Abstract
Females of the hematophagous mosquito species require a vertebrate blood meal to supply amino acids and other nutrients necessary for egg development, serving as the driving force for the spread of many vector-borne diseases in humans. Blood digestion utilizes both early and late phase serine proteases (SPs) that are differentially regulated at the transcriptional and post-transcriptional level. To uncover the regulatory complexity of SPs in the female mosquito midgut, we investigated involvement of miRNAs in regulating the juvenile hormone (JH)-controlled chymotrypsin-like SP, JHA15. We identified regulatory regions complementary to the mosquito-specific miRNA, miR-1890, within the 3′ UTR of JHA15 mRNA. The level of the JHA15 transcript is highest post eclosion and drastically declines post blood meal (PBM), exhibiting an opposite trend to miR-1890 that peaks at 24 h PBM. Depletion of miR-1890 results in defects in blood digestion, ovary development and egg deposition. JHA15 mRNA and protein levels are elevated in female mosquitoes with miR-1890 inhibition. JHA15 RNA interference in the miR-1890 depletion background alleviates miR-1890 depletion phenotypes. The miR-1890 gene is activated by the 20-hydroxyecdysone pathway that involves the ecdysone receptor and the early genes, E74B and Broad Z2. Our study suggests that miR-1890 controls JHA15 mRNA stability in a stage- and tissue- specific manner.
Keywords: digestive protease, ecdysone, juvenile hormone, Mosquito, microRNA
Introduction
Hematophagous female mosquitoes utilize a nutrient-rich vertebrate blood meal for rapid egg development. During successive blood feedings, mosquitoes acquire and transmit pathogens of various harmful human diseases. Disease pathogens enter the female mosquito gut with ingested blood and exploit mosquitoes for obligatory stages of their life cycles. Hence, deciphering the molecular mechanisms governing the gut function and blood digestion is of utmost importance for devising innovative vector and pathogen control methods.
In mosquitoes, multiple enzymes are involved in the digestion of blood. The two major classes of secreted proteases in the blood fed midgut are endoproteases, represented by trypsin-like and chymotrypsin-like serine proteases (SPs), and exopeptidases, that function as aminopeptidases and carboxypeptidases.1 Blood digestion functions in a biphasic fashion, utilizing early and late phase digestive proteases that are specifically regulated at the transcriptional and post-transcriptional levels. In Aedes aegypti, the early trypsin (AaET) gene is expressed and its mRNA accumulated in midgut cells prior to blood feeding, while the synthesis of the AaET protein occurs soon after blood feeding.1-3 Expression of the AaET gene is under the control of juvenile hormone (JH),3 while the amino acid (AA)/target of rapamycin (TOR) pathway regulates AaET translation after blood feeding.4 The gene encoding the chymotrypsin-like SP, JHA15, is also expressed in the Aedes midgut prior to blood feeding, and its expression is controlled by JH.5 Although its mRNA profile is similar to that of AaET, the JHA15 protein is readily detected in the midgut of both non-blood fed and blood fed mosquitoes. The major events of blood digestion occur later after blood feeding. The best-studied example of a late digestive endoprotease is Ae. aegypti Late Trypsin (AaLT). The transcript level of the AaLT gene reaches maximum by 24 h post blood meal (PBM).1,6 It has been shown that insulin-like peptides and TOR regulate expression of the AaLT gene.7 Despite of significant progress in understanding mosquito blood digestion and regulation of digestive enzymes, the entire molecular complexity of regulatory mechanisms governing blood digestion in female mosquitoes has yet to be revealed.
MicroRNAs (miRNAs) are small 22 nt regulatory RNAs that regulate gene expression post-transcriptionally in plants and animals.8 Midgut-specific miRNAs have been identified in Anopheles gambiae.9 In Ae. aegypti, several miRNAs exhibit stage-specific expression, and their levels are enhanced in the midgut of blood-fed females,10 implying miRNA involvement in blood-meal-associated events. The first functional study of a mosquito miRNA revealed that miR-275 is required for blood digestion and egg maturation in Ae. aegypti.11 More recently, the mosquito- and gut-specific miR-1174 was shown to function in sugar absorption, fluid excretion, and blood intake in the gut - further supporting miRNA function in regulating gut functions.12 To identify factors that function in the regulation of digestive SPs in the female mosquito gut, we investigated involvement of miRNAs in regulating JHA15. In this report, we have identified putative miRNA binding sites within the 3′ UTR of JHA15 and examined the role of the mosquito-specific miRNA, miR-1890, in regulating JHA15 in the mosquito gut. In addition, we provide evidence for regulation of miR-1890 by the ecdysone regulatory pathway.
