Abstract
One of the newer classes of targeted cancer therapeutics is monoclonal antibodies. Monoclonal antibody therapeutics are a successful and rapidly expanding drug class due to their high specificity, activity, favourable pharmacokinetics, and standardized manufacturing processes. Antibodies are capable of recruiting the immune system to attack cancer cells through complement-dependent cytotoxicity or antibody dependent cellular cytotoxicity. In an ideal scenario the initial tumor cell destruction induced by administration of a therapeutic antibody can result in uptake of tumor associated antigens by antigen-presenting cells, establishing a prolonged memory effect. Mechanisms of direct tumor cell killing by antibodies include antibody recognition of cell surface bound enzymes to neutralize enzyme activity and signaling, or induction of receptor agonist or antagonist activity. Both approaches result in cellular apoptosis. In another and very direct approach, antibodies are used to deliver drugs to target cells and cause cell death. Such antibody drug conjugates (ADCs) direct cytotoxic compounds to tumor cells, after selective binding to cell surface antigens, internalization, and intracellular drug release. Efficacy and safety of ADCs for cancer therapy has recently been greatly advanced based on innovative approaches for site-specific drug conjugation to the antibody structure. This technology enabled rational optimization of function and pharmacokinetics of the resulting conjugates, and is now beginning to yield therapeutics with defined, uniform molecular characteristics, and unprecedented promise to advance cancer treatment.
Keywords: Cancer, Metastasis, Metabolism pathway, Carcinogenesis
Introduction
Cancer, in its most basic definition, is a class of diseases characterized by abnormal cell growth. Cancer affects essentially all macro-organisms, and paleo-pathologic findings suggest that cancer has existed since long before humans [1]. The earliest known recorded case of cancer, a breast cancer, was found in an ancient Egyptian medical text (the Edwin Smith Papyrus written circa 3000 B.C.), which described the ailment as a grave disease with no treatment [2]. Since its initial identification 5000 years ago, the treatments for cancer, while varying wildly in the specific methods and outcomes, have remained quite consistent. Essentially every medical procedure for cancer throughout history employed surgical and chemotherapeutic intervention, and recognized that early diagnosis and intervention were necessary for successful treatment [3-6]. The causes and progression of cancer, however, remained essentially a complete mystery until around mid-19th century.
Tumor metastasis was originally described by the 1st century Roman physician Anulus Celsus, in his De Medicina encyclopedia of medicine [1]. Celsus described secondary tumours and recurrences of breast cancer in the armpits, and noted that in advanced cases death may be caused by spreading of the disease to distant organs [7]. It took another 2000 years, however, to develop the modern model for cancer progression and metastasis. Campbell De Morgan, after 34 years of clinical study, developed his model for the “focal origin of cancer” [8]. In a series of publications between 1872 and 1874, De Morgan described cancer as a disease that arose locally before spreading, first to the lymph nodes and then throughout the body [9-11]. Furthermore, it was around this time that chemical carcinogens were first discovered in association with occupation risk of lung cancer. Miners, and other industrial workers, where shown to have significantly higher rates of lung cancer, which researchers eventually attributed to the inhalation of arsenic, bismuth and other chemicals in the form of mine dust [12]. In the following decades, the discovery of both x-rays and radioactivity revolutionized the treatment and diagnosis of cancer. Importantly, both of these types of ionizing radiation were eventually also identified as another significant source of carcinogenesis [12].
In the decades following these discoveries, more cancer research took place than during the prior centuries combined. First and foremost, the hypotheses that cancer was caused by demons, moral and religious deviations, and humoral imbalances were largely disabused by this point [13]. The discoveries of the mid to late 19th century coupled with the advent of in vitro and in vivo cancer models resulted in a great advancement in the field of cancer research [5,13,14]. During this time, histopathological staging of tumours was first introduced, a number of new cancers and carcinogens were discovered, and in vitro and in vivo techniques enabled early investigation in carcinogenesis and the biology and biochemistry of cancer cells [13,15,16]. The connection between genetics and cancer, which was first suggested in the mid to late 19th century, was not discovered until the early 20th century with the advent of in vivo cancer biology and genetically controlled animal strains [8,13,17].
One of the most important discoveries of this time was made by German biochemist Otto Warburg in 1924 [18]. He discovered that cancer cells metabolize glucose in a manner that is distinct from the main energy metabolism pathway used by normal cells and tissues. While normal cells derive energy primarily from oxidative phosphorylation through mitochondrial respiration, cancer cells use glycolysis, even in the presence of sufficient oxygen to support mitochondrial oxidative phosphorylation [19-24]. This discovery is the basis for positron emission tomography (PET) imaging of tumours, an invaluable tool in modern cancer diagnosis and treatment, based on the differential uptake of 18F labelled glucose derivatives by cancer cells compared to normal cells [25-29].
Warburg went on to hypothesize that this phenomenon was not just a feature of cellular transformation, but that cancer was caused by mitochondrial damage, resulting in lower oxidative phosphorylation and higher levels of glycolysis [30]. Since then, the cancer research community has largely discredited this hypothesis, stating that the metabolic changes observed in cancer are a result of cellular transformation with the anaerobic tumor microenvironment selecting for increased glycolysis. Down-regulation of oxidative phosphorylation in response to oncogene activation was considered an advantage for tumor cells that could foster adaptation to hypoxic conditions [31-33]. However, Warburg’s hypothesis may have been more appropriate than initially given credit for. During the current renaissance of research into cancer metabolism, there have been a number of studies showing that damaged mitochondria directly facilitate a more aggressive cancer phenotype, and that normalization of mitochondrial function in cancer cells can reduce tumorigenesis and metastatic activity [34-43]. Thus, while mitochondrial dysfunction in conjunction with oncogenic events may not be the exclusive cause of all cancers, as Warburg initially hypothesized; mitochondrial functionality is certainly intimately involved in tumorigenesis and cancer progression [44-46].
The era of the late 19th and early 20th century also provided the very first examples of cancer immunotherapy, another area of cancer research that is currently undergoing a renaissance of research [8,12]. Clinical reports in the late 19th century described occasional spontaneous remission of various cancers when patients co-presented with infectious diseases, notably erysipelas [47]. This phenomenon prompted investigation by William B. Cooley into the infection of cancer patients with various infectious agents, e.g. Serratia marcescens or Streptococcus pyogenes, or administering extracts of these bacteria, in attempt to induce remission [48,49]. The idea behind Cooley’s toxin therapy is that the administered infectious agent or toxin would result in systemic immune activation with a desired side effect of tumor cell destruction by the activated immune system [50,51]. Even though the results and methods gained significant criticism over the years, Coley claimed great success, reporting an estimated 80% 5-year survival rate for diseases with no other treatment options [52-54]. Despite the literally unbelievable success rate, and the eventual discontinuation of Coley’s toxin cancer treatment, this approach was the first example of attempting to artificially induce an immune response as a cancer treatment; the basis for many of today’s promising cancer therapies.
