Abstract
Endogenous thiols undergo rapid and reversible oxidation to disulfides when exposed to oxidants and are, therefore, suitable biomarkers of oxidative stress. However, accurate analysis of thiols in blood is frequently compromised by their artifactual oxidation during sample manipulation, which spuriously elevates the disulfide levels. Here, we describe a validated pre-analytical procedure that prevents both artifactual oxidation of thiols during sample manipulation and their oxidative decay for months in biosamples that are stored at −80°C. Addition of N-ethylmaleimide to blood samples from healthy donors was used to stabilize whole blood, red blood cells, platelets and plasma disulfides, whereas addition of citrate buffer followed by dilution of plasma with H2O was used to stabilize plasma thiols. The concentrations of thiols and disulfides were stable in all biosamples for at least 6 months when analyzed by UV/Vis HPLC at regular intervals. Only 3 ml of blood were needed to perform the analyses of thiols and disulfides in the different blood fractions. This pre-analytical procedure is reliable for use in both animal and human prospective studies. Its ease of implementation makes the method suitable for application to multicenter studies where blood samples are collected by different sites and personnel and are shipped to specific specialized laboratories.
Keywords: Thiols, Disulfides, Blood, Red blood cells, Platelets, Plasma
1. Introduction
The sulfhydryl group (-SH), unless masked within proteins, is the most reactive chemical functionality in cells with a unique ability to undergo very rapid and readily reversible oxidation to disulfides [1]. Sulfhydryl groups are abundant in proteins (P-SH) - primarily as cysteine (Cys) residues - and in low molecular mass (LMM) molecules - including free Cys and glutathione (GSH), with concentrations and redox states that vary substantially between the cellular and extracellular compartments. For example, in human red blood cells (RBCs) glutathione is by far the thiol of highest concentration, with ~99.8% (~2.5 mM) occurring in the reduced form (GSH); only ~0.1% of total glutathione is represented by glutathione disulfide (GSSG) [2,3] and just ~0.05% as S-glutathionylated hemoglobin (HbSSG) [4,5]. Conversely, in human plasma cysteine is by far the thiol of highest concentration, but ~90–95% of it circulates in disulfide form, as either cystine (CySS) or S-cysteinylated albumin (CySS-Alb) [6,7]. The concentrations and oxidation percentages of thiols vary as a function of physiological and pathological conditions and, consequently, these molecules have attracted growing interest as markers of oxidative activity and burden in a variety of disease states [8,9].
Thiols, however, are highly reactive not only in vivo but also ex vivo, and analysis of the thiol redox status in whole blood, blood cells and plasma calls for particular attention to sample handling, in order to avoid artifactual oxidation of the -SH group and error in the calculation of the thiol to disulfide ratios. We have previously demonstrated that thiols must be stabilized immediately after blood collection in order to prevent artificial oxidation. In that context we showed that blood treatment with N-ethylmaleimide (NEM), a membrane permeating agent that rapidly alkylates the – SH group, is effective in avoiding ex vivo artifactual oxidation [3,4,10,11].
As an extension of this observation, here, we describe a validated new pre-analytical procedure that allows stabilization of thiols and disulfides and preservation of their chemical characteristics and concentration for several months after sample collection. This procedure enables precise and accurate measurement of thiols and disulfides in whole blood, RBCs, platelets or plasma for at least 6 months after sample collection.
The protocol represents a technical advancement in this line of research since it can be easily applied to single-center or multi-center basic and clinical prospective studies that often involve extended delays between the times of sample collection and of laboratory analysis.
2. Materials and Methods
Monobromobimane (mBrB) was purchased from Calbiochem, Milan, Italy. HPLC grade solvents were purchased from Mallinckrodt-Baker (Milan, Italy). All other reagents were obtained from Sigma-Aldrich, Milan, Italy. Thiol and disulfide standards (GSH, GSSG, Cys, CySS) were at least 98% pure. NEM was prepared as 0.5 ml aliquots of a 310 mM solution in water and stored at −20°C. Citrate buffer pH 4.3 was prepared by mixing 0.5 M sodium citrate with 0.5 M citric acid and stored at −20°C.
