Abstract
Background
Nitric oxide (NO) is an important antibacterial defense molecule produced by upper airway (sinonasal) epithelial cells. We previously showed that a bitter taste receptor expressed in airway epithelium detects quorum-sensing molecules secreted by Gram-negative bacteria and subsequently triggers bactericidal NO production. We hypothesized that the upper airway epithelium may also be able to detect the Gram-positive aerobe Staphylococcus aureus and mount an NO response.
Methods
Human sinonasal air-liquid interface (ALI) cultures were treated with methicillin-resistant S. aureus (MRSA)-conditioned medium (CM), and NO production was measured using fluorescence imaging. Inhibitors of bitter taste receptor signaling were used to pharmacologically determine if this pathway was involved in the production of NO.
Results
A low-molecular-weight, heat, and protease-stabile product found in MRSA CM induced differential, NO synthase (NOS)-mediated NO production. This response varied markedly between individual patients. The MRSA-stimulated NO production was not dependent on 2 important components of bitter taste signaling: phospholipase C isoform β-2 or the transient receptor potential melastatin isoform 5 (TRPM5) ion channel.
Conclusion
This study shows that a S. aureus product elicits an NO-mediated innate defense response in human upper airway epithelium. The active bacterial product is likely a small, nonpeptide molecule that triggers a pathway independent of bitter taste receptors. Patient variation in the NO response to MRSA product(s), potentially due to genetic differences, might play a role in pathophysiology of Gram-positive upper respiratory infections and/or pathogenesis of chronic rhinosinusitis.
Keywords: chronic rhinosinusitis, innate immunity, Staphylococcus aureus, nitric oxide, infection, epithelial
The upper airway serves as the first line of defense against inhaled pathogens and toxins. Epithelial cells are the key immune regulators of the upper airway, providing innate and adaptive protection.1 The epithelium’s innate immune defense is mediated primarily by mucociliary clearance, which involves the coordinated beating of cilia to propel mucus, and secretion of antimicrobial defense molecules.2, 3 Nitric oxide (NO), a highly reactive and diffusible free radical, is secreted by the epithelium and serves a dual role—it increases ciliary beat frequency through intracellular signaling pathways and diffuses into the mucus where it has antiviral and antibacterial activity.4, 5 There are various isoforms of the enzyme nitric oxide synthase (NOS) that synthesize NO, all of which have been identified in the upper airway.6, 7
Our previous work demonstrated that the bitter taste receptor T2R38, which is expressed in airway epithelium, is able to detect acyl-homoserine lactones (AHLs), quorum-sensing molecules secreted by gram-negative bacteria, and subsequently trigger NO production.8 Quorum-sensing molecules or autoinducers are small diffusible molecules that bacteria use for communicating with each other. These quorum-sensing molecules have the capacity to control expression of bacterial genes and influence biofilm formation, virulence, and antibiotic production, among other things.9, 10 Host-pathogen interactions mediated through bacterial quorum-sensing molecules is an emerging field of research, with previous studies showing that pathogens can modulate host behavior through secreted molecules and, similarly, that hosts can detect quorum-sensing molecules and influence microbe activity, such as through inactivation of AHLs.11–13
Similar to Gram-negative bacteria, Gram-positive bacteria are known to secrete quorum-sensing molecules in response to environmental factors; however, most Gram-positive bacteria signal with small peptides rather than AHLs.9 Host immune recognition of Gram-positive bacteria and subsequent NO production has not previously been shown in the airway. We hypothesized that upper airway epithelium is able to detect the Gram-positive aerobe Staphylococcus aureus and mount an NO response. S. aureus is 1 of the most common bacteria seen in patients with chronic rhinosinusitis (CRS),14 with an increasing prevalence of various antibiotic-resistant forms, including commonly found strains of methicillin-resistant S. aureus (MRSA). MRSA isolates from intranasal cultures of patients with rhinosinusitis were nearly absent before the year 2000 and have risen significantly over the past decade.15 Definitive therapeutic regimens for rhinosinusitis caused by MRSA have not yet been established.16 Thus, a better understanding of MRSA’s interaction with the human host has profound clinical implications.
