ABSTRACT
To secure the functionality of activated macrophages in the innate immune response, efficient life span control is required. Recognition of bacterial lipopolysaccharides (LPS) by toll-like receptor 4 (TLR4) induces downstream signaling pathways, which merge to induce the expression of cytokine genes and anti-apoptotic genes. MicroRNAs (miRNAs) have emerged as important inflammatory response modulators, but information about their functional impact on apoptosis is scarce. To identify miRNAs differentially expressed in response to LPS, cDNA libraries from untreated and LPS-activated murine macrophages were analyzed by deep sequencing and regulated miRNA expression was verified by Northern blotting and qPCR. Employing TargetScanTM we identified CASPASE-3 (CASP-3) mRNA that encodes a key player in apoptosis as potential target of LPS-induced miR-155. LPS-dependent primary macrophage activation revealed TLR4-mediated enhancement of miR-155 expression and CASP-3 mRNA reduction. Endogenous CASP-3 and cleaved CASP-3 protein declined in LPS-activated macrophages. Accumulation of miR-155 and CASP-3 mRNA in miRNA-induced silencing complexes (miRISC) was demonstrated by ARGONAUTE 2 (AGO2) immunoprecipitation. Importantly, specific antagomir transfection effectively reduced mature miR-155 and resulted in significantly elevated CASP-3 mRNA levels in activated macrophages. In vitro translation assays demonstrated that the target site in the CASP-3 mRNA 3'UTR mediates miR-155-dependent Luciferase reporter mRNA destabilization. Strikingly, Annexin V staining of macrophages transfected with antagomir-155 and stimulated with LPS prior to staurosporine (SSP) treatment implied that LPS-induced miR-155 prevents apoptosis through CASP-3 mRNA down-regulation. In conclusion, we report that miR-155-mediated CASP-3 mRNA destabilization in LPS-activated RAW 264.7 macrophages suppresses apoptosis, as a prerequisite to maintain their crucial function in inflammation.
Keywords: Caspase-3 mRNA, LPS, macrophage activation, miR-155, post-transcriptional regulation, TLR4
Introduction
The tight regulation of the innate immune response is substantial for the host defense against invading pathogens. An excessive inflammatory response can lead to tissue damage and inflammatory disorders, such as atherosclerosis or arthritis.1,2 Macrophages sense microbial pathogen infection mainly through the toll-like receptor (TLR) family. TLR4 recognizes lipopolysaccharides (LPS) in the outer membrane of gram-negative bacteria.3 LPS-binding protein (LBP) and CD14 mediate the transfer of LPS to a TLR4/MD-2 heterodimer.4 The LPS-induced dimerization of 2 TLR4/MD-2 complexes leads to the recruitment of intracellular TIR-domain containing proteins, which activate 2 different downstream signaling pathways.5,6 The MyD88-dependent branch activates mitogen-activated protein kinases (MAPKs), e.g. p38, JNK or ERK1/2 and the early NFκB response.7-9 This results in the expression of pro- and anti-inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β and IL-10).10 Adaptor proteins TRAM and TRIF are involved in the MyD88-independent LPS response, which triggers activation of transcription factors IRF-3 and IRF-7, resulting in enhanced interferon expression, and induces the late NFκB reaction.3,11,12 In response to environmental stimuli, inflammatory gene expression is regulated at the transcriptional and post-transcriptional level. Besides trans-acting factors, like RNA-binding proteins (RBPs) or non-coding RNAs that affect mRNA translation and mRNA stability, post-transcriptional regulation comprises alternative splicing and alternative polyadenylation of mRNAs, which encode TLR4 signaling components.1 In murine macrophages and human monocytes MD-2 variants translated from alternatively spliced transcripts, bind to the TLR4, but fail to initiate signaling and thereby inhibit LPS-induced activation.13,14 MiRNAs are small (∼22 nt) endogenous RNA molecules that have emerged as post-transcriptional regulators of biological processes.15 MiRNA processing in the nucleus and the cytoplasm is catalyzed by the RNase III type enzymes DROSHA and DICER and their cofactors.16 In the cytoplasm, the mature miRNA is loaded onto miRISC with an ARGONAUTE (AGO) protein as core component.17,18 To exert their regulatory function, miRNAs guide the complex to their target sequence in the 3'UTR of an mRNA, resulting in translational repression or mRNA degradation.19-21
Besides their complex role in cell differentiation, cancer or apoptosis, miRNAs regulate inflammatory response pathways.22 Several inflammation-related, LPS-responsive miRNAs and mRNA targets have been predicted. MiR-146a was identified as a negative immune response regulator that inhibits the expression of key TLR4-downstream proteins IRAK1 and TRAF6 thereby decreasing inflammatory cytokine expression.23
By deep sequencing of small RNA libraries from untreated and LPS-activated murine RAW 264.7 macrophages we identified miRNAs differentially expressed in response to LPS. We focused on miR-155 to identify new target mRNAs encoding proteins involved in inflammatory response control. In silico target prediction revealed CASP-3 mRNA as a new potential miR-155 target. To address the hypothesis that miR-155 confers a protective function in macrophage immune response, we investigated miR-155-mediated CASP-3 mRNA modulation in LPS-activated RAW 264.7 cells.
Results
Identification of differentially expressed miRNAs in LPS-activated RAW 264.7 cells
To systematically analyze miRNA expression profiles related to the inflammatory response, murine RAW 264.7 macrophages were left untreated or stimulated with LPS for 6 h. Functional LPS concentration and stimulation time were determined before in titration experiments that monitored cytokine mRNA synthesis (e.g., TNF-α, IL-6) and kinase activation (p38 and ERK 1/2).24 For deep sequencing, small RNA libraries were generated from isolated total RNA. MiRNAs differentially expressed in response to LPS were identified and classified through integration of the miR-IntessTM platform and miRBase libraries.25,26 Heatmaps depict miRNAs with ≥ 30 reads, either up- (left panel) or down-regulated (right panel) upon LPS-stimulation. 41 up-regulated and 84 downregulated miRNA candidates were identified (Fig. 1A, Table S2). MiRNA expression in RAW 264.7 cells was validated in Northern blots and by qPCR. To characterize LPS-responsive miRNA candidates, 3 parameters were defined: A minimum of 50 reads, a fold change of at least 1.5 and putative target mRNAs linked to macrophage immune response identified by in silico target prediction. MiRNA candidates that met these criteria are listed in Fig. 1B. MiR-155 showed the highest fold change (40-fold) among the LPS-induced miRNAs (Fig. 2A). Validation of miR-155 expression in RAW 264.7 cells by Northern blotting and qPCR confirmed the significant LPS-induced miR-155 increase that was detected by deep-sequencing (Fig. 2A, B). Another miRNA identified as LPS-induced by deep sequencing, miR-342, served as specificity control. To identify potential target mRNAs encoding proteins involved in the macrophage immune response Target-ScanTM was employed. Interestingly, besides known miR-155 target mRNAs encoding proteins of the TLR4 pathway, like IKKε or TGF-β activated kinase 1 binding protein 2 (TAB2)27,28, the screen predicted CASP-3 mRNA as a putative target of LPS-induced miR-155. Induced by apop-totic signals, CASP-3 is processed by initiator caspases to the active enzyme, which promotes apoptosis by proteolytic substrate cleavage.29,30 The miR-155 binding site in the CASP-3 mRNA 3'UTR is highly conserved between mammalian species, indicating functionality (Fig. 2C).
