Abstract
Hydrogen/deuterium exchange (HDX) mass spectrometry (MS) for protein structural analysis has been adopted for many purposes, including biopharmaceutical development. One of the benefits of examining amide proton exchange by mass spectrometry is that it can readily resolve different exchange regimes, as evidenced by either binomial or bimodal isotope patterns. By careful analysis of the isotope pattern during exchange, more insight can be obtained on protein behavior in solution. However, one must be sure that any observed bimodal isotope patterns are not artifacts of analysis and are reflective of the true behavior in solution. Sample carryover and certain stationary phases are known as potential sources of bimodal artifacts. Here, we describe an additional undocumented source of deuterium loss resulting in artificial bimodal patterns for certain highly charged peptides. We demonstrate that this phenomenon is predominantly due to gas-phase proton exchange between peptides and bulk solvent within the initial stages of high-transmission conjoined ion guides. Minor adjustments of the ion guide settings, as reported here, eliminate the phenomenon without sacrificing signal intensity. Such gas-phase deuterium loss should be appreciated for all HDX-MS studies using such ion optics, even for routine studies not focused on interpreting bimodal spectra.
Keywords: Deuterium exchange, HDX, Binomial, Bimodal, Envelope broadening, Gas-phase proton exchange, Deuterium loss, Intermolecular proton exchange, StepWave, Conjoined ion guide
Graphical abstract
Introduction
Hydrogen-deuterium exchange (HDX) detected by mass spectrometry (MS) is a powerful tool for solution-phase structural analysis of proteins [1]. By examining the kinetics of amide deuterium exchange under native conditions, information can be obtained for understanding protein folding, stability, aggregation, and mapping ligand binding sites [2]. HDX-MS is now used in the biopharmaceutical industry as a tool for research, formulation development, and quality control, among other things [3].One of the advantages of the technique is that it can be applied to study protein systems of great size and complexity thanks to the increasing sensitivity and resolving power of mass spectrometers. Commercial platforms for automated HDX-MS analysis and advancements in ion optics and mass spectrometer performance make the technique available to a broad user base [4].
HDX-MS is often analyzed at the peptide level where the mass envelope for each charge state of each peptide shifts gradually with increasing deuterium uptake over time [5]. In some cases, slow, correlated protein motions can give rise to bimodal isotope patterns [6], which have been observed in numerous protein systems [1, 7]. A simple centroid-based analysis can be insufficient for interpreting H/D exchange data as it does not account for potential bimodal behavior [8]. Accurate assessment of true bimodal isotope patterns in the data can reveal new information regarding the time scale of different protein motions, or the presence of multiple conformational states [5, 9–11]. In response, peak width analysis and bimodal detection have been incorporated into some software for HDX-MS data analysis [12–14].
False bimodal isotope patterns—that is, bimodal isotope patterns that are introduced during the analysis itself and are not reflective of protein conformation—may arise from various sources, including sample handling or LC, and can confound the interpretation of HDX-MS data. For example, insufficient washing of LC columns can cause sample carryover that can lead to false bimodal patterns in the data [15]. Carryover from immobilized pepsin columns can also cause false bimodal patterns [16], and certain stationary-phase chemistries have been linked to non-uniform deuterium loss during online pepsin digestion [17]. Here, we report an additional source of false bimodal isotope patterns arising from non-uniform gas-phase proton exchange within the ion optics of several instruments that have a StepWave ion guide, including the Waters Synapt G2-Si, a quadrupole-time of flight (Q-TOF) mass spectrometer with a traveling wave ion guide (TWIG) between the quadrupole and the TOF for ion mobility separation [18, 19]. The StepWave is a dual stage conjoined ion guide with a DC offset potential to propel ions from the lower to the upper stage and into the subsequent low pressure regions of the mass spectrometer for analysis; neutral species and bulk solvent are left behind in the lower stage and removed by the vacuum system. This configuration allows for a larger ion inlet aperture that lowers limits of detection by roughly an order of magnitude [20]. The lower detection limits are extremely valuable for many applications, especially for HDX-MS of samples present in only small quantities. As we describe below, by optimizing the parameters of the StepWave ion guide, the false bimodal patterns from gas-phase deuterium loss can be eliminated with a minimal loss in overall signal.
