Abstract
Miscanthus has been proposed as a promising crop for phytoremediation due to its high biomass yield and remarkable adaptability to different environments. However, little is known about the resistance of Miscanthus spp. to cadmium (Cd). To determine any differences in resistance of Miscanthus to Cd, we examined plant growth, net photosynthetic rate (Pn), activities of anti-oxidant and C4 photosynthetic enzymes, concentrations of Cd in leaves and roots, and observed the chloroplast structure in three Miscanthus species treated with 0, 10, 50, 100 or 200 μM Cd in solutions. Miscanthus sinensis showed more sensitivity to Cd, including sharp decreases in growth, Pn, PEPC activity and damage to chloroplast structure, and the highest H2O2 and Cd concentrations in leaves and roots after Cd treatments. Miscanthus sacchariflorus showed higher resistance to Cd and better growth, had the highest Pn and phosphoenolpyruvate carboxylase (PEPC) activities and integrative chloroplast structure and the lowest hydrogen peroxide (H2O2) and leaf and root Cd concentrations. The results could play an important role in understanding the mechanisms of Cd tolerance in plants and in application of phytoremediation.
Introduction
Soil cadmium (Cd) pollution has posed a serious threat to our soil quality and food security as well as to human health. The sources of Cd contamination is not only introduced through geogenic processes but also derive from anthropogenic activities, such as the by-product of smelting, mining and refining of metal works [1], industrial waste from electroplating, manufacturing of plastics and paint pigments processes [2] and agriculture pollutions including impurities of fertilizers and irrigation with wastewater [3,4]. The toxicant of Cd is higher than that of organic toxic compounds due to its greater mobility and harder degraded and thus resulting in difficult to remove from the environment [5].
Cd is not a necessary element for plant growth and excess Cd has a series of harmful effects. Cd is known to inhibit plant growth, disorder nutrient uptake, affect chloroplast ultrastructure, inactivate enzymes of carbon dioxide (CO2) fixation, inhibit photosynthesis and damage the structure and function of photosystem II [6–8]. It was also reported that Cd generates oxidative stress in plants through inducing the production of reactive oxygen species (ROS), including superoxide anion radicals (O2–), hydroxyl radicals and hydrogen peroxide (H2O2) [9]. To remove ROS, plants have evolved a series of anti-oxidant enzymatic systems including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) [6]. Fernández et al. [7] showed that the effective Cd detoxification of Dittrichia viscosa (L.) Greuter consisted mainly of promoted APX activity and greater efficiency of CAT and GR.
Among the various strategies adopted for removal of Cd from contaminated soils, phytoremediation has been proposed as an economical, eco-friendly and aesthetically acceptable technology to reduce the risk of soil contamination [10,11]. The hyper-accumulation of heavy metals in some plants has been recorded by many researchers during the last few decades [12,13]. However, many hyper-accumulator plants have slow growth and low biomass, and are difficult to grow and harvest [14]. Miscanthus spp. has been proposed as promising crops for phytoremediation due to high biomass yield and remarkable adaptability to different environments [15,16]. M. sinensis, M. floridulus and M. sacchariflorus are all generally found along river banks, in mountain regions and open waste areas of China. M. × giganteus is widely cultured in Europe as a bio-energy crop, which is a sterile, triploid interspecific hybrid from native across with M. sinensis (diploid) and M. sacchariflorus (tetraploid) [17,18]. It has been reported that Miscanthus sinensis exhibits high resistance to the metal stress of acid soil by excreting citric acid [19]. Miscanthus× giganteus is tolerant not only to chromium (Cr), but also to Cd at certain concentration [20]. Pavel et al. [21] reported that M. sinensis × giganteus be used for the production of renewable biomass on zinc (Zn), Cd and lead (Pb) contaminated soils, and for further increase in biomass and reduction of the metal concentrations of plant tissues upon addition of red mud to these soils. However, the related research on the responses of M. floridulus and M. sacchariflorus to Cd stress is scarce. Moreover, there is a lack of data on Cd tolerance and comparisons among different Miscanthus spp. to evaluate their capacity for phytoremediation. Thus, it is of importance, for removing Cd from contaminated soils, to elucidate the mechanism of Cd resistance of different Miscanthus spp.
Materials and Methods
Ethics statement
The seeds of M. sinensis, M. floridulus and M. sacchariflorus were obtained from the Daming Mountain Scenic Area in Linan, Zhejing Province of China, in 2013. The three Miscanthus species are widely distributed throughout this Scenic Area and the local government departments have no special requirements to protect them. The experiments were also permitted according to the rules of Zhejiang University. So these will not cause any controversy. It is also confirmed that the field studies did not involve endangered or protected species by the institute of plant science, Zhejiang University.
Plant materials and growth conditions
Mature seeds of M. sinensis, M. floridulus and M. sacchariflorus were planted in commercial potting mix in plastic trays, and then allowed to germinate at 28°C in the dark for 3 d. Four weeks after germination, the seedlings were transferred to hydroponic cultures supplied with half-strength Hoagland nutrient solution (pH 6.0). Half-strength Hoagland nutrient solution was used containing the following macronutrients in mM: KNO3, 2.5; Ca(NO3)2·4H2O, 2.5; MgSO4·7H2O, 1.0; NH4H2PO4, 0.5, and the following micronutrients in μM: CuSO4·5H2O, 0.5; ZnSO4·7H2O, 1.0; MnCl2, 1.25; H3BO3, 7.5; (NH4)6Mo7O24, 0.25 and NaFeEDTA 50. To ensure proper growth, the solutions were aerated and renewed weekly. Following 32 days of hydroponic growth, seedlings were subjected to aerated nutrient solution including 0, 10, 50, 100 or 200 μM CdCl2. Each treatment was replicated six times and each replicate included eight seedlings. The solutions were renewed every week. The entire experiment was conducted in an environmentally controlled growth room with a 14 h/26°C day (white fluorescent light intensity of 1200 μmol photons m−2 s−1) and 10 h/22°C night regime with relative humidity kept at 65%.
Growth analysis and Cd contents measurement
Growth such as plant height, root length, aerial part and hypogeal-part dry weight were measured after 16 d of treatment. Plant height and the length of the below ground part (root length) were measured on a centimeter scale. Dry weight was determined after drying the samples in an oven at 80°C to a constant weight. The root:shoot ratio was computed as the hypogeal part divided by the aerial part on a dry weight basis.
For Cd content measurement, roots and shoots were separately harvested, and the roots were washed with deionized water for three times. Then shoots and roots were dried at 80°C for 72 h, weighed, ground to fine powder and 0.2 g of each was digested with nitric acid/H2O2 (30:1, v/v) and total Cd content was measured by inductively coupled plasma atomic emission spectrometer (ICP-AES; Fisons ARL Accuris, Ecublens, Switzerland).