Materials and methods
Sinonasal air-liquid interface cultures
Cultures were prepared as described.8, 17 Briefly, sinonasal mucosal specimens were obtained during surgery from patients undergoing functional endoscopic sinus surgery (FESS) at the Philadelphia Veterans Affairs Medical Center and the Department of Otorhinolaryngology at the University of Pennsylvania. Both institutions’ review boards provided full study approval and informed consent was obtained from patients preoperatively. Patients were excluded if they had a history of systemic diseases such as Wegner’s granulomatosis, sarcoidosis, cystic fibrosis, or immunodeficiencies, or if they had a history of use of antibiotics, oral corticosteroids, or anti-biologics (eg, Xolair) within 1 month of surgery. Air-liquid interface (ALI) cultures were prepared from human sinonasal epithelial cells obtained from enzymatically dissociated human tissue and grown to confluence in tissue culture flasks (75 cm2) using bronchial epithelial basal medium (BEBM; Clonetics, Cambrex, East Rutherford, NJ) and proliferation medium consisting of Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 media containing 100 μg/mL streptomycin and 100 U/mL penicillin for 7 days. Cells were trypsinized and seeded on porous polyester membranes (67 × 104 cells per membrane) in cell culture inserts (Transwell-clear, 12-mm-diameter, 0.4-μm pores; Corning, Acton, MA) coated with type I bovine collagen (30 μg/mL; BD Biosciences, San Jose, CA), 100 μL of coating solution (bovine serum albumin [BSA; 0.1 mg/mL; Sigma-Aldrich, St. Louis, MO] and fibronectin [10 μg/mL; BD Biosciences] in LHC basal medium [Invitrogen, Grand Island, NY]) and placed in a tissue culture laminar flow hood for approximately 12 hours. After 5 days, the culture media from the upper compartment was removed and the epithelium was allowed to differentiate in differentiation medium consisting of 1:1 bronchial epithelial basal medium (BEBM; Clonetics, Cambrex) and DMEM (Invitrogen) with the Clonetics complements for human epidermal growth factor (hEGF; 0.5 ng/mL), bovine pituitary extract (BPE; 0.13 mg/mL), hydrocortisone (0.5 g/mL), triiodothyronine (6.5 g/mL), insulin (5 g/mL), epinephrine (5 g/mL), and transferrin (0.5 g/mL), supplemented with 100 g/mL streptomycin, 100 U/mL penicillin, 0.1 nM retinoic acid (Sigma-Aldrich), and 10% fetal bovine serum (FBS; Sigma-Aldrich) in the basal compartment.
MRSA conditioned medium
For preparation of MRSA conditioned medium (CM), S. aureus strain M2 cultures were grown for 24 hours at 37°C with shaking in lysogeny broth (LB) medium. The 24-hour overnight culture was than diluted to 0.1 optical density (OD) (log phase), and grown under the same conditions for 12 hours. The resultant medium was centrifuged at 2000g for 10 minutes at room temperature and filtered through a 0.2-μm filter. Dialysis of the MRSA CM was performed for approximately 5 hours at 4°C against a 1000-fold excess of LB (changed once after the first 1.5 hours) using a 3500 molecular weight (MW) cutoff dialysis membrane (Spectra/Por; Spectrum Medical Industries Inc., Laguna Hills, CA). Boiled MRSA CM was prepared by heating MRSA CM at 100°C for 1 hour then immediately placing on ice. Trypsinization of MRSA CM was performed by incubating at 37°C for 1 hour with 250 μg/mL trypsin (Life Technologies) followed by incubation at 37°C for 2 hours with 5 mM 4-(2-aminoethyl) benzensulfonyl fluoride hydrochloride, an irreversible serine protease inhibitor (Fisher Scientific, Waltham, MA). All MRSA CM was used at a final concentration of 25%, diluted in Dulbecco’s phosphate-buffered saline (DPBS); this concentration was used because the equivalent concentration of LB medium was the highest concentration that did not have damaging effects on the epithelial cells.