Coinciding with the discontinuation of Coley’s toxin cancer treatments in the 1950s, was the discovery of tumor associated antigens: the next great advancement in the field of cancer immunotherapy [8,55]. The discovery of tumor associated antigens resulted in a massive amount of research over the following decades to identify new tumor associated antigens and develop therapies to take advantage of these new discoveries [56-69]. Unfortunately, efforts towards the development of cancer vaccines, and other immune based cancer therapies over this time period were largely ineffective [8,55,70]. However, the efforts towards the research of tumor associated antigens laid the groundwork for modern monoclonal antibody therapy and other immunotherapies for cancer. Concurrent with this research in cancer immunotherapy, research into carcinogens, tumorigenesis, diagnostic techniques, chemotherapeutics, radiation therapy, cancer classification, and surgical procedures all advanced by leaps and bounds. However, despite the advances in detection, diagnosis, and treatments of cancer, the overall mortality rate remained high, and relatively unchanged from previous generations [13,55].
Despite discovery of cancer as a deadly disease thousands of years ago, characterization of the disease progression, and accumulating knowledge on causes over the course of the last few hundred years, modern cancer treatment faces many of the same issues today as it has throughout history [1,3,4,12,13,55]. Treatment today generally follows the same scheme as it has since humans began attempting to treat this disease: Surgical intervention (if possible) to remove any primary and secondary tumors, and chemotherapeutic intervention to treat inoperable tumours and lesions to halt or prevent disease progression. Importantly, the issue of cancer metastasis still plagues clinicians and patients, without break-through advances toward truly effective therapies that can achieve a cure for patients with advanced cancer. The majority of deaths from cancer are caused not by the primary tumor, but by secondary growths that result from tumor invasion and metastasis, and advanced cancers still have a very poor prognosis [71].
Through advances in research, diagnostic, and surgical approaches, oncologists now have refined, clinically validated chemotherapeutics, combination treatments, and other cancer therapeutics making successful intervention much more common. Cancer survival has steadily increased in Western countries for the last 30 years [72,73]. This can be explained by a better understanding of cancer biology and disease progression, more advanced in vivo and in vitro cancer models, the sequencing of the human genome resulting in a clearer picture of the genetic contributions to cancer, an understanding of onco-genetics, the development of new and more accurate cancer screening techniques, and new, more targeted cancer therapeutics [74-84].
One of the newer classes of targeted cancer therapeutics is monoclonal antibodies. Monoclonal antibody (mAb) therapeutics are a successful and rapidly expanding drug class due to their high specificity, activity, favourable pharmacokinetics, and standardized manufacturing processes [85-94]. The success of monoclonal antibody therapeutics is built on the back of the aforementioned investigation into cancer immunology from the 1950s to the 1970s. Since the discovery of tumor specific antigens, researches attempted to use antibodies as therapeutic agents. Originally, researchers attempted to immunize an animal with cancer cells and then administer serum or polyclonal antibodies in attempt to confer passive immunization [55,62,95]. However, immunogenicity of the serum and/or antibodies, coupled with the irreproducibility of the animal immunizations made this therapy impractical and ineffective [55,62,95]. The development of hybridoma technology in 1975 re-invigorated researches into antibody based cancer therapeutics [96,97]. For the first time, monoclonal antibodies could be isolated and produced. Thus, individual antibody sequences could be cloned and screened for maximal efficacy instead of relying on the unpredictable and often irreproducible process of passive immunization with serum.
After initial issues with the immunogenicity of murine mAbs, and subsequent development of chimeric and humanized antibodies, mAbs have become some of the most successful and efficacious drugs of the last few decades [94,98,99]. There are currently 36 FDA approved monoclonal antibodies for the treatments of various diseases, with 17 for the treatment of cancer. While there are 5 classes of antibodies (immunoglobulin’s) in humans (IgG, IgA, IgE, IgD, and IgM), every currently clinically approved mAb therapeutic is an IgG (the immunoglobulin responsible for antibody-based immunity against pathogens) [100-103]. Assuming the availability of targetable and tumor specific antigen, antibody therapeutics are an ideal cancer drug.
Antibodies (IgGs in particular) are capable of recruiting the immune system to attack whatever they bind. The binding of 2 or more IgG1 molecules to the cancer cell surface results in binding of the C1q protein of the complement system which initiates activation of the complement cascade [104]. Activation of the complement system results in the formation of the membrane attack complex which causes membrane pore development and subsequent cell lysis. Complement activated cell lysis can additionally result in recruitment and activation of certain immune effector cells (macrophages, neutrophils, eosinophils etc.) [104,105]. This process is known as complement-dependent cytotoxicity (CDC). Furthermore, bound IgGs can recruit and activate immune cells directly via Fcγ receptor binding. Natural killer (NK) cells, macrophages, dendritic cells, neutrophils, eosinophils, and other immune cells all express various forms of the Fcγ receptor, enabling them to be recruited and activated to induce antibody dependent cellular cytotoxicity (ADCC) and antibody dependent cellular phagocytosis (ADCP) [106,107]. Moreover, in an ideal scenario the initial tumor cell destruction induced by administration of a therapeutic antibody can result in uptake of tumor associated antigens by antigen-presenting cells establishing a prolonged memory effect [96].
Importantly, many mAb therapeutics have intrinsic anti-cancer activity and exerts their effects without the need of immune activation. For example, several tumor associated antigens are growth factor receptors that are overexpressed at the tumor cell surface, and can be one of the driving forces of unregulated cellular growth as well as promote insensitivity and resistance to chemotherapeutic agents [108]. mAb therapeutics that bind such growth factor receptors are often capable of disrupting ligand binding or receptor signaling, which can potentially inhibit cell proliferation and re-sensitize tumor cells to chemotherapeutics [94,96,109].
Some of the first cell surface receptors to be targeted in this fashion are the EGFR family receptors [108]. Antibodies that target these receptors are among the most potent inhibitors of signal transduction. The specific mode of action of these antibodies can vary. Therapeutically used antibodies cetuximab and panitumumab physically block the interaction between the EGF receptor and its ligand via steric hindrance. This prevents the receptor from assuming the conformation required for dimerization and subsequent cell signaling [110-112]. Others, such as pertuzumab and trastuzumab which target the EGFR family member Her2 do not inhibit ligand binding but disrupt the ability for their target receptors to heterodimerize, thereby preventing receptor signaling [113,114]. Essentially every clinically effective, unconjugated mAb disrupts cell signaling of an overexpressed, proliferation-driving cell surface receptor, as opposed to relying strictly on antibody effector function for activity [96]. However, the efficacy of these antibodies is fully explained by a combination of immune activation and the antibody’s intrinsic ability to inhibit growth factor signaling [112,115,116].