2.1 Blood collection for plasma thiol stabilization tests
Blood (3 ml) was collected from 4 healthy donors into vials containing EDTA and 0.3 ml citrate buffer, and immediately centrifuged at 10,000×g for 30s. A fraction of plasma (0.4 ml) was diluted with an equal volume of H2O and 40 µl citrate buffer and stored at −80°C in 0.1 ml aliquots. The remaining plasma was stored undiluted at −80°C in 0.1 ml aliquots. Two milliliters (2 ml) of blood were collected from the same donors into vials containing EDTA and immediately centrifuged at 10,000×g for 30s. Plasma was stored undiluted at −80°C in 0.1 ml aliquots. Low-molecular mass thiols (LMM-SH) were measured in all the samples at the indicated times. Healthy donors granted informed consent to the research protocol.
2.2 Stability study
For the stability study, blood was obtained from 5 healthy donors (age 25–55 years), who gave oral informed consent at the time of enrollment. About 15 ml of peripheral venous blood were collected from the antecubital vein, in EDTA tubes, and then 12 ml were immediately treated with 1.2 ml of 310 mM NEM (NEM-blood).
2.2.1 Analysis of GSH and GSSG in whole blood
One aliquot of NEM-blood (0.2 ml) was immediately deproteinized by addition of 0.15 ml 15% (w/v) trichloroacetic acid (TCA) and freshly analyzed; eleven aliquots of 0.2 ml blood were stored at −80°C for delayed determination at the indicated times.
2.2.2 Analysis of GSH, GSSG and GSSP in RBCs
Five ml of NEM-blood were centrifuged at 8,000×g for 30s, deprived of plasma (used for plasma analysis of disulfides, see below) and washed twice with saline. RBC pellets were then divided into 24 aliquots of 0.075 ml each. One of these was immediately deproteinized by addition of 0.15 ml 15% (w/v) TCA and freshly analyzed for GSH and GSSG content. A second aliquot was used for analyses of S-glutathionylated proteins (GSSP) both in cytoplasm and in membranes after hemolysis by addition of 5 volumes of 5 mM phosphate buffer, pH 6.5, containing 1 mM NEM. The other 22 aliquots were stored at −80°C for delayed determinations at the indicated times.
2.2.3 Analysis of GSH, GSSG and GSSP in platelets
Five ml of blood-NEM were centrifuged at 4,200×g for 40s to obtain platelet-rich plasma (PRP). PRP was then centrifuged at 8,000×g for 30s to remove plasma, washed once with saline, resuspended in 1 ml of saline, counted for platelet number (by a Beckman coulter series Z) and divided into ten 0.1 ml-aliquots. One aliquot was treated with 0.01 ml of 60% (w/v) TCA and immediately used for determination of GSH, GSSG and GSSP content. The remaining aliquots were stored at −80°C for delayed determinations at indicated times.
2.2.4 Analysis of disulfides in plasma
Plasma separated as described above (see RBC paragraph), was divided into 12 aliquots of 0.1 ml each. One of these was freshly mixed with 0.1 ml of 12% (w/v) TCA and analyzed for disulfide content. The other aliquots were stored at −80°C for delayed determinations at indicated times.
2.2.5 Analysis of thiols in plasma
One ml and five hundreds microliters of blood were immediately treated with 0.15 ml citrate buffer solution, tilted 5 times, and centrifuged at 8,000×g for 30s to obtain plasma. Six hundred microliters of plasma were diluted with 0.6 ml H2O and 0.06 ml citrate solution and divided into 12 aliquots of 0.1 ml each. P-SH and LMM-SH content was immediately determined in one aliquot. The other aliquots were stored at −80°C for further determinations at indicated times.
To study the effect of storage on thiol and disulfide levels in samples that were not treated for stabilization, about 2 ml of blood were collected in EDTA tubes and handled as follows: a) for analysis of whole blood, 2 aliquots of 0.2 ml blood were stored at −80°C for further determinations at the indicated times; b) for analysis of RBCs, 0.5 ml of blood were processed as described above for RBCs isolation and then 2 aliquots of 0.075 ml each were stored at −80°C; c) for analysis of platelets, 1 ml of blood was processed as described above for platelet isolation. Platelets were then resuspended in 0.2 ml of saline and divided into two 0.1 ml-aliquots.
