Abstract
Objective
In the past decade, hereditary forms of motor neuron disease (spinal muscular atrophy and/or amyotrophic lateral sclerosis) are increasingly identified. As advanced genetic testing is performed, molecular diagnosis can be obtained. Identifying new gene mutations can lead to further understanding of disease.
Methods and Results
We report a single case of a patient with early-onset amyotrophic lateral sclerosis, evaluated at University of Texas Health Houston Science Center from 2011–2014. Initial genetic testing did not reveal an etiology in this patient. Through whole-exome sequencing, a VRK1 mutation was identified.
Conclusions and Relevance
We identify a possible new cause of hereditary amyotrophic lateral sclerosis, VRK1 mutation. This case report also expands the phenotypic spectrum of this mutation in neurologic diseases.
Keywords: amyotrophic lateral sclerosis, anterior nerve cell disease, neurogenetics, all neuromuscular disease, motor neuron disease
INTRODUCTION
Many new genetic forms of motor neuron disease are currently recognized due to advances in genomic testing.1 We describe a novel compound heterozygous mutation in the vaccinia-related kinase 1 gene (VRK1) gene possibly leading to early-onset amyotrophic lateral sclerosis. This expands the phenotypic spectrum of VRK1 mutations.2–4
CASE REPORT
A 32-year-old Hispanic man presented with 5 years of progressive, symmetric distal weakness in his lower extremities. He noted progressive loss in calf bulk. Subsequently, he developed dull, aching pain in both legs and falls. He had difficulty walking downstairs. He denied dysphagia, dysarthria, dyspnea, or upper extremity weakness. He was athletically active in high school. There were no family members with similar symptoms. His neurologic examination showed moderate atrophy in the bilateral gastrocnemius, tibialis anterior, and intrinsic muscles of the feet, including extensor digitorum brevis (Fig. 1A). He had pes cavus and hammer toes. Bilateral dorsiflexion and plantarflexion were rated as 4-/5 and 4/5, respectively, on Medical Research Council (MRC) scale. All other tested upper extremity and lower extremity muscles were rated 5. His reflexes were brisk throughout with crossed adductors and Hoff-man’s signs. Bilateral ankle jerks were absent. His mental status and cranial nerve examinations were normal. He had patchy sensory changes to small fiber modalities in lower extremities, large fiber modalities were preserved. Serum creatine kinase (CK) was mildly elevated [CK 347 U/L (normal range 40–265 U/L)]. Electrodiagnostic studies showed normal sensory conductions of bilateral sural, left median, ulnar, and radial nerves. Motor conduction studies of bilateral peroneal/EDB, bilateral tibial, and left median nerves were normal. Needle electromyographic examination showed abnormal spontaneous activity (fibrillations, positive sharp waves, complex repetitive discharges, and fasciculations) and chronic denervation/rein-nervation (large amplitude, long duration polyphasic units with reduced recruitment pattern) in the bilateral tibialis anterior and gastrocnemius muscles. The proximal lower extremity muscles and distal upper extremity muscles (vastus lateralis, semimembranosus, first dorsal interosseous, flexor carpi radialis) showed rare fasciculations and chronic denervation/reinnervation units (long duration, polyphasic units with reduced recruitment pattern). The thoracic myotome (rectus abdominus) did not show abnormal spontaneous activity. The patient declined evaluation of his tongue. Genetic testing for spinobulbar muscular atrophy, SOD 1 mutation, and complete Charcot-Marie-Tooth panel was unrevealing. His magnetic resonance imaging of brain did not show pontine or cerebellar hypoplasia.
FIGURE 1.
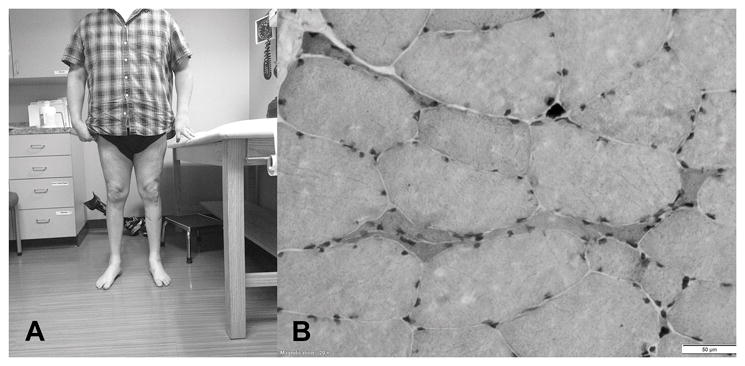
Photograph of distal atrophy and muscle biopsy. A, Patient with noted distal atrophy of the lower extremities in both anterior and posterior compartments. B, Hematoxylin and eosin stains show few atrophic fibers and rare nuclear clumps observed against a background of normal variation in fiber size and shape. The bar at the lower right corner depicts a scale of 50 μm.