Determination of photosynthetic and chlorophyll fluorescence parameters
Photosynthetic parameters of leaves were measured with a Li-6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA). These parameters consisted of net photosynthetic rate (Pn), stomata conductance (gs) and intercellular CO2 concentration (Ci). The data were recorded at 16 d of treatment using the most fully expanded youngest leaves. The light intensities were maintained at 2000 μmol m–2 s–1, and the temperature and external CO2 concentration were maintained at 30°C and 400 μmol L–1, respectively. Five representative plants of each treatment were selected randomly at each measured time-point. For light response curves measurements, a series of light intensities were set as 2500, 2000, 1500, 1200, 800, 600, 400, 300, 200, 100, 50, 30, 10, 0 μmol m–2 s–1 PPFD at an ambient CO2 concentration 400 μmol mol–1 with the LI-COR CO2 mixer. Minimum time and maximum time were respectively set to 1min and 2 min for each given PPFD. Before the measurement, each leaf was adapted at a PPFD of 2500 μmol m–2 s–1 for about 5 min until the stability state of Pn. According to the modified rectangular hyperbola model[22], light compensation point (LCP), the maximum photosynthetic rate (PnMAX), apparent quantum yield (AQY) and dark respiratory rate (DR) were calculated as: P(I) = α·I·(1-β·I)/(1+γ·I)-Rd. Where P(I) is Pn, I is light intensity, Rd is dark respiratory rate, and α, β and γ are coefficients which are independent of I. Once Pn was obtained, the leaf tissue was freeze-clamped quickly at liquid N2 temperature and stored at –80°C for chlorophyll, malondialdehyde (MDA), hydrogen peroxide (H2O2) contents and enzyme activity analysis.
The chlorophyll fluorescence parameters were measured with an chlorophyll fluorescence imaging system (CF imager, Technologica Ltd., Colchester, UK) according to the method of Liu et al. [23] with minor modification. The first fully grown leaves of Miscanthus seedlings treated with different concentrations of Cd were dark-adapted for 20 min with leaf clips, then the leaves were cut off and arranged neatly underneath the fluorometer for recording the minimum fluorescence (F0) and maximum fluorescence (Fm) parameters and getting the false-color images of maximal photochemical efficiency (Fv/Fm) images. The Fv/Fm was calculated as (Fm- F0)/Fm. Then leaves were light-adapted for approximately 15 min prior to measurement of the effective PSII quantum yield [Y(II)] which was calculated as Y(II) = (Fm'-F)/Fm', where Fm' and F were fluorescence at maximum fluorescence and steady-state photosynthesis in the light, respectively.
Determination of photosynthetic pigment contents
Photosynthetic pigments were extracted by soaking 0.1 g of frozen leaf tissues in 80% (v/v) acetone in darkness at room temperature for 45 h. Chlorophyll and carotenoid contents in supernatants were determined with a spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan) at 665, 649 and 470 nm, and calculated using the method of Lichtenthaler and Wellburn [24].
Determination of C4 photosynthetic enzyme activities
The phosphoenolpyruvate carboxylase (PEPC), NADP-malate enzyme (NADP-ME) and NADP-malate dehydrogenase (NADP-MDH) activities of leaves were determined using a commercial chemical assay kit (Jiangsu Keming Biotechnology Institute, Suzhou, China). For the measurement of PEPC and NAD-MDH activities, about 0.1 g of frozen leaf tissues were homogenized in 1 ml buffer I [0.4 M Tris-HCl buffer (pH 8.0), 15 mM EDTA, 10 mM DTT, 5 mM MgCl2 and 2% (w/v) polyethylene pyrrole (PVP)], which is contained in the commercial chemical assay kit, at 4°C with an ice-chilled pestle and mortar, centrifuged at 10,000 rpm at 4°C for 10 min and the supernatant was used for the enzymes activity analysis according to the manufacturer’s instructions. For the measurement of NADP-ME activity, about 0.1 g of frozen leaf tissues were extracted using 1 ml buffer I [0.1 mM KH2PO4/KOH buffer (pH 7.5), 10 mM DTT, 5 mM MgCl2 and 2% (w/v) polyethylene pyrrole (PVP)] according to the above-mentioned method, then analyzed according to the manufacturer’s instructions.
Chloroplast ultrastructure
The chloroplast ultrastructure of bundle sheath cells were observed according to Shao et al. [25]. After 16 days treatment, the fully expanded youngest leaves were immediately fixed in 2.5% (v/v) glutaraldehyde (0.1 mol L–1 phosphate buffer, pH 7.2) for 24 h. Then the samples were post-fixed for 30 min in 1% (v/v) osmium acid, dehydrated in a graded ethanol series (30%–100%, v/v), embedded in Spurr resin and ultrathin-sectioned for transmission electron microscopy (H7650, Hitachi, Tokyo, Japan).
Determination of MDA, H2O2 contents and anti-oxidant enzymes activities
For the determination of SOD, CAT and POD activities, about 0.5 g of frozen leaf tissues were ground at 4°C in a mortar with 5 ml of 50 mM phosphate buffer solution (pH 7.8) containing 1% PVP. The homogenate was centrifuged at 10,000 rpm at 4°C for 30 min. Supernatants were collected for measuring enzyme activities according to Hong et al. [26]. The MDA, H2O2 contents, GR and APX activities, were determined using a commercial chemical assay kit (Jiangsu Keming Biotechnology Institute, Suzhou, China). For the measurement of MDA content and GR and APX activities, about 0.1 g of frozen leaf tissues were homogenized in 1 ml buffer I [50 mM phosphate buffer (pH 7.8), containing 0.1 mM EDTA, 0.5% (w/v) Triton-100 and 2% PVP], which is contained in the commercial chemical assay kit, at 4°C with mortar and pestles, centrifuged at 10,000 rpm at 4°C for 10 min and the supernatant was used for content or enzyme ability analysis according to the manufacturer’s instructions. For the measurement of H2O2 contents, the extraction buffer I was replaced by 1 ml acetone according to the above-mentioned method, then analyzed according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was carried out by one-way or two-way analysis of variance using SPSS (SPSS Inc., USA, version 13.0) and OriginPro (OriginLab Corp., USA, v8.0724). Differences between treatments were evaluated at P < 0.05.
Results
Plant growth and Cd accumulation
The sensitivities of Miscanthus spp. to Cd varies, and roots were more sensitive than shoots (Fig 1 and Table 1). The growth parameters such as root length, dry weight of the hypogeal part and the entire plant dry weight were significantly inhibited (p<0.05) by all Cd concentrations and plant height was significantly decreased (p<0.05) by ≥ 50 μM Cd concentrations in Miscanthus sinensis (Table 1). For M. floridulus there were no significant differences (p<0.05) in root length between 10 μM Cd treatment and control (0 μM Cd), and no significant differences (p<0.05) in plant height, hypogeal part and entire plant between 10 and 50 μM Cd; however, there was a significant difference (p<0.05) in dry weight of aerial parts between all Cd treatments. For M. sacchariflorus, however, in comparison with control 10 μM Cd treatment slightly promoted plant growth according to all growth indexes (Table 1) and 50 μM Cd treatment significantly increased (p<0.05) in dry weight of the hypogeal part and the entire plant, and in root:shoot ratio. Therefore, M. sacchariflorus was more resistant to Cd than the other Miscanthus spp.
Fig 1. Effects of different Cd treatments on seedling growth and morphology of three Miscanthus species after 16 days of Cd treatment.
Growth characteristics of seedlings of (A) M. sinensis, (B) M. floridulus and (C) M. sacchariflorus. Miscanthus seedlings (60-d-old) were grown in Hoagland nutrient solution (pH 6.0) containing 0, 10, 50, 100 and 200 μM CdCl2 for 16 days, respectively. Bar = 10 cm.