Results and Discussion
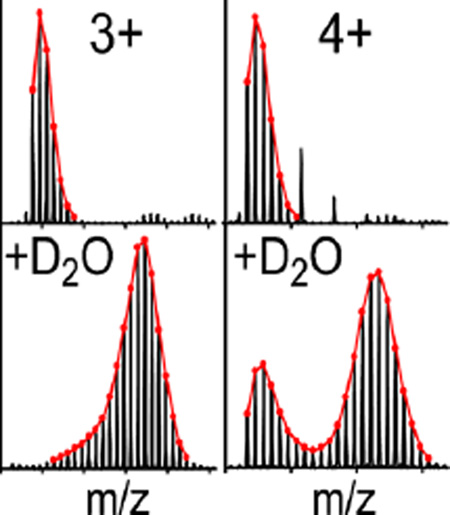
Observation of unexpected false bimodal isotope distributions was first made in a peptide digest of maximally deuterated HIV Env gp140 analyzed on a Synapt G2-Si mass spectrometer. Of the 100 peptides monitored, 19 of them showed an unusual bimodal pattern not previously seen with identical samples analyzed on an earlier generation Synapt G1, a similar Q-TOF mass spectrometer with similar ion mobility optics, but a single inlet TWIG instead of a StepWave [21] (Supplementary Table S1). In particular, the peptide spanning residues 484–507 showed the strongest subpopulation of a low-mass isotope envelope corresponding to the nondeuterated species (Figure 1). The bimodal pattern could not be attributed to carryover from the LC system or the pepsin column, as subsequent blank injections showed less than 2% carryover. What was further puzzling was that the higher charge state ions of the peptide showed considerably more of the nondeuterated population, or lower isotope envelope of the bimodal pattern. Given that these patterns were not observed in the Synapt G1 mass spectrometer, early indications were that the Synapt G2-Si mass spectrometer was responsible for the false bimodal patterns and we sought to identify the exact source.
Figure 1.
Artifactual bimodal isotope patterns caused by MS instrument parameters. Maximally deuterated HIV Env gp140 peptide 484–507 (YKYKVVKIEPLGVAPTRCKRRVVG) showed bimodal isotope patterns in the Synapt G2-Si (bottom row) but not in the Synapt G1 (middle row). The extent of the lower-mass envelope (resulting from deuterium loss) varied among the different observable charge states
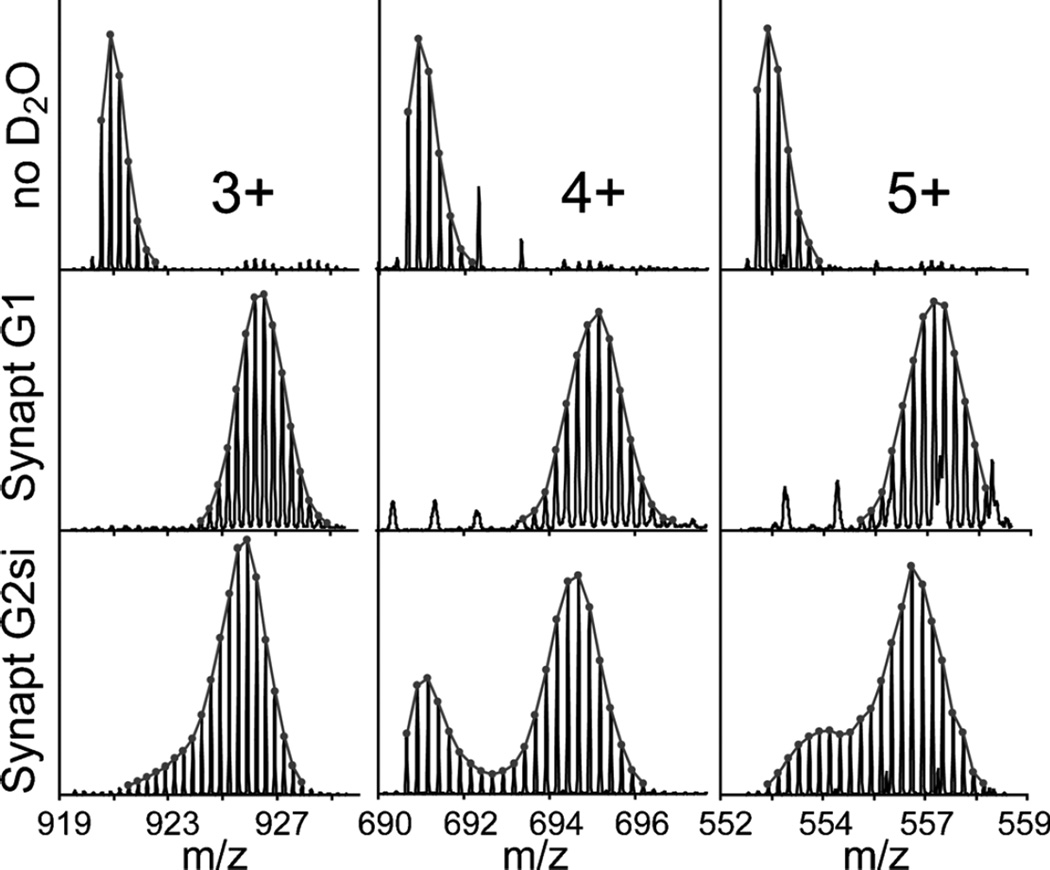
Synthetic peptides similar in sequence to those showing bimodal profiles from the HIV Env data were obtained in order to enable a stable infusion of deuterated peptides for testing the effects various source parameters on the observed isotopic distributions (Supplementary Table S1). Env peptide 497–511 served as a particularly useful reagent for these tests as it also showed a clear bimodal isotope pattern at the higher charge states (Figure 2). In contrast, [Glu1]-fibrinopeptide B (GluFib) co-infused with the synthetic peptides, showed no bimodal distribution (Figure 2). Other commonly used peptides, including bradykinin and angiotensin II, also showed no observable bimodal distributions (data not shown). Ion mobility was enabled in the same Synapt G2-Si and the resulting drift times of maximally deuterated Env 497–511 showed an identical drift time to the lower-mass envelope of the bimodal distribution (Supplementary Figure S1A). Based on this result, we concluded that the bimodal isotope pattern could not be attributed to different gas-phase conformations of the peptide. We also observed a temporal dimension to the deuterium loss and found that while the maximally deuterated component of the bimodal isotope pattern had a very stable signal, the intensity of the nondeuterated species fluctuated greatly during the infusion (Supplementary Figure S1B).