Live-cell imaging of reactive NO production in human ALI cultures
To track cellular NO production, the fluorescent reactive nitrogen species indicator 4-amino-5-methylamino-2′,7′-difluoroflurescein (DAF-FM; Invitrogen/Life-Technologies, Inc.) was imaged as described8 using an IX-81 microscope (×10, 0.3 NA UPlanFLN objective; Olympus, Tokyo, Japan) and the 488-nm argon laser line of a Fluoview FV1000 laser scanning confocal system. Briefly, cells were loaded via the apical side for 90 minutes with DAF-FM-diacetate by incubation in DPBS containing 10 μM DAF-FM-diacetate and 5 μM of the cell-permeant NO scavenger, carboxy-PTIO (cPTIO; Cayman Chemical, Ann Arbor, MI). Cultures were washed 5 times with PBS to remove any unloaded DAF-FM and cPTIO and incubated for 15 minutes with 30 μL of PBS on the apical side. After establishing baseline fluorescence, 30 μL of the treatment was added to the apical side. DAF-FM fluorescence images were acquired at 5-second intervals.
All DAF-FM experiments were performed using Dulbecco’s PBS (DPBS; containing 1.8 mM Ca2+) on the apical side of the cultures and modified 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES)-buffered Hank’s balanced salt solution (HBSS) containing 1× Minimum Essential Medium (MEM) amino acids on the basolateral side. Stock solutions of DAF-FM and cPTIO were prepared at 1000× in dimethylsulfoxide (DMSO) and working solutions were made fresh daily in DPBS. U73122, the phospholipase C (PLC)β-2 inhibitor, and its inactive analogue, U73343 (Cayman Chemical), were used at 5 μM, and the TRPM5 blocker, triphenylphosphine oxide (TPPO; Sigma-Aldrich),18 was used at 100 μM; each of the working solutions was prepared fresh daily in PBS from 1000× DMSO stock solutions. The inhibitors or inactive analogue were added to the apical side of the cultures for 15 minutes prior to DAF imaging and were also contained in the MRSA CM treatments. The NOS inhibitor, L-NG-nitroarginine methyl ester (L-NAME; Cayman Chemical), was used at 100 μM and was prepared fresh daily from a 1000× DMSO stock solution. L-NAME was applied to the apical and basolateral sides 15 minutes prior to treatment with MRSA CM, also containing L-NAME.
Data analysis and statistics
Data were analyzed in Fluoview (Olympus) and statistical analysis was performed in GraphPad Prism (Graph-Pad Software, Inc., La Jolla, CA) with p < 0.05 considered statistically significant. One-way analysis of variance (ANOVA) with Bonferroni posttest was used for multiple comparisons. Unpaired t tests with 2 tails were used for single comparisons. All data are reported as means ± standard deviation (SD). For all figures, 2 asterisks (**) indicates p < 0.01, 1 asterisk (*) indicates p < 0.05, and n.s. indicates no statistical significance. Because of the small sample size normality was not determined knowing that the ANOVA and t tests work well even when the distribution is only approximately Gaussian.