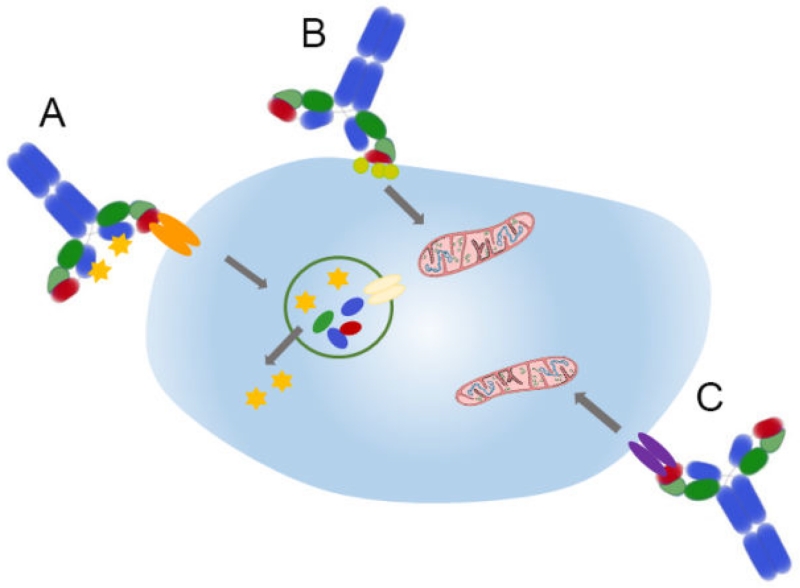
Generally, mechanisms of direct tumor cell killing by antibodies (Figure 1) may include antibody recognition of cell surface bound enzymes to neutralize enzyme activity and signaling. In other cases, antibodies can induce receptor agonist or antagonist activity. The result of both approaches is induction of cellular apoptosis. In another and very direct approach, antibodies are used to deliver drugs to target cells and cause cell death. Such antibody drug conjugates (ADCs) direct cytotoxic compounds to tumor cells after selective binding to cell surface antigens, internalization, and intracellular drug release. In this way, ADCs take advantage of the desirable specificity and pharmacokinetics of immunoglobulins as a means to deliver highly cytotoxic drug payloads [117,118]. This is done by conjugating cytotoxic drugs onto an antibody, so when the antibody binds its target cell and is subsequently internalized, the toxic drug is released and able to kill the cell [119]. ADCs offer significant promise as therapeutics because they are able to deliver highly toxic drugs very specifically to the tumor cells. Thus, untargeted toxicities associated with the use of free drugs are greatly reduced, and poor therapeutic indices associated with conventional chemotherapies can be significantly improved [120]. Many biotech and pharmaceutical companies are researching and pursuing ADCs as potential cancer therapeutics. There are currently over 30 ADC drugs in clinical trials for cancer therapy, in addition to two FDA approved ADCs (Kadcyla and Adcetris) being administered to patients [120-122]. Kadcyla is an ADC of trastuzumab and emtansine, the tubulin binding drug DM1 that potently blocks cell proliferation. This ADC is approved for breast cancer patients with Her2 positive tumours after prior treatment with trastuzumab alone and a taxane. Adcetris is an ADC of anti-CD30 and the highly toxic anti-mitotic agent auristatin E. Adcetris is being used for treatment of classical Hodgkin lymphoma and systemic anaplastic large cell lymphoma after other chemotherapies have failed.
Figure 1.
Mechanisms of direct tumor cell killing by antibodies. A: Antibody drug conjugates deliver cytotoxic drugs after selective binding to tumor cell surface antigens, internalization, and intracellular drug release; B: Antibodies against surface bound enzymes can neutralize enzyme activity and signaling, leading to apoptosis (symbolized by the mitochondrion); C: Antibodies can induce receptor agonist or antagonist activity and thereby cause apoptosis.
There are distinct advantages to ADCs over unconjugated mAbs. Unconjugated mAbs, while they can induce an immune response and often disrupt cancer cell signaling, tend to require combination therapy with conventional chemotherapeutics to be efficacious [123,124]. Additionally, in pre-clinical research and in clinical trials the efficacy of ADCs was shown to be significantly better than unconjugated mAbs (or conventional chemotherapy) [119,125-127]. Importantly, the high specificity of the drug delivery mechanism allows for the application of drugs that would be otherwise too toxic to administer to a cancer patient [125,128-130]. Despite the use of extremely cytotoxic drugs, ADCs have proven to have toxicity profiles better than conventional chemotherapeutics [127,131,132].
The primary drawback to ADCs is their inherent complexity. Each ADC is comprised of at least 3 components: the antibody, a linker, and the drug. Each of these components can be varied, and the efficacy and safety profile of the resulting therapeutic can change drastically [128,133]. An additional confounding factor with the production of ADCs is using the commonly utilized conjugation methods of lysine amidation, or maleimide coupling of cysteines from reduced interchain disulfide bonds, resulting in a heterogeneous mixture of both location and number of conjugates per antibody molecule (usually resulting in a mixture of products comprising between 0 and 8 distinct conjugates) [134-136]. This can negatively affect both the toxicity of the ADC as well as its binding affinity [137-139]. An example is gemtuzumab ozogamicin, a previously FDA approved ADC consisting of anti-CD33 and a calicheamicin as a potent anti-tumor antibiotic. This ADC, tried against acute myelogenous leukemia, used random lysine conjugation and had to be pulled from the market because of toxicity and lack of efficacy [140].
A critical current area of research looking to improve upon some the issues plaguing ADC production and clinical use, is site-specific conjugation. Site-specific conjugation allows for control over the conjugate:antibody stoichiometry and the location of conjugation, which enables the generation of a homogenous product [140-142]. There are three primary methods to accomplish site specific conjugation: engineered cysteine residues, bio-orthogonal reaction with unnatural amino acids, and through enzymatic conjugation with glycotransferases [139,141-143]. Importantly, it has been recently shown that the site location, and conjugate:antibody stoichiometry can have a profound impact on conjugate stability and therapeutic activity of ADCs [144,145]. Thus, use of site-specific conjugation methods allows for optimization of therapeutic activity and toxicity profile of an ADC that would have been otherwise impossible with conventional conjugation chemistries.
Therapeutic antibody-drug conjugates, however, are not the only clinically relevant application of immunoconjugation. Radio-immunconjugates have been used in the treatment and diagnosis of various cancers [146-148]. Oligonucleotide-antibody conjugates have been used in highly sensitive immuno-PCR for the detection of circulating tumor cells (CTCs) or other rare cells, in the generation of highly sensitive antibody arrays, and for the generation of multimeric antibody constructs [149-153]. Bi-specific antibody constructs have been produced via conjugation to either gain synergistic activity against multiple tumor associated antigens, or to recruit and activate cytotoxic T-cells for cancer therapy [154-161]. Antibody conjugation has also been used to circumvent the often procedurally intensive process of antibody selection, by using the conjugated molecule for the specificity, and rely on the antibody scaffold to provide the desired effector function and pharmacokinetics [162-169].