Figure 2.
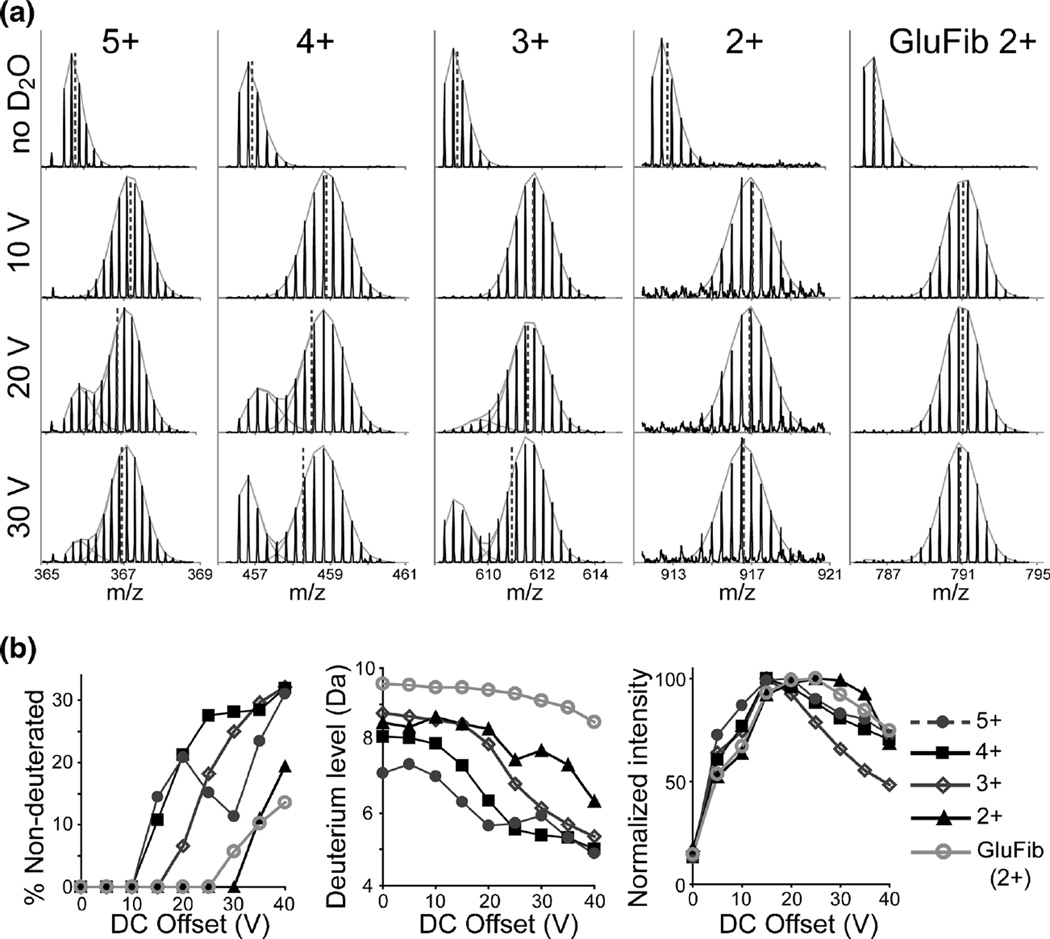
Effect of the StepWave DC offset potential on deuterium loss. (a) Mass spectra of the 5+, 4+, 3+, 2+ charge states of synthetic peptide Env 497–511 (APTKAKRRVVQREKR) and the 2+ ion of GluFib. Spectra for the undeuterated form (at top) and maximally deuterated states with various StepWave DC offsets (10, 20, or 30 V) are shown. Dashed lines show the position of the centroid, and gray fits show the binomial distributions used to estimate the content of non-deuterated species. (b) Percent of the nondeuterated population (left), overall deuterium level (center), and the normalized signal intensity (right) as a function of DC offset voltage for various charge states
A number of parameters within the mass spectrometer were tested for their effect on the bimodal isotope patterns. Reducing the ion current by either lowering the capillary voltage or infusing a more dilute sample had no effect on the bimodal patterns. A lower infusion flow rate of 10 µL/min (typical flow rate for HDX-MS is ~40 µL/min) resulted in a slight decrease in the presence of the nondeuterated species, but also led to lower deuterium levels overall, presumably due to more time spent near the high temperature of the source needle (Supplementary Figure S2A). Infusions with higher flow rates (up to 200 µL/min) did not affect the presence of the nondeuterated species. Using a higher desolvation gas flow and desolvation temperature led to slightly lower overall deuterium levels, but did not affect the bimodal pattern (Supplementary Figure S1B). A higher cone gas flow of 90 L/h (standard is ~15 L/h) mildly decreased the abundance of the nondeuterated species without affecting overall signal intensity. Offsetting the angle of the spray needle also moderately reduced the abundance of the nondeuterated species (data not shown), but with a drastic loss in signal intensity. Adjustments to any of the TriWave (trap, ion mobility cell, and transfer ion guides), or the TOF settings had no detectable effect on the bimodal patterns observed.