Results
Identification of putative miRNA binding sites within the 3′ UTR of JHA15
Typically, miRNA functional analysis focuses on the characterization of a miRNA and subsequent identification of functionally relevant targets. An alternative approach for analyzing miRNA-target interaction is to first identify putative miRNA binding sites within a gene's 3′ UTR region, and then functionally characterize these putative interactions. Because of the presumed role of JHA15 in blood digestion and the existing knowledge about regulation of expression, we analyzed the 3′ UTR of JHA15 (AAEL001703), encoding an early digestive protease, for the presence of putative miRNA binding sites. To identify putative miRNA binding sites within the 3′ UTR of JHA15, we took a multi-algorithm approach using 4 different target-prediction programs: TargetScan,13 PITA,14 miRANDA15 and RNAhybrid.16 Analysis with TargetScan, PITA and RNAhybrid revealed that the 3′ UTR of JHA15 contains putative binding sites for several miRNAs, including 3 conserved miRNAs (miR-34–3p, miR-137 and miR-315–5p) and the mosquito-specific miRNA miR-1890 (Table 1). miRanda did not predict an interaction between JHA15 and any Ae. aegypti miRNA. The JHA15 3′ UTR was identified to contain a strong 7-mer seed match site at positions 1–7 for miR-1890, with a putative complementary match site at positions 17–20 on the 3′ end of the miRNA (Table 1). Because miR-1890 is a mosquito-specific miRNA, we hypothesized that this miRNA may play a role in mosquito-specific events, such as blood digestion. Therefore, we assessed the 3′ UTR of JHA15 for its response to miR-1890 in vitro. The JHA15 3′ UTR was cloned downstream of the Renilla translational stop codon within the psiCheck-2 vector to generate 3′ UTR-fused luciferase reporters. When transfected into Drosophila S2 cells along with the miR-1890 mimic, the luciferase reporter containing the full-length JHA15 3′ UTR yielded 64.59% luciferase activity compared with the negative control mimic and no-mimic control samples (Fig. 1A).
Table 1.
Putative miRNA binding sites within the 3′ UTR of JHA15
| miRNA | Programi | mfeii | Target Site |
|---|---|---|---|
| miR-34–3p | TS; RH | -16.8 kcal/mol | 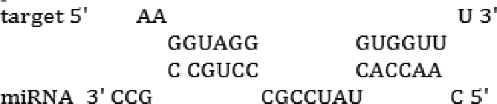 |
| miR-137 | TS; PITA; RH | -16.8 kcal/mol | 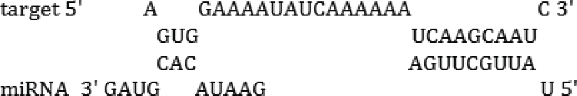 |
| miR-315–5p | TS; RH | -15.3 kcal/mol | 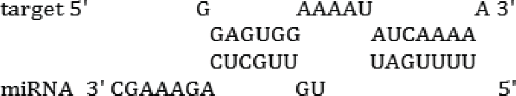 |
| miR-1890 | TS; RH | -14.2 kcal/mol |  |
miRanda did not identify any putative miRNA binding sites in JHA15.
Minimal free energy (mfe) values based off RNA hybrid predictions.
Program Abbreviation; TS, TargetScann; PITA, Probability Interaction by Target Accessibility; RH, RNA hybrid.
Figure 1.
JHA15 and miR-1890 display opposing expression profiles. (A) Dual Luciferase Reporter Assay for JHA15. Data represents the percent activity (Δ Fold Activity * 100) average ± SEM of triplicate samples. Percentage shown. (B) Relative expression profile of mature miR-1890 in the female mosquito midgut. Relative expression was analyzed at the following time-points: 0–6, 24, 48 and 72 h post eclosion (PE), and 6, 12, 24, 36, 48 h post blood meal (PBM). (C) Relative expression profile of JHA15 in the female mosquito midgut. Relative expression was analyzed at the following time-points: 0–6, 24, 48 and 72 h post eclosion (PE), and 6, 12, 24, 36, 48 h PBM. (B-C) Data represents 3 biological replicates with 3 technical replicates and are illustrated as average ± SEM.
Expression analysis of miR-1890 and JHA15 in the female mosquito midgut
To compare miR-1890 and JHA15 expression, we produced a thorough time-course expression analysis of mature miR-1890 and JHA15 in the adult female mosquito midgut by measuring relative levels of mature miR-1890 expression by quantitative real-time (qRT)-PCR analysis. We monitored the abundance of mature miR-1890 in the midgut using 9 time points collected over the first reproductive cycle. We obtained total RNA samples from female mosquito midguts at 0–6, 24, 48, and 72 h post eclosion (PE) and at 6, 12, 24, 36, and 48 h PBM. Mature miR-1890 peaked in abundance 24 h PBM and declined sharply by 36 h PBM (Fig. 1B). These results suggest that the high expression of miR-1890 in the female mosquito midgut may play an important role in tuning events associated with blood digestive processes. We also monitored the expression of JHA15 mRNA in the midgut during the same developmental time. As previously reported, JHA15 expression is highest PE and drastically declines PBM5 (Fig. 1C), the latter coinciding with miR-1890 peak abundance PBM (Fig. 1B). This correlation in profiles further suggests JHA15 regulation by miR-1890 in the female mosquito midgut.