Figure 1.
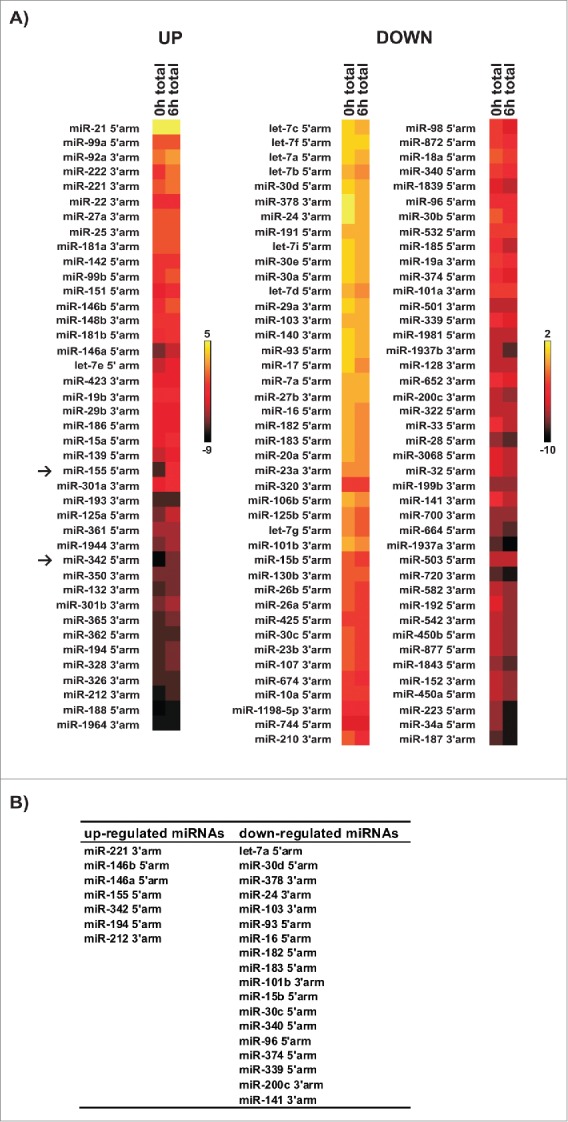
Identification of differentially expressed miRNAs in LPS-induced RAW 264.7 cells. (A) Total RNA was isolated from untreated [0 h total] and LPS-activated [6 h total] RAW 264.7 cells. CDNA libraries were generated and analyzed by deep-sequencing. Heat maps represent relative cloning frequencies of miRNAs (% of reads, log2 scale). Yellow color indicates high and dark red low miRNA expression. With the threshold set to 30 reads, 41 up-regulated and 84 down-regulated miRNAs were identified in response to LPS. (B) Overview of mature miRNAs selected for validation by Northern blot and qPCR analysis. For 7 upregulated and 18 downregulated miRNAs with ≥50 reads, a ≥1.5-fold change was detected and inflammation-related putative target mRNAs were identified.
Figure 2.

MiR-155 is significantly elevated in LPS-induced RAW 264.7 cells. (A) Upon LPS treatment, deep sequencing revealed that mature miR-155 (5'arm) was most strongly induced (B) Relative cloning frequencies (% of reads, log2 scale) of up-regulated mature miR-155 and miR-342 (5'arm). Northern blot (middle panel) and qPCR analysis (right panel) confirmed significantly increased miR-155 expression, endogenous U6 snRNA served as a control. P-values (Student's t-test): **P < 0.01, ***P < 0.001. (C) CASP-3 mRNA was identified as a potential miR-155 target employing TargetScanTM. CASP-3 mRNA 3'UTR alignment identifies a highly conserved miR-155 target site (boxed) in mouse, man and dog. Sequences were analyzed with Clustal W2.62
Analysis of the LPS response in primary macrophages
In order to investigate whether miR-155 expression is TLR4-dependent, the LPS response in C57BL/6 wild type (wt)- and TLR4−/−- bone marrow derive macrophages (BMDM) was initially analyzed. Since primary cells respond earlier to LPS, their induction was reduced to 2 h.24 Upon LPS stimulation wt-BMDM displayed a strong activation of the kinases p38 and ERK 1/2 after 10 min (Fig. 3A lane 2) that was clearly diminished in TLR4-/--BMDM (Fig. 3A lane 6-8), indicating TLR4-dependent kinase phosphorylation. Synthesis of pro-inflammatory cytokine mRNAs TNF-α, IL-1β, IL-6 and anti-inflammatory cytokine IL-10 mRNA was induced in a time dependent manner (Fig. 3B). A strong decrease of LPS-induced cytokine mRNA synthesis was observed in TLR4-/--BMDM compared to wt-BMDM (Fig. 3B). Strikingly, emphasizing TLR4-dependence, miR-155 expression decreased from approximately 40-fold activation (Fig. 3C left panel) in wt-BMDM to less than 2-fold in TLR4-/--BMDM (Fig. 3C right panel), whereas miR-342 was not affected (Fig. 3C left and right panel). After 2 h LPS activation CASP-3 mRNA was reduced in wt-BMDM, but not in TLR4-/--BMDM (Fig. 3D), which did not exhibit elevated miR-155 expression (Fig. 3C, right panel). Our data suggest that elevated miR-155 expression, which results in CASP-3 mRNA reduction in LPS treated cells, is TLR-4-dependent.
Figure 3.

LPS response of primary murine macrophages (BMDM). (A) Cytoplasmic extracts of BMDM from wild type (wt) and TLR4−/− mice treated with LPS for the indicated times were analyzed with specific antibodies. TLR4-dependent MAP kinase ERK 1/2 and p38 phosphorylation was detected in BMDM of wt (lanes 1 - 4) and TLR4−/− mice (lanes 5 - 8), VINCULIN served as control. (B) Induction of mRNAs encoding pro-inflammatory cytokines TNFα, IL-1β, IL-6 and anti-inflammatory cytokine IL-10 determined by qPCR, was analyzed in BMDM of wt and TLR4−/− mice, treated with LPS as indicated. P-values (Student's t-test): **P < 0.01. (C) Induction of mature miR-155 and miR-342 in 2 h LPS treated wt and TLR4−/− BMDM analyzed by qPCR was determined as fold-change relative to endogenous U6 snRNA. (D) QPCR analysis of CASP-3 mRNA in wt and TLR4−/− BMDM in response to 2 h LPS stimulation normalized to endogenous NDUFV1 mRNA.
Association of potential miR-155 target mRNAs with functional miRISC
AGO2 immunoprecipitation was performed from cytoplasmic extracts of untreated RAW 264.7 cells and after 6 h LPS exposure to elucidate whether predicted target mRNAs specifically accumulate in functional miRISC (Fig. 4A). Importantly, CASP-3 mRNA co-precipitated with AGO2, as well as the miR-155 target mRNAs IKKε and TAB2,27,28 while GAPDH control mRNA did not (Fig. 4B lane 3 and 4). Interestingly, after LPS treatment CASP-3 mRNA decreased in the input and AGO2 immunoprecipitation (Fig. 4B lane 2 and 4). This suggests that in LPS-activated macrophages CASP-3 mRNA is destabilized resulting in reduced miRISC association. In comparison, IKKε mRNA was strongly increased in both input and the AGO2 immunoprecipitation (Fig. 4B lane 2 and 4). Analysis of specific miR-155 association with miRISC revealed that LPS treatment efficiently enhanced miR-155 co-precipitation with AGO2 (Fig. 4C). These results strongly suggest that both CASP-3 mRNA and miR-155 interact with AGO2 in LPS-activated macrophages.
