Abstract
An operationally simple and general method for copper-catalyzed, aminoquinoline-assisted amination of β-C(sp2)-H bonds of benzoic acid derivatives is reported. The reaction employs Cu(OAc)2 or (CuOH)2CO3 catalysts, an amine coupling partner, and oxygen from air as a terminal oxidant. Exceptionally high generality with respect to amine coupling partners is observed. Specifically, primary and secondary aliphatic and aromatic amines, heterocycles, such as indoles, pyrazole, and carbazole, sulfonamides, as well as electron-deficient aromatic and heteroaromatic amines are competent coupling components.
Graphical Abstract

1. INTRODUCTION
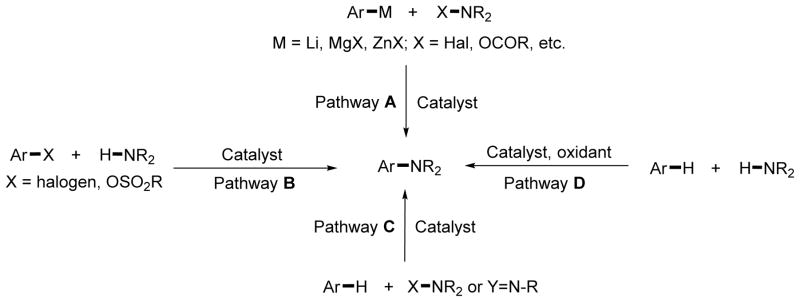
Aryl amine functionality can be found in biologically important substances and functional materials.1 As a consequence, their synthesis has attracted substantial attention. Methods for aryl amine synthesis by intermolecular formation of an aryl-nitrogen bond are summarized in Scheme 1. Electrophilic amination reactions can be performed under very mild conditions, but they require the presence of two functionalized coupling components: arylmetal and an oxidized nitrogen source (Scheme 1, pathway A).2 Currently, the most general method for aryl amine synthesis involves palladium- and copper-catalyzed aryl halide amination that originates in the Goldberg reaction (Scheme 1, pathway B).3 This process typically delivers one product regioisomer, a feature that is advantageous compared to most nondirected C–H bond aminations that afford product mixtures. A very large number of both coupling components are available commercially, making this reaction a backbone of many pharmaceutically important amine syntheses. However, the presence of a functionalized aryl halide is required. In pathway C, an arene C–H bond is functionalized by an electrophilic amination reagent, which may be either a nitrene source or an amine possessing a leaving group.4 Although C–H bond coupling components are widely available, only a very limited number of electrophilic nitrogen sources are commercially accessible. Currently, most examples of C–H bond aminations feature reactivity by pathway C. Iridium, rhodium, and cobalt catalysts have been used in the amination of directing-group-containing arenes with azides, acetoxycarbamates, and dioxazolones.4a–d Ellman has shown that aryl azides can be reacted with imines under rhodium catalysis to afford acridines.4e Nakamura has used an aminoquinoline directing group to couple arenes with secondary chloroamines under iron catalysis, and Miura has disclosed copper-catalyzed reactions of heterocycles with chloroamines.4f,g Nitrosobenzenes, azodicarboxylates, and hydroxysuccinimide derivatives have been used as electrophilic nitrogen sources.4h–k Ackermann has reported that azides can be coupled with arenes under ruthenium catalysis.4l Hydroxyl-amine derivatives have been used as sp2 C–H bond aminating reagents by a number of groups.4m–o
Scheme 1.
Arylamine Syntheses via Cross-Coupling