Depletion of miR-1890 results in misregulation of JHA15 and abnormal blood digestion
To determine the function of miR-1890 in the adult female mosquito, we inhibited miR-1890 using a sequence-specific antisense oligonucleotide known as an antagomir. We designed an antagomir consisting of the reverse complement of miR-1890 (1890Ant), as well as a randomly scrambled "missense" antagomir (MsAnt) for a control. Female mosquitoes were microinjected at a dose of 50 pmol per mosquito of the 1890Ant or MsAnt at 12 h PE. To evaluate the efficiency of the miR-1890Ant, we performed qRT-PCR to monitor endogenous levels of mature miR-1890. Antagomirs designed to target miR-1890 successfully down-regulated mature miR-1890 in the female mosquito by 24 h PBM when compared to the MsAnt-treated and non-injected controls (Fig. 2A). There was no change in the relative expression levels of other miRNAs, such as mature miR-275, in the 1890Ant-treated females (Fig. 2B). Further, JHA15 transcript and protein levels were dramatically enhanced in the 1890Ant-treated female midguts 24 h PBM, indicative that miR-1890 targets JHA14 in vivo (Fig. 2C-D).
Figure 2.

miR-1890 depletion results in increased JHA15 levels. (A) Percent relative expression of miR-1890 in the female mosquito midgut 24 h post blood meal (PBM). (B) Mature miR-275 percent relative expression in the female mosquito midgut 24 h PBM. (C) JHA15 transcript levels increases in miR-1890-depleted female mosquito midguts. (A-C) Data represents 3 biological replicates with 3 technical replicates and are illustrated as average ± SEM, * P< 0.05; ** P < 0.01. (D) Western blot analyses utilizing antibodies against JHA15 in 1890Ant-treated, MsAnt-treated and non-injected female mosquito midguts. Beta-actin was used as a loading control.
Female mosquitoes treated with the 1890Ant were screened for phenotypic manifestations PBM. First, we evaluated the efficiency of blood feeding and state of blood digestion at 24 h PBM. Less than 70% of the 1890Ant-treated females took blood, compared with over 90% of MsAnt-treated and non-injected females (Fig. S1A). In nearly 40% of the blood-fed 1890Ant-treated females, blood remained partially undigested in the midgut (red bolus) 24 h PBM (Fig. 3A, Fig. S1B); however, at the same developmental time normal digestion (dark brown bolus) was observed in the MsAnt-treated (Fig. 3B) and non-injected (Fig. 3C) mosquitoes. Blood-fed females treated with the 1890Ant failed to fully develop mature ovaries during the first gonadotrophic cycle compared to the MsAnt-treated and non-injected controls (Fig. 3D). Ovarian follicle growth was reduced in nearly 50% of the miR-1890-depleted females compared to control mosquitoes, with an average primary follicle length of 180.71 µm (Fig. 3E, Fig. S1C). Ovaries from MsAnt-treated females were similar to those in non-injected female mosquitoes at 24 h PBM, with primary follicles reaching 218.84 µm in length on average (Fig. 3E). In addition, 1890Ant-treated females displayed reduce fecundity, laying a significantly reduced number of eggs per mosquito as compared to controls, with an average of 53.7 eggs per mosquito (Fig. 3F). MsAnt-treated females laid a similar number of eggs as non-injected female mosquitoes, with 92.2 and 99.97 eggs per female on average, respectively (Fig. 3F). Pre-vitellogenic ovary development, host seeking behavior, blood digestion and longevity were unaffected in 1890Ant-treated females, and displayed no other adverse phenotypes when compared to MsAnt-treated and non-injected controls.
Figure 3.
miR-1890 depletion results in impaired blood digestion and egg development (A-C) Midguts 24 h post blood meal (PBM) of (A) miR-1890 antagomir (1890Ant)-treated, (B) MsAnt-treated, and (C) non-injected females. Images (A-C) obtained under the same conditions using the Leica M165FC stereo microscope. (D) Female mosquito ovaries 24 h PBM. Ovaries were visualized using the Leica M165FC stereo microscope. (E) Average follicle size of 1890Ant-treated, MsAnt-treated and non-injected mosquitoes 24 h PBM. Measurements were made using the Leica M165FC stereo microscope. (F) Egg numbers per female mosquito for 1890Ant-treated, MsAnt-treated and non-injected mosquitoes. (E-F) Data represents 3 biological replicates with 3 technical replicates and are illustrated as average ± SEM, * P< 0.05; ** P < 0.01.
JHA15 RNA interference knockdown rescues miR-1890 depletion phenotype
Next, we conducted phenotypic rescue experiments through JHA15 RNA interference (RNAi) in female mosquitoes with the 1890Ant background. It was expected that the RNAi-mediated knockdown of the physiologically relevant target of miR-1890 would alleviate the adverse phenotypes caused by miR-1890 depletion. This method has proved successful in validating mosquito miRNA targets.12,17 dsRNA designed to target JHA15 transcripts successfully depleted JHA15 transcript levels in female mosquitoes (Fig. S2A). We co-injected 50 pmol of the 1890Ant or MsAnt and 0.5 μg of JHA15 (iJHA15) or luciferase (iLuc) dsRNA into each female mosquito at 12 h PE. Females treated with the 1890Ant and luciferase dsRNA control displayed reduced follicle size and egg-deposition phenotypes characteristic of 1890Ant–treated mosquitoes, while no adverse phenotypes were observed in the MsAnt/iJHA15-treated and non-injected control mosquitoes at 24 h PBM (Fig. 4A-B). However, injection of JHA15 dsRNA and the 1890Ant partially restored the ovary-development and egg deposition in the 1890Ant/iJHA15-treated mosquitoes at 24 h PBM (Fig. 4A-B). Hence, the phenotype rescue by JHA15 RNAi in miR-1890–depleted female mosquitoes confirms that JHA15 is an authentic target of miR-1890 in vivo.