In contrast, settings for the StepWave ion guide had a significant impact on the observed deuterium profiles, most notably the DC offset potential (Figure 2). Generally, higher DC offset caused more of the nondeuterated species to appear. One exception was observed with the 5+ ion of the Env 497–511 peptide for which the intensity of the nondeuterated species was reduced between 20–30 V, but then gained intensity again above 30 V. With lower DC offset voltage (<10 V) all of the bimodal isotope patterns were eliminated, leaving the expected isotope pattern for the maximally deuterated peptide. However, the field created by the DC offset is necessary for efficient ion transfer in the StepWave, and the lower DC offset (<10 V) resulted in nearly a 50% loss in overall signal.
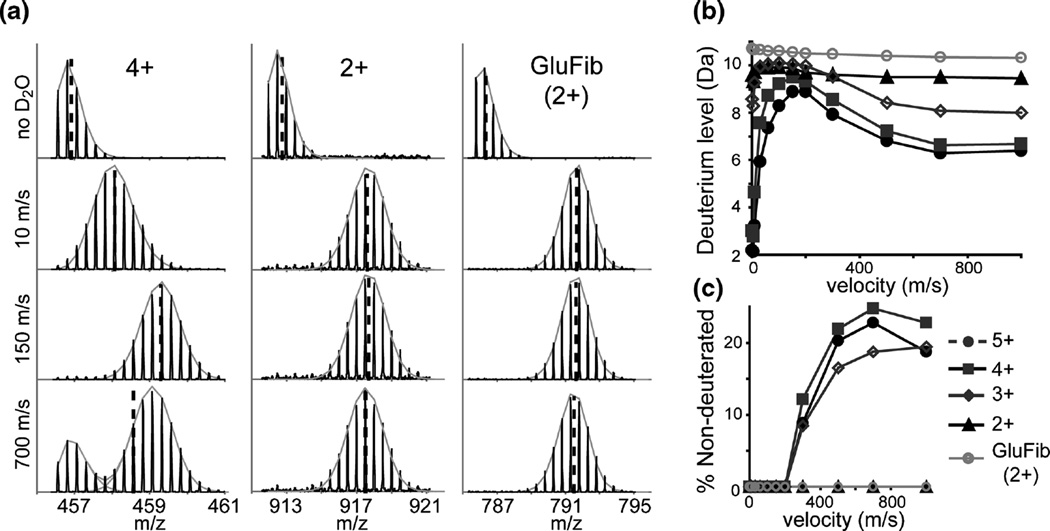
The second parameter that had a major effect on the bimodal isotope pattern was the traveling wave (TW) height and velocity within the StepWave ion guide. Raising the TW height to 30 V for the upper and lower stages of the StepWave helped reduce the magnitude of the nondeuterated population without a significant loss in signal intensity (data not shown). The TW velocity in the upper stage of the StepWave ion guide showed a strong effect on the deuteration profile (Figure 3). Surprisingly, at very low velocities (<10 m/s) there was a lower m/z envelope corresponding to a uniform loss in deuterium (Figure 3a), but only for the higher charge states. As the speed was increased, a higher m/z envelope was observed, suggesting more deuterium was retained. At speeds above 300 m/s, a second, lower mass, nondeuterated species emerged, but again, only for the higher charge states.
Figure 3.
Effects of the T-wave velocity in the upper stage of the StepWave. (a) Mass spectra of HIV Env 497–511 (4+), (2+), and GluFib (2+) undeuterated and maximally deuterated at a wave velocity of 10, 150, and 700 m/s. Centroid positions are shown with dashed lines and the gray fits show binomial distributions used to estimate the relative percentage of nondeuterated species. Average deuterium level (b) and percentage of nondeuterated species (c) are shown as a function of the traveling wave velocity
To test whether the deuterium loss was due to exchange with lingering water molecules in the StepWave, we manipulated the isotopic environment of the solvent surrounding the deuterated peptide. When protonated Env 497–511 was rapidly diluted into deuterated quench solution and infused directly into the Synapt G2-Si in a 98% D2O solution, there was the expected high degree of deuteration (due to the exchange-in process occurring at labile positions during infusion). There was no evident bimodal pattern, but the overall deuterium levels at either low (5 V) or high (40 V) DC offset were different, with the 40 V setting leading to a higher observed deuterium level (Supplementary Figure S3). When the same peptide was boiled in D2O to maximally deuterate all exchangeable sites, and then infused into the instrument in the 98% D2O solution, there was no difference in the level of deuteration at either DC offset setting (Supplementary Figure S3). These results suggest that when infused in deuterated buffer, the protonated sites were exchanging for deuterium more efficiently under high, but not low, DC offset settings. Therefore, bulk solvent co-infused with the sample contributes to gas-phase proton exchange, and results in deuterium loss when deuterated peptides are analyzed in aqueous buffer (Figures 1, 2, and 3).
