in girls, and this association represents a cardiometabolic risk factor.
Introduction
Ferritin, a ubiquitous intracellular protein essential for the regulation of iron homeostasis, is a clinical biomarker widely used to estimate body iron status. Iron is a transition metal capable of causing oxidative stress and tissue damage by catalyzing the formation of free radicals [1]. Increasing evidence has shown that moderately increased iron stores, resulting in elevated serum ferritin levels, are associated with various metabolic risk factors [2].
Previously, several studies including large cross-sectional studies have shown that increased serum ferritin levels in adults are associated with central obesity [3,4], metabolic syndrome (MS) [5–8], dyslipidemia [9], hypertension [10], insulin resistance [11,12], diabetes mellitus [7,13], and nonalcoholic fatty liver disease [14].
In particular, dyslipidemia, defined as excessive lipids in the blood, may begin in childhood, and is a causal factor of early atherosclerosis and premature cardiovascular diseases (CVD) in young adults [15–18]. Moreover, some studies have demonstrated an association between smoking status, hypertension, obesity, atherosclerosis and dyslipidemia in children and adolescents as well as in adults [19,20].
The levels of lipid parameters and the detection rate of dyslipidemia have increased among children and adolescents in Asian countries including South Korea [21–23]. This trend appears to have been caused by the adoption of a more Westernized diet rich in fat, sugar, and cholesterol following recent economic development [21–23]. Because dyslipidemia is considered an important modifiable risk factor for CVD [16,17], the relationship between dyslipidemia and serum ferritin levels may serve as a predictive factor for cardiometabolic disease in adolescents. While a previous study has shown an association between serum ferritin levels and obesity in Korean male adolescents [24], few studies have examined the relationship between serum ferritin levels and dyslipidemia. Therefore, the aim of this study was to investigate the association between serum ferritin levels and dyslipidemia in South Korean adolescents.
Methods
Survey overview and study subjects
This study was based on data obtained from the 2009–2010 Korean National Health and Nutrition Examination Survey (KNHANES), the third year of the KNHANES IV (2007–2009) survey, and the first year of the KNHANES V(2010–2012) survey. The KNHANES conducted by the Korean Ministry of Health and Welfare. This survey was a nationwide representative study that used a stratified, multistage, probability sampling design for the selection of household units and consisted of 3 components: the health interview, nutrition, and health examination surveys.
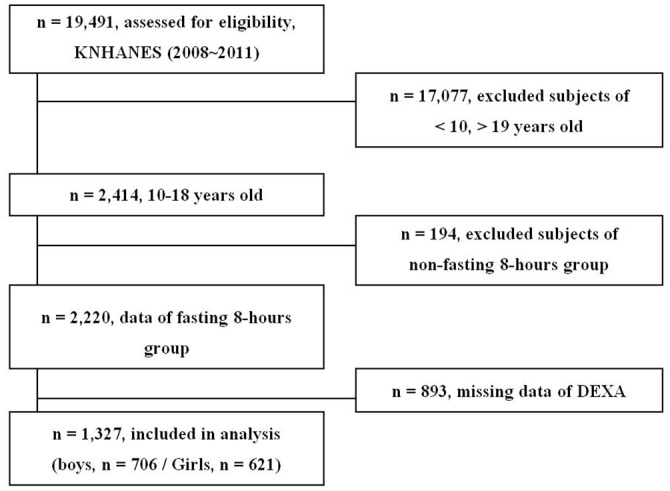
A total of 19,491 people participated in the 2009–2010 KNHANES. The present analysis was restricted to 2,414 adolescents aged 10–18 years; 5 individuals who had chronic hepatitis B virus were excluded. The standard anemia cutoff values associated with age were applied to exclude adolescents with anemia or iron deficiency (Fig 1). Consequently, 64 participants were not included in the study. Finally, 466 subjects with missing data were also excluded. The total number of participants eligible for inclusion in the final analysis was 1,879 (1,026 boys and 853 girls; Fig 2).
Fig 1. Standard anemia cutoff values associated with age.
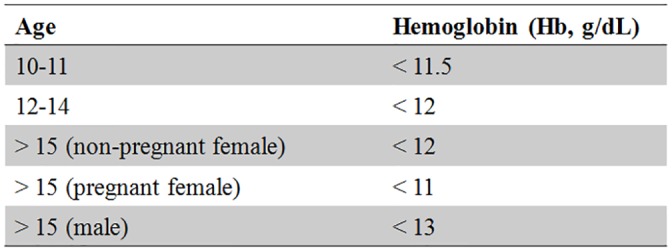
Fig 2. Flow diagram showing the inclusion and exclusion criteria.
This study was conducted according to the principles of Helsinki and the study protocol was reviewed and approved by the KCDC (Korea Centers for Disease Control and Prevention)’s institutional review board (IRB approval number: 2009-01CON-03-2C, 2010-02CON-21-C). Written informed consent was obtained from all subjects. The Institutional Review Board of the KCDC approved the study protocol.