Due to antibody specificity and target selectivity, no single antibody or antibody conjugate will be broadly applicable to cancer treatment. Thus, in order to exploit the extremely high selectivity of therapeutic antibodies, tumor associated antigens must be identified and their exclusive expression on tumours has to be validated to avoid off-target toxicity [170-175]. This is particularly important for ADCs, as these can be extremely effective at eliminating target cells identified by the antibody moiety. Unless a ubiquitous tumor associated antigen is discovered, each different type of cancer requires the development of an antibody specific to cell surface antigens expressed on that cancer. This requirement applies to each individual disease type. For example, breast cancer is a highly heterogeneous disease, with no less than 5 distinct molecular subtypes [176-180]. Only the HER2-enriched subtype currently has an antibody therapeutic as an available treatment (though ER+ breast cancer relies on anti-hormone therapy targeting estrogen as a disease-driving ligand) [181-186]. Breast tumours that do not express the estrogen receptor, the progesterone receptor, and are HER2-negative, are referred to as “triple-negative” which accounts for approximately 15%-20% of diagnosed breast cancers [178,187,188]. Triple negative breast cancer is generally the most aggressive subtype, has a particularly poor outcome, and there are no antibody therapeutics or targeted therapies available to treat this subtype of breast cancer [178,187,189-192]. Considering the need for targeted treatments of otherwise hard-to-manage and particularly aggressive types of cancer in general, identification of antigens specific for those tumor cells would revolutionize cancer therapy.
While there are many techniques for the discovery of antibodies against important disease-associated antigens, finding antibodies that bind defined functional epitopes with high specificity and affinity is not trivial. This is particularly true if one attempts to identify antibodies against an unknown target protein on a target cell type or cell line. Classical hybridoma mAb development approaches require animal immunization, B-cell fusion, clonal screening, and antibody gene cloning and sequencing, before a hybridoma derived mAb can begin preclinical testing as a potential therapeutic [97,193,194]. Additionally, many hybridoma derived mAbs will have to be humanized before clinical application [195]. Antibody display based approaches circumvent many of the issues associated with animal immunization and hybridoma development. Antibody display allows for the direct selection of fully human antibodies from recombinant antibody libraries, and the display systems are designed such that much of the cell and molecular biology associated with hybridoma development is unnecessary [196]. This significant improvement over classical mAb development approaches can be enhanced by smartly designed selection strategies, multiple and diversified rounds of selection, and clonal antibody screening [196-198]. Identification of antibodies with function blocking properties, or selection of antibodies that merely recognize epitopes unique to the target tumor cell type with high selectivity, should enable specific development of targeted antibody therapies that can effectively interfere with cancer progression, eliminate cancer recurrence, or prevent spreading of particularly aggressive cancer cell types. Deep analysis of antibody-antigen relationships, antigen distribution, and identification of patient target groups will lead to the development of novel targeted therapies for many cancer types that urgently require effective treatment approaches.
Acknowledgments
Studies in the Felding lab were supported by NIH grants R01CA170737, R01CA170140, R21CA198595 (to B Felding), CDMRP DoD grants W81XWH-13-1-0401 W81XWH-14-1-0381 (to B Felding), California Breast Cancer Research Program grant 18IB-0022 (to B Felding), and donations from the Plotkin-Weiss Family Foundation and the Bharwani Family.
References
- 1.Hajdu SI. A note from history: landmarks in history of cancer, part 1. Cancer. 2011;117:1097–1102. doi: 10.1002/cncr.25553. [DOI] [PubMed] [Google Scholar]
- 2.Breasted JH. The Edwin Smith Surgical Papyrus. University of Chicago; 1930. pp. 363–369. [Google Scholar]
- 3.Hajdu SI. A note from history: landmarks in history of cancer, part 2. Cancer. 2011;117:2811–2820. doi: 10.1002/cncr.25825. [DOI] [PubMed] [Google Scholar]
- 4.Hajdu SI. A note from history: landmarks in history of cancer, part 3. Cancer. 2012;118:1155–1168. doi: 10.1002/cncr.26320. [DOI] [PubMed] [Google Scholar]
- 5.Papac RJ. Origins of cancer therapy. Yale J Biol Med. 2001;74:391–398. [PMC free article] [PubMed] [Google Scholar]
- 6.Hajdu SI. 2000 years of chemotherapy of tumors. Cancer. 2005;103:1097–1102. doi: 10.1002/cncr.20908. [DOI] [PubMed] [Google Scholar]
- 7.Piperno D. Surgery in De Medicina of Celsus. Ann Chir. 1998;52:568–570. [PubMed] [Google Scholar]
- 8.Grange JM, Stanford JL, Stanford CA. Campbell De Morgan’s ‘Observations on cancer’, and their relevance today. J R Soc Med. 2002;95:296–299. doi: 10.1258/jrsm.95.6.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Morgan C. Considered with Reference to the Treatment of Disease. Churchill; London: 1872. The Origin of Cancer. [Google Scholar]
- 10.De Morgan C. On cancer. Restrospect of Medicine. 1874;69:34–39. [Google Scholar]
- 11.De Morgan C. Observations on cancer. Lancet. 1874:323–329. [Google Scholar]
- 12.Hajdu SI. A note from history: landmarks in history of cancer, part 4. Cancer. 2012;118:4914–4928. doi: 10.1002/cncr.27509. [DOI] [PubMed] [Google Scholar]
- 13.Hajdu SI, Darvishian F. A note from history: landmarks in history of cancer, part 5. Cancer. 2013;119:1450–1466. doi: 10.1002/cncr.27889. [DOI] [PubMed] [Google Scholar]
- 14.Talmadge JE, Fidler IJ. AACR Centennial Series: The Biology of Cancer Metastasis: Historical Perspective. Cancer Research. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrel A. Essential characteristics of a malignant cell. Journal of the American Medical Association. 1925;84:157–158. [Google Scholar]
- 16.Losee JR, Ebeling AH. THE CULTIVATION OF HUMAN SARCOMATOUS TISSUE IN VITRO. J Exp Med. 1914;20:140–148. doi: 10.1084/jem.20.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.STRONG LC. Genetic concept for the origin of cancer: historical review. Ann N Y Acad Sci. 1958;71:810–838. doi: 10.1111/j.1749-6632.1958.tb46811.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 19.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warburg O. Is aerobic glycolysis specific to tumours? Biochemische Zeitschrift. 1929;204:482–483. [Google Scholar]
- 21.Warburg O, Negelein E. On the absorption spectrum of the respiratory enzyme. Biochemische Zeitschrift. 1929;214:64–100. [Google Scholar]
- 22.Warburg O, Negelein E. On the absorption spectrum of the retina respiratory enzyme. Biochemische Zeitschrift. 1929;214:101–106. [Google Scholar]
- 23.Warburg O. Improved method of measurement of respiration and glycolosis. Biochemische Zeitschrift. 1924;152:51–63. [Google Scholar]
- 24.Warburg O. On the metabolism of cancer cells. Naturwissenschaften. 1924;12:1131–1137. [Google Scholar]