To determine whether this type of gas-phase deuterium loss was unique to the StepWave conjoined ion guide, or whether it could occur in other high-pressure ion guides, we infused deuterated Env 497–511 into a Synapt G1, which has a single TWIG source. The results revealed no bimodal isotope patterns for any charge state under standard (default) source settings; however, at higher source TW heights and exceedingly fast source TW velocities there was an observable loss of deuterium in the TWIG (Supplementary Figure S4A). Therefore, while not observed under standard conditions, moderate deuterium loss can also occur on other source ion guides. We speculate that the lower degree of deuterium loss in the Synapt G1 is, at least in part, due to the smaller ion inlet aperture (and correspondingly lower sensitivity) on the SynaptG1 (compared with the G2-Si) allowing much less solvent and analyte ions into the primary source ion guide and limiting gas-phase proton exchange with the co-infused solvent.
In the course of this work, we infused Env 497–511 into four different Synapt G2-Si instruments. All showed identical behavior with respect to the appearance of bimodal isotope patterns attributed to deuterium loss within the StepWave ion guide. To test whether this was specific to the Synapt G2-Si StepWave, we also performed the infusion on a Xevo TQ-S triple quadrupole mass spectrometer (Waters) equipped with a similar StepWave ion guide. Though the conditions of the StepWave were slightly altered, at standard DC offset potentials and high TW velocities a bimodal isotope pattern was also observed for the higher charge states (Supplementary Figure S4B).
The resolution of HDX-MS has been advanced by ion transfer dissociation (such as ETD), which can provide residue specific deuterium exchange information [22]. For effective ETD analysis, several source parameters, including the wave velocity in the TWIG of a Waters Synapt, needed to be tuned to minimize intramolecular proton migration (“scrambling”) [23]. Interestingly, the study by Rand et al. [23] found that higher charge state ions were more susceptible to source-induced scrambling on a Synapt G2 mass spectrometer. Peptide P1 (HHHHHHIIKIIK), which is commonly used to measure the degree of scrambling during ETD analysis [24] was also examined in our Synapt G2-Si to detect gas-phase deuterium loss within the StepWave ion guide. Under standard settings, peak broadening of the deuterated envelope was observed for the higher charge states and was alleviated by adjusting the StepWave settings (described below) (Supplementary Figure S5). We therefore suspect that the StepWave settings will also have a major impact on scrambling and should be considered for HDX-MS studies incorporating the use of ETD.
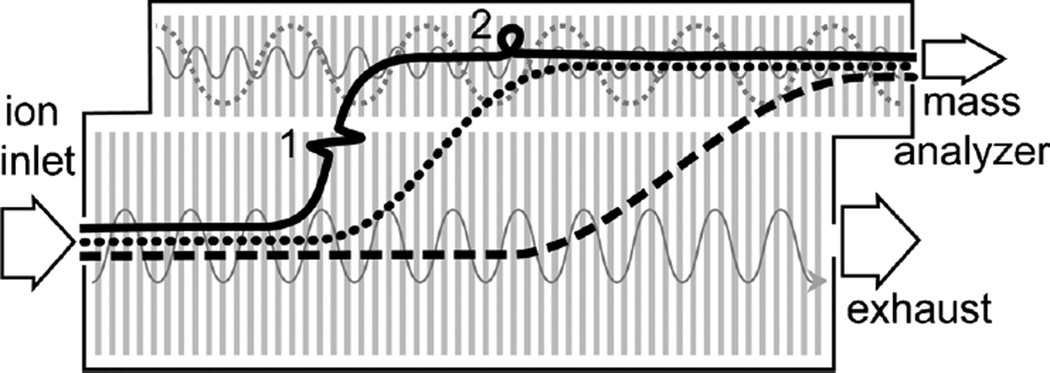
The role of the step in the StepWave is to separate the charged species from neutrals and bulk solvent in the conjoined ion guide by the use of a DC offset potential between the upper and lower stages (Figure 4). Based on our findings, we hypothesize that with deuterated peptides, the excitation from the DC offset potential leads to deuterium loss for a subset of the ions. We suspect that proton exchange within the StepWave occurs with bulk co-infused solvent as higher desolvation temperature and gas flow, which should accelerate desolvation, did not affect the level of deuterium loss. The intermittent nature of the deuterium loss (Supplementary Figure S1B) would be consistent with the changes in pressure that can vary both spatially through the ion guide and temporally throughout the course of infusion, which would then influence the gas-phase exchange with bulk solvent.