Sociodemographic and lifestyle variables
The sociodemographic and lifestyle variables that were regarded as confounding factors in this study were age, alcohol use, smoking status, physical activity, household income level, and menarche in girls. Health survey of alcohol use, smoking status, physical activity was done by self-reported questionnaire. Questionnaires for alcohol use and smoking status include the evaluation of frequency, quantity, dependence and addiction. Based on drinking of alcohol within one year, subjects were classified as drinkers and non-drinkers. Also, subjects were categorized as non-smokers or smokers according to smoking within a month. Participants recalled their level of physical activities for a week by the days and the duration. Subjects were categorized into 3 groups according to their physical activity levels as follows: sedentary, < 600 metabolic equivalents (METs)/week; minimally active, 600–3000 METs/week; and health-enhancing physical activity, > 3000 METs/week (Sedentary; walking more than 10minutes/ minimally active; Slowly swimming, tennis doubles, volleyball, badminton, table tennis, carrying light weight objects / health-enhancing physical activity; Jogging, hiking, fast bike riding, fast swimming, football, basketball, jump rope, squash, tennis singles, carrying heavy objects) [25]. Monthly household income level was divided into 2 groups: those in the lowest quartile and those above the lowest quartile.
Anthropometric and biochemical measurements
Height and weight measurements were performed with the participants wearing light clothing and no shoes. The body mass index (BMI) of the participants was calculated as their weight in kilograms divided by the square of their height in meters. Blood pressure was measured with subjects in the sitting position following a 5-minute rest period. Blood pressure was measured on the right arm on 3 occasions with a mercury sphygmomanometer and the final 2 blood pressure readings were averaged. Waist circumference was measured once and it was measured midway between the costal margin and iliac crest at the end of a normal expiration. Blood samples were obtained in the morning following an overnight fast. The serum concentrations of fasting blood glucose (FBG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG) were measured enzymatically using a Hitachi automatic analyzer 7600 (Tokyo, Japan). Serum ferritin, 25-hydroxyvitaminD (25[OH]D) and insulin levels were measured by immunoradiometric assays using a 1470 Wizard gamma-counter (Perkin-Elmer, Turku, Finland). Blood Hb levels were measured using the XE-2100D (Sysmex, Tokyo, Japan). All clinical analyses were performed by the Neodin Medical Institute, a laboratory certified by the Korean Ministry of Health and Welfare.
Nutritional assessment
Daily nutritional intakes were assessed by the 24-h recall method. The 24-hour-recall foods were recorded for 24 hours prior to the study by once. The 24h recalls were spread throughout the week including week and weekend days. Participants filled out food intake questionnaires for 24 hours prior to the study including food name, weight, volume, materials, manufacturers, whether processed or not. Information of food materials accessed by eye measurement and the volume measuring tools was used to increase the accuracy in the intake investigation. The information was based on the memories of participants but written by using unified measuring tools [26]. Daily intake of total energy, protein, fat and calcium were calculated by using a food database developed for the KNHANES and the food composition table published by the National Rural Living Science Institute under the Rural Development Administration.
Definition of dyslipidemia and insulin resistance of adolescents
Subjects were categorized into quartiles by their lipid parameters, which were classified according to age and gender. For TC, LDL-C, and TG concentrations, those in the highest quartile were defined as the dyslipidemia group, and for HDL-C values, those in the lowest quartile were assigned to the low HDL-C group. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by following equation:
Participants were categorized into quartile groups according to HOMA-IR and FSI levels with age-specific cutoff values for boys and girls. The values were based on the 2009–2010 KNHANES survey.
Statistical analysis
General baseline characteristics were presented as the mean ± standard error (SE) or as a percentage ±SE. The differences in baseline characteristics between the boys and girls were assessed by using the Student’s t-test for continuous variables or the chi-square test for categorical variables. Multivariable linear regression analysis was performed to assess the correlation between ferritin levels, and dyslipidemia parameters and insulin resistance in 3 multivariable adjusted models. Multivariable logistic regression analysis was used to determine odds ratios (ORs) and 95% confidence intervals (CIs) for dyslipidemia in accordance with serum ferritin levels. Model 1 was adjusted for age. Model 2 was adjusted for height, weight, and age. Model 3 was adjusted for age; height; weight; place; smoking status; drinking; physical activity; serum 25(OH)D levels; total daily energy intake; fat, protein, calcium intakes; and menarche in girls. For each dyslipidemic parameter, the ORs for the highest tertile group of serum ferritin levels were calculated by using the lowest tertile as a reference. Serum ferritin levels were estimated by analysis of covariance (ANCOVA) after adjusting for age, height, and weight, following consideration of the number of dyslipidemic parameters satisfied. The SAS survey procedure (version 9.2; SAS Institute, Cary, N.C., U.S.A.) was used for statistical analyses. P < 0.05 was considered statistically significant.