Figure 4.

JHA15 RNAi partially rescues miR-1890 depletion phenotypes. (A) JHA15 RNAi alleviated ovary development phenotype in miR-1890 antagomir (1890Ant)-treated female mosquitoes. (B) JHA15 RNAi alleviated egg deposition phenotype in miR-1890Ant-treated female mosquitoes. Data represents 3 biological replicates with 3 technical replicates and are illustrated as average ± SEM, *P< 0.05; **P < 0.01.
miR-1890 is regulated by the ecdysone signaling pathway
The mature miR-1890 levels exhibited a peak of the midgut expression at 24 h PBM and declined sharply by 36 h PBM, suggesting a possible involvement of regulatory signals activated after a blood meal. To elucidate the regulatory signals involved in the transcriptional regulation of miR-1890, we investigated the effects of insulin and 20-hydroxyecdysone (20E) on mature miR-1890 levels in the midgut. Digestive systems that included midguts were isolated from 4-day old female mosquitoes and incubated in vitro with the presence or absence of specific factors in a culture medium. We then evaluated the level of mature miR-1890 from our in vitro experiments. Midguts incubated in the medium with amino acids (AAs) or medium alone showed a low, baseline level expression of miR-1890 (Fig. 5A). Insulin with AAs did not increase miR-1890 levels; however, the miR-1890 expression rose significantly after incubation in medium containing both AAs and 20E (Fig. 5A). This indicated that 20E, but not insulin, likely regulates miR-1890 levels in the mosquito midgut.
Figure 5.

miR-1890 responds to 20E, while JHA15 is regulated by JH. (A) Mature miR-1890 expression from in vitro midgut. Midguts were isolated from non-blood fed female mosquitoes 72 post eclosion (PE) and incubated under indicated conditions in vitro for 6 h. (B) Mature miR-1890 expression in vivo in Ecdysone Receptor (EcR) and Insulin Receptor (iInR) RNAi treatments 24 h post blood meal (PBM). (C) Mature miR-1890 expression in vivo in E74B and Broad RNAi treatments PBM. (D) JHA15 expression in vivo in Methoprene Tolerant (iMet) RNAi-treated non-blood fed female mosquitoes. (B-D) RNAi for Luciferase (iLuc) served as a control. (A-D) Data represents 3 biological replicates with 3 technical replicates and are illustrated as average ± SEM, * P< 0.05; ** P < 0.01; *** P < 0.001.
To substantiate in vitro experiments, we investigated the in vivo effect of RNAi depletions of potential factors involved in regulation of the miR-1890 in the mosquito midgut. We performed RNAi-mediated depletion of the ecdysone receptor (EcR) and insulin receptor (InR) in vivo (Fig. S2). The dsRNA for EcR was designed using the region common to both EcR-A and EcR-B isoforms. We injected female mosquitoes with EcR, InR or Luc dsRNA at 12 h PE and assayed the level of mature miR-1890 in EcR and InR-depleted female mosquitoes 24 h PBM. We found that miR-1890 levels are substantially decreased in the EcR-depleted females while remaining unchanged in InR RNAi-treated mosquitoes compared to the iLuc and non-injected controls (Fig. 5B, S2B-C). Thus, in vivo RNAi experiment results were in agreement with those from the in vitro tissue culture, strongly suggesting that the 20E-signaling pathway controls miR-1890 expression PBM.
To further investigate the possible influence of the 20E regulatory cascade on miR-1890 in the female mosquito midgut, we performed RNAi depletions in vivo of 2 early genes of the 20E genetic hierarchy– E74B and broad (Br). The dsRNA for Br was designed using the region common of all Br isoforms (Br-cm), while E74 dsRNA was specific to the E74B isoform. We injected female mosquitoes with E74B, Br-cm or Luc dsRNA at 12 h PE and assayed the level of mature miR-1890 at 24 h PBM. Females treated with E74B dsRNA, but not Br-cm dsRNA, displayed a significant reduction in miR-1890 expression compared to iLuc and non-injected controls (Fig. 5C, S2D-E). We used a pattern-search approach to identify the presence of putative E74 and Br isoform binding sites within the promoter of miR-1890. Analysis of the 2-kb region upstream of the miR-1890 precursor sequence revealed the presence of several putative E74 (C/AGGAA) and Br-Z2 (TTTATCATTT) binding motifs at positions 1581–1608. Taken together, these results suggest that miR-1890 is regulated by the 20E-signaling cascade, likely through an interaction with E74B and the miR-1890 promoter, as it has been demonstrated for the vitellogenin gene.18 Using the Br-cm probe was insufficient to establish the involvement of Br-Z2 in regulating miR-1890 gene expression.