- 25.Foo SS. Molecular imaging of tumour hypoxia in non-small cell lung cancer (NSCLC) with F-18-fluoromisonidazole positron emission tomography (F-18-FMISO PET) Journal of Nuclear Medicine. 2003;44:133–134. [Google Scholar]
- 26.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 27.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 28.Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, et al. In vivo validation of 3 ′ deoxy-3 ′-[F-18]fluorothymidine ([F-18]FLT) as a proliferation imaging tracer in humans: Correlation of [F-18]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clinical Cancer Research. 2002;8:3315–3323. [PubMed] [Google Scholar]
- 29.Wahl RL, Hutchins GD, Buchsbaum DJ, Liebert M, Grossman HB, et al. 18f-2-Deoxy-2-Fluoro-D-Glucose Uptake into Human Tumor Xenografts - Feasibility Studies for Cancer Imaging with Positron-Emission Tomography. Cancer. 1991;67:1544–1550. doi: 10.1002/1097-0142(19910315)67:6<1544::aid-cncr2820670614>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.WARBURG O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 31.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 32.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 34.Santidrian AF, Matsuno-Yagi A, Ritland M, Seo BB, LeBoeuf SE, et al. Mitochondrial complex I activity and NAD(+)/NADH balance regulate breast cancer progression. Journal of Clinical Investigation. 2013;123:1068–1081. doi: 10.1172/JCI64264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25:4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 36.Carew JS, Zhou Y, Albitar M, Carew JD, Keating MJ, et al. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia. 2003;17:1437–1447. doi: 10.1038/sj.leu.2403043. [DOI] [PubMed] [Google Scholar]
- 37.Kulawiec M, Owens KM, Singh KK. Cancer cell mitochondria confer apoptosis resistance and promote metastasis. Cancer Biology & Therapy. 2009;8:1378–1385. doi: 10.4161/cbt.8.14.8751. [DOI] [PubMed] [Google Scholar]
- 38.Ma YW, Bai RK, Trieu R, Wong LJ. Mitochondrial dysfunction in human breast cancer cells and their transmitochondrial cybrids. Biochimica Et Biophysica Acta-Bioenergetics. 2010;1797:29–37. doi: 10.1016/j.bbabio.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrella P, Xiao Y, Fliss M, Sanchez-Cespedes M, Mazzarelli P, et al. Detection of mitochondrial DNA mutations in primary breast cancer and fine-needle aspirates. Cancer Res. 2001;61:7623–7626. [PubMed] [Google Scholar]
- 40.Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci U S A. 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20:291–293. doi: 10.1038/3108. [DOI] [PubMed] [Google Scholar]
- 42.Shen L, Wei J, Chen T, He J, Qu J, et al. Evaluating mitochondrial DNA in patients with breast cancer and benign breast disease. Journal of Cancer Research and Clinical Oncology. 2011;137:669–675. doi: 10.1007/s00432-010-0912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 44.Samudio I, Fiegl M, Andreeff M. Mitochondrial Uncoupling and the Warburg Effect: Molecular Basis for the Reprogramming of Cancer Cell Metabolism. Cancer Research. 2009;69:2163–2166. doi: 10.1158/0008-5472.CAN-08-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frezza C, Gottlieb E. Mitochondria in cancer: not just innocent bystanders. Semin Cancer Biol. 2009;19:4–11. doi: 10.1016/j.semcancer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Isidoro A, Casado E, Redondo A, Acebo P, Espinosa E, et al. Breast carcinomas fulfill the Warburg hypothesis and provide metabolic markers of cancer prognosis. Carcinogenesis. 2005;26:2095–2104. doi: 10.1093/carcin/bgi188. [DOI] [PubMed] [Google Scholar]
- 47.Bruns P. Die Heilwirkung des Erysipels auf Geschwulste. Beitr Kiln Chir. 1887;3:443–446. [Google Scholar]
- 48.Coley WB. The Treatment of Malignant-Tumors by Repeated Inoculations of Erysipelas - with a Report of 10 Original Cases. Clinical Orthopaedics and Related Research. 1991;262:3–11. [PubMed] [Google Scholar]
- 49.Coley WB. Treatment of inoperable malignant tumour with the toxins of erysipelas and Bacillus prodigiosus. Trans Am Surg Assoc. 1894;12:183–196. [Google Scholar]
- 50.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- 51.Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64:529–564. doi: 10.1016/0163-7258(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 52.Nauts HC, Fowler GA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley’s mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl. 1953;276:1–103. [PubMed] [Google Scholar]
- 53.Nauts HC, McLaren JR. Coley toxins--the first century. Adv Exp Med Biol. 1990;267:483–500. doi: 10.1007/978-1-4684-5766-7_52. [DOI] [PubMed] [Google Scholar]
- 54.Zacharski LR, Sukhatme VP. Coley’s toxin revisited: immunotherapy or plasminogen activator therapy of cancer? J Thromb Haemost. 2005;3:424–427. doi: 10.1111/j.1538-7836.2005.01110.x. [DOI] [PubMed] [Google Scholar]
- 55.Hajdu SI, Vadmal M. A note from history: Landmarks in history of cancer, Part 6. Cancer. 2013;119:4058–4082. doi: 10.1002/cncr.28319. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham TJ, Olson KB, Laffin R, Horton J, Sullivan J. Treatment of advanced cancer with active immunization. Cancer. 1969;24:932–937. doi: 10.1002/1097-0142(196911)24:5<932::aid-cncr2820240510>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 57.FOLEY EJ. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953;13:835–837. [PubMed] [Google Scholar]
- 58.Gold P. Citation Classic - Specific Carcinoembryonic Antigens of the Human Digestive-System. Current Contents/Clinical Practice. 1980;48:10–10. [Google Scholar]
- 59.GRAHAM JB, GRAHAM RM. Autogenous vaccine in cancer patients. Surg Gynecol Obstet. 1962;114:1–4. [PubMed] [Google Scholar]
- 60.Gross L. Intradermal immunization of C3H mice against a sarcoma that originated in an animal of the same line. Cancer Research. 1943;3:326–333. [Google Scholar]
- 61.Klein G, Klein E, Clifford P. Search for Host Defenses in Burkitt Lymphoma - Membrane Immunofluorescence Tests on Biopsies and Tissue Culture Lines. Cancer Research. 1967;27:2510–2520. [PubMed] [Google Scholar]
- 62.Laszlo J, Buckley CE, 3rd, Amos DB. Infusion of isologous immune plasma in chronic lymphocytic leukemia. Blood. 1968;31:104–110. [PubMed] [Google Scholar]
- 63.Mathé G, Amiel JL, Schwarzenberg L, Schneider M, Cattan A, et al. Active immunotherapy for acute lymphoblastic leukaemia. Lancet. 1969;1:697–699. doi: 10.1016/s0140-6736(69)92648-8. [DOI] [PubMed] [Google Scholar]
- 64.Mathe G, Amiel JL, Schwarzenberg L, Schneider M, Hayat M, et al. Preliminary Result of a New Protocol for Active Immunotherapy of Acute Lymphoblastic Leukaemia - Inhibition of Immunotherapeutic Effect by Vincristine or Adamantadine. Revue Europeenne D Etudes Cliniques Et Biologique. 1971;16:216–224. [PubMed] [Google Scholar]
- 65.Morton DL, Malmgren RA, Holmes EC, Ketcham AS. Demonstration of antibodies against human malignant melanoma by immunofluorescence. Surgery. 1968;64:233–240. [PubMed] [Google Scholar]
- 66.Murray G. Experiments in Immunity in Cancer. Journal of Bone and Joint Surgery. American Volume. 1959;41:759–759. [Google Scholar]