Result
Baseline characteristics of study subjects
The clinical features of the study participants are listed in Table 1 of the 1,879 participants, 853 (45.4%) were girls. There were no significant differences between the mean ages of the girls and boys (P = 0.135). Compared with the girls, the boys had higher mean values for Hb, hematocrit, serum ferritin, height, weight, BMI, systolic and diastolic blood pressure, aspartate aminotransferase, and alanine aminotransferase (P < 0.001 for each value). The levels of total cholesterol, LDL-C, and HDL-C were all higher in girls (P < 0.001). However, there was no gender difference in TG levels. Furthermore, fasting insulin and HOMA-IR were all higher in girls (P < 0.05).
Table 1. Principal clinical characteristics of Korean adolescents in the 2009–2010 Korean National Health and Nutrition Examination Survey.
| Boys | Girls | P-value* | |
|---|---|---|---|
| (n = 1026) | (n = 853) | ||
| Place (Rural,yes, %) † | 83.6(2.7) | 82(3.4) | 0.492 |
| Smoking (yes, %) | 19.3(1.6) | 6.6(1.2) | <0.001 |
| Alcohol intake (yes, %) | 25.9(1.7) | 17.6(2) | 0.002 |
| Physical activity (%)** | <0.001 | ||
| Sedentary | 31.6(1.6) | 43.6(2.1) | |
| Minimally active | 41.6(1.9) | 44.5(2.1) | |
| Health-enhancing activity | 26.8(1.6) | 11.8(1.5) | |
| Monthly income (lowest quartile, %) | 14.5(1.6) | 14.3(1.9) | 0.912 |
| Menarche (yes) | NA | 76.3(2) | . |
| Age (years) | 14.2±0.1 | 14±0.1 | 0.135 |
| Height (cm) | 165.4±0.5 | 157.3±0.4 | <0.001 |
| Weight (kg) | 58.1±0.6 | 50.3±0.5 | <0.001 |
| BMI (kg/m2) | 21±0.1 | 20.2±0.2 | 0.001 |
| SBP (mmHg) | 110±0.4 | 104.2±0.4 | <0.001 |
| DBP (mmHg) | 68.9±0.4 | 66.8±0.4 | <0.001 |
| FBG (mg/dL) | 89.3±0.3 | 88.3±0.3 | 0.002 |
| TC (mg/dL) | 152.9±1.2 | 163.1±1 | <0.001 |
| LDL-C (mg/dL) | 83.9±1.1 | 90.2±0.9 | <0.001 |
| HDL-C (mg/dL) | 52.1±0.4 | 55.5±0.5 | <0.001 |
| TG (mg/dL) | 73.7(70.6–77) | 77.2(74.2–80.3) | 0.084 |
| 25(OH)D3 (ng/mL) | 17.1±0.3 | 16.3±0.3 | 0.006 |
| FSI (μIU/mL) | 12(11.7–12.4) | 12.9(12.4–13.4) | 0.005 |
| HOMA-IR | 2.6(2.6–2.7) | 2.8(2.7–2.9) | 0.031 |
| Hemoglobin (g/dL) | 14.6±0 | 13.4±0 | <0.001 |
| Hematocrit (%) | 43.3±0.1 | 40.1±0.1 | <0.001 |
| Serum ferritin (ng/mL) | 42.7(40.7,44.9) | 26.9(25.4,28.6) | <0.001 |
| ALT (IU/L) | 14.7(14.1–15.3) | 10.9(10.6–11.2) | <0.001 |
| AST (IU/L) | 19.4(19–19.8) | 17(16.7–17.3) | <0.001 |
| Daily energy intake (kcal) | 2290.9±39.3 | 1843.6±35.7 | <0.001 |
| Daily protein intake (%) | 14.3±0.2 | 13.7±0.2 | 0.013 |
| Daily fat intake (%) | 22.6±0.3 | 22.6±0.4 | 0.923 |
| Daily intake of calcium (mg) | 539.4±15.7 | 450.6±13.8 | <0.001 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; 25(OH)D3, 25-hydroxyvitamin D3; FSI, fasting serum insulin; HOMA-IR, homeostasis model assessment of insulin resistance; ALT, alanine transaminase; AST, aspartate transaminase; NA, not applicable.
Data are presented as mean ± standard error (SE) or percentage ±SE.
TG, AST, ALT, FSI levels, and HOMA-IR were tested after logarithmic transformation.
* P-values were obtained by using the chi-square test or Student’s t-test.
** Study subjects were categorized according to their physical activity levels as follows: sedentary, < 600 metabolic equivalents (METs)/week; minimally active, 600–3000METs/week; and health-enhancing physical activity, > 3000METs/week.
† Place means the area of residence and was categorized into rural and urban area. Urban area included metropolitan cities and province such as ward and county were classified into rural area.
Relationship of serum ferritin level with lipid parameters and HOMA–IR
Table 2 shows the relationship of serum ferritin levels with lipid parameters and HOMA-IR. In boys, total cholesterol, LDL-C, and TG concentrations were positively correlated with serum ferritin levels. HDL-C values were negatively associated with serum ferritin levels in both boys and girls. In girls, there was no positive correlation between serum ferritin levels and TC, LDL, or TG concentrations. There was no significant statistical association between HOMA-IR and any of the lipid parameters in the multivariable-adjusted models.