The JHA15 expression profile is closely correlated to the changing titers of JH in the female mosquito (Fig. 1C). Moreover, JH involvement in JHA15 regulation is supported by in vitro analysis that indicates JHA15 responsiveness to topical application of increasing doses of JHIII and the JH analog methoprene.5 To support this work, we performed RNAi depletions in vivo of the JH receptor, Methoprene-tolerant (Met) (Fig. S2F).18 We injected female mosquitoes with Met or Luc dsRNA at 12 h PE and assayed the level of JHA15 at 4 d post injection. Indeed, dsRNA mediated Met depletions in female mosquitoes resulted in a decreased expression of JHA15 compared to iLuc and non-injected controls (Fig. 5D), further supporting JHA15 regulation by the JH signaling cascade.
Discussion
In order to better understand the post-transcriptional regulation of SPs we searched for regulatory links between mosquito gut SPs and miRNAs. Using a multi-algorithm approach, followed by in vitro cell culture assays, we identified a putative miR-1890 binding site within the 3′ UTR of JHA15. JHA15 and mature miR-1890 display opposing expression profiles; the level of the JHA15 transcript is highest PE and declines soon after blood feeding, while mature miR-1890 is induced after blood feeding reaching its peak at 24 PMB. Systemic depletion of miR-1890 results in increased JHA15 transcript and protein PBM, resulting in impaired blood digestion in the gut, followed by decreased egg development and deposition. JHA15 RNAi in the 1890Ant background partially alleviated the ovary development and egg laying phenotypes observed in the miR-1890-depleted females. These results suggest that miR-1890 plays an important role in mosquito blood digestion by regulating JHA15 in the female mosquito. Tissue- and stage-specific systems, such as Aedes midgut carboxypeptide-GAL4/UAS, to investigate the function of miR-1890 may prove beneficial in providing spatiotemporal resolution of miR-1890 function.19
Transcription of JHA15 is known to be upregulated PE by JH in the midgut of adult female mosquitoes, and its transcriptional profile of JHA15 is similar to that of AaET. While the AaET protein is produced PBM,2,3 the JHA15 protein can be detected in the midgut before blood feeding.5 Presence of mature miR-1890 is induced by 20E PBM, coinciding with the decrease in JHA15 transcripts. The nature of JHA15 expression may indicate miR-1890 functions to fine-tune the JHA15 transcript and protein levels PBM, and enhanced JHA15 levels disrupts proper blood digestion. Digestive enzymes function redundantly and cooperatively in the female mosquito midgut; enhanced JHA15 levels in the midgut due to miR-1890 depletion could interfere with the function of other digestive enzymes required for proper blood digestion.
Insulin-like peptides have been shown to function in the regulation of late phase trypsin-like gene expression and blood meal digestion7 and carboxypeptidase.19 RNAi knockdown of InR delays AaLT gene expression and activity. Furthermore, 20E is a major regulator of reproductive events PBM; however, its role in regulating digestive enzymes is not understood. Several insect miRNAs have been shown to be regulated by 20E20-24 and insulin25-27 signaling pathways. Because mature miR-1890 levels are enhanced PBM, we investigated the role of insulin and 20E signaling pathways in regulating miR-1890 expression. In vitro midgut culture assays and RNAi experiments in vivo revealed miR-1890 is induced by the 20E-signaling cascade but not insulin. These results provide insight into the transcriptional and post-transcriptional control of SPs in the female mosquito. Taken together, these results suggest that the 20E-regulated miR-1890 targets the JH-regulated JHA15 in the female mosquito to regulate blood digestive processes.
Expression analysis and discovery of miRNAs in mosquitoes has revealed several mosquito-specific miRNAs, including miR-1890.9,10,28-32 Phenotypic divergence among animal species may be due in part to lineage- and species-specific regulation of gene expression by miRNAs. miRNAs restricted to mosquitoes may contribute to mosquito-specific functions and, as such, have potential for contributing to the development of novel mosquito control approaches. Mosquito-specific miRNAs have been shown to play important roles in blood digestion12 and host-pathogen interactions33-36 in female mosquitoes. In this study, we identified that another mosquito-specific miRNA, miR-1890, targets the juvenile hormone-regulated chymotrypsin-like SP, JHA15, in the midgut of the female mosquito. These studies indicate that these mosquito-specific miRNAs play key roles in mosquito-specific physiological functions, including blood-meal-associated events.
Materials and Methods
Computational miRNA target prediction
The 156 base pair (bp) 3′ UTR of JHA15 was retrieved from the Vectorbase database. The genebuild (AaegL1.3) of the Ae. aegypti genome, with well-annotated gene sequences and 3′ UTR database was utilized. The JHA15 3′ UTR was analyzed with several target prediction tools, including TargetScan,13 Probability Interaction of Target Accessibility (PITA),14 miRANDA15 and RNAhybrid.16 The miRNA prediction programs were used to determine if any of the 124 available Ae. aegypti miRNAs have a putative binding site within the 3′ UTR of JHA15.