Potentially, the most general and efficient method for amine synthesis is represented by pathway D, where the arene C–H bond is coupled with an amine N–H bond.5 Both the arene and amine coupling components are readily available, allowing for rapid synthesis of structurally diverse arylamines. Optimally, amine synthesis by pathway D should meet the following conditions: First, the scope of the reactants (amines and arenes) that can be used in the coupling reaction has to be general. Second, the reaction should be catalyzed by an abundant first-row transition metal. Third, the oxidant in this reaction has to be cheap and readily available; preferably, oxygen from air should be employed. Fourth, the reaction has to be regioselective. Although significant advances have been made in recent years, limitations of C–H amination methodology are apparent. In most cases, either second- or third-row transition metal catalysis is employed. The use of first-row transition metal catalysis is relatively rare. Furthermore, reactions usually employ expensive oxidants such as silver or iodonium salts, are limited to cyclic secondary amine coupling components, and lack generality. Notably, the first copper-promoted directed amination of arene C–H bonds was reported by Yu in 2006 (Scheme 2A).5a Several other groups have later shown similar reactivity of 2-phenylpyridine derivatives.5b,c Yu has subsequently employed copper salts in bidentate auxiliary-directed amination of diverse aromatic compounds with tosylamides, aromatic amides, and electron-poor anilines (Scheme 2B).5d
Scheme 2.
Copper-Catalyzed Arene Amination
Chen, Zhang, and Liu have reported efficient picolinamide- and aminoquinoline-directed, copper- and nickel-catalyzed amination of sp2 C–H bonds by simple amines.5e,f These reactions appear to be limited to secondary amines; the best results are obtained with cyclic amines, and they require either PhI(OAc)2 oxidant or excess second-row transition metal (silver) additive. Several papers report amination of simple arenes by amines in the presence of iodine-based oxidants.5g,h Specifically, Suna has developed an outstanding method for regioselective amination of electron-rich arenes with structurally diverse amines. The reaction uses a copper catalyst and employs a stoichiometric iodine(III) oxidant.5g Mori discovered that azoles can be coupled with secondary amines in the presence of a copper catalyst under oxygen atmosphere.5i Subsequently, amination of acidic polyfluoroarenes was demonstrated.5j
In 2013, we reported a method for copper-catalyzed, direct, aminoquinoline- and picolinamide-assisted6 amination of β-sp2 C–H bonds of benzoic acid derivatives and γ-sp2 C–H bonds of benzylamine derivatives (Scheme 2C).6d The reaction employed a Cu(OAc)2 catalyst in conjunction with a Ag2CO3 cocatalyst, amine coupling partner, NMP or DMSO solvent, and NMO oxidant. Although the reaction showed high generality and functional group tolerance, several limitations were apparent. First, only primary and secondary aliphatic amines were reactive under the reported conditions. Second, the reaction required a silver cocatalyst. Third, N-methylmorpholine oxide was used as a terminal oxidant. We report here a method for copper-catalyzed or -promoted amination of sp2 C–H bonds of aminoquinoline benzamides that employs oxygen from air as a terminal oxidant and allows the use of an exceptionally wide range of amine coupling partners. Specifically, primary and secondary aliphatic and aromatic amines, sulfonamides, heterocycles, and guanidines are reactive.
2. Method Development Considerations
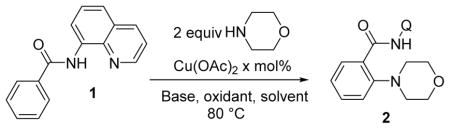
We have previously observed that amination of 8-aminoquinoline benzamide with morpholine gave reasonable conversion to product if oxygen was used as the terminal oxidant.6d Thus, it should be possible to use air or oxygen instead of the somewhat expensive NMO oxidant. Reaction optimization studies are presented in Table 1.
Table 1.
Optimization of Reaction Conditionsa