Figure 4.
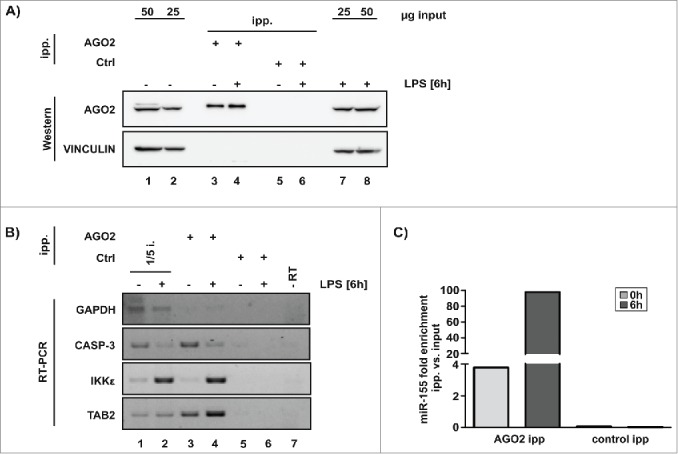
miRISC core component AGO2 associates with miR-155 and target mRNAs. (A) Specific AGO2 immunoprecipitation from cytoplasmic extracts of untreated RAW 264.7 cells and after 6 h LPS treatment, VINCULIN served as control. (B) Analysis of predicted miR-155 targets IKKε, TAB2 and CASP-3 mRNA in the AGO2-precipitation by RT-PCR, GAPDH mRNA served as negative control. (C) Determination of miR-155 in the AGO2-precipitation by qPCR, C. elegans miR-39 was used for normalization.
Expression of CASP-3 is reduced in LPS-activated macrophages
A time-dependent decline of CASP-3 mRNA correlated with an increasing miR-155 level in LPS-activated macrophages (Fig. 5A). When RAW 264.7 cells were treated with LPS for 6 h, CASP-3 mRNA was significantly reduced to 35 % compared to untreated macrophages (Fig. 5B, left panel), in agreement with the decline of CASP-3 mRNA in the input and AGO2 immunoprecipitation (Fig. 4B). Consistently, both endogenous full-length CASP-3 and cleaved CASP-3 protein were reduced in cytoplasmic extracts of LPS-activated cells to 66 % and 59 %, respectively (Fig. 5B, right panel). Again this suggests that CASP-3 mRNA stability declines in the inflammatory response. In contrast, analysis of IKKε mRNA and protein revealed that although IKKε mRNA significantly increased upon LPS activation, IKKε protein declined to 55 % (Fig. 5C). This indicates that IKKε mRNA was not destabilized by miR-155, but its translation was inhibited.
Figure 5.

CASP-3 expression is reduced in LPS-induced RAW 264.7 cells. (A) Time course of miR-155 expression and CASP-3 mRNA level in LPS treated RAW 264.7 cells. (B, C) Analysis of CASP-3 mRNA (B) and IKKε mRNA (C) in untreated or 6 h LPS-exposed RAW 264.7 cells by qPCR, normalized to NDUFV1 mRNA (left panel). CASP-3, as well as cleaved CASP-3 and IKKε from cytoplasmic extracts were detected with specific antibodies, VINCULIN served as control (right panel). P-values (Student's t-test): **P < 0.01, ***P < 0.001.
MiR-155 antagomir elevates CASP-3 mRNA in activated macrophages
Transfection of an antagomir against mature miR-155 resulted in a significant reduction of miR-155 in LPS-activated RAW 264.7 cells. Specifically, the impact of the antagomir against miR-155, but not against miR-12231 that functions as control (antagomir-ctrl.) strongly diminished the LPS-dependent CASP-3 mRNA downregulation (Fig. 6A and 6B). Moreover, antagomir-155 abolished the CASP-3 protein reduction in LPS-activated macrophages compared to non-activated cells, whereas antagomir-ctrl. did not (Fig. 6C). This supports the conclusion that miR-155 affects CASP-3 mRNA translation and/or stability during macrophage LPS-response.
Figure 6.

Antagomir-155 elevates CASP-3 expression in LPS-induced RAW 264.7 cells. RAW 264.7 cells transfected with antagomir-155 were harvested 48 h post transfection, liver-specific miR-122 antagomir served as control (ctrl.). Cells were left untreated or exposed to LPS 6 h prior to harvesting. (A) Change of mature miR-155 expression in RAW 264.7 cells treated as indicated, determined by qPCR normalized to U6 snRNA. (B) CASP-3 mRNA level in RAW 264.7 cells treated as indicated, determined by qPCR normalized to NDUFV1 mRNA. (C) Analysis of CASP-3 with a specific antibody, VINCULIN served as control. (D) RAW 264.7 cells were transfected with fLUC mRNA, bearing the wild type (wt) or mutated (mut) miR-155 target site of CASP-3 mRNA 3'UTR, Renilla mRNA (rLUC) for normalization and miRNA mimics as indicated. FLUC activity was normalized to rLUC activity. P-values (Student's t-test): *P < 0.05, **P < 0.01, ***P < 0.001.
To further evaluate the impact of miR-155 on CASP-3 mRNA, we transfected unstimulated RAW 264.7 cells with 5'capped (m7GpppG-cap) and 3'polyadenylated (A98) fLUC-CASP-3 reporter mRNAs together with a control miRNA mimic (ctrl.) or the miR-155 mimic. The mRNAs contained either the CASP-3 mRNA 3'UTR with the single miR-155 target site (wt) or a mutated variant (mut). As an internal control, 5'cap-Renilla-poly(A98) mRNA (rLUC) was co-transfected. In the presence of the miR-155 mimic the translational activity of the fLUC-CASP-3 reporter mRNA bearing the functional miRNA-155 target site was clearly reduced to 66 % compared to the mutated version that was not affected (Fig. 6D). This result is comparable to the endogenous CASP-3 level in LPS-activated RAW 264.7 cells when miR-155 is induced (Fig. 6C). The data demonstrate that the single miR-155 target site in the CASP-3 mRNA 3'UTR mediates miR-155 dependent CASP-3 modulation in RAW 264.7 cells.