Table 1. Growth and dry weight of three Miscanthus species in response to Cd stress for 16 days.
Values represent mean ± SD (n = 4). Differences letters indicate significant differences (P < 0.05) between Cd levels.
| Cultivars | Cadmium (μM) | Plant height (cm) | Root length (cm) | Dry weight (g per plant) | Root-shoot ratio | ||
|---|---|---|---|---|---|---|---|
| Aerial part | Hypogeal part | Entire plant | |||||
| M. sinensis | 0 | 89.33±1.53a | 23.00±1.73a | 4.06±0.09a | 1.10±0.09a | 5.16±0.04a | 0.271±0.021a |
| 10 | 87.67±2.08a | 20.17±0.76b | 3.96±0.11a | 0.89±0.05b | 4.85±0.16b | 0.225±0.020c | |
| 50 | 85.12±1.52b | 16.67±0.58c | 3.72±0.08b | 0.82±0.01c | 4.54±0.20c | 0.220±0.014d | |
| 100 | 77.33±1.53c | 14.37±0.58d | 3.17±0.07c | 0.74±0.01d | 3.91±0.18d | 0.233±0.020b | |
| 200 | 73.00±2.65d | 12.00±1.00e | 2.44±0.25d | 0.57±0.03e | 3.02±0.22e | 0.234±0.010b | |
| M. floridulus | 0 | 87.23±1.37a | 16.00±1.00a | 3.47±0.36a | 0.81±0.04a | 4.28±0.07a | 0.232±0.010c |
| 10 | 83.57±2.04b | 15.33±0.58a | 3.08±0.17b | 0.72±0.07b | 3.80±0.19b | 0.234±0.030c | |
| 50 | 82.00±1.73b | 14.17±0.76b | 2.98±0.07c | 0.70±0.04b | 3.68±0.22b | 0.235±0.017c | |
| 100 | 76.38±1.53c | 13.13±1.21c | 2.68±0.04d | 0.65±0.03c | 3.33±0.04c | 0.243±0.024b | |
| 200 | 72.33±1.53d | 11.93±0.90d | 2.15±0.13e | 0.55±0.09d | 2.70±0.07d | 0.256±0.033a | |
| M. Sacchariflorus | 0 | 93.33±1.15a | 17.67±1.53a | 5.41±1.16a | 1.52±0.32b | 6.93±1.45b | 0.281±0.029d |
| 10 | 94.56±1.03a | 18.00±1.00a | 5.72±1.18a | 1.65±0.35b | 7.37±1.92a | 0.288±0.028d | |
| 50 | 91.63±1.53a | 18.12±1.26a | 5.20±0.74a | 2.25±0.18a | 7.45±1.12a | 0.432±0.010a | |
| 100 | 86.78±1.53b | 15.33±1.15b | 4.60±0.09b | 1.82±0.26b | 6.42±0.18c | 0.396±0.064b | |
| 200 | 82.16±2.08c | 10.50±0.50c | 2.97±0.29c | 0.99±0.12c | 3.96±0.36d | 0.333±0.037c | |
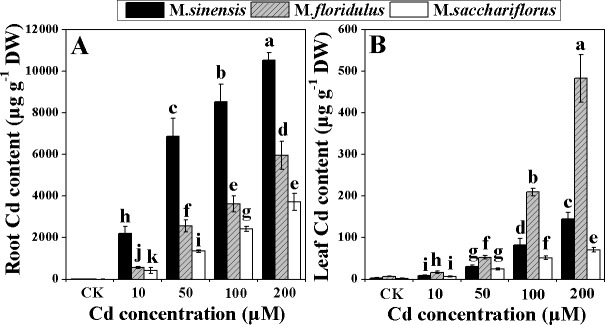
The Cd contents of roots and leaves of Miscanthus spp. were extremely different, although they significantly increased (p<0.05) with increasing Cd concentration (Fig 2A and 2B). In roots, M. sinensis exhibited the highest Cd concentration, followed by M. floridulus and then M. sacchariflorus (Fig 2A). In leaves, M. floridulus had the highest Cd concentration, then M. sinensis and M. sacchariflorus (Fig 2B). Under 200 μM Cd treatment, Cd concentrations in leaves of M. sinensis, M. floridulus and M. sacchariflorus were 146, 210 and 71 μg g–1 dry weight, respectively, while correspondingly in roots they were 10.55, 5.96 and 3.72 mg g–1 dry weight. These results suggested that the Cd mainly accumulated in roots in M. sinensis and was transported to leaves in M. floridulus, while M. sacchariflorus accumulate less Cd in total.
Fig 2.
Change in root Cd content (A) and leaf Cd content (B). Data are mean ± SD (n = 3). Different letters indicate a significant difference at P < 0.05.
Photosynthesis
Photosynthetic parameters among the three Miscanthus species significantly differed (p<0.05) with increasing exogenous Cd concentrations (Fig 3). When treated with 10 μM Cd, Pn and gs of M. sinensis and M. floridulus were significantly depressed (p<0.05) to about one-third of their control values and continuously decreased to 10–20% of controls under 200 μM Cd treatment. However, there were no significant decreases (p<0.05) in Pn and gs of M. sacchariflorus for Cd concentrations < 50 μM, and it maintained about one-third of Pn and gs of controls even when treated with 200 μM Cd (Fig 3A and 3B). Ci enhanced with increasing Cd concentrations for M. sinensis and M. floridulus, but did not change for M. sacchariflorus (Fig 3C). Pn in three Miscanthus species also decreased significantly with increasing Cd concentrations in different light conditions (S1 Fig). Under PAR of 2500 μmol m-2 s-1, the Pn of M. sinensis was decreased by 10.7, 61, 76.9 and 83% in the 10, 50, 100 and 200 μM Cd treatments, respectively (S1A Fig); it was decreased by 36.6, 53.7, 57.4 and 85.1% in M. floridulus (S1B Fig) and 10, 38.7, 54.9 and 84.3% in M. sacchariflorus, respectively (S1C Fig). Similar trends for the effect of different Cd concentrations on the gs of the three Miscanthus species were observed (S1D–S1F Fig) in different light conditions. According to the light response curve (S1 Fig) it was observed that LCP sharply raised (Fig 3D) and AQY dramatically decreased (p<0.05) in all species with increased Cd concentrations (Fig 3E), but the degree of increase in LCP and decrease in AQY was least in M. sacchariflorus (Fig 3D and 3E). Moreover, DR gradually increased and reached a maximum for 50–100 μM Cd treatments in all Miscanthus spp. (Fig 3F).
Fig 3. Changes in photosynthetic parameters.