Figure 4.
Cartoon of suspected origin of deuterium loss in the StepWave ion guide on the Synapt G2-Si mass spectrometer. Based on the results of this study, the following hypotheses emerge. Under standard conditions (black solid lines) certain ions can: 1) gain enough energy from the rapid transfer between the lower and upper stages to induce inter-molecular proton exchange with the residual bulk water; 2) “roll-over” the traveling wave (gray solid line) in the upper stage of the ion guide to induce inter-molecular proton exchange. When run with a lower DC offset voltage (black dashed line) the ions transfer from the lower to the upper stage under milder conditions resulting in reduced deuterium loss, at the cost of signal intensity due to the loss in transfer efficiency. By using a moderate DC offset voltage (black dotted line) along with larger wave height and slower traveling wave velocity in the upper stage (gray dotted line) both transfer and roll-over induced deuterium loss are vastly reduced, with minimal loss in overall signal intensity
A second hypothesis regarding deuterium loss in the conjoined ion guide is that it is the result of a “roll-over” effect. A traveling wave is used to propel ions through the ion guide and into the subsequent parts of the spectrometer. When the traveling wave moves too quickly (Figure 3), some ions cannot keep up with the wave and roll over the wave, resulting in them spending more time in the ion guide [25]. In the presence of the co-infused H2O that presumably exists in the ion guide, such activation can apparently also lead to intermolecular proton exchange. By slowing down the wave velocity and increasing the height of the wave, it is possible to avoid roll-over induced deuterium loss without affecting the transmission efficiency of ions. As illustrated in Figure 4, by tuning the DC offset (which minimizes energy input into ions during transit step), and the TW velocities and heights (to reduce roll-over effects), an optimal set of StepWave parameters for HDX-MS can be established. These settings were fine tuned on the Synapt G2-Si to alleviate all ion guide-induced deuterium loss for every problematic peptide, while maintaining overall signal intensity (Table 1). Re-analysis of the identical HIV Env gp140 samples with the optimized HDX-MS source settings showed no evidence of bimodal isotope patterns for any of the peptides and the overall decrease in signal was, on average, less than 10%.
Table 1.
Optimal StepWave and Source Settings for HDX-MS Studies on the Synapt G2-Si
| Parameter | Default | Optimal |
|---|---|---|
| Height in (V) | 15 | 30 |
| Velocity in (m/s) | 300 | 300 |
| Height out (V) | 15 | 30 |
| Velocity out (m/s) | 300 | 200 |
| DC offset (V) | 25 | 15 |
| Aperture 1 offset (V) | 3 | 3 |
| Aperture 2 offset (V) | 0 | 0 |
| rf Voltage (V) | 300 | 300 |
| Cone gas flow rate (L/h) | 15 | 90 |
At present, we are not sure why only a subset of peptides are susceptible to gas-phase deuterium loss. In our observation, deuterium loss was most predominant for higher charge states of peptides that contain a large number of basic residues (Supplementary Table S1). Sequence and gas-phase conformation may also play a role in the reactions that lead to loss of deuterium with this type of ion optics. We note that even with the optimal StepWave settings, there can still be a slight difference in the extent of deuterium incorporation for different charge states of the same peptide, something which was not seen in the Synapt G1 data. A similar effect was also observed with the infusions of the P1 peptide, which could not be attributed to detector saturation artifacts or signal overlap (Supplementary Figure S5). Therefore, there is still minor variation in the extent of deuterium loss within the StepWave ion guide among various charge states for a subset of peptides. For this reason, averaging deuterium levels among different charge states may sometimes increase the error of the measurement when deuterium losses such as we have described here play a role. As is the case with all HDX-MS studies, be they bimodal isotope patterns or the more common binomial patterns, vigilance is required to ensure that artifacts do not confound the data interpretation.
Conclusions
The significant increase in ion transmission as well as the reduction in limits of detection provided by the Waters StepWave ion guide design are attributed in part to the increased sample cone aperture size and increased primary ion guide pressure. However, the increased pressure provides a region where gas-phase proton exchange between analyte ions and the co-infused water occurs under certain conditions. Such effects should be considered for HDX-MS studies, especially when interpreting bimodal isotope patterns. With standard source conditions on a Waters Synapt G2-Si equipped with a StepWave ion guide, 19% of the peptides examined displayed some level of distortion in the deuterated mass envelope, ranging from subtle broadening to obvious bimodal behavior. By adjusting the ion guide parameters, as described here, it is possible to easily eliminate gas-phase deuterium loss artifacts, enabling accurate deuterium analysis without compromising signal intensity.