Results
MRSA CM induces differential NO production in patients
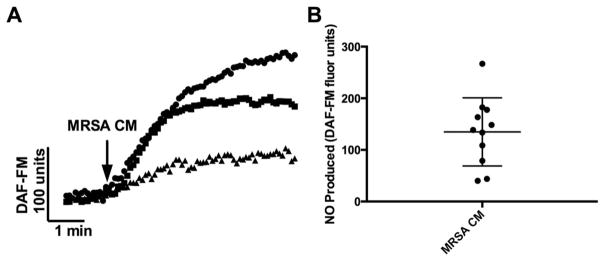
Our previous work showed that NO production was induced by Gram-negative AHL quorum-sensing molecules and was mediated by the bitter taste receptor T2R38.8 We first sought to determine if S. aureus, a Gram-positive bacterium, also triggered a NO response in sinonasal epithelium. Using the fluorescent probe DAF-FM, which reacts with NO-derived reactive nitrogen species to form a fluorescent benzotriazole, we measured the cellular production of NO after treatment with MRSA CM. We found that MRSA CM stimulation resulted in production of NO-derived reactive nitrogen species (DAF-FM fluorescence increase) over the course of 5 minutes (Fig. 1). Interestingly, we found that the degree of NO production varied markedly between patients (Fig. 1). The average DAF-FM fluorescence increase was 134.8 ± 19.91 DAF-FM units (n = 11 patients, 1 culture per patient).
FIGURE 1.
MRSA CM induces differential NO production in patients. (A) Representative traces of DAF-FM fluorescence in 3 different patients (1 culture each, each symbol [square, circle, triangle] represents a culture from a different patient). (B) Average DAF-FM fluorescence (n = 11 patients, 1 culture per patient) of cultures stimulated with MRSA CM is 134.8 ± 19.91. Graph shows mean ± SD. CM = conditioned medium; DAF-FM = 4-amino-5-methylamino-2′,7′-difluoroflurescein; MRSA = methicillin-resistant Staphylococcus aureus; NO = nitric oxide.
MRSA CM induces NOS-mediated NO production through a low-molecular-weight, heat- and protease-stable product
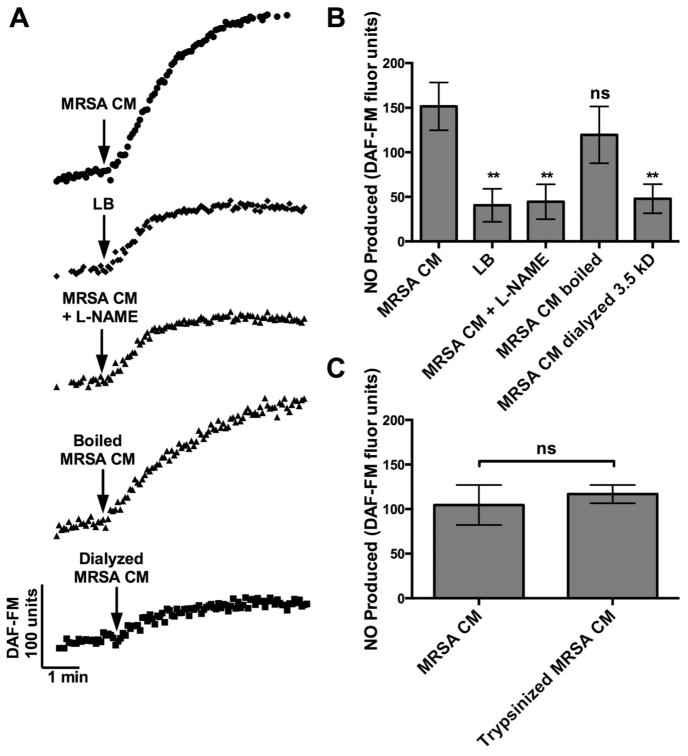
To further characterize the NO response, we compared the magnitude of DAF-FM fluorescence for MRSA CM to the bacteria growth media LB and in the presence of the NOS inhibitor L-NAME. DAF-FM fluorescence increase for MRSA CM was significantly different from LB and was also significantly decreased in the presence of L-NAME (Fig. 2B). Specifically, the mean DAF-FM increase for MRSA CM (151.5 ± 26.75) was >3-fold higher than LB (40.58 ± 18.44, p < 0.01) and MRSA CM with L-NAME (44.51 ± 19.51; p < 0.01). In the presence of L-NAME, the DAF-FM fluorescence increase for MRSA CM was decreased to approximately the level of LB, showing that a MRSA product in the CM increased DAF-FM fluorescence specifically via NOS-mediated NO production.