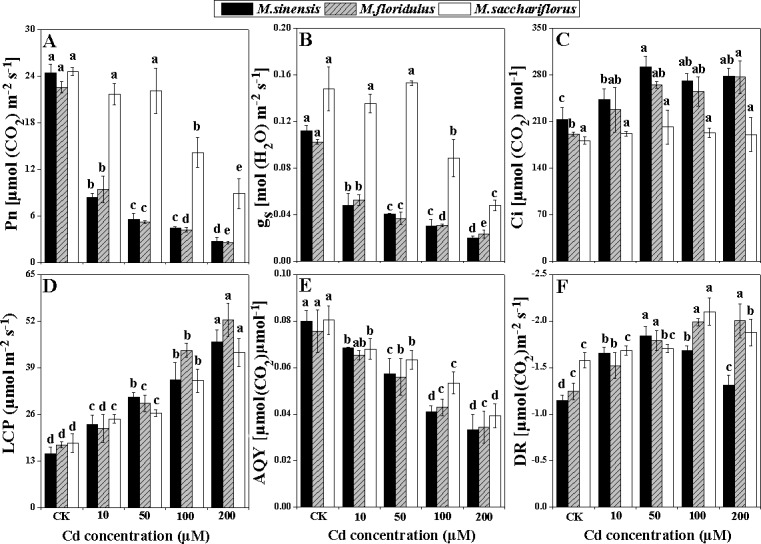
(A) Net photosynthetic rate (Pn), (B) stomata conductance (gs), (C) intercellular CO2 concentration (Ci), (D) light compensation point (LCP), (E) apparent quantum yield (AQY), (F) dark respiratory rate (DR). Data are mean ± SD (n = 3). Different letters indicate a significant difference at P < 0.05.
Photosynthetic pigment contents
Chlorophyll and carotenoid contents were significantly decreased (p<0.05) by increased Cd concentrations for all Miscanthus spp., and the reductions were always lower in M. sacchariflorus than for the other species (Fig 4). Miscanthus sinensis and M. floridulus showed similar decreases in chlorophyll and carotenoid contents, especially at 100 and 200 μM Cd treatments. The chlorophyll contents and carotenoid contents of M. sinensis decreased by 57.7% and 48.6%, respectively and had corresponding decreases in M. floridulus of 56.8% and 44.8% in comparison to control, whereas 36.6% and 20.8% reduction was noted in M. sacchariflorus under 200 μM Cd stress (Fig 4A and 4B).
Fig 4. Change in photosynthetic pigments content.
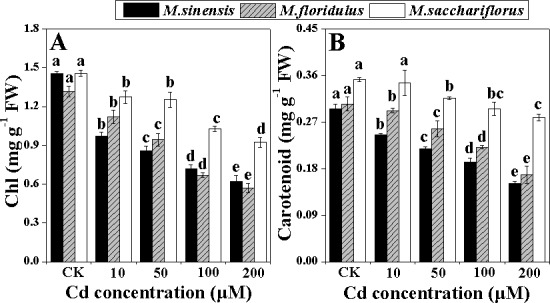
(A) Chlorophyll (Ch) and (B) carotenoid. Data are mean ± SD (n = 3). Different letters indicate a significant difference at P < 0.05.
C4 photosynthetic enzymes activities and Chloroplast structure
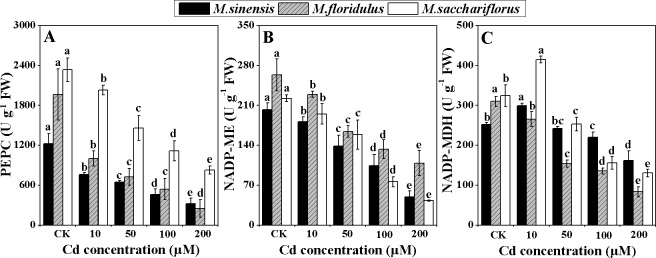
PEPC activity differed markedly between the Miscanthus spp., although it significantly decreased (p<0.05) under Cd stress. The highest activity was in M. sacchariflorus, whereas the lowest in M. sinensis (Fig 5A). The inhibitory effect of Cd on PEPC activity was more evident for M. sinensis and M. floridulus than for M. sacchariflorus. The PEPC activities were decreased by 37.9%, 46.9%, 62.5% and 74.5% in M. sinensis, respectively and 49.1%, 63.0%, 72.5% and 87.2% in M. floridulus, respectively, in comparison to control, whereas 13.0%, 37.5%, 52.2% and 64.5% reduction was found in M. sacchariflorus with increasing Cd concentrations, respectively (Fig 5A). NADP-ME activity also decreased significantly (p<0.05) for all Miscanthus spp. under Cd stress with slightly higher activity for M. floridulus than the other species for all Cd concentrations (Fig 5B). The greatest reduction in NADP-ME activity was for 100 and 200 μM Cd treatments in M. sacchariflorus, with inhibition ratios of 68.1% and 81.6%, respectively (Fig 5B). Low concentration of exogenous Cd (10 μM) enhanced the NADP-MDH activity in M. sinensis and M. sacchariflorus, and all Cd concentrations resulted in significantly decreased (p<0.05) NADP-MDH activity in M. floridulus (Fig 5C). In addition, NADP-MDH activity was significantly inhibited (p<0.05) by 50, 100 and 200 μM Cd treatments in M. sinensis and M. sacchariflorus; resulting in reductions of 50.3%, 56.2% and 73.1% in M. sinensis, respectively; and correspondingly 22.1%, 52.0% and 59.7% in M. sacchariflorus (Fig 5C).
Fig 5. Change in C4 photosynthetic enzymes activities.
(A) Phosphoenolpyruvate carboxylase (PEPC), (B) NADP-malate enzyme (NADP-ME), (C) NADP-malate dehydrogenase (NADP-MDH). Data are mean ± SD (n = 3). Different letters indicate a significant difference at P < 0.05.
The structural changes in chloroplasts markedly differed between Miscanthus spp. under Cd stress (Fig 6 and S2 Fig). With zero Cd treatment, chloroplasts in all species showed well-developed structures with normal granal and stromal thylakoids and some small osmiophilic globules (Fig 6A–6C). Treatment ≥ 10 μM Cd dramatically increased production of starch grains and enlarged osmiophilic globules in M. sinensis (Fig 6D, 6G and 6J); 100 μM Cd caused accumulation of small starch grains and enlargement of osmiophilic globules in M. floridulus (Fig 6K and 6N); but in M. sacchariflorus, only 200 μM Cd resulted in accumulation of small starch grains and enlargement osmiophilic globules (Fig 6L and 6O). The chloroplast envelope became indistinct in M. sinensis treated with ≥ 50 μM Cd (Fig 6G, 6J and 6M) and in M. floridulus treated with ≥ 100 μM Cd. Higher concentrations of exogenous Cd caused the granal and stromal lamellae of chloroplasts to condense and a loss of connection between both lamellae in M sinensis (Fig 6G, 6J and 6M) and M. floridulus (Fig 6N). The chloroplast structure in M. sacchariflorus did not change significantly for all Cd concentrations.
Fig 6. The chloroplast ultrastructure in bundle sheath cells.