| |||||
|---|---|---|---|---|---|
| entry | x | oxidant | solvent | time, h | yield, % |
| 1b | 20 | NMO | DMF | 20 | 33 |
| 2b | 20 | NMO | pyridine | 20 | 38 |
| 3 | 20 | NMO | pyridine | 16 | 68 |
| 4 | 20 | air | pyridine | 18 | 53 |
| 5 | 20 | air | pyridine | 6 | 56 |
| 6 | 20 | air | pyridine | 6 | 87 |
| 7 | 30 | air | pyridine | 6 | 92 |
| 8c | 30 | air | pyridine | 6 | quant |
Amide (0.1 mmol), copper(II) acetate (x mol % relative to amide), solvent (entries 1–5, 1 mL; entries 6–8, 0.2 mL), NMO (2 equiv) or air (~3.5 mL). Yield determined by GC analysis of reaction mixtures with 2,6-diisopropyl-naphthalene as the internal standard.
One equiv of K2CO3 was added. Q = 8-quinolinyl.
Three equiv of amine was used.
The reaction performed in DMF using the NMO oxidant and 20 mol % Cu(OAc)2 gave 33% conversion to product (entry 1). Slightly higher conversion was obtained by employing pyridine solvent (entry 2). Omission of base was fruitful, and 68% conversion was observed (entry 3). Replacing the NMO oxidant with air slightly decreased the yield of the product; however, a respectable 53% conversion was obtained in 16 h (entry 4). Decreasing the reaction time to 6 h slightly increased the yield, showing that some product decomposition occurs (entry 5). Decreasing the amount of solvent to 0.2 mL from 1mL increased the reaction yield (entry 6). The use of 30 mol % Cu(OAc)2 gave 92% yield (entry 7). Finally, if the amount of morpholine was increased from 2 to 3 equiv, quantitative conversion to 2 was observed (entry 8). Thus, optimal reaction conditions for employing secondary amine aminating reagents include 0.3 equiv of Cu(OAc)2 catalyst, air oxidant, and pyridine solvent at elevated temperature.
3. RESULTS
3.1. Reaction Scope with Respect to Aminoquinoline Benzamides
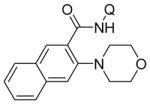
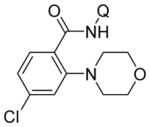
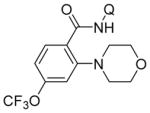
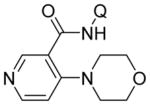
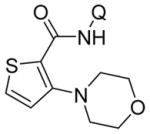
Aminoquinoline benzamide amination with morpholine is presented in Table 2. Both electron-rich (entries 2, 8, 9, 14) and electron-poor substrates (entries 3–7, 10–12) afforded products in good to excellent yields. In contrast to other copper-catalyzed, aminoquinoline-directed C–H bond functionalizations, the amination selectively afforded monosubstitution products at the less sterically hindered position (entries 2, 7, 8). Only trace amounts of diamination products were observed in crude reaction mixtures. Reactions showed excellent functional group tolerance. Chloro (entry 3), cyano (entry 4), trifluoromethoxy (entry 5), trifluoromethylthio (entry 6), nitro (entry 8), vinyl (entry 9), pyrazole (entry 13), and sulfonamide functionalities (entry 10) were all compatible with the reaction conditions. Importantly, heterocycles such as pyridine (entries 11 and 12) and thiophene (entry 14) afforded amination products in good yields. Probenecid7 amide was reacted with morpholine to give the amination product in 76% yield (entry 10), which shows the relevance of the methodology to the functionalization of drug-like molecules. Aminoquinoline 3-fluorobenzamide reacted to give an approximate 1:1 mixture of separable isomeric products (entry 15). The reactions could be run on a 5 mmol scale without loss of yield (entry 10). The generality of amination should allow for a rapid parallel synthesis of drug analogues.
Table 2.
Reaction with Morpholinea
| |||
|---|---|---|---|
| entry | Ar | product | yield, % |
| 1 | Ph |