FIGURE 2.
MRSA CM induces NOS-mediated NO production through a low molecular weight, heat and protease stabile product. (A) Representative traces of DAF-FM fluorescence for MRSA CM, LB, MRSA CM + L-NAME (NOS inhibitor), boiled MRSA CM (100°C, 1 hour) and dialyzed MRSA CM (in LB, 3.5 MWCO, 5 hours). (B) Bar graphs of average DAF-FM fluorescence (n = 3 cultures; 3 patients for each condition) of cultures stimulated with MRSA CM, LB, MRSA CM + L-NAME, boiled MRSA CM, and dialyzed MRSA CM. DAF-FM fluorescence increases were 151.5 ± 26.75 (MRSA CM), vs 40.58 ± 18.44 (LB; p < 0.01 by 1-way ANOVA with Bonferroni post-test), vs 44.51 ± 19.51 (MRSA CM + L-NAME; p < 0.01), vs 119.6 ± 31.78 (boiled MRSA CM; n.s.), and vs 47.92 ± 16.26 (dialyzed MRSA CM; p < 0.01). (C) Bar graphs of average DAF-FM fluorescence (n = 3 cultures; 3 patients for each condition) of cultures stimulated with MRSA CM alone or MRSA CM digested with trypsin (trypsinized MRSA CM). DAF-FM fluorescence increases were 104.7 ± 22.45 (MRSA CM) vs 116.9 ± 10.29 (trypsinized MRSA CM; p = 0.4401). Graph shows mean ± SD. **p < 0.01. ANOVA = analysis of variance; CM = conditioned medium; DAF-FM = 4-amino-5-methylamino-2′,7′-difluoroflurescein; LB = lysogeny broth; L-NAME = L-NG-nitroarginine methyl ester; MRSA = methicillin-resistant Staphylococcus aureus; MWCO = molecular weight cutoff; NO = nitric oxide; NOS = NO synthase; n.s. = no statistical significance; SD = standard deviation.
We also compared the DAF-FM fluorescence increase for MRSA CM with CM that was boiled to denature any proteins or dialyzed to remove low-molecular-weight (<3500 Da) products (Fig. 2B). The MRSA CM was not significantly affected by boiling (119.6 ± 31.78), but dialysis reduced its potency approximately 3-fold (47.92 ± 16.26; p < 0.01). Therefore, the MRSA CM appears to contain a low-molecular-weight and heat-stable product that can induce NO production. Typically, proteins with secondary or tertiary structure are denatured by heat (although not an absolute), so this result suggested that the MRSA product was not a protein. To further test for this, we treated the MRSA CM with the protease trypsin. There was no significant difference between DAF-FM fluorescence increases for MRSA CM with and without trypsinization (Fig. 2C). The average DAF-FM fluorescence (n = 3 cultures from 3 patients for each condition) was 104.7 ± 22.45 (MRSA CM alone) and 116.9 ± 10.29 (MRSA CM after trypsin digestion), respectively (p = 0.4401). As a control, trypsin alone tested at the concentration used had no effects on NO production (data not shown).