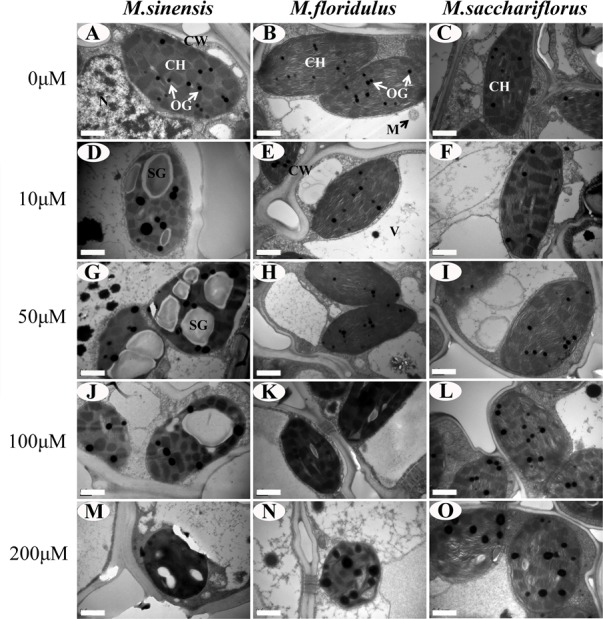
(A,D,G,J,M) M. sinensis; (B,E,H,K,N) M. floridulus; and (C,F,I,L,O) M. sacchariflorus. Note the differences in number of osmiophilic globules (OG, indicated by arrows) between different Cd treatments. Abbreviations: CH, chloroplast; CW, cell wall; SG, starch grains; M, mitochondria; V, vacuole.
Contents of MDA and H2O2
The MDA accumulation increased in all the three species under Cd stress. The degrees of increment varied for the 10, 50, 100 and 200 μM Cd treatments, with 43%, 53%, 67% and 91% in M. sinensis, respectively; and correspondingly 29%, 37%, 48% and 64% in M. floridulus; and 11%, 25%, 39% and 55% in M. sacchariflorus (Fig 7A). The H2O2 contents also increased under Cd stress in all the three Miscanthus spp.. As shown in Fig 7B, they were the highest in M. sinensis with about three times the control values for 200 μM Cd and only about two times greater for both M. floridulus and M. sacchariflorus treated with 200 μM Cd.
Fig 7.
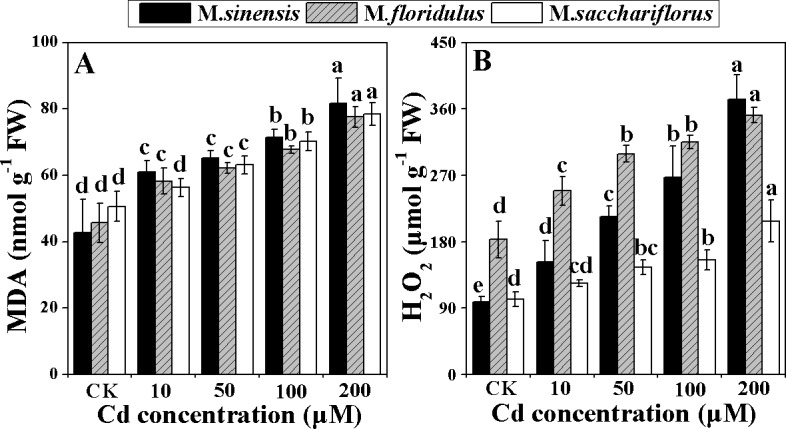
Change in (A) malondialdehyde (MDA) and (B) hydrogen peroxide (H2O2) content. Data are mean ± SD (n = 3). Different letters indicate a significant difference at P < 0.05.
Activities of anti-oxidant enzymes
All anti-oxidant enzymes including SOD, CAT, POD, APX and GR had markedly increased activity in leaves of Miscanthus spp. treated with Cd (Fig 8A–8E). SOD activity in leaves showed a higher increasing trend in M. sinensis and M. sacchariflorus than in M. floridulus under Cd stress. Compared to controls, 200 μM Cd treatment resulted in SOD activity of 7.5 times higher in M. sinensis and 3.6 times in M. sacchariflorus (Fig 8A). CAT activity in M. sinensis increased significantly (p<0.05) with Cd treatment up to 50 μM Cd, and then decreased with further increasing Cd concentrations; while in M. floridulus and M. sacchariflorus, CAT activity raised continuously with increasing Cd levels, to about twice the control values for 200 μM Cd treatment (Fig 8B). POD activities also varied among the species (Fig 8C) and were much higher in M. sacchariflorus than in M. sinensis and M. floridulus. Cd treatments significantly promoted (p<0.05) POD activities, especially in M. sacchariflorus, but only high Cd concentrations (100 and 200 μM) significantly up-regulated (p<0.05) POD activity in M. floridulus. APX activities in M. sacchariflorus were greatly enhanced by Cd treatments and reached a peak for M. sinensis at 100 μM Cd (Fig 8D). GR activity was lower in M. sacchariflorus than in M. sinensis and M. floridulus with increasing Cd concentrations, except for 200 μM Cd treatment where GR activity increased by 2.4, 1.2 and 1.4 times, respectively, compared with their controls (Fig 8E).
Fig 8.
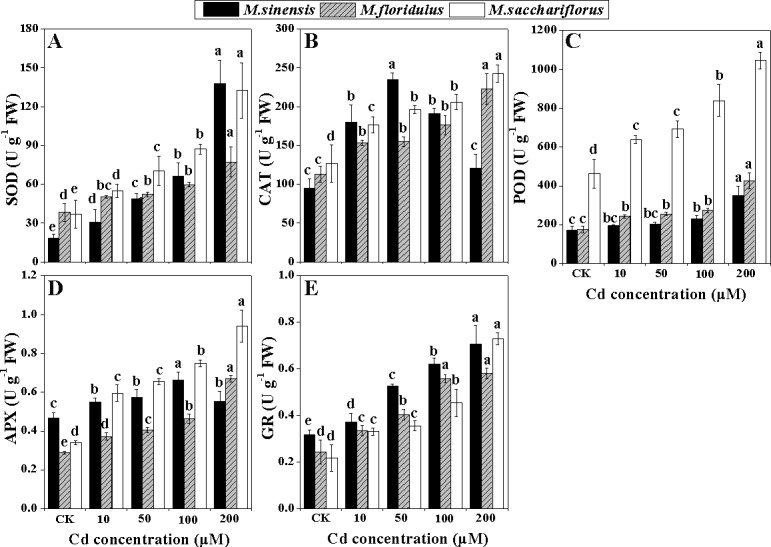
Change in activities of antioxidant enzymes (A) Superoxide dismutase (SOD), (B) catalase (CAT), (C) peroxidase (POD), (D) ascorbate peroxidase (APX) and (E) glutathione reductase (GR). Data are mean ± SD (n = 3). Different letters indicate a significant difference at P < 0.05.
Discussion
Different response of growth and Pn in Miscanthus spp. to exogenous Cd concentrations
Cd is a trace pollutant that is toxic to plants, animals and humans. In the present study, all Cd levels negatively influenced plant growth of M. sinensis and M. floridulus, causing significant reductions (p<0.05) in plant growth and dry biomass, while, there was a slight increase in growth of M. sacchariflorus at < 50 μM Cd and lower degree of reduction compared to M. sinensis and M. floridulus at 200 μM Cd, suggesting that M. sacchariflorus had greater Cd stress tolerance than M. sinensis and M. floridulus. It has also been reported that plant genotypes differ in their tolerance to Cd toxicity [6]. At high Cd concentrations, the leaves of M. sinensis and M. floridulus became yellow and roots became soft and brown, while the leaves remained green and the roots white for M. sacchariflorus, even at 200 μM Cd (Fig 1), thus further confirming greater Cd tolerance of M. sacchariflorus among the three species. Arduini et al. [15] find that, even for long-term, low Cd (0.5 mg L-1) application stimulates Miscanthus growth. Gill et al. [27] reported that at 25 mg kg-1 soil Cd, co-ordination of S and N metabolism can still complement to the antioxidant mechanism to protect the growth and photosynthesis of Lepidium sativum plants. However, high Cd doses (50–100 μM) cause growth inhibition and even plant death owing to inhibiting photosynthesis, respiration, water and nutrient uptake [28,29].