- 67.Nadler SH, Moore GE. Immunotherapy of malignant disease. Arch Surg. 1969;99:376–381. doi: 10.1001/archsurg.1969.01340150084016. [DOI] [PubMed] [Google Scholar]
- 68.Old LJ, Boyse EA, Clarke DA, Carswell EA. Antigenic Properties of Chemically Induced Tumors. Annals of the New York Academy of Sciences. 1962;101:80–106. [Google Scholar]
- 69.WOODRUFF MF, NOLAN B. PRELIMINARY OBSERVATIONS ON TREATMENT OF ADVANCED CANCER BY INJECTION OF ALLOGENEIC SPLEEN CELLS. Lancet. 1963;2:426–429. doi: 10.1016/s0140-6736(63)92171-8. [DOI] [PubMed] [Google Scholar]
- 70.Takeuchi S. A new look at the history of tumor immunotherapy--for its fruitful future through overcoming the widespread cynicism. Hum Cell. 1996;9:1–10. [PubMed] [Google Scholar]
- 71.Biddle A, Mackenzie IC. Cancer stem cells and EMT in carcinoma. Cancer and Metastasis Reviews. 2012;31:285–293. doi: 10.1007/s10555-012-9345-0. [DOI] [PubMed] [Google Scholar]
- 72.Coleman MP, Forman D, Bryant H, Butler J, Ravhet B, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jemal A, Center MM, DeSantis C, Ward EM. Global Patterns of Cancer Incidence and Mortality Rates and Trends. Cancer Epidemiology Biomarkers & Prevention. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 74.Chiao PJ, Bischoff FZ, Strong LC, Tainsky MA. The current state of oncogenes and cancer: experimental approaches for analyzing oncogenetic events in human cancer. Cancer Metastasis Rev. 1990;9:63–80. doi: 10.1007/BF00047589. [DOI] [PubMed] [Google Scholar]
- 75.Early Breast Cancer Trialists’ Collaborative Group Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. No authors listed. [PubMed] [Google Scholar]
- 76.Green ED, Guyer MS, National Human Genome Research Institute Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470:204–213. doi: 10.1038/nature09764. [DOI] [PubMed] [Google Scholar]
- 77.Khalkhali I, Mena I, Diggles L. Review of imaging techniques for the diagnosis of breast cancer: a new role of prone scintimammography using technetium-99m sestamibi. Eur J Nucl Med. 1994;21:357–362. doi: 10.1007/BF00947973. [DOI] [PubMed] [Google Scholar]
- 78.Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 79.Lander ES. Initial impact of the sequencing of the human genome. Nature. 2011;470:187–197. doi: 10.1038/nature09792. [DOI] [PubMed] [Google Scholar]
- 80.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 81.Sawaya GF, Brown AD, Washington AE, Garber AM. Clinical practice. Current approaches to cervical-cancer screening. N Engl J Med. 2001;344:1603–1607. doi: 10.1056/NEJM200105243442107. [DOI] [PubMed] [Google Scholar]
- 82.Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–297. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 83.Scheuer L, Kauff N, Robson M, Kelly B, Barakat R, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260–1268. doi: 10.1200/JCO.2002.20.5.1260. [DOI] [PubMed] [Google Scholar]
- 84.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 85.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A. 2008;105:967–972. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, et al. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol. 1997;15:637–640. doi: 10.1038/nbt0797-637. [DOI] [PubMed] [Google Scholar]
- 87.Kacskovics I, Kis Z, Mayer B, West AP, Jr, Tiangco NE, et al. FcRn mediates elongated serum half-life of human IgG in cattle. Int Immunol. 2006;18:525–536. doi: 10.1093/intimm/dxh393. [DOI] [PubMed] [Google Scholar]
- 88.Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des Devel Ther. 2009;3:7–16. [PMC free article] [PubMed] [Google Scholar]
- 89.Nelson AL, Reichert JM. Development trends for therapeutic antibody fragments. Nat Biotechnol. 2009;27:331–337. doi: 10.1038/nbt0409-331. [DOI] [PubMed] [Google Scholar]
- 90.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- 91.Sarmay G, Lund J, Rozsnyay Z, Gergely J, Jefferis R. Mapping and comparison of the interaction sites on the Fc region of IgG responsible for triggering antibody dependent cellular cytotoxicity (ADCC) through different types of human Fc gamma receptor. Mol Immunol. 1992;29:633–639. doi: 10.1016/0161-5890(92)90200-h. [DOI] [PubMed] [Google Scholar]
- 92.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 93.Strohl WR. Optimization of Fc-mediated effector functions of monoclonal antibodies. Curr Opin Biotechnol. 2009;20:685–691. doi: 10.1016/j.copbio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 94.Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- 95.Rosenberg SA, Terry WD. Passive immunotherapy of cancer in animals and man. Adv Cancer Res. 1977;25:323–388. doi: 10.1016/s0065-230x(08)60637-5. [DOI] [PubMed] [Google Scholar]
- 96.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 97.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 98.Badger CC, Anasetti C, Davis J, Bernstein ID. Treatment of malignancy with unmodified antibody. Pathol Immunopathol Res. 1987;6:419–434. doi: 10.1159/000157067. [DOI] [PubMed] [Google Scholar]
- 99.Khazaeli MB, Conry RM, LoBuglio AF. Human immune response to monoclonal antibodies. J Immunother Emphasis Tumor Immunol. 1994;15:42–52. doi: 10.1097/00002371-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 100.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 101.Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7:1401–1413. doi: 10.1517/14712598.7.9.1401. [DOI] [PubMed] [Google Scholar]
- 102.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys. 2012;526:159–166. doi: 10.1016/j.abb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 103.Phipps RP, Mitchell GF, Mandel TE, Tew JG. Antibody isotypes mediating antigen retention in passively immunized mice. Immunology. 1980;40:459–466. [PMC free article] [PubMed] [Google Scholar]
- 104.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 105.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 106.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 107.Strohl WR. Optimization of Fc-mediated effector functions of monoclonal antibodies. Curr Opin Biotechnol. 2009;20:685–691. doi: 10.1016/j.copbio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 108.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 109.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li SQ, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 111.Sunada H, Magun BE, Mendelsohn J, Macleod CL. Monoclonal-Antibody against Epidermal Growth-Factor Receptor Is Internalized without Stimulating Receptor Phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:3825–3829. doi: 10.1073/pnas.83.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PH, et al. Human IgG2 Antibodies against Epidermal Growth Factor Receptor Effectively Trigger Antibody-Dependent Cellular Cytotoxicity but, in Contrast to IgG Only by Cells of Myeloid Lineage. Journal of Immunology. 2010;184:512–520. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- 113.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 114.Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer and Metastasis Reviews. 2005;24:487–499. doi: 10.1007/s10555-005-6192-2. [DOI] [PubMed] [Google Scholar]
- 116.Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist. 2007;12:1084–1095. doi: 10.1634/theoncologist.12-9-1084. [DOI] [PubMed] [Google Scholar]