Methods
HIV consensus peptides were obtained from the NIH AIDS reagent program. 99.9% D2O was obtained from Cambridge isotope labs (Tewksbury, MA, USA). Peptide P1 (HHHHHHIIKIIK-NH2) was purchased from Anaspec (Fremont, CA, USA), and [Glu1]-fibrinopeptide B (GluFib), Angiotensin II, and bradykinin were purchased from Sigma Aldrich (St. Louis, MO, USA). Peptides were resuspended in Optima LC-MS water (Fisher Scientific, Pittsburgh, PA, USA)) for a stock concentration of 2 mg/mL. HIV Env gp140 SOSIP.664 expression, purification, and HDX-MS analysis were performed as described previously [21].
For LC-MS analyses, 20 pmol of denatured and maximally deuterated frozen aliquots of HIV Env gp140 SOSIP.664 were manually injected onto a Waters HDX manager coupled with a Synapt G2-Si. Samples were loaded onto custom prepared pepsin beads (POROS AL20; Life Technologies, Carlsbad, CA, USA) [26], at 150 µL/min and trapped onto a Vanguard 2.1 ×5 mm C18 BEH column for 5 min (Waters). Peptides were then separated with a 1 × 100 mm C18 BEH 1.7 µm column (Waters, Milford, MA, USA) using a linear gradient of 5 to 40% B (A: 2% ACN, 0.1% FA, 0.04% TFA; B: 100% ACN 0.1% FA) over 8 min at 40 µL/min. MS scans were collected every second using a source temperature of 80 °C and desolvation temperature of 150 °C. Other source parameters were left as default unless otherwise stated. A lock mass solution of leucine enkephalin was sampled every 90 s to maintain mass accuracy. A third switching valve was introduced to allow for stringent washing of the trap and pepsin columns between injections to minimize sample carryover (less than 2%) [15, 16].
For direct infusions, Env peptides were diluted 50-fold into 95% D2O containing 10 mM ammonium bicarbonate pH 8.0, and 0.02 mg/mL GluFib, Angiotensin II, or bradykinin. The solution was heated to 85 °C for 5 min for complete deuterium exchange and kept at 4 °C until MS analysis. Deuterium measurements were initiated by diluting 3 µLs of the deuterated peptide solution 100-fold into cold quench buffer (0.1% FA, 0.04% TFA, 100% H2O, final pH 2.5). The solution was immediately loaded onto a 200 µL injection loop and pumped with the identical quench buffer isocratically at 40 µL/min to push the sample from the loop into the mass spectrometer. The injection loop and lines were kept under ice to minimize deuterium loss during the infusion. Source settings were adjusted to test effects on the deuterated spectra after the signal intensity stabilized. In-exchange measurements were performed identically except the stock peptide mixture was diluted 200-fold into ice-cold 98% D2O, 0.1% FA, 0.04% TFA, and immediately injected onto the loop. Deuterium incorporation analysis and bimodal deconvolution were performed with HX-Express v2 [14].
Supplementary Material
Acknowledgments
The authors thank Kevin Giles, Natalie K. Garcia, Elizabeth A. Komives, Ross F. Lawrence, and Scott J. Edgar for assistance with data collection and data interpretation. The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 Consensus Subtype B Env Peptide Set. This work was supported by NIH grants R01-GM099989 (K.K.L.) and R01-GM101135 (J.R.E.) as well as the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (CAVD) grant OPP1033102 (K.K.L.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s13361-015-1330-8) contains supplementary material, which is available to authorized users.
References
- 1.Pirrone GF, Iacob RE, Engen JR. Applications of hydrogen/deuterium exchange MS from 2012 to 2014. Anal. Chem. 2015;87(1):99–118. doi: 10.1021/ac5040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcsisin SR, Engen JR. Hydrogen exchange mass spectrometry: what is it and what can it tell us? Anal. Bioanal. Chem. 2010;397(3):967–972. doi: 10.1007/s00216-010-3556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houde D, Berkowitz SA, Engen JR. The utility of hydrogen/deuterium exchange mass spectrometry in biopharmaceutical comparability studies. J. Pharm. Sci. 2011;100(6):2071–2086. doi: 10.1002/jps.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacob RE, Engen JR. Hydrogen exchange mass spectrometry: are we out of the quicksand? J. Am. Soc. Mass Spectrom. 2012;23(6):1003–1010. doi: 10.1007/s13361-012-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Smith DL. Determination of amide hydrogen exchange by mass spectrometry: a new tool for protein structure elucidation. Protein Sci. 1993;2(4):522–531. doi: 10.1002/pro.5560020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miranker A, Robinson CV, Radford SE, Aplin RT, Dobson CM. Detection of transient protein folding populations by mass spectrometry. Science. 1993;262(5135):896–900. doi: 10.1126/science.8235611. [DOI] [PubMed] [Google Scholar]