Table 2. Multivariable linear regression analysis to determine the association of serum ferritin levels with lipid parameters and the homeostasis model assessment of insulin resistance by gender.
| Response variable | Model | Boys | Girls | ||||
|---|---|---|---|---|---|---|---|
| B | SE | P-value* | B | SE | P-value* | ||
| TC | Model 1 | 9.783 | 2.002 | <0.001 | -0.327 | 1.648 | 0.843 |
| Model 2 | 8.7 | 1.964 | <0.001 | -0.45 | 1.588 | 0.777 | |
| Model 3 | 8.36 | 2.014 | <0.001 | -0.676 | 1.67 | 0.686 | |
| HDL-C | Model 1 | -2.55 | 0.724 | 0.001 | -2.115 | 0.704 | 0.003 |
| Model 2 | -2.129 | 0.695 | 0.002 | -2.193 | 0.64 | <0.001 | |
| Model 3 | -2.137 | 0.695 | 0.002 | -2.116 | 0.657 | 0.002 | |
| LDL-C | Model 1 | 10.126 | 1.72 | <0.001 | 1.235 | 1.415 | 0.384 |
| Model 2 | 9.203 | 1.688 | <0.001 | 1.173 | 1.292 | 0.365 | |
| Model 3 | 9.043 | 1.726 | <0.001 | 0.815 | 1.365 | 0.551 | |
| TG** | Model 1 | 0.124 | 0.037 | 0.001 | 0.007 | 0.033 | 0.824 |
| Model 2 | 0.095 | 0.037 | 0.012 | 0.008 | 0.031 | 0.804 | |
| Model 3 | 0.085 | 0.037 | 0.023 | 0.011 | 0.032 | 0.737 | |
| HOMA-IR** | Model 1 | 0.02 | 0.03 | 0.508 | 0.008 | 0.035 | 0.819 |
| Model 2 | -0.022 | 0.026 | 0.385 | 0.013 | 0.03 | 0.664 | |
| Model 3 | -0.021 | 0.026 | 0.419 | 0.018 | 0.03 | 0.562 | |
Abbreviations: B, unstandardized regression coefficient; SE, standard error; TC,total cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; HOMA-IR, homeostasis model assessment of insulin resistance.
* P-values were obtained by multivariable linear regression analyses after adjusting for age (Model 1); age, height, and weight (Model 2); and age, height, weight, place, smoking status, drinking, physical activity, serum 25(OH)D level, total daily energy intake, fat intake, protein intake, calcium intake, and menarche in girls (Model 3).
** TG levels and HOMA-IR were tested after logarithmic transformation.
Adjusted ORs and 95%CIs for dyslipidemia according to serum ferritin levels
Table 3 shows the adjusted ORs and 95% CIs for dyslipidemic parameters according to each increase in log-transformed serum ferritin levels. In boys, the ORs for high TC, high LDL-C, and low HDL-C concentrations were significantly increased in the highest tertile group of serum ferritin levels. The ORs for high TG values in the highest tertile group of serum ferritin levels were also significantly increased in Models 1 and 2, but not in Model 3. However, this trend was not observed in girls. Only the ORs for low HDL-C values were significantly increased in all multivariable-adjusted models in both genders. The ORs for high HOMA-IR were only significant in Model 1 in boys.
Table 3. Adjusted odds ratios for dyslipidemic parameters according to each increase in log-transformed serum ferritin levels.
| Model | Response variables | Boys | Girls | ||
|---|---|---|---|---|---|
| OR (95% CI)* | P-value | OR (95% CI)* | P-value | ||
| Model 1 | High TC | 1.733(1.321–2.274) | < 0.001 | 0.948(0.687–1.308) | 0.745 |
| Low HDL-C | 1.917(1.387–2.648) | < 0.001 | 1.793(1.336–2.406) | <0.001 | |
| High LDL | 2.053(1.564–2.694) | < 0.001 | 1.246(0.917–1.693) | 0.16 | |
| High TG | 1.558(1.137–2.135) | 0.006 | 0.987(0.722–1.35) | 0.936 | |
| High HOMA-IR | 1.420(1.035–1.948) | 0.03 | 0.939(0.682–1.294) | 0.702 | |
| Model 2 | High TC | 1.529(1.159–2.017) | 0.003 | 0.937(0.687–1.276) | 0.679 |
| Low HDL-C | 1.786(1.268–2.515) | 0.001 | 1.828(1.341–2.492) | <0.001 | |
| High LDL | 1.824(1.374–2.422) | < 0.001 | 1.231(0.919–1.649) | 0.164 | |
| High TG | 1.409(1.022–1.941) | 0.036 | 0.961(0.714–1.295) | 0.794 | |
| High HOMA-IR | 1.124(0.805–1.567) | 0.493 | 0.888(0.635–1.243) | 0.489 | |
| Model 3 | High TC | 1.485(1.065–2.07) | 0.02 | 0.901(0.655–1.238) | 0.519 |
| Low HDL-C | 1.636(1.124–2.379) | 0.01 | 1.866(1.308–2.662) | <0.001 | |
| High LDL | 1.882(1.337–2.648) | < 0.001 | 1.144(0.840–1.558) | 0.393 | |
| High TG | 1.299(0.908–1.859) | 0.153 | 1.161(0.853–1.580) | 0.342 | |
| High HOMA-IR | 0.926(0.606–1.414) | 0.722 | 1.002(0.694–1.446) | 0.991 |
Abbreviations: OR, odds ratio; CI, confidential intervals; TC, total cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; HOMA-IR, homeostasis model assessment of insulin resistance.