The inhibitory effect of Cd on Pn, gs and chlorophyll content was more evident in M. sinensis and M. floridulus than M. sacchariflorus. The growth inhibition may be a consequence of Cd interference with the main metabolic processes such as photosynthesis and translocation of photosynthetic products and nutrient elements [30]. In the present study, the decrease in whole plant dry weight was in accordance with the decrease of Pn (Fig 3A and Table 1), suggesting that Pn played an important role in biomass accumulation during Cd stress. The Cd-induced reduction in Pn and AQY (Fig 3A and 3E) could be partially due to the decrease in gs and chlorophyll content of the Miscanthus species (Figs 3B and 4A), as reported for maize (Zea mays L.) [31, 32] and sugarcane (Saccharum officinarum L.) [33]. The mechanism of photosynthetic response involves both stomatal and non-stomatal effects under environmental stress in C4 crops [34–36]. The results showed that the decrease in Pn was accompanied by increasing Ci concentration in M. sinensis and M. floridulus, suggesting that the factor limiting photosynthesis was mainly non-stomatal under Cd stress [34–37]. However, such changes were absent in M. sacchariflorus, implying different mechanisms for Pn depression due to Cd in different Miscanthus spp.
Difference in Cd accumulation and transfer is relative to resistance among Miscanthus spp.
Gill et al. [6] reported that the uptake and transport of Cd differed with plant species and genotypes. Cd accumulation in leaves directly leads to damage to the photosynthetic apparatus and decreases in Pn [28,29]. The different concentrations of Cd in roots and leaves of Miscanthus spp., even for the same concentration of exogenous Cd treatment (Fig 2A and 2B) reflects the difference in absorption by roots and transport from roots to shoot, and explains the difference in resistance of Miscanthus spp. to Cd. The highest Cd concentration in roots (Fig 2A) and medium Cd concentration in leaves (Fig 2B) indicated restricted transport and more absorption for M. sinensis. The highest Cd concentrationin leaves (Fig 2B) and medium Cd concentration in roots (Fig 2A) suggested stronger transport and absorption for M. floridulus. The lowest Cd concentrations both in leaves and in roots confirmed much less absorption of exogenous Cd for M. sacchariflorus (Fig 2) and this low absorption is not only a characteristic but could be the main cause of the higher resistance of M. sacchariflorus to Cd.
The decrease in Pn was due to lower activities of C4 photosynthetic enzymes and damage to chloroplast structure
Exogenous Cd treatment resulted in depression of Pn and AQY of all species, and the depression was much greater in M. sinensis and M. floridulus (Fig 3A and 3E). To determine the reason for this depression in Miscanthus spp. under different Cd concentrations, we determined the activities of key enzymes of the C4 photosynthetic pathway—PEPC, NADP-ME and NADP-MDH—that participate in the process of concentrating CO2 in C4 photosynthesis [38]. We found significant decreases (p<0.05) in PEPC, NADP-ME and NADP-MDH activity in all Miscanthus spp. exposed to Cd stress. However, PEPC activity was much higher in M. sacchariflorus than in the other two species for all Cd concentrations (Fig 5A). This was consistent with the highest Pn and higher Cd tolerance in M. sacchariflorus. The Pn of Miscanthus spp. were closely related to PEPC content rather than ribulose 1,5 bisphosphate carboxylase/oxygenase (Rubisco) under higher nitrogen content [36]. Moreover, in maize leaves it was found to be inactivated by Cd [39]. The NADP-ME is a key enzyme in the NADP-ME subtype of C4 plants and helps enrich the CO2 for Rubisco, thus lowering photorespiration and improving photosynthetic efficiency [40]. NADP-MDH is particularly abundant in C4 plants, where it functions photosynthetically in the NADP-dependent reduction of oxaloacetate to malate [41]. It was also reported that higher MDH activity and malate accumulation in companion with higher Pn were found in the drought-resistant Sorghum bicolor genotype compared with a sensitive genotype [42]. In the present study, the higher activity of NADP-MDH in M. sacchariflorus under Cd stress favored conversion of oxaloacetate to malate (Fig 5C) which is then transported into adjacent bundle sheath cells to enhance Calvin cycle in bundle sheath cells [43]. The increase in malate synthesis can cause a significant increase in root malate exudation, thus improving toxic metal resistance in C3 plants [44,45], but it is still unclear in C4 plant whether or not the malate, except for C3 CO2 fixation in leaf, can be transported from leaf to root. If a part of malate resulted from the higher activity of NADP-MDH in M. sacchariflorus under Cd stress can be transported out of leaf and reaches to root, it is possible to confer high Cd tolerance in this plants.
Chloroplast ultrastructure could provide important information concerning the biochemical properties of the thylakoids, which suffer the greatest changes during adverse environmental conditions, such as salt [46], drought [47] or heavy metal [48] stresses. The decrease of Pn is related to changes in the membrane structure of chloroplasts [49] and degradation of chloroplasts [48]. The production of starch grains, enlargement of osmiophilic globules and loss of the chloroplast envelope showed large differences among the Miscanthus species under various Cd concentrations (Fig 6D, 6G and 6J–6O). Cd caused the grana and stroma lamellae of chloroplasts to condense and the loss of connection between both lamellae in M. sinensis (Fig 6G, 6J and 6M) and M. floridulus (Fig 6N) but did not induce significant change in M. sacchariflorus. These results not only indicated the different resistance of Miscanthus spp. to Cd, but also confirmed that the different decreases in Pn, photosynthetic pigment, the maximal photochemical efficiency of PSII (Fv/Fm) and effective PSII quantum yield [Y(II)] (S3 Fig) resulted from damage to chloroplasts.
Stronger anti-oxidant system may alleviate the damage to photosynthetic apparatus
Cd exposure initially results in severe oxidative stress, which in turn caused lipid peroxidation and H2O2 accumulation [9]. MDA is a product of lipid peroxidation and is considered an indicator of oxidative damage [50]. The present study showed that MDA accumulation increased most in M. sinensis under all Cd stress (Fig 7A), indicating that Cd induced stronger peroxidation and caused more serious damage to the cell membrane in M. sinensis. A certain amount of H2O2 accumulation during Cd stress may act as an oxidative agent and a local or systemic signal that activates various anti-oxidant enzymes, but over-accumulation of H2O2 induces peroxidative reactions that damage plant cells [51]. The SOD catalyzes the O2– dismutation reaction to form H2O2. CAT and POD could catalyze H2O2 into water and oxygen, alleviating the oxidative damage caused by H2O2. However, in chloroplasts, H2O2 is restricted by the ascorbate–glutathione (ASH–GSH) cycle, where APX uses ASH as a hydrogen donor and GR catalyzes the NADPH-dependent reduction of oxidized glutathione (GSSG) to reduced GSH [52]. The activities of these enzymes were increased by Cd stress in wheat and tobacco [53,54]. In the present study, M. sinensis showed a greater increase in SOD activity (Fig 8A), but lesser increase in POD and APX activities (Fig 8C and 8D), resulting in greater H2O2 accumulation (Fig 7B) and the most serious damage to chloroplasts (Fig 6D, 6G, 6J and 6M), compared with the other Miscanthus spp. In M. sacchariflorus, for all concentrations of Cd treatment, the SOD, POD and APX activities were higher (Fig 8A, 8C and 8D) and the H2O2 accumulations much lower than that in the other Miscanthus spp. (Fig 7B). These results indicated that M. sacchariflorus possessed a better anti-oxidative system, which could scavenge ROS and maintain integrity of the chloroplast (Fig 6F, 6I, 6L and 6O). The results support the view that genotypic difference in the anti-oxidative system could partially account for the genotypic difference in Cd accumulation, tolerance and an increase in tolerance to Cd stress is positively correlated with anti-oxidant capacity [55].