CASP-3 mRNA is destabilized by miR-155
To get a first insight into the mechanism of miR-155-dependent CASP-3 and IKKε synthesis regulation (Fig. 5), we developed an in vitro translation system employing cytoplasmic extracts of unstimulated and LPS-activated RAW 264.7 cells (Fig. 7). As it has been shown in other cellular contexts, the development of an in vitro system is important to understand the molecular mechanisms of miRNA function.32,33 For in vitro translation experiments 5'capped and 3'polyadenylated (A98) fLUC-CASP-3 or fLUC-IKKε mRNA bearing the respective target mRNA 3'UTR with either the individual wt or mut miR-155 target site, were used (Fig. 7A and C). As an internal control rLUC was added to each reaction. The importance of a pre-incubation step unfavorable for mRNA translation, which we included to allow active RISC formation, has been demonstrated in other cell-free systems used to study miRNA function.32,33 In cytoplasmic extracts of unstimulated RAW 264.7 cells, where miR-155 is absent (Fig. 2A and B, Fig. 7E, right panel) fLUC reporter mRNAs bearing either the wt or mut versions were translated with similar efficiencies (Fig. 7B and D, left panel). To confirm LPS-mediated activation of macrophages, which were used to prepare in vitro translation extracts, induction of TNF-α mRNA, IL-10 mRNA and miR-155 expression was monitored (Fig. 7E, left panel). In cytoplasmic extracts of LPS-induced cells, translation of fLUC reporter mRNAs with either the wt or mut CASP-3- (Fig. 7B, middle panel) or IKKε miRNA-155 target site (Fig. 7D, middle panel) clearly demonstrated reduced relative fLUC activity in the presence of the single wt target site (CASP-3 to 65% and IKKε to 56%, respectively). Importantly, the reduction of relative fLUC activity for both mRNAs bearing the respective wt sequence was comparable to the endogenous levels of CASP-3 and IKKε detected in LPS-activated RAW 264.7 cells (compare Fig. 7B and D, middle panel with Fig. 5B and C, right panel). Notably, a similar 50 % repression was also observed for reporter mRNAs with a single miR-2 binding site in a D. melanogaster cell-free translation system.34 To analyze the impact of miR-155 on mRNA stability, we determined the amount of fLUC-CASP-3 and fLUC-IKKε mRNA following in vitro translation. Remarkably, relative fLUC CASP-3 mRNA stability significantly declined in the presence of the wt miR-155 target site while no difference in relative fLUC IKKε mRNA stability was observed (Fig. 7B and D, right panel). These results strongly indicate that miR-155-mediated modulation of CASP-3 expression involves mRNA destabilization, while IKKε mRNA is translationally repressed, consistent with the data observed for the endogenous mRNAs (Fig. 5B and C).
Figure 7.
CASP-3 3'UTR mediates miR-155-dependent mRNA degradation in vitro. Alignment of the wild type (wt) miR-155 target site in CASP-3 mRNA 3'UTR (A) and IKKε mRNA 3'UTR (C) or mutated (mut) variants inserted in fLUC reporter mRNAs. (B, D) In vitro translation of fLUC mRNAs bearing the wt or mut miR-155 target site in CASP-3 mRNA 3'UTR (B) and IKKε mRNA 3'UTR (D) in cytoplasmic extracts of untreated (left panel) or LPS-induced (middle panel) RAW 264.7 cells. Total RNA was extracted from 3 independent in vitro translation reactions with CAT mRNA as spike-in control. FLUC and rLUC mRNA recovery before and after translation was determined by qPCR and fLUC mRNA stability was normalized to rLUC mRNA stability (right panel). (E) Cytokine mRNA expression (left panel) and miR-155 response (right panel) in untreated and LPS-activated RAW 264.7 cells used to prepare translation competent cytoplasmic extracts, analyzed by RT-PCR or qPCR, respectively. P-values (Student's t-test) *P < 0.05, ** P < 0.01.
LPS-induced miR-155 diminishes macrophage susceptibility to apoptosis
To address a protective function of miR-155 during the macrophage immune response, apoptotic cell death was induced by the microbial alkaloid staurosporine (SSP) in RAW 264.7 cells.
Initially, miR-155 and CASP-3 mRNA expression was analyzed in RAW 264.7 cells, which were left untreated or induced with LPS prior to 500 nM SSP exposure (Fig. S1). Compared to untreated RAW 264.7 cells, LPS induction resulted in a 50-fold increase of miR-155 and mediated a significant CASP-3 mRNA decrease. When cells were treated with SSP, miR-155 expression was not elevated and CASP-3 mRNA increased (Fig. S1A and B). In RAW 264.7 cells pre-treated with LPS prior to SSP incubation, induction of miR-155 increased 17-fold, while CASP‑3 mRNA declined, compared to cells only treated with SSP (Fig. S1A and B). To analyze apoptosis of RAW 264.7 cells, Annexin V and 7-Amino-Actinomycin (7-AAD) staining was performed and monitored by flow cytometry. Strikingly, the percentage of Annexin V positive cells was reduced when RAW 264.7 cells were pre-treated with LPS prior to apoptosis induction by SSP (Fig. S1C and E). The increase of CASP-3 activity in SSP-treated compared to LPS-induced cells was strongly reduced when LPS pre-incubation preceded SSP-mediated apoptosis induction (Fig. S1D). These results indicate that LPS-induced miR-155 might diminish the susceptibility of RAW 264.7 cells to SSP-triggered apoptotic cell death, most presumably by down-regulating CASP-3 mRNA.
To evaluate the specific effect of miR-155 on SSP-induced apoptosis, RAW 264.7 cells were transfected either with antagomir-155 or the non-specific antagomir-ctrl. As observed before, detection of miR-155 and CASP-3 mRNA levels demonstrated a significant increase of miR-155 (Fig. 8A) and a decrease of CASP-3 mRNA (Fig. 8B) in antagomir-ctrl. transfected and LPS-induced cells. Upon antagomir-155 transfection, miR-155 significantly declined while the CASP-3 mRNA level is elevated (Fig. 8A and B). In LPS- and SSP-treated RAW 264.7 cells, which were transfected with antagomir-ctrl., miR-155 exhibited a 19-fold increase and CASP-3 mRNA declined. When LPS- and SSP-treated cells were transfected instead with antagomir-155, miR-155 expression decreased 4-fold and the CASP-3 mRNA level is elevated (Fig. 8A and B).
Figure 8.
LPS-triggered miR-155 diminishes staurosporine-induced apoptosis of RAW 264.7 cells. (A–D) RAW 264.7 cells harvested 48 h after transfection with antagomir-155 or liver-specific antagomir-122 (antagomir-ctrl.). Where indicated, cells were incubated with LPS for 30 min followed by stimulation with SSP for 6 h prior to harvesting. (A) miR-155 expression and (B) CASP-3 mRNA level were determined by qPCR normalized to U6 snRNA or NDUFV1 mRNA, respectively. (C) RAW 264.7 cells stained with APC-Annexin V and 7-AAD were analyzed by flow cytometry. Percentage of Annexin V positive cells determined in 3 independent experiments. (D) CASP-3 activity in cytoplasmic extracts of RAW 264.7 cells. P-values (Student's t-test) *P < 0.05, ** P < 0.01, ***P < 0.001.
Flow cytometric analysis revealed a significant decrease of Annexin V positive cells when RAW 264.7 cells transfected with antagomir-ctrl. were stimulated with LPS prior to SSP incubation (Fig. 8C), in accordance with Fig. S1C. Interestingly, the number of Annexin V positive cells increased significantly when LPS- and SSP-treated cells were transfected with antagomir-155 (Fig. 8C). Consistent with this observation, analysis of CASP-3 activity in cytoplasmic extracts of antagomir-155 transfected RAW 264.7 cells treated with LPS and SSP indicated a significant increase of CASP-3 activity compared to cells transfected with antagomir-ctrl. (Fig 8D).
From these results we conclude that inhibition of miR-155 in LPS-induced and SSP-treated RAW 264.7 cells leads to an elevated CASP-3 mRNA level and the resulting increase in CASP-3 enhances SSP-mediated apoptosis.