* Odds ratios and 95% confidence intervals were obtained by multivariable logistic regression analysis after adjusting for age (Model 1); age, height, and weight (Model 2); and age, height, weight, place, smoking status, drinking, physical activity, serum 25(OH)D level, total daily energy intake, fat intake, protein intake, calcium intake, and menarche in girls (Model 3).
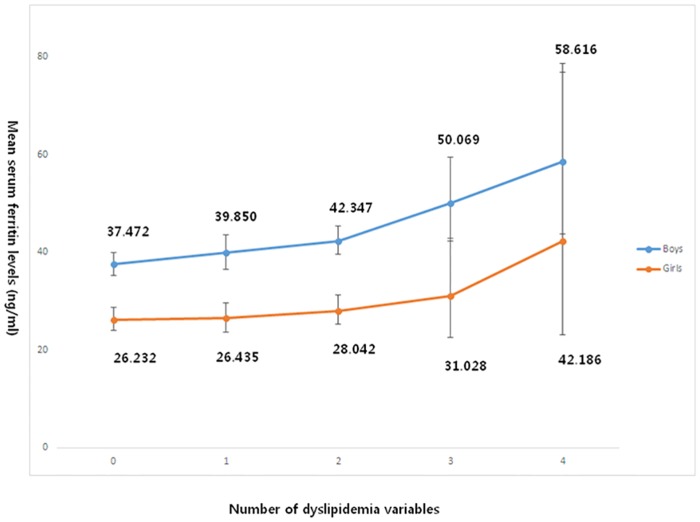
Estimated serum ferritin levels according to the number of dyslipidemic parameters
Fig 3 shows the trend in serum ferritin levels according to the number of satisfied dyslipidemic parameters, which were estimated by ANCOVA after adjustments for age, height, weight, place, smoking status, drinking, physical activity, serum 25(OH)D levels, total daily energy intake, fat, protein, calcium intakes and menarche in girls. The mean serum ferritin levels significantly increased as the number of satisfied dyslipidemic parameters accumulated in both genders (boys, P < 0.001; girls, P < 0.06).
Fig 3. Analysis of covariance of serum ferritin levels according to the number of satisfied dyslipidemia variables in age-, height-, weight-, place-, smoking status-, drinking-, physical activity-, serum 25(OH)D levels-, total daily energy intake-, fat-, protein-, calcium intakes; and menarche in girls-adjusted model (boys, P < 0.001; girls, P = 0.06).
Discussion
The major finding of this study was that serum ferritin levels were associated with prevalent dyslipidemia in a large cross-sectional study of Korean adolescents from the 2009–2010 KNHANES. There was a strong positive association between TC, LDL-C, and TG, and serum ferritin levels in boys, while there was a significant negative correlation between HDL-C and serum ferritin levels in both genders.
As a protein of iron-storage, the serum ferritin is a tool for the evaluation of common disease states, such as iron deficiency anemia. And also it is able to evaluating hereditary and acquired iron overload conditions, such as hereditary hemochromatosis, thalassemias, hemoglobinopathy and chronic transfusion therapy [27]. So, diseases that might affect the iron storage are possible determinant of serum ferritin levels. Furthermore serum ferritin is also recognized as an acute phase marker of inflammation such as chronic kidney disease [28], rheumatoid arthritis and other autoimmune disorders [29]. And many studies had shown the serum ferritin as a determinant of myocardiac ischemia [30], metabolic syndrome [31], Non-alcoholic fatty liver disease and hyperinsulinemia [32].
In addition, iron is a transition metal capable of causing oxidative stress-induced tissue damage by catalyzing the conversion of hydrogen peroxide to free radicals that attack cellular membranes, proteins, and DNA [1,8,12,33], leading to derangements in glucose homeostasis such as insulin resistance and pancreatic β-cell dysfunction through iron toxicity [34,35]. Chronic oxidative stress is associated with disruption of mitochondrial β-oxidation of long chain fatty acids and β-cell dysfunction in the pancreas [36,37]. Abnormal mitochondrial β-oxidation leads to hypertriglyceridemia, and excessive triglyceride accumulation in muscle and liver tissue, while pancreatic β-cell dysfunction results in hyperglycemia due to insufficient insulin production [36,37].