Conclusions
The present study revealed the effects of Cd on plant growth, photosynthesis characteristics, chloroplast ultrastructure, Cd-uptake and translocation and physiological responses of three Miscanthus species. The results showed that the effects of different Cd concentrations on growth and Pn in Miscanthus spp. differed. The inhibitory effect of Cd on growth characteristics was more evident for M. sinensis whereas, least for M. sacchariflorus. The resistance of M. sacchariflorus to Cd was mainly due to a lower Cd absorption and translocation, thus keeping more effective activities of C4 photosynthetic enzymes and better chloroplast structure. Furthermore, hyperactivity of anti-oxidant enzymes also played an important role in protecting M. sacchariflorus from Cd toxicity.
Supporting Information
(TIF)
Bar = 2μm.
(TIF)
(TIF)
Acknowledgments
We thank Dr. Yanhua Qi for assistance in improving the written English. We also thank Dr. Claudia Cosio who carefully reviewed our manuscript and put forward many valuable suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present research was financially supported by the National Science and Technology Support Plan of China (2012BAC09B01, DAJ) and the National Natural Science Foundation of China (31371591, DAJ and 31470683, BSZ).
References
- 1.Dudka S, Adriano DC (1997) Environmental impacts of metal ore mining and processing: a review. J Environ qual 26: 590–602. [Google Scholar]
- 2.Misra V, Pandey SD (2005) Hazardous waste, impact on health and environment for development of better waste management strategies in future in India. Environ Int 31: 417–431. [DOI] [PubMed] [Google Scholar]
- 3.Bernhard Zarcinas A, Ishak CF, Mike McLaughlin J, Cozens G (2004) Heavy Metals in Soils and Crops in Southeast Asia 1. Peninsular Malaysia. Environ Geochem Health 26: 343–357. [DOI] [PubMed] [Google Scholar]
- 4.Ji P, Sun T, Song Y, Ackland ML, Liu Y (2011) Strategies for enhancing the phytoremediation of cadmium-contaminated agricultural soils by Solanum nigrum L. Environ Pollut 159: 762–768. 10.1016/j.envpol.2010.11.029 [DOI] [PubMed] [Google Scholar]
- 5.Hassan Z, Aarts MGM (2011) Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environ Exp Bot 72: 53–63. [Google Scholar]
- 6.Gill SS, Khan NA, Tuteja N (2011) Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars: an evaluation of the role of antioxidant machinery. Plant Signal Behav 6: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández R, Bertrand A, Reis R, Mourato MP, Martins LL, González A (2013) Growth and physiological responses to cadmium stress of two populations of Dittrichia viscosa (L.) Greuter. J Hazard Mater 244: 555–562. 10.1016/j.jhazmat.2012.10.044 [DOI] [PubMed] [Google Scholar]
- 8.Khan AL, Waqas M, Hussain J, Al-Harrasi A, Lee IJ (2014) Fungal endophyte Penicillium janthinellum LK5 can reduce cadmium toxicity in Solanum lycopersicum (Sitiens and Rhe). Biol Fertil Soils 50: 75–85. [Google Scholar]
- 9.Cui W, Gao C, Fang P, Lin G, Shen W (2013) Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Hazard Mater 260: 715–724. 10.1016/j.jhazmat.2013.06.032 [DOI] [PubMed] [Google Scholar]
- 10.Chaney RL, Malik M, Li YM, Brown SL, Brewer EP, Angle JS, et al. (1997) Phytoremediation of soil metals. Curr Opin Biotech 8: 279–284. [DOI] [PubMed] [Google Scholar]
- 11.Bauddh K, Singh RP (2012) Cadmium tolerance and its phytoremediation by two oil yielding plants Ricinus communis (L.) and Brassica juncea (L.) from the contaminated soil. Int J Phytoremediat14: 772–785. [DOI] [PubMed] [Google Scholar]
- 12.Deniau AX, Pieper B, Ten Bookum WM, Lindhout P, Aarts MGM, Schat H (2006) QTL analysis of cadmium and zinc accumulation in the heavy metal hyperaccumulator Thlaspi caerulescens. Theor Appl Genet 113: 907–920. [DOI] [PubMed] [Google Scholar]
- 13.Bianconi D, Pietrini F, Massacci A, Iannelli MA (2013) Uptake of Cadmium by Lemna minor, a (hyper-) accumulator plant involved in phytoremediation applications. E3S Web of Conferences, EDP Sciences 1: 13002. [Google Scholar]
- 14.Zhuang X, Chen J, Shim H, Bai Z (2007) New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int 33: 406–413. [DOI] [PubMed] [Google Scholar]
- 15.Arduini I, Masoni A, Mariotti M, Ercoli L (2004) Low cadmium application increase Miscanthus growth and cadmium translocation. Environ Exp Bot 52: 89–100. [Google Scholar]
- 16.Jørgensen U (2011) Benefits versus risks of growing biofuel crops, the case of Miscanthus. Curr Opin Environ Sust 3: 24–30. [Google Scholar]
- 17.Hodkinson TR, Chase MW, Lledo MD, Salamin N, Renvoize SA (2002) Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL-F intergenic spacers. J Plant Res 115: 381–392. [DOI] [PubMed] [Google Scholar]
- 18.Feng XP, Lourgant K, Castric V, Saumitou-Laprade P, Zheng BS, Jiang D, et al. (2014) The Discovery of natural Miscanthus accessions related to Miscanthus giganteus using chloroplast DNA. Cop Sci 54: 1645–1655. [Google Scholar]
- 19.Kayama M (2001) Comparison of the Aluminum tolerance of Miscanthus sinensis Anderss. and Miscanthus sacchariflorus Bentham in hydroculture. Int J Plant Sci 165: 1025–1031. [Google Scholar]
- 20.Stewart JR, Toma Y, Fernández F, Nishiwakis A, Yamada T, Bollero G (2009) The ecology and agronomy of Miscanthus sinensis, a species important to bioenergy crop development, in its native range in Japan: a review. GCB Bioenergy 1: 126–153. [Google Scholar]
- 21.Pavel PB, Puschenreiter M, Wenzel WW, Diacu E, Barbu CH (2014) Aided phytostabilization using Miscanthus sinensis × giganteus on heavy metal-contaminated soils. Sci Total Environ 479: 125–131. 10.1016/j.scitotenv.2014.01.097 [DOI] [PubMed] [Google Scholar]
- 22.Ye ZP (2007) A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica 45: 637–640. [Google Scholar]
- 23.Liu H, Zhang S, Zhang X, Chen C (2015) Growth inhibition and effect on photosystem by three imidazolium chloride ionic liquids in rice seedlings. J Hazard Mater 286: 440–448. 10.1016/j.jhazmat.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 24.Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem F: F4.3. [Google Scholar]