MRSA CM–induced NO generation is not specific to the T2R taste-signaling pathway
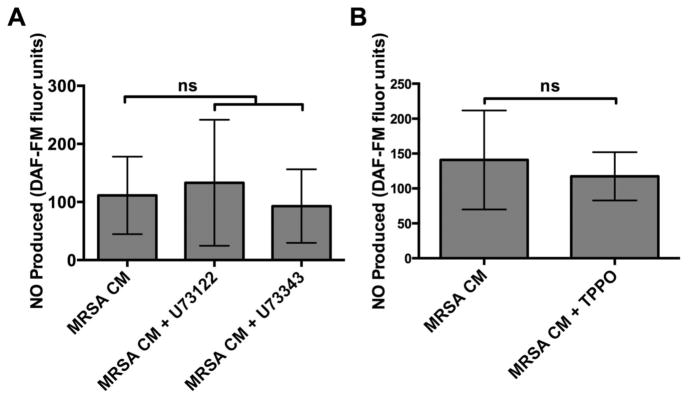
We tested if the NO response seen with MRSA CM was T2R-dependent, as shown in our previous work with T2R38 and Pseudomonas aeruginosa AHLs.8 We found that NO generation was not blocked by an inhibitor of phospholipase C isoform β-2 (PLCβ-2), which activates Ca2+ signaling downstream of bitter taste receptors. The average DAF-FM fluorescence increases (n = 4 to 10 cultures; 4 to 10 patients for each condition) of cultures was 133.2 ± 108.5 with MRSA CM alone, 93.0 ± 63.27 in the presence of the PLCβ-2 inhibitor U73122, and 111.3 ± 66.81 in the presence of its inactive analogue U73343 (Fig. 3A).
FIGURE 3.
MRSA CM-induced NO generation is not blocked by inhibitors of PLCβ-2 dependent Ca2+ signaling or inhibitors of TRPM5-dependent Ca2+ signaling. (A) Bar graphs of average DAF-FM fluorescence (n = 4–10 cultures; 4–10 patients for each condition) of cultures stimulated with MRSA CM alone or in the presence of U73122 (PLCβ-2 inhibitor) or U73343 (inactive analogue). After stimulation with MRSA CM, DAF-FM fluorescence increases were 111.3 ± 66.81 (MRSA CM + U73343) vs 133.2 ± 108.5 (MRSA CM; ns by 1-way ANOVA with Bonferroni post-test), vs 93.0 ± 63.27 (MRSA CM + U73122; ns). (B) Bar graphs of average DAF-FM fluorescence (n = 4–6 cultures; 4–6 patients for each condition) of cultures stimulated with MRSA CM alone or in the presence of TRPM5 blocker (MRSA CM + TPPO). After stimulation with MRSA CM, DAF-FM fluorescence increases were 140.8 ± 70.96 (MRSA CM) vs 117.4 ± 34.58 (MRSA CM + TPPO; p = 0.2662). Graph shows mean ± SD. ANOVA = analysis of variance; CM = conditioned medium; DAF-FM = 4-amino-5-methylamino-2′,7′-difluoroflurescein; MRSA = methicillin-resistant Staphylococcus aureus; NO = nitric oxide; ns = no statistical significance; PLC = phospholipase C; SD = standard deviation; TPPO = triphenylphosphine oxide; TRPM5 = transient receptor potential melastatin isoform 5.
We also found that there was no significant difference between DAF-FM fluorescence increases for MRSA CM and in the presence of triphenylphosphine oxide (TPPO), an inhibitor of the transient receptor potential melastatin isoform 5 (TRPM5) ion channel, another important component of T2R bitter taste signaling. The average DAF-FM fluorescence (n = 4 to 6 cultures; 4 to 6 patients for each condition) of cultures stimulated with MRSA CM alone or in the presence of TPPO (MRSA CM + TPPO) was 140.8 ± 70.96 and 117.4 ± 34.58, respectively (Fig. 3B; p = 0.2662). Together, the results for the PLCβ-2 and TRPM5 inhibitors strongly suggest that the NO generation is not specific to the T2R taste signaling pathway.
Discussion
To our knowledge, this is the first study showing NO production in differentiated human sinonasal epithelial cells in response to S. aureus product(s). This novel, inter-kingdom signaling and NO production is of critical importance. S. aureus is 1 of the most prominent pathogens associated with CRS,14 and NO mediates numerous protective effects in the airway, namely increased mucociliary clearance and antimicrobial effects.4, 5 We have previously shown that NO produced by sinonasal ALI cultures in vitro, as seen here, can have direct bactericidal effects.8 The NO response to S. aureus shown in this study may have evolved in humans as a means of preventing a commonly encountered pathogen from running rampant in the upper airway. In this study we focused on MRSA; however, we do not feel that the presence of the mecA gene encoding a different penicillin binding protein and conferring resistance substantially alters our findings with regard to methicillin sensitive S. aureus.