|
95 |
| 2 | 2-naphthyl |
|
62 |
| 3 | 4-ClC6H4 |
|
71 |
| 4 | 4-NCC6H4 |

|
78 |
| 5 | 4-CF3OC6H4 |
|
87 |
| 6 | 4-CF3SC6H4 |

|
86 |
| 7 | 3-NO2C6H4 |

|
73 |
| 8 | 3-CH3OC6H4 |
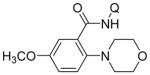
|
72 |
| 9 | 4-vinylC6H4 |
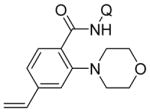
|
78 |
| 10 | 4-SO2NPr2-C6H4 |
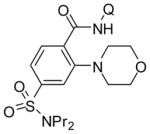
|
76 74b |
| 11 | 4-pyridyl |
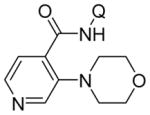
|
75 |
| 12 | 3-pyridyl |
|
77 |
| 13 | 4-(3,5-di-methyl-1H-pyrazol-1-yl)C6H4 |

|
90 |
| 14 | 2-thio-phenyl |
|
65 |
| 15c | 3-F-C6H4 |

|
39 |

|
36 | ||
Amide (1.0 mmol), Cu(OAc)2 (0.3 mmol), pyridine (2.0 mL), morpholine (3.0 mmol), 5–36 h, 80–110 °C. Yields are isolated yields. Please see Supporting Information for details.
Reaction scale of 5 mmol.
Approximately 1:1 ratio of isomers formed.
3.2. Reaction Scope with Respect to Secondary Amines
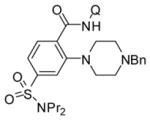
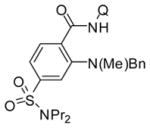
The reaction conditions optimized for coupling with morpholine are applicable for amination with other aliphatic secondary amines (Table 3). All reactions were carried out by using probenecid amide as the C–H bond coupling component. Six-(entries 1–4) and five-membered rings (entry 5) as well as acyclic amines (entries 6 and 7) gave products in acceptable to excellent yields. Tertiary amine (entries 1 and 3), amide (entries 2 and 4), and heterocycle (entries 2 and 5) functionalities were compatible with the reaction conditions. The amide N–H bond was not reactive under these conditions (entry 4). Interestingly, we were able to couple nornicotine with probenecid amide in acceptable yield (entry 5).
Table 3.
Amination with Secondary Aminesa

| |||
|---|---|---|---|
| entry | amine | product | yield, % |
| 1 | N-Bn-piperazine |
|
63 |
| 2 | N-2-furoyl-piperazine |
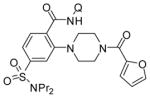
|
64 |
| 3 | 4-morpholinyl-piperidine |

|
73 |
| 4 | 4-(N-Boc)-amino-piperidine |

|
67 |
| 5 | nornicotine |

|
47 |
| 6 | methylbenzylamine |
|
73 |
| 7 | 4-MeO-methylbenzylamine |

|
77 |
Amide (1.0 mmol), Cu(OAc)2 (0.3 mmol), pyridine (2.0 mL), amine (3.0 mmol), 5–36 h, 110 °C. Yields are isolated yields. Please see Supporting Information for details. PMB = p-methoxybenzyl.
3.3. Reaction Scope with Respect to Primary Aliphatic Amines and Anilines
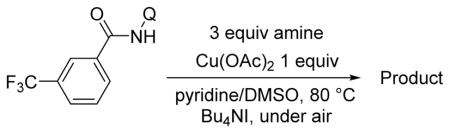
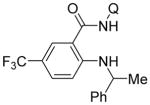
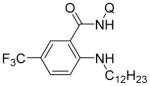
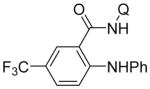
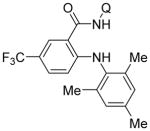
Copper-catalyzed amination of aromatic C–H bonds with secondary amines has been demonstrated in several systems.5a–f,6d We have previously shown that hindered primary amines could be coupled with aminoquinoline benzamides to give amination products in modest yields.6d For nonhindered primary aliphatic amines, low coupling yields were obtained, and aromatic amines were unreactive. Yu recently disclosed copper-promoted, bidentate ligand-directed amination with electron-poor anilines and sulfonamides.5d However, no examples of electron-rich aniline use were demonstrated. We were pleased to discover that, with slight modifications, our amination reaction could be used to couple primary amines with sp2 C–H bonds (Table 4). In this case, pyridine/DMSO solvent mixture afforded the best results. One equiv of copper(II) acetate is required, presumably because the amination product forms a strong chelate with copper, preventing the dissociation needed for achieving catalytic turnover. Hindered aliphatic amines possessing secondary and tertiary substituents were reactive, affording products in acceptable to good yields (entries 1–4). Benzylic primary amines were compatible with the amination conditions (entry 4). n-Dodecylamine could be coupled in 44% yield (entry 5). Simple aniline (entry 6) as well as electron-rich, hindered trimethylaniline (entry 7) gave products in 46–48% yields.
Table 4.
Amination with Primary Aminesa
| |||
|---|---|---|---|
| entry | amine | product | yield, % |
| 1 | isopropyl-amine |