Discussion
In a deep sequencing approach we searched for miRNAs differentially expressed in response to LPS in untreated and LPS-activated murine macrophages. Applying in silico target prediction we identified 7 upregulated miRNAs with a minimum of 50 reads, at least 1.5-fold increased expression and putative target mRNAs linked to the macrophage immune response. Among them miR-155 exhibited the highest LPS-induced increase in expression (Fig. 1). Induction of miR-155 in response to the cytokines IFN-β and IFN-γ, as well as LPS has been reported in monocytes, dendritic cells and macrophages.23,27,35,36 We focused on miR-155 to characterize new target mRNAs that are involved in inflammatory response regulation in RAW 264.7 cells. Interestingly, in silico miRNA-155 target prediction revealed CASP-3 mRNA as putative target (Fig. 2), in addition to known mRNAs encoding TLR4 pathway components, like IKKε or TAB2.27,28
Besides its crucial function in the regulation of immunological reactions, miR-155 is linked to cancer37-39 and apoptosis. MiR-155 up-regulation in MCF-7 cells correlated with impeded tumor suppressor mRNA expression, elevated proliferation and reduced apoptosis,40 miR-155 inhibition in Jurkat cells diminished viability and induced apoptosis41 and mice treated with antisense miR-155 showed attenuated tumorigenesis resulting from increased apoptosis and inhibited proliferation.42
To address our hypothesis that miR-155 expression is linked to apoptosis protection in the macrophage immune response, we analyzed the potential modulation of CASP-3 expression by miRNA-155 in LPS-activated RAW 264.7 cells. Importantly, LPS-activation of primary macrophages revealed TLR4-dependent activation of miR-155 expression43 and CASP-3 mRNA reduction (Fig. 3). In AGO2 immunoprecipitations we demonstrated that miR-155 and CASP-3 mRNA accumulated in functional miRISC (Fig. 4). Interestingly, the increase of miR-155 in LPS-activated macrophages correlated with a decline of CASP-3 mRNA to 35 % (Fig. 5). Consistently, both endogenous full-length and cleaved CASP-3 were reduced in cytoplasmic extracts of LPS-activated cells (Fig. 5). In contrast, analysis of IKKε mRNA, described as miR-155 target,28,44 and IKKε protein revealed that IKKε protein declined upon LPS activation although IKKε mRNA was significantly increased (Fig. 5). These results indicate that miR-155 does not destabilize IKKε mRNA, but inhibits its translation. Ectopic miRNA-155 overexpression or inhibition did not clearly elucidate the mechanism underlying miR-155 mediated IKKε repression.45,46 Interestingly, by transfecting LPS-activated macrophages with antagomir-155 we could effectively reduce mature miR-155 expression and observed significantly elevated CASP-3 mRNA (Fig. 6). In vivo, translation of the fLUC reporter mRNA bearing the functional miRNA-155 target site was clearly reduced in the presence of miR-155 mimic, compared to the mutated target site variant, demonstrating that the single miR-155 target site in the CASP-3 mRNA 3'UTR mediates miR-155 dependent CASP-3 modulation in RAW 264.7 cells (Fig. 6). In vitro translation assays established in cytoplasmic extracts of LPS-induced RAW 264.7 cells, demonstrated that modulation of CASP-3 expression by miR-155 involves mRNA destabilization, while IKKε mRNA remains stable when IKKε synthesis is repressed (Fig. 7). The significant inhibition of fLUC reporter mRNA translation mediated by the individual miR-155 target site in the 3'UTR of CASP-3 and IKKε mRNA was exclusively dependent on endogenous, LPS-induced miR-155 (Fig. 7). These results confirm the concept that specific miRNAs function as post-transcriptional modulators of gene expression in infla-mmation.47,48
In murine osteoblasts SSP-induced apoptosis was shown to increase caspase-3-like protease activity, suggesting that SSP mediates apoptotic cell death via CASP-3 activation49 by a so far unsolved mechanism. Strikingly, Annexin V staining of macrophages, which were stimulated with LPS prior to SSP treatment, implied that LPS prevents apoptosis, most likely through miR-155-mediated CASP-3 mRNA down-regulation. Antagomir-155-mediated inhibition of miR-155 in RAW 264.7 cells treated with LPS and SSP revealed that the percentage of Annexin V positive cells increases compared to antagomir-ctrl. transfected cells. This indicates that repression of miR-155 reverses the protective effect of LPS-dependent miR-155 induction (Fig. 8 and Fig. S1). Anti-apoptotic genes, which support apoptotic stimuli resistance, are activated in LPS-induced macrophages besides inflammatory cytokine genes.50,51 Fas-associated death domain protein (FADD) mRNA represents a validated miR-155 target52 and miR-155-dependent FADD downregulation in RAW 264.7 cells contributed to the decline of induced apoptosis.53
In this study we identified CASP-3 mRNA as a new miRNA-155 target in LPS-activated RAW 264.7 macrophages. Our data suggest that through destabilization of CASP-3 mRNA miR-155 contributes to the prolonged life span of activated macrophages and has a protective role to sustain macrophage function in inflammation response.
Methods
Sequences of primers for cloning, RT-PCR and qPCR and, sequences of Northern blot probes, as well as antagomirs (all Eurofins Genomics) and mRNA mimics (biomers.net) are listed in the Supplemental Material (Table S1).
Plasmid construction
For cloning of Firefly LUCIFERASE (fLUC) reporter constructs 466 bp of the CASP-3 and 464 bp or of the IKKε 3'UTR, each containing the miR-155 binding site were cloned into Pst1/Xho1 (NEB, #R0140S, #R0146S) in the pBluescript II KS (+)-LUC poly(A), which was described previously in.24 To generate reporter constructs containing 4 bp substitutions in the respective miR-155 target site, site-directed mutagenesis was performed. The Renilla LUCIFERASE control plasmid (rLUC) was provided by R. Thermann.33
Cell culture and LPS treatment
RAW 264.7 cells (ATCC, TIB-71) were grown in DMEM (Life Technologies# 31966047) supplemented with 10 % heat inactivated FBS (Biochrom, #S0615), penicillin and streptomycin (Life Technologies, #15140–122). Bone marrow derived macrophages (BMDM) from wild type (wt) C57BL/6 or TLR4 knockout mice54 and L929 cells were prepared and cultured as described in.24 For LPS-activation, RAW 264.7 cells were stimulated with 10 ng/ml E. coli LPS (serotype 0111:B4, Sigma-Aldrich, #L2630) for 6 h and BMDM with 5 ng/ml highly purified Salmonella minnesota LPS for 2 h (minimal effective concentration) unless stated otherwise. To induce apoptosis, RAW 264.7 cells were pre-incubated with or without LPS for 30 min followed by 500 nM SSP stimulation (Applichem, #A7626) for 6 h.
Cytoplasmic extract preparation
RAW 264.7 cell extract was prepared as described previously.24,55
In vitro transcription
MRNA for in vitro translation and extraction controls was transcribed with T7 MEGAscript® Kit (life technologies, #AM1334) in the presence of 6 mM m7GpppG cap (KEDAR).