- 117.Wang W, Wang EQ, Balthasar JP. Monoclonal Antibody Pharmacokinetics and Pharmacodynamics. Clinical Pharmacology & Therapeutics. 2008;84:548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 118.Xie H, Blättler WA. In vivo behaviour of antibody-drug conjugates for the targeted treatment of cancer. Expert Opin Biol Ther. 2006;6:281–291. doi: 10.1517/14712598.6.3.281. [DOI] [PubMed] [Google Scholar]
- 119.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 120.Leal M, Sapra P, Hurvitz SA, Senter P, Wahl A, et al. Antibody-drug conjugates: an emerging modality for the treatment of cancer. Ann N Y Acad Sci. 2014;1321:41–54. doi: 10.1111/nyas.12499. [DOI] [PubMed] [Google Scholar]
- 121.Mullard A. Maturing antibody-drug conjugate pipeline hits 30. Nat Rev Drug Discov. 2013;12:329–332. doi: 10.1038/nrd4009. [DOI] [PubMed] [Google Scholar]
- 122.Sassoon I, Blanc V. Antibody-drug conjugate (ADC) clinical pipeline: a review. Methods Mol Biol. 2013;1045:1–27. doi: 10.1007/978-1-62703-541-5_1. [DOI] [PubMed] [Google Scholar]
- 123.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 124.Senter PD. Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol. 2009;13:235–244. doi: 10.1016/j.cbpa.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 125.Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A. 2012;109:16101–16106. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chari RV, Miller ML, Widdison WC. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed Engl. 2014;53:3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- 127.Verma S, Miles D, Gianni L, Krop IE, Welslau M, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Flygare JA, Pillow TH, Aristoff P. Antibody-drug conjugates for the treatment of cancer. Chem Biol Drug Des. 2013;81:113–121. doi: 10.1111/cbdd.12085. [DOI] [PubMed] [Google Scholar]
- 129.Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 130.Maderna A. Discovery of new auristatins for the use on antibody drug conjugates for the treatment of cancer. Abstracts of Papers of the American Chemical Society. 2013:246. [Google Scholar]
- 131.Girish S, Gupta M, Wang B, Lu D, Krop IE, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 2012;69:1229–1240. doi: 10.1007/s00280-011-1817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 133.Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 2010;21:5–13. doi: 10.1021/bc9002019. [DOI] [PubMed] [Google Scholar]
- 134.Lazar AC, Wang L, Blättler WA, Amphlett G, Lambert JM, et al. Analysis of the composition of immunoconjugates using size-exclusion chromatography coupled to mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:1806–1814. doi: 10.1002/rcm.1987. [DOI] [PubMed] [Google Scholar]
- 135.Wang L, Amphlett G, Blättler WA, Lambert JM, Zhang W. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM by mass spectrometry. Protein Sci. 2005;14:2436–2446. doi: 10.1110/ps.051478705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang L, Amphlett G, Lambert JM, Blättler W, Zhang W. Structural characterization of a recombinant monoclonal antibody by electrospray time-of-flight mass spectrometry. Pharm Res. 2005;22:1338–1349. doi: 10.1007/s11095-005-5267-7. [DOI] [PubMed] [Google Scholar]
- 137.Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10:7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 138.Junutula JR, Bhakta S, Raab H, Ervin KE, Eigenbrot C, et al. Rapid identification of reactive cysteine residues for site-specific labeling of antibody-Fabs. J Immunol Methods. 2008;332:41–52. doi: 10.1016/j.jim.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 139.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26:925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 140.Kim CH, Axup JY, Schultz PG. Protein conjugation with genetically encoded unnatural amino acids. Curr Opin Chem Biol. 2013;17:412–419. doi: 10.1016/j.cbpa.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hallam TJ, Smider VV. Unnatural amino acids in novel antibody conjugates. Future Med Chem. 2014;6:1309–1324. doi: 10.4155/fmc.14.79. [DOI] [PubMed] [Google Scholar]
- 142.Panowksi S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. MAbs. 2014;6:34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu Z, Ramakrishnan B, Li J, Wang Y, Feng Y, et al. Site-specific antibody-drug conjugation through an engineered glycotransferase and a chemically reactive sugar. MAbs. 2014;6:1190–1200. doi: 10.4161/mabs.29889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shen BQ, Xu K, Liu L, Raab H, Bhakta S, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30:184–189. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- 145.Agarwal P, Kudirka R, Albers AE, Barfield RM, de Hart GW, et al. Hydrazino-Pictet-Spengler ligation as a biocompatible method for the generation of stable protein conjugates. Bioconjug Chem. 2013;24:846–851. doi: 10.1021/bc400042a. [DOI] [PubMed] [Google Scholar]
- 146.Kraeber-Bodéré F, Bodet-Milin C, Rousseau C, Eugène T, Pallardy A, et al. Radioimmunoconjugates for the treatment of cancer. Semin Oncol. 2014;41:613–622. doi: 10.1053/j.seminoncol.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 147.Medley CD, Gruenhagen J, Yehl P, Chetwyn NP. Detection of residual biocides in antibody drug conjugates for ImmunoPET imaging. Analytical Methods. 2014;6:6635–6640. [Google Scholar]
- 148.Melendez-Alafort L. New Tc-99m-radioimmunoconjugates for pancreatic carcinoma detection. Nuclear Medicine and Biology. 2014;41:624–624. [Google Scholar]
- 149.Bailey RC, Kwong GA, Radu CG, Witte ON, Heath JR. DNA-encoded antibody libraries: a unified platform for multiplexed cell sorting and detection of genes and proteins. J Am Chem Soc. 2007;129:1959–1967. doi: 10.1021/ja065930i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hutchins BM, Kazane SA, Staflin K, Forsyth JS, Felding-Habermann B, et al. Selective formation of covalent protein heterodimers with an unnatural amino acid. Chem Biol. 2011;18:299–303. doi: 10.1016/j.chembiol.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kazane SA, Sok D, Cho EH, Uson ML, Kuhn P, et al. Site-specific DNA-antibody conjugates for specific and sensitive immuno-PCR. Proc Natl Acad Sci U S A. 2012;109:3731–3736. doi: 10.1073/pnas.1120682109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ladd J, Boozer C, Yu Q, Chen S, Homola J, et al. DNA-directed protein immobilization on mixed self-assembled monolayers via a streptavidin bridge. Langmuir. 2004;20:8090–8095. doi: 10.1021/la049867r. [DOI] [PubMed] [Google Scholar]
- 153.Kazane SA, Axup JY, Kim CH, Ciobanu M, Wold ED, et al. Self-assembled antibody multimers through peptide nucleic acid conjugation. J Am Chem Soc. 2013;135:340–346. doi: 10.1021/ja309505c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim CH, Axup JY, Lawson BR, Yun H, Tardif V, et al. Bispecific small molecule-antibody conjugate targeting prostate cancer. Proc Natl Acad Sci U S A. 2013;110:17796–17801. doi: 10.1073/pnas.1316026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kranz DM, Manning TC, Rund LA, Cho BK, Gruber MM, et al. Targeting tumor cells with bispecific antibodies and T cells. J Control Release. 1998;53:77–84. doi: 10.1016/s0168-3659(97)00239-3. [DOI] [PubMed] [Google Scholar]