Cell culture and luciferase reporter assay
in vitro target validation was performed as previously described.12,17 Drosophila Schneider 2 (S2) cells (Invitrogen) were kept at 28°C in Schneider's Drosophila medium (Gibco, 21720–024) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, 10082139) and 1× Antibiotic-Antimycotic (Gibco, 15240–062). Luciferase constructs were made by inserting the JHA15 3′ RACE fragment containing the putative miR-1890 binding site into the multiple cloning region located downstream of the Renilla translational stop codon within the psiCheck-2 vector (Promega, C8021). 100ng of psiCheck-2 reporters and synthetic aae-miR-1890 miScript miRNA Mimic (Qiagen, MSY0014247) or All Stars Negative Control siRNA (Qiagen, SI03650318) at a final concentration of 100nm were co-transfected into Drosophila S2 cells using Attractene Transfection Reagent (Qiagen, 301005). A no mimic treatment was also performed. Dual Luciferase Reporter assay was completed 48 h post-transfection using the Dual Luciferase Reporter Assay System (Promega, E1910). Firefly luciferase in the psiCheck-2 Vector was used for normalization of Renilla luciferase expression. Treatments were made in triplicate, and transfections were repeated 3 times.
Mosquito rearing
The Ae. aegypti wild-type UGAL/Rockefeller strain and transgenic lines were reared at 27 °C and 80% humidity with unlimited access to 10% sugar solution and water until blood feeding, as described previously.37 Blood feeding of adult female mosquitoes was performed using White Leghorn chickens.
Antagomir and dsRNA treatments
Antagomirs were obtained from Dharmacon using the RNA module for custom single-stranded RNA synthesis, which can be found at: http://dharmacon.gelifesciences.com/rnai-and-custom-rna-synthesis/custom-rna-synthesis/single-strand-rna-synthesis/. The antagomir to miR-1890 (1890Ant) was designed with the following sequence and modifications as follows: 5′ mU.*.mA.*.mU.mC.mC.mA.mG.mA.mC.mC.mU.mA.mA.mU.mC.mA.mA.mA.mG.mA.mU.mU.*.mU.*.mC.*.mA.*.mG.*mC –Chl 3′. A scrambled antagomir termed “missense” (MsAnt) was used as a control as previously described.11 “*” is a Phosphorothioate backbone instead of the usual PO backbone. “m” is an OCH3 group on the 2′ end of the base instead of the usual OH group. A 3′ cholesterol (Chl) group was added to each antagomir to enhance potency. Mosquitoes were CO2 anesthetized 12 h PE and microinjected into the thorax at a dose of 200 μM in a volume of 0.25 μL (50 pmol). Mosquitoes were allowed to recover for 3 to 4 d before blood feeding.
Double-stranded RNA (dsRNA) was produced using the MEGAscript kit (Ambion, AM1333) as described previously.37 The luciferase gene was used to generate control iLuc dsRNA. After dsRNA synthesis, samples were subjected to phenol/chloroform extraction and ethanol precipitation. At 12 h PE, female mosquitoes were microinjected into the thorax with 0.5ug (0.25 ul of 2 ug/ul) dsRNA. For rescue experiments, mosquitoes were co-injected with 0.25 uL of an antagomir/dsRNA mixture with a final concentration of 200 uM antagomir and 2 ug/ul dsRNA. Knockdown was confirmed via semi-quantitative PCR.
Total RNA extraction and Real-Time PCR
MicroRNA expression analysis was measured quantitatively as previously described.11,17 TRIzol Reagent (Invitrogen, 15596–018) was utilized for total RNA extractions according to the manufacturer's protocol. Total RNA was treated with DNase I (Invitrogen) before cDNA synthesis using the miScript II RT Kit (Qiagen, 218161). miRNA expression was analyzed quantitatively via the miScript SYBR Green PCR kit (Qiagen, 218073) according to manufacturer's protocol. Quantitative measurements were performed in triplicate and normalized to the internal control of the housekeeping genes: S7 ribosomal protein (RPS7), actin.
Immunoblot
Protein analysis of JHA15 was done as described previously.17 Blood was removed from female mosquito midguts 24 h PBM and homogenized in lysis buffer (50 mM Tris HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1× phosphatase inhibitor from Sigma P2850, and 1× protease inhibitor from Sigma P8340). Total protein was quantified using the Bio-Rad Protein Assay (Bio-Rad, 500–0006). Ten ug protein was boiled in LDS (4X) NuPage sample buffer (Invitrogen, NP0007) with 10X sample reducing agent (Invitrogen, NP0004) for 5–10 min and run on 4–12% Tris-Glycine gels (Invitrogen, EC6038BOX) before being transferred to PVDF membranes (Invitrogen, LC2002). For detection of JHA15, JHA15 antisera5 was partially purified by affinity chromatography using Antibody Purification Kit Protein G (Abcam, ab128747) and used at 1:10,000 dilution; followed by the secondary anti-Rat-HRP (Fisher Scientific, 31218) at 1:2000 dilution. For detection of Actin, β-actin monoclonal antibody (Sigma, A5316) was used at 1:5000 dilution followed by the secondary anti-mouse-HRP (Sigma, A9044) at 1:2000 dilution.