Previously, several studies have suggested apositive relationship between serum ferritin levels and metabolic risk factors in adults. Kang et al. reported that increased serum ferritin levels were associated with high TG and FBG concentrations in men and women [5]. Yoo et al. confirmed that low HDL-C values and MS were associated with high serum ferritin levels in Korean non-obese young women [8]. Similarly, Lee et al. suggested that high serum ferritin levels were linked to MS in women and to diabetes mellitus in men and premenopausal women [7]. Moreover, in 2004, Jehn et al. conducted a cross-sectional study of 6,044 U.S. adults. In this study, they observed a positive correlation between serum ferritin levels and the prevalence of MS components such as TG and plasma glucose in men and women [6]. In 2013, in China, Li et al. showed an association between serum ferritin levels and MS in 8,441 Chinese adults, ≥ 18 years. MS was most prevalent in men and women in the highest quartile group for serum ferritin levels, and included those with high TG and low HDL-C concentrations [38]. Chang et al. also suggested that high serum ferritin levels were significantly associated with FBG and TG values [39]. Our research extended these previous results by studying adolescents.
Although some large cross-sectional studies have suggested an association between ferritin levels, and dyslipidemia and some metabolic diseases, few studies have focused on adolescents. Recently, Lee et al. reported that children in a group with high HOMA-IR had greater total iron-binding capacity and serum transferrin levels, and were more likely to develop MS, butthey could not demonstrate an association between serum ferritin levels and dyslipidemia parameters [40]. A study byJeon et al. also examined dyslipidemia parameters in Korean adolescents, and observed a link between serum ferritin levels and obesity, but not between serum ferritin levels and MS components [24]. In contrast to previous studies, we observed an association between serum ferritin levels and dyslipidemia, especially in boys.
Interestingly, the present study identified a gender difference in the association of serum ferritin levels with lipid parameters. In boys, dyslipidemia parameters such as TC, LDL-C, HDL-C, and TG were all significantly associated with serum ferritin levels. Conversely, in girls, serum ferritin levels were only significantly associated with low HDL-C concentrations. Gender differences were seenin other studies on adults previously. However, many studies have suggested that serum ferritin levels appear to be correlated with metabolic risk factors or disease in women rather than men [7,8]. In agreement with our study, Han et al. showed that elevated serum ferritin levels were positively associated with MS, obesity, and diabetes in men, but not in women [41]. Although the underlying cause of gender differences in adolescents is unclear, intrinsic sexual dimorphisms at the molecular and cellular levels, and the effects of different sex steroid hormones may play a part [42]. In addition, the transient physiological increase in insulin resistance during puberty could lead to increased activity of the insulin-like growth factor-I/growth hormone axis [43] or sex hormones [44]. In adults, men have more visceral fat accumulation and lower plasma adiponectin, which contribute to gender differences in insulin sensitivity and vulnerability to cardiovascular disease [45]. Women are intrinsically more insulin resistant than men, and this may be due to specific sex-linked gene expression which affects metabolic control elements (e.g., signaling pathway and substrate shuttling elements, and receptors). Sex hormones, and environmental and life-style factors augment women’s susceptibility, in ways that may be genetically predetermined [42].
The present study has several limitations. First, because we used a cross-sectional study design, it was difficult to examine the causal relationship between serum ferritin levels and dyslipidemia. Second, we could not exclude subjects with active infection or inflammation, or those with elevated inflammatory markers such as white blood cell counts or C-reactive protein. These could be potential confounding factors and affect our results. Third, serum iron, transferrin, and total iron-binding capacity were not measured in conjunction with serum ferritin levels in the 2009–2010 KNHANES. Fourth, regarding alcohol consumption, no dose related effect can be taken into account as only a dichotomous variable was coded, classifying subjects as drinkers or non-drinkers. The same applies to smoking status and it could be the limitation of this study. Despite these limitations, it is worth noting that this study was conducted using nationwide representative data from a survey based on a stratified, multistage, probability sampling design. Furthermore, few studies have examined the relationship between serum ferritin levels and metabolic risk factors in adolescents. To our knowledge, this study is the first to report an association between serum ferritin levels and various dyslipidemia parameters in adolescents using recent nationally representative data.
In conclusion, serum ferritin levels were significantly associated with major dyslipidemia parameters, more prominently in boys than in girls, and this association represents a cardiometabolic risk factor.
Abbreviations
- ALT
alanine aminotransferase
- ANCOVA
analysis of covariance
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular diseases
- FBG
fasting blood glucose
- Hb
hemoglobin
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment of insulin resistance
- KCDC
Korean Centers for Disease Control
- KNHANES
Korea National Health Examination and Nutrition Survey
- LDL-C
low-density lipoprotein cholesterol
- METs
metabolic equivalents
- MS
metabolic syndrome
- OR
odds ratio
- SE
standard error
- TC
total cholesterol
- TG
triglyceride
- 25(OH)D
25-hydroxyvitamin D
Data Availability
All data are third party and belong to the Korea National Health and Nutrition Examination Survey. To access this data: 1. Please go to the following website [https://knhanes.cdc.go.kr]. 2. Select language “ English” 3. Click “Survey data” and “Data downloads” 4. Enter your E-mail address for creating your account 5. You can download original data of KNHANES IV and V.