- 7.Weis DD, Wales TE, Engen JR, Hotchko M, Ten Eyck LF. Identification and characterization of EX1 kinetics in H/D exchange mass spectrometry by peak width analysis. J. Am. Soc. Mass Spectrom. 2006;17(11):1498–1509. doi: 10.1016/j.jasms.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Ramachandran P, Kumar R, Gross ML. H/D exchange centroid monitoring is insufficient to show differences in the behavior of protein states. J. Am. Soc. Mass Spectrom. 2013;24(3):450–453. doi: 10.1007/s13361-012-0555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Post CB, Smith DL. Amide hydrogen exchange determined by mass spectrometry: application to rabbit muscle aldolase. Biochemistry. 1996;35(3):779–791. doi: 10.1021/bi952227q. [DOI] [PubMed] [Google Scholar]
- 10.Englander SW. Hydrogen exchange and mass spectrometry: a historical perspective. J. Am. Soc. Mass Spectrom. 2006;17(11):1481–1489. doi: 10.1016/j.jasms.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaltashov IA, Bobst CE, Abzalimov RR, Berkowitz SA, Houde D. Conformation and dynamics of biopharmaceuticals: transition of mass spectrometry-based tools from academe to industry. J. Am. Soc. Mass Spectrom. 2010;21(3):323–337. doi: 10.1016/j.jasms.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weis DD, Engen JR, Kass IJ. Semi-automated data processing of hydrogen exchange mass spectra using HX-Express. J. Am. Soc. Mass Spectrom. 2006;17(12):1700–1703. doi: 10.1016/j.jasms.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Kan ZY, Mayne L, Chetty PS, Englander SW. ExMS: data analysis for HX-MS experiments. J. Am. Soc. Mass Spectrom. 2011;22(11):1906–1915. doi: 10.1007/s13361-011-0236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttman M, Weis DD, Engen JR, Lee KK. Analysis of overlapped and noisy hydrogen/deuterium exchange mass spectra. J. Am. Soc. Mass Spectrom. 2013;24(12):1906–1912. doi: 10.1007/s13361-013-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang J, Rand KD, Beuning PJ, Engen JR. False EX1 signatures caused by sample carryover during HXMS analyses. Int. J. Mass Spectrom. 2011;302(1/3):19–25. doi: 10.1016/j.ijms.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumdar R, Manikwar P, Hickey JM, Arora J, Middaugh CR, Volkin DB, Weis DD. Minimizing carry-over in an online pepsin digestion system used for the H/D exchange mass spectrometric analysis of an IgG1 monoclonal antibody. J. Am. Soc. Mass Spectrom. 2012;23(12):2140–2148. doi: 10.1007/s13361-012-0485-9. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Kaveti S, Engen JR. Extensive deuterium back-exchange in certain immobilized pepsin columns used for H/D exchange mass spectrometry. Anal. Chem. 2006;78(5):1719–1723. doi: 10.1021/ac0518497. [DOI] [PubMed] [Google Scholar]
- 18.Pringle SD, Giles K, Wildgoose JL, Williams JP, Slade SE, Thalassinos K, Bateman RH, Bowers MT, Scriven JH. An investigation of the mobility separation of some peptide and protein ions using a new hybrid quadrupole/travelling wave IMS/oa-ToF Instrument. Int. J. Mass Spectrom. 2007;261:1–12. [Google Scholar]
- 19.Shvartsburg AA, Smith RD. Fundamentals of traveling wave ion mobility spectrometry. Anal. Chem. 2008;80(24):9689–9699. doi: 10.1021/ac8016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters: Dramatically enhanced analytical sensitivity with the use of novel StepWave ion transfer technology in the SYNAPT G2-S system. Waters Application Note Literature No. 720003964EN. 2011 [Google Scholar]
- 21.Guttman M, Garcia NK, Cupo A, Matsui T, Julien JP, Sanders RW, Wilson IA, Moore J, Lee KK. CD4-induced activation in a soluble HIV-1 Env trimer. Structure. 2014;22:974–984. doi: 10.1016/j.str.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rand KD, Zehl M, Jensen ON, Jorgensen TJ. Protein hydrogen exchange measured at single-residue resolution by electron transfer dissociation mass spectrometry. Anal. Chem. 2009;81(14):5577–5584. doi: 10.1021/ac9008447. [DOI] [PubMed] [Google Scholar]
- 23.Rand KD, Pringle SD, Morris M, Engen JR, Brown JM. ETD in a traveling wave ion guide at tuned Z-spray ion source conditions allows for site-specific hydrogen/deuterium exchange measurements. J. Am. Soc. Mass Spectrom. 2011;22(10):1784–1793. doi: 10.1007/s13361-011-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rand KD, Jorgensen TJ. Development of a peptide probe for the occurrence of hydrogen (1H/2H) scrambling upon gas-phase fragmentation. Anal. Chem. 2007;79(22):8686–8693. doi: 10.1021/ac0710782. [DOI] [PubMed] [Google Scholar]
- 25.Giles K, Pringle SD, Worthington KR, Little D, Wildgoose JL, Bateman RH. Applications of a traveling wave-based radio-frequency-only stacked ring ion guide. Rapid Commun. Mass Spectrom. 2004;18(20):2401–2414. doi: 10.1002/rcm.1641. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Pan H, Smith DL. Hydrogen exchange-mass spectrometry: optimization of digestion conditions. Mol. Cell. Proteom. 2002;1(2):132–138. doi: 10.1074/mcp.m100009-mcp200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.