Muscle Pathology
The left vastus lateralis biopsy showed few atrophic fibers and rare nuclear clumps, and few angular fibers had increased staining with nonspecific esterase. These findings are consistent with neurogenic atrophy without evidence of reinnervation (Fig. 1B).
Genomic Sequencing
The patient underwent clinical whole-exome sequencing performed by Baylor College of Medicine Human Medical Genetic Laboratory.
RESULTS
Mutation Analysis
Analysis of the patient’s genomic sequence data revealed 2 novel variants in VRK1:chr14:97,313,663 (c.356A > G), and chr14:97,326,965 (c.961C > T) resulting in p.H119R and p.R321C, respectively. Both mutations were predicted to be pathogenic by available bioinformatics tools. The subsequent parental studies demonstrated that mutations were inherited from both parents who were heterozygous asymptomatic carriers.
DISCUSSION
This patient with adult-onset, distal lower extremity predominant, progressive weakness with upper and lower motor neuron signs would meet revised El Escorial criteria for clinically probable amyotrophic lateral sclerosis.5 His early onset and slow progression over 5 years led to evaluation for hereditary causes of motor neuron disease. On exome capture, he displayed compound heterozygous missense mutations in VRK1, which are suspected to be disease causing. This gene was initially implicated in a rare form of pontocerebellar hypoplasia (PCH) in an Ashkenazi Jewish family who were found with homozygous p.R358X mutation and in 4 Iranian siblings positive for homozygous p.R133C mutation.2,4 Anterior horn cell degeneration, brisk reflexes, and PCH were noted in the Ashkenazi Jewish family.2 More recently, 3 pediatric patients from 2 unrelated families with sensory-motor axonal neuropathy and microcephaly (but no evidence of PCH) were found with pathogenic VRK1 compound heterozygous (p.V236M; p. R89Q) and homozygous p.R358X mutations, respectively.3
VRK1 encodes for a serine/threonine kinase which interacts closely with p53, forming an autoregulatory loop.6 This action of VRK1 is intriguing as p53 interacts directly with SMN1 and has been postulated as an etiology in motor neuron cell death.7 Neuropath-ological studies have shown elevated p53 in dying anterior horn cells in patients with amyotrophic lateral sclerosis.8 However, VRK1 is ubiquitously expressed, playing a role in nuclear envelope proteins.6 Therefore, the reason for this selected involvement of anterior horn cells in our patient despite ubiquitous expression is difficult to explain.2–4 As further unclassified patients with neurologic disease undergo advanced genomic testing, the phenotypic spectrum of VRK1 mutations may continue to broaden.
Acknowledgments
Wiszniewski is supported by a K23NS078056 grant from the NINDS. K. Sheikh is supported by the National Institute of Neurological Disorders and Stroke and the National Institutes of Health (Grant R01NS42888, R01NS54962, R21NS087467).
Footnotes
The authors report no conflicts of interest.
References
- 1.Wee CD, Kong L, Sumner CJ. The genetics of spinal muscular atrophies. Curr Opin Neurol. 2010;23:450–458. doi: 10.1097/WCO.0b013e32833e1765. [DOI] [PubMed] [Google Scholar]
- 2.Renbaum P, Kellerman E, Jaron R, et al. Spinal muscular atrophy with pontocerebellar hypoplasia is caused by a mutation in the VRK1 gene. Am J Hum Genet. 2009;85:281–289. doi: 10.1016/j.ajhg.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzaga-Jauregui C, Lotze T, Jamal L, et al. Mutations in VRK1 associated with complex motor and sensory axonal neuropathy plus microcephaly. JAMA Neurol. 2013;70:1491–1498. doi: 10.1001/jamaneurol.2013.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najmabadi H, Hu H, Garshasbi M, et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- 5.Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord. 2000;5:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 6.Valbuena A, Vega FM, Blanco S, et al. p53 downregulates its activating vaccinia-related kinase 1, forming a new autoregulatory loop. Mol Cell Biol. 2006;26:4782–4793. doi: 10.1128/MCB.00069-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin LJ. p53 is abnormally elevated and active in the CNS of patients with amyotrophic lateral sclerosis. Neurobiol Dis. 2000;7:613–622. doi: 10.1006/nbdi.2000.0314. [DOI] [PubMed] [Google Scholar]
- 8.Young PJ, Day PM, Zhou J, et al. A direct interaction between the survival motor neuron protein and p53 and its relationship to spinal muscular atrophy. J Biol Chem. 2002;277:2852–2859. doi: 10.1074/jbc.M108769200. [DOI] [PubMed] [Google Scholar]