|
58 |
| 2 | 3-pentylamine |

|
57 |
| 3 | 1-adamantyl-amine |

|
40 |
| 4 | 1-methylbenzylamine |
|
54 |
| 5 | 1-dodecylamine |
|
44 |
| 6 | aniline |
|
46 |
| 7 | 2,4,6-tri-methylaniline |
|
48 |
Amide (1.0 mmol), Cu(OAc)2 (1.0 mmol), pyridine/DMSO (1:1, 2.0 mL), amine (3.0 mmol), Bu4NI (1.0 mmol), 5–36 h, 110 °C. Yields are isolated yields. Please see Supporting Information for details.
3.4. Amination with Heterocycles
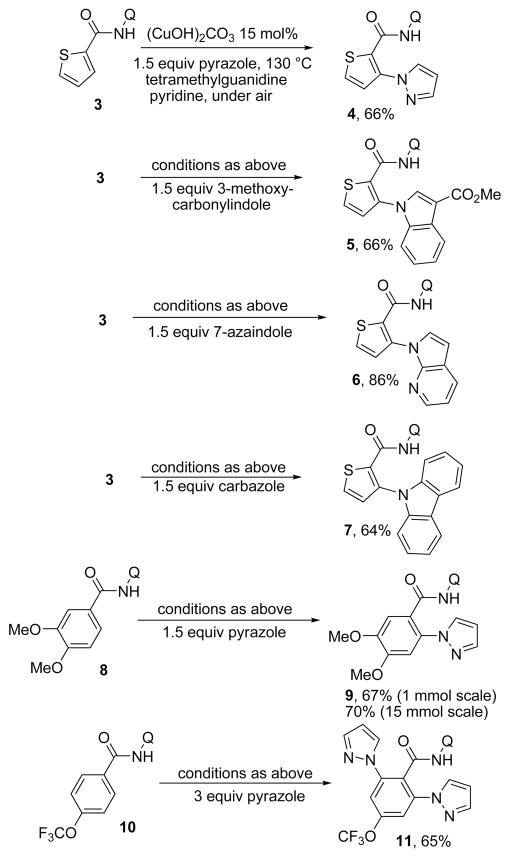
Amination attempts using heterocycles under the conditions of Tables 2–4 gave low conversions to products. We speculated that the lack of reactivity was due to low nucleophilicity of heterocycles. If an equilibrium concentration of the heterocycle anion could be formed, perhaps assisted by copper coordination, then amination would be successful. The acidities of pyrazole, carbazole, and indole (pKa = ~20 in DMSO solvent)8 were sufficient for successful cross-coupling reactions (Scheme 3). Thus, thiophenecarboxylic amide 3 could be coupled with pyrazole and substituted indoles to afford amination products 4–6 in good yields in the presence of tetramethylguanidine base. Furthermore, electron-rich and -poor aminoquinoline benzamides were competent coupling partners, giving products 9 and 11 in the reaction with pyrazole. By using excess pyrazole, double amination product 11 was formed in good yield, which is in contrast to reactions described earlier where only monoamination was observed. The reactions proceeded best if copper basic carbonate (malachite) catalyst was employed. The reaction scale can be increased from 1 to 15 mmol without loss of yield as shown in the preparation of 9.
Scheme 3.
Amination with Heterocycles
3.5. Amination with Sulfonamides and Electron-Poor Anilines
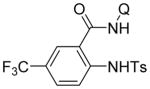
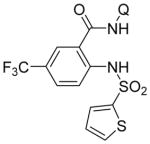
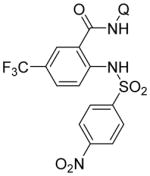
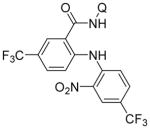
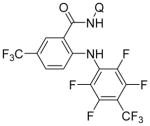
Following logic used in aminations with acidic heterocycles, we speculated that the addition of tetramethylguanidine base should allow for amination with sulfonamides and acidic aniline derivatives. Gratifyingly, this reasoning was successful as shown in Table 5. Tosylamide (entry 1), thiophenesulfonamide (entry 2), and 4-nitrobenzenesulfonamide (entry 3) were successfully coupled with aminoquinoline trifluoromethylbenzamide to give products in excellent yields. Electron-deficient anilines were reactive as well. 2-Nitro-4-trifluoromethyl-aniline (entry 4), perfluoro-p-toluidine (entry 5), and 2-amino-5-chloropyridine (entry 6) reacted to afford coupling products in moderate to good yields.
Table 5.
Amination with Electron-Poor Substancesa

| |||
|---|---|---|---|
| entry | RNH2 | product | yield, % |
| 1 | TsNH2 |
|
82 |
| 2 | 2-thiophene-SO2NH2 |
|
77 |
| 3 | 4-NO2C6H4-SO2NH2 |
|
81 |
| 4 | 2-NO2-4-CF3C6H3NH2 |
|
62 |
| 5 | 4-CF3C6F4NH2 |
|
62 |
| 6 | 2-amino-5-chloropyridine |