In all patient ALI cultures evaluated, there was a rapid rise in intracellular NO, occurring within seconds of exposure to MRSA CM and typically lasting for at least 5 minutes, not seen in the LB control–treated cultures. Nevertheless, there was wide variability seen between patients, with some mounting a more robust NO response to the same concentration of bacterial CM. Our previous work showed that common genetic polymorphisms in the TAS2R38 gene were linked to significant differences in the ability of upper respiratory cells to produce NO in response to Gram-negative quorum-sensing molecules and subsequently kill bacteria,8 and the nonprotective polymorphism was shown to be an independent risk factor for CRS.19 In the present study, we also show variable NO response in patients, which raises the question of whether there is a genetic basis for the variability seen here. It is interesting that in the human population, 24% of individuals are persistently colonized with S. aureus, 32% are intermittently colonized, and the remaining 44% are never colonized.20 Could the differential NO produced seen from individual to individual define persistent vs intermittent vs noncolonizers? Future identification of a genetic basis for the individual differences in S. aureus–stimulated NO production may reveal a novel and important risk factor for upper airway diseases involving S. aureus or other Gram-positive bacteria.
Unlike our previous work, the response to MRSA CM was independent of the T2R38-mediated NO pathway triggered by Gram-negative AHL quorum-sensing molecules8; the pharmacological inhibition used in this study ruled out T2Rs entirely as the activators of the NO production by NOS. Future identification of a human receptor, either extracellular or intracellular, responsible for binding the microbial product and the pathway involved will be essential for identifying the basis of patient-to-patient variability observed here. This may also reveal potentially novel therapeutic modalities to stimulate bactericidal NO production that could be used in clinical practice.
The other component of this inter-kingdom signaling pathway of tremendous interest is the S. aureus product. We showed that the bacterial product inducing the response was less than 3.5 kD in size and heat stable. The activity of the product was not altered by boiling, suggesting that it was not a protein with secondary structure. Furthermore, trypsinization of the MRSA CM caused no change in activity, strongly indicating that the microbial product was not a peptide. However, if the product did not contain any arginine or lysine sites, where trypsin cleaves, then it may possibly have retained its activity.21
The product inducing NO production was present in the medium when bacteria were growing at log phase. Future studies are needed to investigate whether it is accumulated in a cell-density-dependent manner, similar to quorum-sensing molecules. As with most Gram-positive bacteria, the known quorum-sensing systems utilized by S. aureus, such as those involved in regulating biofilm maturation, utilize secreted peptides for gene regulation.9, 22 Identification of the small molecule eliciting the response observed here will shed light on its potential quorum-sensing role in S. aureus and possibly other Gram-positive bacteria. Because processes regulated by quorum sensing, including colonization, virulence, and biofilm formation, all play a role in CRS, these future studies may reveal novel insights in the pathophysiology of Gram-positive infection in this disease.
Conclusion
The present study demonstrates that a S. aureus product elicits an NO-mediated innate defense pathway in upper airway epithelium that varies between patients. The active bacterial product is likely a small, nonpeptide molecule that triggers an NO pathway independent of T2R signaling. The heterogeneous response to the same pathogen product may contribute to the complex interplay between genes and environment that predisposes some CRS patients to Gram-positive infections. Once the active bacterial product and its human target are identified, clinical studies will be necessary to quantify its impact on otorhinolaryngology practice.
Acknowledgments
Funding sources for the study: U.S. Public Health Service (USPHS) (R01DC013588 to N.A.C.).
Footnotes
Potential conflict of interest: None provided.
Presented at the ARS Spring Meeting on April 24, 2015, in Boston, MA.