In vitro translation
Translation reactions contained 45-57 μg cytoplasmic extract, 100 μM amino acids, 16 mM Hepes pH 7.6, 2.5 mM Mg(CH3CO2), 60 mM K(CH3CO2), 80 μg/ml tRNA, 0.8 mM ATP, 0.1 mM GTP, 40 μg/ml creatine kinase, 20 mM creatine phosphate, 1.25 fmol of 5'capped fLUC CASP-3 transcript and 0.125 fmol 5'capped rLUC or 0.625 fmol of 5'capped fLUC IKKε and 0.0625 fmol 5'capped rLUC transcript, respectively. Translation reactions were incubated for 30 min at 37°C. Prior to translation, the reaction was incubated at 16°C for 20 min. Luciferase activity was measured using the Dual-Luciferase® Reporter System (Promega, #E1910). For determination of transcript stabilities, the indicated fLUC and rLUC mRNAs were incubated on ice or translated as described above. For RNA preparation with Trizol, reactions were spiked-in with 0.5 fmol CAT mRNA as extraction control in qPCR experiments. Recovery of fLUC and rLUC mRNAs before and after translation was determined by qPCR and fLUC stability was normalized to rLUC stability.
Small RNA cloning and sequencing
Total RNA was isolated from untreated or LPS-activated RAW 264.7 cells and small RNA libraries were generated as published previously.56 Libraries were sequenced by Fasteris SA (Plan-les-Ouates) on an Illumia SolexaTM sequencer (Genome Analyzer IIx). Employing the miR-IntessTM analysis platform based on miRBase version 16,26 miRNAs were identified and classified from the cloned small RNA populations25 by InteRNA Genomics B.V. (Utrecht). For prediction the TargetScanTM database (Release 5.2) was used.
RNA preparation and quantitative real-time PCR
Total RNA was prepared using Trizol. For miRNA analysis, cDNA was generated using the miScript II RT Kit (Qiagen, #218161). To determine mRNA expression, RNA was reverse transcribed with the Maxima H Minus First Strand cDNA synthesis kit (Thermo Scientific, #K1682) or random primers and M-MLV RT (Promega, #M5301) according to.57 QPCR was performed on a StepOnePlus (Life Technologies) with the miScript SYBR® Green PCR Kit (Qiagen, #218073) for miRNA expression analysis and Power SYBR Green PCR Master Mix (Life Technologies, #4368702) to study target mRNA expression. U6 snRNA (miRNAs), NDUFV1 (Fig. 3D, Fig. 5, Figs. 6B, 8B), RPS7 (Fig. 3B), cel-miR-39 (Fig. 4C) or CAT (Fig. 7B and D) served as controls for normalization, respectively. Data were analyzed using the ΔΔ CT method.58
Northern blotting
Northern blotting was performed as previously described.56,59 Total RNA was separated on a 12 % urea gel. Northern blot signals were visualized through exposure on a screen followed by scanning on a development machine (OPTIMAX, Protec).
AGO2 immunoprecipitation
AGO2 immunoprecipitation was modified according to Beitzinger and Meister.60 6.25 μg of AGO2 or GST control antibody per mg cytoplasmic extract were incubated with 50 μl protein G-agarose beads (Roche, #11719416001) overnight at 4°C in 500 μl PBS on a rotating wheel. Unless noted otherwise all steps were performed at 4°C, incubation steps were performed under rotation and wash steps imply 2 min on a rotating wheel followed by centrifugation at 1.500 g. Beads were centrifuged and washed once with 1 ml of IP buffer.61 2.5 mg of cytoplasmic extracts were incubated with the beads overnight in 500 μl IP buffer. After incubation the beads were gently washed 5 times with IP buffer and in a last wash step with PBS. For protein analysis 100 μl of bead suspension was centrifuged. Beads were resuspended in SDS loading dye, denatured and Western blotting was performed. The remaining 900 μl of bead suspension were used for RNA analysis as described before.60 Following Proteinase K treatment (Life Technologies, #EO0491), input samples and beads were spiked-in with 25 fmol CAT RNA and 25 fmol cel-miR-39 prior to RNA extraction. Co-precipitated mRNAs were analyzed by RT-PCR according to24 using primers directed against the respective mRNA 3'UTRs. Association of miR-155 was determined by qPCR.
Transfection of antagomirs
RAW 264.7 cells (1×106 in DMEM without FBS and antibiotics) were electroporated at 360 V and 500 μF (GenePulser II, BioRad) with 160 pmol of chemically modified antisense oligonucleotides (Eurofins Genomics) against mature miR-155 or liver-specific miR-122 as control. Antagomirs were designed according to31 containing 2'O methylated RNA bases and a partial phosphorthionate backbone. RAW 264.7 cells were harvested 48 h post transfection unless stated otherwise. LPS stimulation of transfected cells was performed 6 h prior to cell harvesting. To analyze the effect of miR-155 on apoptosis, RAW 264.7 cells were transfected as described above. Prior to harvesting the cells were pre-incubated with or without LPS for 30 min followed by treatment with 500 nM SSP for 6 h.
RNA transfection
RAW 264.7 cells (1×106 in DMEM without FBS and antibiotics) were electroporated at 360 V and 500 μF (GenePulser II, BioRad) with 1.25 pmol of 5'capped fLUC CASP-3 transcript and 0.125 pmol 5'capped rLUC together with 100-fold molar excess of miR-122 mimic (mimic-ctrl.) or miR-155 mimic (biomers.net). RAW 264.7 cells were lysed 3 h after transfection in 1x passive lysis buffer and Luciferase activity was measured using the Dual-Luciferase® Reporter System (Promega, #E1910).
Immunoblotting
Western blotting was performed as described,61 blots were analyzed on a LAS-4000 system (GE Healthcare Life Sciences) and quantified using MultiGauge version 3.2 (FUJIFILM).
Antibodies
Antibodies were purchased from Cell Signaling (CASP-3 #9660, IKKε #2905,), Santa Cruz (CASP-3, cleaved CASP-3 #sc-56053, GST #sc-138), Sigma Aldrich (VINCULIN #V9131) and Wako Chemicals (AGO2 #018–22021).
CASP-3 activity
12.5 μg of cytoplasmic extracts from RAW 264.7 cells were employed to determine CASP-3 activity using the Caspase-Glo®3/7 Assay System (Promega, # G8091).
Flow cytometry
RAW 264.7 cells were incubated with 5 μl APC-Annexin V (BD Biosciences, #556547) 15 min prior to harvesting. Cells were washed twice with PBS containing 1.8 mM CaCl2 and harvested in FACS buffer (2 mM EDTA, 0.1 % BSA, 3.8 mM CaCl2 in PBS). 10 μl of 7-AAD (Sigma-Aldrich, #A9400) were added to the cells prior to flow cytometry analysis on an LSRFortessa (BD Biosciences). Data were analyzed using FlowLogic (Inivai) and Flowjo (Tree Star). Presented are the percentages of Annexin V positive cells of living cells.
Supplementary Material
Disclosure of potential conflicts of interests
No potential conflicts of interests were disclosed.
Acknowledgments
We thank Klaus Brandenburg for purified Salmonella minnesota LPS and Sven Burgdorf for L929 cells. The work was supported by a grant (IZKF E6-4) from the Interdisciplinary Center for Clinical Research, Faculty of Medicine, RWTH Aachen to A.O.-L. and D.H.O. R.D.S acknowledged a grant from the RWTH Aachen Graduate Program.