|
48 |
Benzamide (1.0 mmol), (CuOH)2CO3 (0.5 mmol), pyridine (2.0 mL), amine (1.5 mmol), tetramethylguanidine (1.2 mmol) 5–8 h, 130 °C. Yields are isolated yields. Please see Supporting Information for details.
3.6. Amination with Tetramethylguanidine
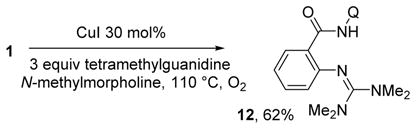
In some of the reactions using tetramethylguanidine base, a minor amount of a side product was formed. Isolation and spectroscopic characterization showed that cross-coupling of tetramethylguanidine with aminoquinoline amide occurred. After optimization of the reactions conditions, 12 was isolated in 62% yield (eq 1).
 |
(1) |
3.7. Competition Experiments
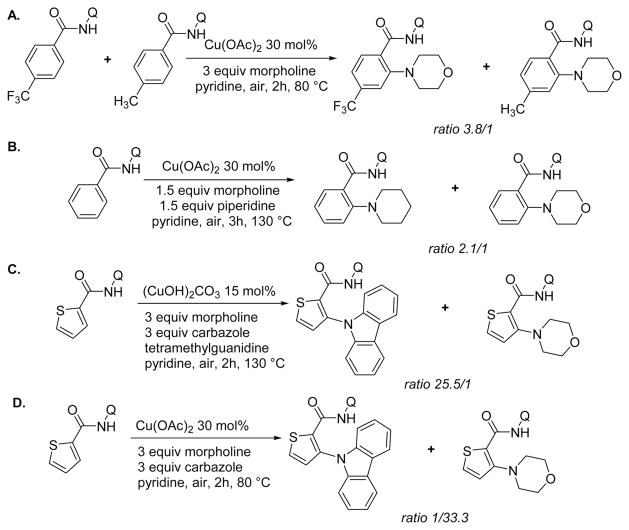
Several competition experiments were performed to probe the reactivity of substrates (Scheme 4). First, the influence of amide electronics was investigated. Competitive morpholination of 3-methylbenzoic amide and 3-trifluoromethylbenzamide derivatives afforded a 3.8:1 ratio of the products favoring the electron-deficient amide (Scheme 4A). Second, a competition experiment in coupling of morpholine and piperidine with aminoquinoline benzamide showed that coupling with more nucleophilic amine was favored (Scheme 4B). Third, thiophenecarboxylic acid aminoquinoline amide was reacted with morpholine and carbazole under the conditions of Scheme 3. Carbazole was coupled selectively, and only a trace amount of coupling with morpholine was observed (Scheme 4C). Fourth, the same coupling was performed under the conditions of Table 2. In this case, selective coupling of morpholine was observed (Scheme 4D).
Scheme 4.
Competition Experiments
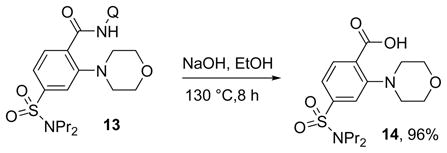
3.8. Directing Group Cleavage
The aminoquinoline directing group can be cleaved by base hydrolysis (eq 2). An ortho-aminated probenecid derivative was isolated in nearly quantitative yield.
 |
(2) |
4. SUMMARY
We have developed an operationally simple and general method for copper-catalyzed, aminoquinoline-assisted amination of β-C(sp2)-H bonds of benzoic acid derivatives. The reaction employs Cu(OAc)2 or (CuOH)2CO3 catalysts, an amine coupling partner, pyridine or pyridine/DMSO solvent, and oxygen from air as a terminal oxidant. Significant differences from earlier methods for C-H/N-H couplings include utilization of an inexpensive first-row transition metal catalyst in conjunction with air oxidant and high generality with respect to amine coupling partners. Specifically, primary and secondary aliphatic and aromatic amines, heterocycles, such as indoles, pyrazole, and carbazole, sulfonamides, and electron-deficient aromatic and heteroaromatic amines as well as guanidines are competent coupling components. Furthermore, the reaction possesses high functional group tolerance.
5. EXPERIMENTAL SECTION
General Procedure for Coupling of Aminoquinoline Amides with Secondary Amines