References
- 1.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fokkens WJ, Scheeren RA. Upper airway defence mechanisms. Paediatr Respir Rev. 2000;1:336–341. doi: 10.1053/prrv.2000.0073. [DOI] [PubMed] [Google Scholar]
- 3.Antunes MB, Gudis DA, Cohen NA. Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29:631–643. doi: 10.1016/j.iac.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun. 1993;191:83–88. doi: 10.1006/bbrc.1993.1187. [DOI] [PubMed] [Google Scholar]
- 5.Sahin G, Klimek L, Mullol J, Hormann K, Walther LE, Pfaar O. Nitric oxide: a promising methodological approach in airway diseases. Int Arch Allergy Immunol. 2011;156:352–361. doi: 10.1159/000324678. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg JO, Farkas-Szallasi T, Weitzberg E, et al. High nitric oxide production in human paranasal sinuses. Nat Med. 1995;1:370–373. doi: 10.1038/nm0495-370. [DOI] [PubMed] [Google Scholar]
- 7.Lundberg JO, Rinder J, Weitzberg E, Lundberg JM, Alving K. Nasally exhaled nitric oxide in humans originates mainly in the paranasal sinuses. Acta Physiol Scand. 1994;152:431–432. doi: 10.1111/j.1748-1716.1994.tb09826.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell RJ, Lee SK, Kim T, Ghim CM. Microbial linguistics: perspectives and applications of microbial cell-to-cell communication. BMB Rep. 2011;44:1–10. doi: 10.5483/BMBRep.2011.44.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Rumbaugh KP, Kaufmann GF. Exploitation of host signaling pathways by microbial quorum sensing signals. Curr Opin Microbiol. 2012;15:162–168. doi: 10.1016/j.mib.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Pacheco AR, Sperandio V. Inter-kingdom signaling: chemical language between bacteria and host. Curr Opin Microbiol. 2009;12:192–198. doi: 10.1016/j.mib.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amara N, Krom BP, Kaufmann GF, Meijler MM. Macromolecular inhibition of quorum sensing: enzymes, antibodies, and beyond. Chem Rev. 2011;111:195–208. doi: 10.1021/cr100101c. [DOI] [PubMed] [Google Scholar]
- 14.Hamilos DL. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014;133:640–653. e4. doi: 10.1016/j.jaci.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rujanavej V, Soudry E, Banaei N, Baron EJ, Hwang PH, Nayak JV. Trends in incidence and susceptibility among methicillin-resistant Staphylococcus aureus isolated from intranasal cultures associated with rhinosinusitis. Am J Rhinol Allergy. 2013;27:134–137. doi: 10.2500/ajra.2013.27.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCoul ED, Jourdy DN, Schaberg MR, Anand VK. Methicillin-resistant Staphylococcus aureus sinusitis in nonhospitalized patients: a systematic review of prevalence and treatment outcomes. Laryngoscope. 2012;122:2125–2131. doi: 10.1002/lary.23435. [DOI] [PubMed] [Google Scholar]
- 17.Lai Y, Chen B, Shi J, Palmer JN, Kennedy DW, Cohen NA. Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2011;128:1207–1215. e1. doi: 10.1016/j.jaci.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Palmer RK, Atwal K, Bakaj I, et al. Triphenylphosphine oxide is a potent and selective inhibitor of the transient receptor potential melastatin-5 ion channel. Assay Drug Dev Technol. 2010;8:703–713. doi: 10.1089/adt.2010.0334. [DOI] [PubMed] [Google Scholar]
- 19.Adappa ND, Zhang Z, Palmer JN, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4:3–7. doi: 10.1002/alr.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthukrishnan G, Lamers RP, Ellis A, et al. Longitudinal genetic analyses of Staphylococcus aureus nasal carriage dynamics in a diverse population. BMC Infect Dis. 2013;13:221. doi: 10.1186/1471-2334-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keil B. Specificity of Proteolysis. Berlin: Springer-Verlag; 1992. [Google Scholar]
- 22.Otto M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med. 2013;64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]