Funding Statement
The authors have no support or funding to report.
References
- 1.Andrews NC (1999) Disorders of iron metabolism. N Engl J Med 341: 1986–1995. [DOI] [PubMed] [Google Scholar]
- 2.Wolff SP (1993) Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull 49: 642–652. [DOI] [PubMed] [Google Scholar]
- 3.Gillum RF (2001) Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men—the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord 25: 639–645. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki T, Nakajima A, Yoneda M, Yamada Y, Mukasa K, Fujita K, et al. (2005) Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care 28: 2486–2491. [DOI] [PubMed] [Google Scholar]
- 5.Kang HT, Linton JA, Shim JY (2012) Serum ferritin level is associated with the prevalence of metabolic syndrome in Korean adults: the 2007–2008 Korean National Health and Nutrition Examination Survey. Clin Chim Acta 413: 636–641. 10.1016/j.cca.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 6.Jehn M, Clark JM, Guallar E (2004) Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care 27: 2422–2428. [DOI] [PubMed] [Google Scholar]
- 7.Lee BK, Kim Y, Kim YI (2011) Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism 60: 1416–1424. 10.1016/j.metabol.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 8.Yoo KD, Ko SH, Park JE, Ahn YB, Yim HW, Lee WC, et al. (2012) High serum ferritin levels are associated with metabolic risk factors in non-obese Korean young adults: Korean National Health and Nutrition Examination Survey (KNHANES) IV. Clin Endocrinol (Oxf) 77: 233–240. [DOI] [PubMed] [Google Scholar]
- 9.Williams MJ, Poulton R, Williams S (2002) Relationship of serum ferritin with cardiovascular risk factors and inflammation in young men and women. Atherosclerosis 165: 179–184. [DOI] [PubMed] [Google Scholar]
- 10.Piperno A, Trombini P, Gelosa M, Mauri V, Pecci V, Vergani A, et al. (2002) Increased serum ferritin is common in men with essential hypertension. J Hypertens 20: 1513–1518. [DOI] [PubMed] [Google Scholar]
- 11.Kim CH, Kim HK, Bae SJ, Park JY, Lee KU (2011) Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism 60: 414–420. 10.1016/j.metabol.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 12.Pham NM, Nanri A, Yi S, Kurotani K, Akter S, Foo LH, et al. (2013) Serum ferritin is associated with markers of insulin resistance in Japanese men but not in women. Metabolism 62: 561–567. 10.1016/j.metabol.2012.07.025 [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Cogswell ME (1999) Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care 22: 1978–1983. [DOI] [PubMed] [Google Scholar]
- 14.Kim CW, Chang Y, Sung E, Shin H, Ryu S (2012) Serum ferritin levels predict incident non-alcoholic fatty liver disease in healthy Korean men. Metabolism 61: 1182–1188. 10.1016/j.metabol.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 15.(1992) National Cholesterol Education Program (NCEP): highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics 89: 495–501. [PubMed] [Google Scholar]
- 16.Daniels SR, Greer FR (2008) Lipid screening and cardiovascular health in childhood. Pediatrics 122: 198–208. 10.1542/peds.2008-1349 [DOI] [PubMed] [Google Scholar]
- 17.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K (2003) American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation 107: 1562–1566. [DOI] [PubMed] [Google Scholar]
- 18.Children NCEPEPoBCLi (1991) Highlights of the report of the expert panel on blood cholesterol levels in children and adolescents: US Dept. of Health and Human Services, Public Health Service, National Institutes of Health. [Google Scholar]
- 19.McGill HC Jr, McMahan CA, Zieske AW, Malcom GT, Tracy RE, Strong JP (2001) Effects of nonlipid risk factors on atherosclerosis in youth with a favorable lipoprotein profile. Circulation 103: 1546–1550. [DOI] [PubMed] [Google Scholar]
- 20.McGill HC Jr, McMahan CA, Zieske AW, Sloop GD, Walcott JV, Troxclair DA, et al. (2000) Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol 20: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 21.Kouda K, Nakamura H, Tokunaga R, Takeuchi H (2004) Trends in levels of cholesterol in Japanese children from 1993 through 2001. J Epidemiol 14: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HM, Park J, Kim HS, Kim DH, Park SH (2006) Obesity and cardiovascular risk factors in Korean children and adolescents aged 10–18 years from the Korean National Health and Nutrition Examination Survey, 1998 and 2001. Am J Epidemiol 164: 787–793. [DOI] [PubMed] [Google Scholar]
- 23.Liao Y, Liu Y, Mi J, Tang C, Du J (2008) Risk factors for dyslipidemia in Chinese children. Acta Paediatr 97: 1449–1453. 10.1111/j.1651-2227.2008.00946.x [DOI] [PubMed] [Google Scholar]