References
- 1. Carpenter S, Ricci EP, Mercier BC, Moore MJ, Fitzgerald KA. Post-transcriptional regulation of gene expression in innate immunity. Nat Rev Immunol 2014; 14:361–76; PMID:24854588; http://dx.doi.org/ 10.1038/nri3682 [DOI] [PubMed] [Google Scholar]
- 2. Tan Y, Kagan JC. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol Cell 2014; 54:212–23; PMID:24766885; http://dx.doi.org/ 10.1016/j.molcel.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takeda K, Akira S. TLR signaling pathways. Semin Immunol 2004; 16:3–9; PMID:14751757; http://dx.doi.org/ 10.1016/j.smim.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 4. Gioannini TL, Weiss JP. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res 2007; 39:249–60; PMID:17917069; http://dx.doi.org/ 10.1007/s12026-007-0069-0 [DOI] [PubMed] [Google Scholar]
- 5. Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 1999; 189:1777–82; PMID:10359581; http://dx.doi.org/ 10.1084/jem.189.11.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009; 458:1191–5; PMID:19252480; http://dx.doi.org/ 10.1038/nature07830 [DOI] [PubMed] [Google Scholar]
- 7. Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 1996; 16:1247–55; PMID:8622669; http://dx.doi.org/ 10.1128/MCB.16.3.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swantek JL, Cobb MH, Geppert TD. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor α (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol Cell Biol 1997; 17:6274–82; PMID:9343388; http://dx.doi.org/ 10.1128/MCB.17.11.6274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci 2000; 20:4563–72; PMID:10844026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ulloa L, Tracey KJ. The "cytokine profile:" a code for sepsis. Trends Mol Med 2005; 11:56–63; PMID:15694867; http://dx.doi.org/ 10.1016/j.molmed.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 11. Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003; 301:640–3; PMID:12855817; http://dx.doi.org/ 10.1126/science.1087262 [DOI] [PubMed] [Google Scholar]
- 12. amamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 2003; 4:1144–50; PMID:14556004; http://dx.doi.org/ 10.1038/ni986 [DOI] [PubMed] [Google Scholar]
- 13. Ohta S, Bahrun U, Tanaka M, Kimoto M. Identification of a novel isoform of MD-2 that downregulates lipopolysaccharide signaling. Biochem Biophys Res Commun 2004; 323:1103–8; PMID:15381113; http://dx.doi.org/ 10.1016/j.bbrc.2004.08.203 [DOI] [PubMed] [Google Scholar]
- 14. Gray P, Michelsen KS, Sirois CM, Lowe E, Shimada K, Crother TR, Chen S, Brikos C, Bulut Y, Latz E, et al. Identification of a novel human MD-2 splice variant that negatively regulates Lipopolysaccharide-induced TLR4 signaling. J Immunol 2010; 184:6359–66; PMID:20435923; http://dx.doi.org/ 10.4049/jimmunol.0903543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215–33; PMID:19167326; http://dx.doi.org/ 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010; 11:597–610; PMID:20661255 [DOI] [PubMed] [Google Scholar]
- 17. Meister G. Argonaute proteins: functional insights and emerging roles. Nat Rev Genet 2013; 14:447–59; PMID:23732335; http://dx.doi.org/ 10.1038/nrg3462 [DOI] [PubMed] [Google Scholar]
- 18. Dueck A, Meister G. Assembly and function of small RNA - argonaute protein complexes. Biol Chem 2014; 395:611–29; PMID:24603840; http://dx.doi.org/ 10.1515/hsz-2014-0116 [DOI] [PubMed] [Google Scholar]
- 19. Braun JE, Huntzinger E, Izaurralde E. The role of GW182 proteins in miRNA-mediated gene silencing. Adv Exp Med Biol 2013; 768:147–63; PMID:23224969; http://dx.doi.org/ 10.1007/978-1-4614-5107-5_9 [DOI] [PubMed] [Google Scholar]
- 20. Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011; 12:99–110; PMID:21245828; http://dx.doi.org/ 10.1038/nrg2936 [DOI] [PubMed] [Google Scholar]
- 21. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 2010; 79:351–79; PMID:20533884; http://dx.doi.org/ 10.1146/annurev-biochem-060308-103103 [DOI] [PubMed] [Google Scholar]
- 22. Boldin MP, Baltimore D. MicroRNAs, new effectors and regulators of NF-kappaB. Immunol Rev 2012; 246:205–20; PMID:22435557; http://dx.doi.org/ 10.1111/j.1600-065X.2011.01089.x [DOI] [PubMed] [Google Scholar]
- 23. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006; 103:12481–6; PMID:16885212; http://dx.doi.org/ 10.1073/pnas.0605298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liepelt A, Mossanen JC, Denecke B, Heymann F, De Santis R, Tacke F, Marx G, Ostareck DH, Ostareck-Lederer A. Translation control of TAK1 mRNA by hnRNP K modulates LPS-induced macrophage activation. RNA 2014; 20:899–911; PMID:24751651; http://dx.doi.org/ 10.1261/rna.042788.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell 2007; 28:328–36; PMID:17964270; http://dx.doi.org/ 10.1016/j.molcel.2007.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res 2008; 36:D154–8; PMID:17991681; http://dx.doi.org/ 10.1093/nar/gkm952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A 2009; 106:2735–40; PMID:19193853; http://dx.doi.org/ 10.1073/pnas.0811073106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 2007; 179:5082–9; PMID:17911593; http://dx.doi.org/ 10.4049/jimmunol.179.8.5082 [DOI] [PubMed] [Google Scholar]
- 29. Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999; 6:99–104; PMID:10200555; http://dx.doi.org/ 10.1038/sj.cdd.4400476 [DOI] [PubMed] [Google Scholar]
- 30. Han Z, Hendrickson EA, Bremner TA, Wyche JH. A sequential two-step mechanism for the production of the mature p17:p12 form of caspase-3 in vitro. J Biol Chem 1997; 272:13432–6; PMID:9148968; http://dx.doi.org/ 10.1074/jbc.272.20.13432 [DOI] [PubMed] [Google Scholar]
- 31. Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature 2005; 438:685–9; PMID:16258535; http://dx.doi.org/ 10.1038/nature04303 [DOI] [PubMed] [Google Scholar]
- 32. Mathonnet G, Fabian MR, Svitkin YV, Parsyan A, Huck L, Murata T, Biffo S, Merrick WC, Darzynkiewicz E, Pillai RS, et al. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science 2007; 317:1764–7; PMID:17656684; http://dx.doi.org/ 10.1126/science.1146067 [DOI] [PubMed] [Google Scholar]
- 33. Thermann R, Hentze MW. Drosophila miR2 induces pseudo-polysomes and inhibits translation initiation. Nature 2007; 447:875–8; PMID:17507927; http://dx.doi.org/ 10.1038/nature05878 [DOI] [PubMed] [Google Scholar]
- 34. Moretti F, Kaiser C, Zdanowicz-Specht A, Hentze MW. PABP and the poly(A) tail augment microRNA repression by facilitated miRISC binding. Nat Struct Mol Biol 2012; 19:603–8; PMID:22635249; http://dx.doi.org/ 10.1038/nsmb.2309 [DOI] [PubMed] [Google Scholar]