- 25.Shao QS, Wang HZ, Guo HP, Zhou AC, Huang YQ, Sun YL, et al. (2014) Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PloS One 9: e85996 10.1371/journal.pone.0085996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong CT, Guo HP, Fang J, Ren W, Wang TF, Ji MC, et al. (2014) Physiological and biochemical responses of Miscanthus sacchariflorus to salt stress. Adv Mater Res 1051: 333–340. [Google Scholar]
- 27.Gill SS, Khan NA, Tuteja N. (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182: 112–120. 10.1016/j.plantsci.2011.04.018 [DOI] [PubMed] [Google Scholar]
- 28.Dias MC, Monteiro C, Moutinho-Pereira J, Correia C, Gonçalves B, Santos C (2013) Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol Plant 35: 1281–1289. [Google Scholar]
- 29.Xue ZC, Gao HY, Zhang LT (2013) Effects of cadmium on growth, photosynthetic rate and chlorophyll content in leaves of soybean seedlings. Biol Plant 57: 587–590. [Google Scholar]
- 30.Hou W, Chen X, Song G, Wang Q, Chang CC (2007) Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol Biochem 45: 62–69. [DOI] [PubMed] [Google Scholar]
- 31.Efthimiadou A, Bilalis D, Karkanis A, Froud-Williams B, Eleftherochorinos I (2009) Effects of cultural system (organic and conventional) on growth, photosynthesis and yield components of sweet corn (Zea mays L.) under semi-arid environment. Not Bot Horti Agrobo 37: 104–111. [Google Scholar]
- 32.Wang H, Zhao SC, Liu RL, Zhou W, Jin JY (2009) Changes of photosynthetic activities of maize (Zea mays L.) seedlings in response to cadmium stress. Photosynthetica 47: 277–283. [Google Scholar]
- 33.Suriyan CU, Chalermpol K (2009) Proline accumulation, photosynthetic abilities and growth characters of sugarcane (Saccharum officinarum L.) plantlets in response to iso-osmotic salt and water-deficit stress. Agr Sci China 8: 51–58. [Google Scholar]
- 34.Ghannoum O (2009) C4 photosynthesis and water stress. Ann Bot 103: 635–644. 10.1093/aob/mcn093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markelz RC, Strellner RS, Leakey AD (2011) Impairment of C4 photosynthesis by drought is exacerbated by limiting nitrogen and ameliorated by elevated [CO2] in maize. J Exp Bot 62: 3235–3246. 10.1093/jxb/err056 [DOI] [PubMed] [Google Scholar]
- 36.Feng XP, Chen Y, Qi YH, Yu CL, Zheng BS, Brancourt-Hulmel M, et al. (2012) Nitrogen enhanced photosynthesis of Miscanthus by increasing stomatal conductance and phosphoenolpyruvate carboxylase concentration. Photosynthetica 50: 577–586. [Google Scholar]
- 37.Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33: 317–345. [Google Scholar]
- 38.Doubnerová V, Ryšlavá H (2011) What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci 180: 575–583. 10.1016/j.plantsci.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 39.Vojtěchová M, Leblová S (1991) Uptake of lead and cadmium by maize seedlings and the effect of heavy metals on the activity of phosphoenolpyruvate carboxylase isolated from maize. Biol Plantarum 33: 386–394. [Google Scholar]
- 40.Edwards GE, Nakamoto H, Burnell JN, Hatch MD (1985) Pyruvate, Pi dikinase and NADP-malate dehydrogenase in C4 photosynthesis: properties and mechanism of light/dark regulation. Annu Rev Plant Physiol 36: 255–286. [Google Scholar]
- 41.Jacquot JPP, Buchanan BB, Martin F, Vidal J (1981) Enzyme regulation in C4 photosynthesis. Plant Physiol 68: 300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beyel V, Brüggemann W (2005) Differential inhibition of photosynthesis during pre‐flowering drought stress in Sorghum bicolor genotypes with different senescence traits. Physiol Plantarum 124: 249–259. [Google Scholar]
- 43.Ray TB, Black CC (1979) The C4 pathway and its regulation. Photosynthesis II. Springer Berlin Heidelberg 1979: 77–101. [Google Scholar]
- 44.Chen Z, Sun L, Liu P, Liu G, Tian J, Liao H (2015) Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiol 167: 176–188. 10.1104/pp.114.251017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Shi DC (2010) Photosynthesis, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress. Photosynthetica 48: 127–134. [Google Scholar]
- 46.He Y, Yu CL, Zhou L, Chen Y, Liu A, Jin JH, et al. (2014) Rubisco decrease is involved in chloroplast protrusion and Rubisco-containing body formation in soybean (Glycine max.) under salt stress. Plant Physiol Biochem 74: 118–124. 10.1016/j.plaphy.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 47.Grigorova B, Vassileva V, Klimchuk D, Vaseva I, Demirevska K, Feller U (2012) Drought, high temperature, and their combination affect ultrastructure of chloroplasts and mitochondria in wheat (Triticum aestivum L.) leaves. J Plant Interact 7: 204–213. [Google Scholar]
- 48.Daud MK, Quiling H, Lei M, Ali B, Zhu SJ (2015) Ultrastructural, metabolic and proteomic changes in leaves of upland cotton in response to cadmium stress. Chemosphere 120: 309–320. 10.1016/j.chemosphere.2014.07.060 [DOI] [PubMed] [Google Scholar]
- 49.Andosch A, Affenzeller MJ, Lütz C, Lütz-Meindl U (2012) A freshwater green alga under cadmium stress: ameliorating calcium effects on ultrastructure and photosynthesis in the unicellular model Micrasterias. J Plant Physiol 169: 1489–1500. 10.1016/j.jplph.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 50.Sekmen AH, Turkan I, Tanyolac ZO, Ozfidan C, Dinc A (2012) Different antioxidant defense responses to salt stress during germination and vegetative stages of endemic halophyte Gypsophila oblanceolata Bark. Environ Exp Bot 77: 63–76. [Google Scholar]
- 51.Cho UH, Seo NH (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168: 113–120. [Google Scholar]
- 52.Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155: 2–18. 10.1104/pp.110.167569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin R, Wang X, Luo Y, Du W, Guo H, Yin D (2007) Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere 69: 89–98. [DOI] [PubMed] [Google Scholar]
- 54.Liu WX, Shang SH, Feng X, Zhang GP, Wu FB (2015) Modulation of exogenous selenium in cadmium-induced changes in antioxidative metabolism, cadmium uptake, and photosynthetic performance in the 2 tobacco genotypes differing in cadmium tolerance. Environ Toxicol Chem 34: 92–99. 10.1002/etc.2760 [DOI] [PubMed] [Google Scholar]
- 55.Srivastava RK, Pandey P, Rajpoot R, Rani A, Dubey RS (2014) Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251: 1047–1065. 10.1007/s00709-014-0614-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Bar = 2μm.
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.