- 156.Kularatne SA, Deshmukh V, Gymnopoulos M, Biroc SL, Xia J, et al. Recruiting cytotoxic T cells to folate-receptor-positive cancer cells. Angew Chem Int Ed Engl. 2013;52:12101–12104. doi: 10.1002/anie.201306866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmacol Ther. 2012;136:334–342. doi: 10.1016/j.pharmthera.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 158.Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol. 2013;17:385–392. doi: 10.1016/j.cbpa.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 159.Friedman M, Lindström S, Ekerljung L, Andersson-Svahn H, Carlsson J, et al. Engineering and characterization of a bispecific HER2 × EGFR-binding affibody molecule. Biotechnol Appl Biochem. 2009;54:121–131. doi: 10.1042/BA20090096. [DOI] [PubMed] [Google Scholar]
- 160.Müller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs. 2010;24:89–98. doi: 10.2165/11530960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 161.Rossi DL, Rossi EA, Cardillo TM, Goldenberg DM, Chang CH., 2 A new class of bispecific antibodies to redirect T cells for cancer immunotherapy. MAbs. 2014;6:381–391. doi: 10.4161/mabs.27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bower KE, Lam SN, Oates BD, Del Rosario JR, Corner E, et al. Evolution of Potent and Stable Placental-Growth-Factor-1-Targeting CovX-Bodies from Phage Display Peptide Discovery. Journal of Medicinal Chemistry. 2011;54:1256–1265. doi: 10.1021/jm101226k. [DOI] [PubMed] [Google Scholar]
- 163.Coronella J, Li L, Johnson K, Shepherd SP, Murphy R, et al. Selective Activity Against Proliferating Tumor Endothelial Cells by CVX-2 A Thrombospondin-1 Mimetic CovX-Body (TM) Anticancer Research. 2009;29:2243–2252. [PubMed] [Google Scholar]
- 164.Doppalapudi VR, Tryder N, Li L, Aja T, Griffith D, et al. Chemically programmed antibodies: Endothelin receptor targeting CovX-Bodies (TM) Bioorganic & Medicinal Chemistry Letters. 2007;17:501–506. doi: 10.1016/j.bmcl.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 165.Li L, Leedom TA, Do J, Huang H, Lai J, et al. Antitumor efficacy of a thrombospondin 1 mimetic CovX-body. Transl Oncol. 2011;4:249–257. doi: 10.1593/tlo.11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li LS, Rader C, Matsushita M, Das S, Barbas CF, 3rd, et al. Chemical adaptor immunotherapy: design, synthesis, and evaluation of novel integrin-targeting devices. J Med Chem. 2004;47:5630–5640. doi: 10.1021/jm049666k. [DOI] [PubMed] [Google Scholar]
- 167.Popkov M, Rader C, Gonzalez B, Sinha SC, Barbas CF., 3rd Small molecule drug activity in melanoma models may be dramatically enhanced with an antibody effector. Int J Cancer. 2006;119:1194–1207. doi: 10.1002/ijc.21924. [DOI] [PubMed] [Google Scholar]
- 168.Rader C, Turner JM, Heine A, Shabat D, Sinha SC, et al. A humanized aldolase antibody for selective chemotherapy and adaptor immunotherapy. Journal of Molecular Biology. 2003;332:889–899. doi: 10.1016/s0022-2836(03)00992-6. [DOI] [PubMed] [Google Scholar]
- 169.Li H, Lu Y, Piao L, Wu J, Yang X, et al. Folate-immunoglobulin G as an anticancer therapeutic antibody. Bioconjug Chem. 2010;21:961–968. doi: 10.1021/bc900545h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Abulkhair OAM, Gasmelseed A, AL Otaibi S. Trastuzumab associated cardiotoxicity: Who is at risk? Journal of Clinical Oncology. 2013;31:15. [Google Scholar]
- 171.Altena R, Boekhout AH. A randomized, pharmacologic intervention study evaluating the effect of the angiotensin II-receptor blocker candesartan versus placebo to prevent trastuzumab-associated cardiotoxicity in women with early breast cancer. Journal of Clinical Oncology. 2010;28:15s. [Google Scholar]
- 172.Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95:1592–1600. doi: 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- 173.Portera CC, Walshe JM, Rosing DR, Denduluri N, Berman AW, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with trastuzumab-insensitive human epidermal growth factor receptor 2-positive metastatic breast cancer. Clinical Cancer Research. 2008;14:2710–2716. doi: 10.1158/1078-0432.CCR-07-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sparano JA. Cardiac toxicity of trastuzumab (Herceptin): implications for the design of adjuvant trials. Semin Oncol. 2001;28:20–27. doi: 10.1016/s0093-7754(01)90189-7. [DOI] [PubMed] [Google Scholar]
- 175.Van Hasselt JG, Boekhout AH, Beijnen JH, Schellens JH, Huitema AD. Population Pharmacokinetic-Pharmacodynamic Analysis of Trastuzumab-Associated Cardiotoxicity. Clinical Pharmacology & Therapeutics. 2011;90:126–132. doi: 10.1038/clpt.2011.74. [DOI] [PubMed] [Google Scholar]
- 176.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 177.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 178.Prat A, Parker JS, Karginova O, Fan C, Livasy C, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fisher B, Dignam J, Wolmark N, Decillius R, Emir B, et al. Tamoxifen and Chemotherapy for Lymph Node-Negative, Estrogen Receptor-Positive Breast Cancer. JNCI Journal of the National Cancer Institute. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 182.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 183.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, et al. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonism of This Interaction by Tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 184.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 185.van de Rijn M, Gilks CB. Applications of microarrays to histopathology. Histopathology. 2004;44:97–108. doi: 10.1111/j.1365-2559.2004.01766.x. [DOI] [PubMed] [Google Scholar]
- 186.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 187.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 188.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 189.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 190.Fan C, Oh DS, Wessels L, Weigelt B, Nuyten DS, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 191.Trivers KF, Lund MJ, Porter PL, Liff JM, Flagg EW, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20:1071–1082. doi: 10.1007/s10552-009-9331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 193.Jin A, Ozawa T, Tajiri K, Obata T, Kondo S, et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody-secreting cells from human peripheral blood. Nat Med. 2009;15:1088–1092. doi: 10.1038/nm.1966. [DOI] [PubMed] [Google Scholar]
- 194.Love JC, Ronan JL, Grotenbreg GM, van der Veen AG, Ploegh HL. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nature Biotechnology. 2006;24:703–707. doi: 10.1038/nbt1210. [DOI] [PubMed] [Google Scholar]
- 195.Clark M. Antibody humanization: a case of the ‘Emperor’s new clothes’? Immunol Today. 2000;21:397–402. doi: 10.1016/s0167-5699(00)01680-7. [DOI] [PubMed] [Google Scholar]
- 196.Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–1116. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- 197.Levitan B. Stochastic modeling and optimization of phage display. J Mol Biol. 1998;277:893–916. doi: 10.1006/jmbi.1997.1555. [DOI] [PubMed] [Google Scholar]
- 198.Mao H, Graziano JJ, Chase TM, Bentley CA, Bazirgan OA, et al. Spatially addressed combinatorial protein libraries for recombinant antibody discovery and optimization. Nat Biotechnol. 2010;28:1195–1202. doi: 10.1038/nbt.1694. [DOI] [PubMed] [Google Scholar]