- 24.Jeon YJ, Jung IA, Kim SH, Cho WK, Jeong SH, Cho KS, et al. (2013) Serum ferritin level is higher in male adolescents with obesity: results from the Korean National Health and Nutrition Examination Survey 2010. Ann Pediatr Endocrinol Metab 18: 141–147. 10.6065/apem.2013.18.3.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun MY (2012) Validity and reliability of korean version of international physical activity questionnaire short form in the elderly. Korean J Fam Med 33: 144–151. 10.4082/kjfm.2012.33.3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DW, Song S, Lee JE, Oh K, Shim J, Kweon S, et al. (2015) Reproducibility and validity of an FFQ developed for the Korea National Health and Nutrition Examination Survey (KNHANES). Public Health Nutr 18: 1369–1377. 10.1017/S1368980014001712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV (2010) Serum ferritin: Past, present and future. Biochim Biophys Acta 1800: 760–769. 10.1016/j.bbagen.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH (2006) The fascinating but deceptive ferritin: to measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 1 Suppl 1: S9–18. [DOI] [PubMed] [Google Scholar]
- 29.Zandman-Goddard G, Shoenfeld Y (2007) Ferritin in autoimmune diseases. Autoimmun Rev 6: 457–463. [DOI] [PubMed] [Google Scholar]
- 30.Yalta K, Sivri N, Yalta T, Yetkin E (2011) Serum ferritin: a potential determinant of myocardial ischemic burden in the setting of ischemic conditions? Int J Cardiol 153: 225–226. 10.1016/j.ijcard.2011.09.057 [DOI] [PubMed] [Google Scholar]
- 31.Tsimihodimos V, Gazi I, Kalaitzidis R, Elisaf M, Siamopoulos KC (2006) Increased serum ferritin concentrations and liver enzyme activities in patients with metabolic syndrome. Metab Syndr Relat Disord 4: 196–203. 10.1089/met.2006.4.196 [DOI] [PubMed] [Google Scholar]
- 32.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R (2007) NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol 46: 700–707. [DOI] [PubMed] [Google Scholar]
- 33.Park SK, Ryoo JH, Kim MG, Shin JY (2012) Association of serum ferritin and the development of metabolic syndrome in middle-aged Korean men: a 5-year follow-up study. Diabetes Care 35: 2521–2526. 10.2337/dc12-0543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson JG, Lindquist JH, Grambow SC, Crook ED, Maher JF (2003) Potential role of increased iron stores in diabetes. Am J Med Sci 325: 332–339. [DOI] [PubMed] [Google Scholar]
- 35.Ferrannini E (2000) Insulin resistance, iron, and the liver. Lancet 355: 2181–2182. [DOI] [PubMed] [Google Scholar]
- 36.Yao D, Shi W, Gou Y, Zhou X, Yee Aw T, Zhou Y, et al. (2005) Fatty acid-mediated intracellular iron translocation: a synergistic mechanism of oxidative injury. Free Radic Biol Med 39: 1385–1398. [DOI] [PubMed] [Google Scholar]
- 37.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Wang R, Luo D, Li S, Xiao C (2013) Association between serum ferritin levels and risk of the metabolic syndrome in Chinese adults: a population study. PLoS One 8: e74168 10.1371/journal.pone.0074168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang JS, Lin SM, Huang TC, Chao JC, Chen YC, Pan WH, et al. (2013) Serum ferritin and risk of the metabolic syndrome: a population-based study. Asia Pac J Clin Nutr 22: 400–407. 10.6133/apjcn.2013.22.3.07 [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Jang HB, Park JE, Park KH, Kang JH, Park SI, et al. (2014) Relationship between Serum Levels of Body Iron Parameters and Insulin Resistance and Metabolic Syndrome in Korean Children. Osong Public Health Res Perspect 5: 204–210. 10.1016/j.phrp.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han LL, Wang YX, Li J, Zhang XL, Bian C, Wang H, et al. (2014) Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res 58: 2189–2195. 10.1002/mnfr.201400088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mittendorfer B (2005) Insulin resistance: sex matters. Curr Opin Clin Nutr Metab Care 8: 367–372. [DOI] [PubMed] [Google Scholar]
- 43.Moran A, Jacobs DR Jr, Steinberger J, Cohen P, Hong CP, Prineas R, et al. (2002) Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. J Clin Endocrinol Metab 87: 4817–4820. [DOI] [PubMed] [Google Scholar]
- 44.Moriarty-Kelsey M, Harwood JE, Travers SH, Zeitler PS, Nadeau KJ (2010) Testosterone, obesity and insulin resistance in young males: evidence for an association between gonadal dysfunction and insulin resistance during puberty. J Pediatr Endocrinol Metab 23: 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. (2002) Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 51: 2734–2741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are third party and belong to the Korea National Health and Nutrition Examination Survey. To access this data: 1. Please go to the following website [https://knhanes.cdc.go.kr]. 2. Select language “ English” 3. Click “Survey data” and “Data downloads” 4. Enter your E-mail address for creating your account 5. You can download original data of KNHANES IV and V.