Aminoquinoline amide (1.0 mmol) and copper acetate (0.3mmol) were dissolved in pyridine (2mL) in a 100mL heavy wall pressure vessel equipped with a magnetic stir bar. To this mixture was added secondary amine (3.0 mmol). The closed vessel was placed in a preheated oil bath set to 80–110 °C. After the reaction reached completion, it was cooled to room temperature; EDTA (0.3 mmol) was added, and themixture was stirred for an additional 30min. Subsequently, the reaction was diluted with dichloromethane (50 mL), combined with SiO2 (15 mL), and evaporated to give a free-flowing solid. After column chromatography on silica gel in an appropriate solvent, the fractions containing the product were combined and concentrated. The residue was dried under reduced pressure to yield the pure product.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (Chair E-0044) and NIGMS (Grant No. R01GM077635) for supporting this research.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b01117.
Detailed experimental procedures and characterization data for new compounds (PDF)
References
- 1.(a) Manfredi N, Cecconi B, Abbotto A. Eur J Org Chem. 2014;2014:7069. [Google Scholar]; (b) Novak M, Zhang Y. Adv Phys Org Chem. 2012;46:121. [Google Scholar]
- 2.Review: Corpet M, Gosmini C. Synthesis. 2014;46:2258.
- 3.(a) Surry DS, Buchwald SL. Angew Chem, Int Ed. 2008;47:6338. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hartwig JF. Acc Chem Res. 2008;41:1534. doi: 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Okano K, Tokuyama H, Fukuyama T. Chem Commun. 2014;50:13650. doi: 10.1039/c4cc03895a. [DOI] [PubMed] [Google Scholar]
- 4.(a) Patel P, Chang S. ACS Catal. 2015;5:853. [Google Scholar]; (b) Park J, Chang S. Angew Chem, Int Ed. 2015;54:14103. doi: 10.1002/anie.201505820. [DOI] [PubMed] [Google Scholar]; (c) Shin K, Baek Y, Chang S. Angew Chem, Int Ed. 2013;52:8031. doi: 10.1002/anie.201302784. [DOI] [PubMed] [Google Scholar]; (d) Yu S, Wan B, Li X. Org Lett. 2013;15:3706. doi: 10.1021/ol401569u. [DOI] [PubMed] [Google Scholar]; (e) Lian Y, Hummel JR, Bergman RG, Ellman JA. J Am Chem Soc. 2013;135:12548. doi: 10.1021/ja406131a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Matsubara T, Asako S, Ilies L, Nakamura E. J Am Chem Soc. 2014;136:646. doi: 10.1021/ja412521k. [DOI] [PubMed] [Google Scholar]; (g) Kawano T, Hirano K, Satoh T, Miura M. J Am Chem Soc. 2010;132:6900. doi: 10.1021/ja101939r. [DOI] [PubMed] [Google Scholar]; (h) Zhou B, Du J, Yang Y, Feng H, Li Y. Org Lett. 2013;15:6302. doi: 10.1021/ol403187t. [DOI] [PubMed] [Google Scholar]; (i) Gu L, Neo BS, Zhang Y. Org Lett. 2011;13:1872. doi: 10.1021/ol200373q. [DOI] [PubMed] [Google Scholar]; (j) Foo K, Sella E, Thomé I, Eastgate MD, Baran PS. J Am Chem Soc. 2014;136:5279. doi: 10.1021/ja501879c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Yadav MR, Shankar M, Ramesh E, Ghosh K, Sahoo AK. Org Lett. 2015;17:1886. doi: 10.1021/acs.orglett.5b00570. [DOI] [PubMed] [Google Scholar]; (l) Thirunavukkarasu VS, Raghuvanshi K, Ackermann L. Org Lett. 2013;15:3286. doi: 10.1021/ol401321q. [DOI] [PubMed] [Google Scholar]; (m) Dong Z, Dong G. J Am Chem Soc. 2013;135:18350. doi: 10.1021/ja410823e. [DOI] [PubMed] [Google Scholar]; (n) Ng KH, Chan ASC, Yu WY. J Am Chem Soc. 2010;132:12862. doi: 10.1021/ja106364r. [DOI] [PubMed] [Google Scholar]; (o) Sun K, Li Y, Xiong T, Zhang J, Zhang Q. J Am Chem Soc. 2011;133:1694. doi: 10.1021/ja1101695. [DOI] [PubMed] [Google Scholar]; (p) Zhu D, Yang G, He J, Chu L, Chen G, Gong W, Chen K, Eastgate MD, Yu JQ. Angew Chem, Int Ed. 2015;54:2497. doi: 10.1002/anie.201408651. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Hao XS, Goodhue CE, Yu JQ. J Am Chem Soc. 2006;128:6790. doi: 10.1021/ja061715q.John A, Nicholas KM. J Org Chem. 2011;76:4158. doi: 10.1021/jo200409h.Uemura T, Imoto S, Chatani N. Chem Lett. 2006;35:842.Shang M, Sun SZ, Dai HX, Yu JQ. J Am Chem Soc. 2014;136:3354. doi: 10.1021/ja412880r.Li Q, Zhang SY, He G, Ai Z, Nack WA, Chen G. Org Lett. 2014;16:1764. doi: 10.1021/ol500464x.Yan Q, Chen Z, Yu W, Yin H, Liu Z, Zhang Y. Org Lett. 2015;17:2482. doi: 10.1021/acs.orglett.5b00990.Berzina B, Sokolovs I, Suna E. ACS Catal. 2015;5:7008.Manna S, Serebrennikova PO, Utepova IA, Antonchick AP, Chupakhin ON. Org Lett. 2015;17:4588. doi: 10.1021/acs.orglett.5b02320.Monguchi D, Fujiwara T, Furukawa H, Mori A. Org Lett. 2009;11:1607. doi: 10.1021/ol900298e.Zhao H, Wang M, Su W, Hong M. Adv Synth Catal. 2010;352:1301.Other relevant examples: Yoo EJ, Ma S, Mei TS, Chan KSL, Yu JQ. J Am Chem Soc. 2011;133:7652. doi: 10.1021/ja202563w.Kim H, Shin K, Chang S. J Am Chem Soc. 2014;136:5904. doi: 10.1021/ja502270y.Shrestha R, Mukherjee P, Tan Y, Litman ZC, Hartwig JF. J Am Chem Soc. 2013;135:8480. doi: 10.1021/ja4032677.Xiao B, Gong T-J, Xu J, Liu Z-J, Liu L. J Am Chem Soc. 2011;133:1466. doi: 10.1021/ja108450m.Marchetti L, Kantak A, Davis R, DeBoef B. Org Lett. 2015;17:358. doi: 10.1021/ol5034805.Louillat ML, Biafora A, Legros F, Patureau FW. Angew Chem, Int Ed. 2014;53:3505. doi: 10.1002/anie.201308601.Thu HY, Yu WY, Che CM. J Am Chem Soc. 2006;128:9048. doi: 10.1021/ja062856v.Copper-catalyzed sp3 C-H amination: Gephart RT, III, Warren TH. Organometallics. 2012;31:7728.Reviews: Shin K, Kim H, Chang S. Acc Chem Res. 2015;48:1040. doi: 10.1021/acs.accounts.5b00020.Thirunavukkarasu VS, Kozhushkov SI, Ackermann L. Chem Commun. 2014;50:29. doi: 10.1039/c3cc47028h.Louillat ML, Patureau FW. Chem Soc Rev. 2014;43:901. doi: 10.1039/c3cs60318k.Jiao J, Murakami K, Itami K. ACS Catal. 2016;6:610.
- 6.(a) Zaitsev VG, Shabashov D, Daugulis O. J Am Chem Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; (b) Shabashov D, Daugulis O. J Am Chem Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Daugulis O, Roane J, Tran LD. Acc Chem Res. 2015;48:1053. doi: 10.1021/ar5004626. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tran LD, Roane J, Daugulis O. Angew Chem, Int Ed. 2013;52:6043. doi: 10.1002/anie.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Rouquet G, Chatani N. Angew Chem, Int Ed. 2013;52:11726. doi: 10.1002/anie.201301451. [DOI] [PubMed] [Google Scholar]
- 7.Robbins N, Koch SE, Tranter M, Rubinstein J. Cardiovasc Toxicol. 2012;12:1. doi: 10.1007/s12012-011-9145-8. [DOI] [PubMed] [Google Scholar]
- 8.Bordwell FG. Acc Chem Res. 1988;21:456. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.