In vitro midgut culture
The in vitro midgut culture was performed as previously described.19 Midguts were dissected from mosquitoes 4 d after microinjection in Aedes physiological saline (APS) buffer and incubated in a complete culture medium supplemented with amino acids and 20-hydroxyecdysone (concentration used for all experiments, 1 μM; Sigma, H5142) for 6 h. Midguts were harvested and RNA was extracted as described above.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Jinsong Zhu (Virginia Tech) for providing the JHA15 antibody. This work was supported by National Institutes of Health Grants R01 AI113729 and R01 AI036959 (to A.S.R.).
References
- 1.Isoe J, Rascón AA Jr, Kunz S, Miesfeld RL. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem Mol Biol 2009; 39:903-12; PMID:19883761; http://dx.doi.org/ 10.1016/j.ibmb.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noriega FG, Wang XY, Pennington JE, Barillas-Mury CV, Wells MA. Early trypsin, a female-specific midgut protease in Aedes aegypti: isolation, aminoterminal sequence determination, and cloning and sequencing of the gene. Insect Biochem Mol Biol 1996; 26:119-26; PMID:8882654; http://dx.doi.org/ 10.1016/0965-1748(95)00068-2 [DOI] [PubMed] [Google Scholar]
- 3.Noriega FG, Shah DK, Wells MA. Juvenile hormone controls early trypsin gene transcription in the midgut of Aedes aegypti. Insect Mol Biol 1997; 6:63-6; PMID:9013256; http://dx.doi.org/ 10.1046/j.1365-2583.1997.00154.x [DOI] [PubMed] [Google Scholar]
- 4.Brandon MC, Pennington JE, Isoe J, Zamora J, Schillinger AS, Miesfeld RL. TOR signaling is required for amino acid stimulation of early trypsin protein synthesis in the midgut of Aedes aegypti mosquitoes. Insect Biochem Mol Biol 2008; 38:916-22; PMID:18708143; http://dx.doi.org/ 10.1016/j.ibmb.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian G, Raikhel AS, Zhu J. Characterization of a juvenile hormone-regulated chymotrypsin-like serine protease gene in Aedes aegypti mosquito. Insect Biochem Mol Biol 2008; 38:190-200; PMID:18207080; http://dx.doi.org/ 10.1016/j.ibmb.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barillas-Mury C, Wells MA. Cloning and sequencing of the blood meal-induced late trypsin gene from the mosquito Aedes aegypti and characterization of the upstream regulatory region. Insect Mol Biol 1993; 2:7-12; PMID:9087537; http://dx.doi.org/ 10.1111/j.1365-2583.1993.tb00119.x [DOI] [PubMed] [Google Scholar]
- 7.Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS One 2011; 6:e20401; PMID:21647424; http://dx.doi.org/ 10.1371/journal.pone.0020401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas K, Raikhel AS. Insect microRNAs: biogenesis, expression profiling and biological functions. Insect Biochem Mol Biol 2013; 43:24-38; PMID:23165178; http://dx.doi.org/ 10.1016/j.ibmb.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter F, Edaye S, Hüttenhofer A, Brunel C. Anopheles gambiae miRNAs as actors of defence reaction against Plasmodium invasion. Nucl Acids Res 2007; 35:6953-62; PMID:17933784; http://dx.doi.org/ 10.1093/nar/gkm686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Mead EA, Liang S, Tu Z. Direct sequencing and expression analysis of a large number of miRNAs in Aedes aegypti and a multi-species survey of novel mosquito miRNAs. BMC Genomics 2009; 10:581; PMID:19961592; http://dx.doi.org/ 10.1186/1471-2164-10-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant B, Macdonald W, Raikhel AS. microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proc Natl Acad Sci USA 2010; 107:22391-8; PMID:21115818; http://dx.doi.org/ 10.1073/pnas.1016230107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Lucas KJ, Roy S, Ha J, Raikhel AS. Mosquito-specific microRNA-1174 targets serine hydroxymethyltransferase to control key functions in the gut. Proc Natl Acad Sci U S A 2014; 111:14460-5; PMID:25246546; http://dx.doi.org/ 10.1073/pnas.1416278111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 2003; 115:787-98; PMID:14697198; http://dx.doi.org/ 10.1016/S0092-8674(03)01018-3 [DOI] [PubMed] [Google Scholar]
- 14.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet 2007; 39:1278-84; PMID:17893677; http://dx.doi.org/ 10.1038/ng2135 [DOI] [PubMed] [Google Scholar]
- 15.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol 2003; 5:R1; PMID:14709173; http://dx.doi.org/ 10.1186/gb-2003-5-1-r1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krüger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucl Acids Res 2006; 34:W451-4; PMID:16845047; http://dx.doi.org/ 10.1093/nar/gkl243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas KJ, Roy S, Ha J, Gervaise AL, Kokoza VA, Raikhel AS. MicroRNA-8 targets the Wingless signaling pathway in the female mosquito fat body to regulate reproductive processes. Proc Natl Acad Sci U S A 2015; 12:1440-5; http://dx.doi.org/ 10.1073/pnas.1424408112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou Z, Saha TT, Roy S, Shin SW, Backman TW, Girke T, White KP, Raikhel AS. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Natl Acad Sci U S A 2013; 110:E2173-81; PMID:23633570; http://dx.doi.org/ 10.1073/pnas.1305293110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao B, Kokoza VA, Saha TT, Wang S, Roy S, Raikhel AS. Regulation of the gut-specific carboxypeptidase: A study using the binary Gal4/UAS system in the mosquito Aedes aegypti. Insect Biochem Mol Biol 2014; 54C:1-10; http://dx.doi.org/ 10.1016/j.ibmb.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varghese J, Cohen SM. microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila. Genes Dev 2007; 21:2277-82.; PMID:17761811; http://dx.doi.org/ 10.1101/gad.439807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Liang Z, Liang Y, Pang R, Zhang W. Conserved microRNAs miR-8-5p and miR-2a-3p modulate chitin biosynthesis in response to 20-hydroxyecdysone signaling in the brown planthopper, Nilaparvata lugens. Insect Biochem Mol Biol 2013; 43:839-48; PMID:23796434; http://dx.doi.org/ 10.1016/j.ibmb.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Ge X, Li Z, Wang Y, Song Q, Stanley DW, Tan A, Huang Y. MicroRNA-281 regulates the expression of ecdysone receptor (EcR) isoform B in the silkworm, Bombyx mori. Insect Biochem Mol Biol 2013; 43:692-700; PMID:23707601; http://dx.doi.org/ 10.1016/j.ibmb.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 23.Fagegaltier D, König A, Gordon A, Lai EC, Gingeras TR, Hannon GJ, Shcherbata HR. A genome-wide survey of sexually dimorphic expression of Drosophila miRNAs identifies the steroid hormone-induced miRNA let-7 as a regulator of sexual identity. Genetics 2014; 198:647-68; PMID:25081570; http://dx.doi.org/ 10.1534/genetics.114.169268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin H, Kim VN, Hyun S. Conserved microRNA miR-8 controls body size in response to steroid signaling in Drosophila. Genes Dev 2012; 26:1427-32; PMID:22751499; http://dx.doi.org/ 10.1101/gad.192872.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell 2009; 139:1096-108; PMID:20005803; http://dx.doi.org/ 10.1016/j.cell.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 26.Foronda D, Weng R, Verma P, Chen YW, Cohen SM. Coordination of insulin and Notch pathway activities by microRNA miR-305 mediates adaptive homeostasis in the intestinal stem cells of the Drosophila gut. Genes Dev 2014; 28:2421-31; PMID:25367037; http://dx.doi.org/ 10.1101/gad.241588.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varghese J, Lim SF, Cohen SM. Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe. Genes Dev 2010; 24:2748-53; PMID:21159815; http://dx.doi.org/ 10.1101/gad.1995910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mead EA, Tu Z. Cloning, characterization and expression of microRNAs from the Asian malaria mosquito, Anopheles stephensi. BMC Genomics 2008; 9:244; PMID:18500992; http://dx.doi.org/ 10.1186/1471-2164-9-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skalsky RL, Vanlandingham DL, Scholle F, Higgs S, Cullen BR. Identification of microRNAs expressed in two mosquito vectors, Aedes albopictus and Culex quinquefasciatus. BMC Genomics 2010; 11:119; PMID:20167119; http://dx.doi.org/ 10.1186/1471-2164-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biryukova I, Ye T, Levashina E. Transcriptome-wide analysis of microRNA expression in the malaria mosquito Anopheles gambiae. BMC Genomics 2014; 15:557; PMID:24997592; http://dx.doi.org/ 10.1186/1471-2164-15-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu W, Criscione F, Liang S, Tu Z. MicroRNAs of two medically important mosquito species: Aedes aegypti and Anopheles stephensi. Insect Mol Biol 2015; 24:240-52; PMID:25420875; http://dx.doi.org/ 10.1111/imb.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maharaj PD, Widen SG, Huang J, Wood TG, Thangamani S. Discovery of mosquito saliva microRNAs during CHIKV infection. PLoS Negl Trop Dis 2015; 9:e0003386; PMID:25612225; http://dx.doi.org/ 10.1371/journal.pntd.0003386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hussain M, Frentiu FD, Moreira LA, O'Neill SL, Asgari S. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc Natl Acad Sci U S A 2011; 108:9250-5; PMID:21576469; http://dx.doi.org/ 10.1073/pnas.1105469108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang G, Hussain M, O'Neill SL, Asgari S. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc Natl Acad Sci U S A 2013; 110 10276-81; PMID:23733960; http://dx.doi.org/ 10.1073/pnas.1303603110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang G, Hussain M, Asgari S. Regulation of arginine methyltransferase 3 by a Wolbachia-induced microRNA in Aedes aegypti and its effect on Wolbachia and dengue virus replication. Insect Biochem Mol Biol 2014; 53:81-8; PMID:25158106; http://dx.doi.org/ 10.1016/j.ibmb.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 36.Slonchak A, Hussain M, Torres S, Asgari S, Khromykh AA. Expression of mosquito microRNA Aae-miR-2940-5p is downregulated in response to West Nile virus infection to restrict viral replication. J Virol 2014; 88:8457-67; PMID:24829359; http://dx.doi.org/ 10.1128/JVI.00317-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol 2007; 37:1317-26; PMID:17967350; http://dx.doi.org/ 10.1016/j.ibmb.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