- 35. O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A 2007; 104:1604–9; PMID:17242365; http://dx.doi.org/ 10.1073/pnas.0610731104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dueck A, Eichner A, Sixt M, Meister G. A miR-155-dependent microRNA hierarchy in dendritic cell maturation and macrophage activation. FEBS Lett 2014; 588(4):632–40; PMID:24444604 [DOI] [PubMed] [Google Scholar]
- 37. Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta 2009; 1792:497–505; PMID:19268705; http://dx.doi.org/ 10.1016/j.bbadis.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 38. Kluiver J, Poppema S, de Jong D, Blokzijl T, Harms G, Jacobs S, Kroesen BJ, van den Berg A. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J Pathol 2005; 207:243–9; PMID:16041695; http://dx.doi.org/ 10.1002/path.1825 [DOI] [PubMed] [Google Scholar]
- 39. O'Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med 2008; 205:585–94; PMID:18299402; http://dx.doi.org/ 10.1084/jem.20072108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang CM, Zhao J, Deng HY. MiR-155 promotes proliferation of human breast cancer MCF-7 cells through targeting tumor protein 53-induced nuclear protein 1. J Biomed Sci 2013; 20:79; PMID:24152184; http://dx.doi.org/ 10.1186/1423-0127-20-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alizadeh S, Kaviani S, Soleimani M, Abroun S, Kashani-Khatib Z, Asgharzadeh A, Dargahi H, Mousavi R. Mir-55 inhibition can reduce cell proliferation and induce apoptosis in Jurkat (Acute T cell Leukemia) cell line. Iran J Ped Hematol Oncol 2014; 4:141–50; PMID:25598954 [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng SR, Guo GL, Zhai Q, Zou ZY, Zhang W. Effects of miR-155 antisense oligonucleotide on breast carcinoma cell line MDA-MB-157 and implanted tumors. Asian Pac J Cancer Prev 2013; 14:2361–6; PMID:23725141; http://dx.doi.org/ 10.7314/APJCP.2013.14.4.2361 [DOI] [PubMed] [Google Scholar]
- 43. Schulte LN, Westermann AJ, Vogel J. Differential activation and functional specialization of miR-146 and miR-155 in innate immune sensing. Nucleic Acids Res 2013; 41:542–53; PMID:23143100; http://dx.doi.org/ 10.1093/nar/gks1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu F, Weidmer A, Liu CG, Volinia S, Croce CM, Lieberman PM. Epstein-Barr virus-induced miR-155 attenuates NF-kappaB signaling and stabilizes latent virus persistence. J Virol 2008; 82:10436–43; PMID:18753206; http://dx.doi.org/ 10.1128/JVI.00752-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Long L, Yu P, Liu Y, Wang S, Li R, Shi J, Zhang X, Li Y, Sun X, Zhou B, et al. Upregulated microRNA-155 expression in peripheral blood mononuclear cells and fibroblast-like synoviocytes in rheumatoid arthritis. Clin Dev Immunol 2013; 2013:296139; PMID:24151514; http://dx.doi.org/ 10.1155/2013/296139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G, Shi Y, Wang F, Wu Y, Tong WD, et al. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis 2009; 200:916–25; PMID:19650740; http://dx.doi.org/ 10.1086/605443 [DOI] [PubMed] [Google Scholar]
- 47. O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol 2012; 30:295–312; PMID:22224773; http://dx.doi.org/ 10.1146/annurev-immunol-020711-075013 [DOI] [PubMed] [Google Scholar]
- 48. Marques-Rocha JL, Samblas M, Milagro FI, Bressan J, Martinez JA, Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J 2015; 29:3595–611; PMID:26065857; http://dx.doi.org/ 10.1096/fj.14-260323 [DOI] [PubMed] [Google Scholar]
- 49. Chae HJ, Kang JS, Byun JO, Han KS, Kim DU, Oh SM, Kim HM, Chae SW, Kim HR. Molecular mechanism of staurosporine-induced apoptosis in osteoblasts. Pharmacol Res 2000; 42:373–81; PMID:10987998; http://dx.doi.org/ 10.1006/phrs.2000.0700 [DOI] [PubMed] [Google Scholar]
- 50. Conte D, Holcik M, Lefebvre CA, Lacasse E, Picketts DJ, Wright KE, Korneluk RG. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol Cell Biol 2006; 26:699–708; PMID:16382159; http://dx.doi.org/ 10.1128/MCB.26.2.699-708.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manna SK, Aggarwal BB. Lipopolysaccharide inhibits TNF-induced apoptosis: role of nuclear factor-kappaB activation and reactive oxygen intermediates. J Immunol 1999; 162:1510–8; PMID:9973408 [PubMed] [Google Scholar]
- 52. Tili E, Croce CM, Michaille JJ. miR-155: on the crosstalk between inflammation and cancer. Int Rev Immunol 2009; 28:264–84; PMID:19811312; http://dx.doi.org/ 10.1080/08830180903093796 [DOI] [PubMed] [Google Scholar]
- 53. Zhu GF, Yang LX, Guo RW, Liu H, Shi YK, Wang H, Ye JS, Yang ZH, Liang X. miR-155 inhibits oxidized low-density lipoprotein-induced apoptosis of RAW264.7 cells. Mol Cell Biochem 2013; 382:253–61; PMID:23797321; http://dx.doi.org/ 10.1007/s11010-013-1741-4 [DOI] [PubMed] [Google Scholar]
- 54. Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 1999; 162:3749–52; PMID:10201887 [PubMed] [Google Scholar]
- 55. Barton DJ, Flanegan JB. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol 1993; 67:822–31; PMID:8380467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dueck A, Ziegler C, Eichner A, Berezikov E, Meister G. microRNAs associated with the different human Argonaute proteins. Nucleic Acids Res 2012; 40:9850–62; PMID:22844086; http://dx.doi.org/ 10.1093/nar/gks705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naarmann IS, Harnisch C, Flach N, Kremmer E, Kuhn H, Ostareck DH, Ostareck-Lederer A. mRNA silencing in human erythroid cell maturation: heterogeneous nuclear ribonucleoprotein K controls the expression of its regulator c-Src. J Biol Chem 2008; 283:18461–72; PMID:18441016; http://dx.doi.org/ 10.1074/jbc.M710328200 [DOI] [PubMed] [Google Scholar]
- 58. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–8; http://dx.doi.org/ 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 59. Pall GS, Hamilton AJ. Improved northern blot method for enhanced detection of small RNA. Nat Protoc 2008; 3:1077–84; PMID:18536652; http://dx.doi.org/ 10.1038/nprot.2008.67 [DOI] [PubMed] [Google Scholar]
- 60. Beitzinger M, Meister G. Experimental identification of microRNA targets by immunoprecipitation of Argonaute protein complexes. Methods Mol Biol 2011; 732:153–67; PMID:21431712; http://dx.doi.org/ 10.1007/978-1-61779-083-6_12 [DOI] [PubMed] [Google Scholar]
- 61. Naarmann IS, Harnisch C, Muller-Newen G, Urlaub H, Ostareck-Lederer A, Ostareck DH. DDX6 recruits translational silenced human reticulocyte 15-lipoxygenase mRNA to RNP granules. RNA 2010; 16:2189–204; PMID:20884783; http://dx.doi.org/ 10.1261/rna.2211110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007; 23:2947–8; PMID:17846036; http://dx.doi.org/ 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




