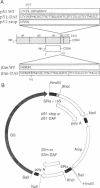
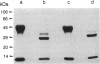
Abstract
Maternal transport of immunoglobulin to the newborn mammal is important for immune defense during the first weeks of independent life. Receptors for the Fc portion of IgG mediate the transfer of immunoglobulin from milk to the bloodstream of newborn mice and rats, by passage through intestinal epithelial cells. Neonatal Fc receptors (FcRn) isolated from intestinal epithelial cells of suckling rats bear a striking resemblance to class I histocompatibility molecules. The heavy chain of FcRn has sequence similarity in three extracellular domains to the corresponding domains of class I molecules, and the light chain of both types of molecules is beta 2-microglobulin. To facilitate biochemical characterization and crystallization of FcRn, we have expressed a secreted form, as well as two different lipid-linked forms solubilizable by phospholipase treatment. The lipid-linked forms are heterodimers consisting of beta 2-microglobulin and the extracellular portion of the heavy chain and are anchored to the membrane by a phosphatidylinositol linkage attached to either the heavy chain or beta 2-microglobulin. Cells expressing either lipid-linked form bind rat Fc, reproducing the known physiological pH dependence of binding. Secreted FcRn has been purified in yields up to 40 mg/liter from cell supernatants. Circular dichroism spectra of soluble FcRn appear similar to spectra of class I MHC molecules, suggesting that the similarities in primary sequence extend also to a similarity in secondary structure. Soluble FcRn crystallizes in a form amenable to a structure determination by x-ray diffraction methods, which will ultimately allow a detailed comparison of the two types of molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernabeu C., van de Rijn M., Lerch P. G., Terhorst C. P. Beta 2-microglobulin from serum associates with MHC class I antigens on the surface of cultured cells. Nature. 1984 Apr 12;308(5960):642–645. doi: 10.1038/308642a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N., Davitz M. A., Nussenzweig V., Martin D. W., Jr Signal for attachment of a phospholipid membrane anchor in decay accelerating factor. Science. 1987 Nov 27;238(4831):1280–1283. doi: 10.1126/science.2446389. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Gorga J. C., Dong A., Manning M. C., Woody R. W., Caughey W. S., Strominger J. L. Comparison of the secondary structures of human class I and class II major histocompatibility complex antigens by Fourier transform infrared and circular dichroism spectroscopy. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2321–2325. doi: 10.1073/pnas.86.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Groves M. L., Greenberg R. Complete amino acid sequence of bovine beta 2-microglobulin. J Biol Chem. 1982 Mar 10;257(5):2619–2626. [PubMed] [Google Scholar]
- Johnson W. C., Jr Protein secondary structure and circular dichroism: a practical guide. Proteins. 1990;7(3):205–214. doi: 10.1002/prot.340070302. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Protein secondary structure and circular dichroism: a practical guide. Proteins. 1990;7(3):205–214. doi: 10.1002/prot.340070302. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lancet D., Parham P., Strominger J. L. Heavy chain of HLA-A and HLA-B antigens is conformationally labile: a possible role for beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3844–3848. doi: 10.1073/pnas.76.8.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. Y., Devaux B., Green A., Sagerström C., Elliott J. F., Davis M. M. Expression of T cell antigen receptor heterodimers in a lipid-linked form. Science. 1990 Aug 10;249(4969):677–679. doi: 10.1126/science.1696397. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McKnight C. J., Briggs M. S., Gierasch L. M. Functional and nonfunctional LamB signal sequences can be distinguished by their biophysical properties. J Biol Chem. 1989 Oct 15;264(29):17293–17297. [PubMed] [Google Scholar]
- Parham P. Purification of immunologically active HLA-A and -B antigens by a series of monoclonal antibody columns. J Biol Chem. 1979 Sep 25;254(18):8709–8712. [PubMed] [Google Scholar]
- Schumacher T. N., Heemels M. T., Neefjes J. J., Kast W. M., Melief C. J., Ploegh H. L. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990 Aug 10;62(3):563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. Cloning and expression of the neonatal rat intestinal Fc receptor, a major histocompatibility complex class I antigen homolog. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):571–580. doi: 10.1101/sqb.1989.054.01.068. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Rees A. R. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985 Jul;15(7):733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- Sundelin J., Björck L., Lögdberg L. The complete amino acid sequence of rat beta 2-microglobulin. Scand J Immunol. 1988 Feb;27(2):195–199. doi: 10.1111/j.1365-3083.1988.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Geier S. S., Uehara H., Nathenson S. G. Secondary structure of the murine histocompatibility alloantigen H-2Kb: relationship between heavy chain, beta 2-microglobulin, and antigenic reactivity. Biochemistry. 1985 Jun 4;24(12):3002–3006. doi: 10.1021/bi00333a029. [DOI] [PubMed] [Google Scholar]