2.3 Standard protocol to stabilize thiols/disulfides in whole blood and single blood components
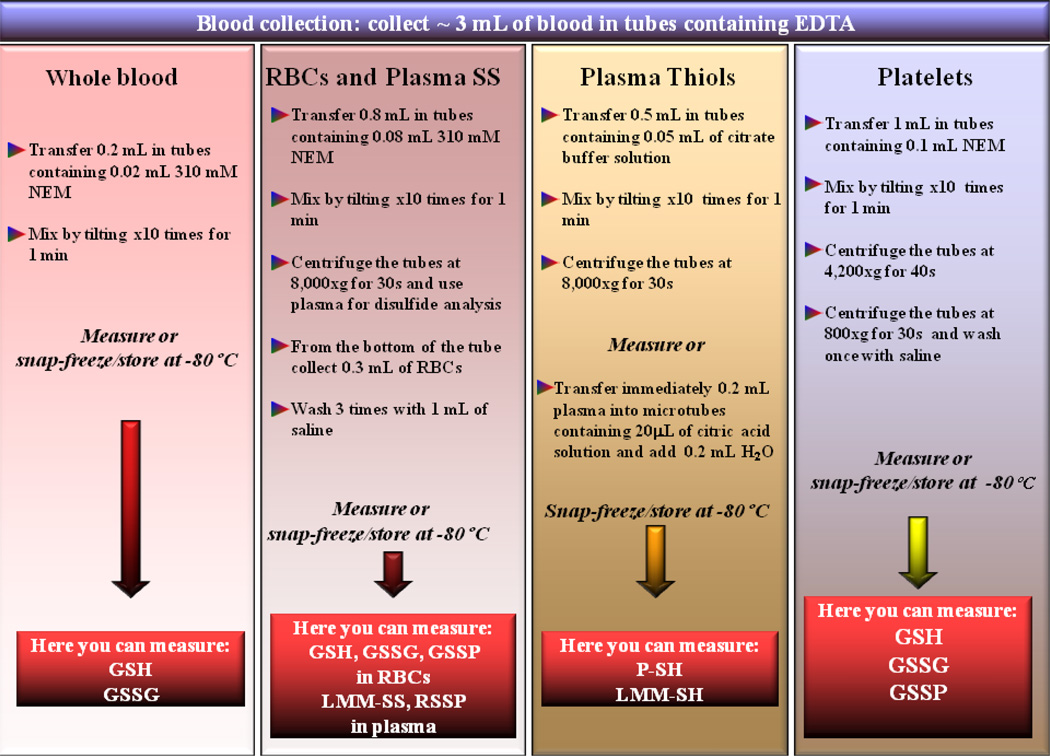
Three ml of blood were collected from 5 healthy people (age 32–49 years) in EDTA Vacutainer tubes, tilted 3–4 times and then immediately treated to stabilize thiols and disulfides. Respective volumes and further details are described in Scheme 1.
Scheme 1. Suggested procedure for thiol and disulfide analysis in whole blood, red blood cells, plasma and platelets.
Whole blood, red blood cell and platelet thiols and disulfides are stabilized by treatment of blood with NEM immediately after the collection. Red blood cells and platelets are then separated and either analyzed or stored at −80°C until analysis. The plasma samples obtained by purification of RBCs, and therefore already treated with NEM, are used for disulfide analyses. Plasma thiols are stabilized by treatment of whole blood with citrate buffer solution. Once plasma is separated, it can be either freshly measured or further diluted with H2O and citrate buffer before its storage at −80°C.
2.4 Validation of the procedure
The accuracy, precision, and recovery of the above methods were tested in both RBCs and plasma. For this purpose, RBCs were hemolyzed with water (1:3, v/v), LMM-SH were removed by gel filtration (Pharmacia PD10 columns, equilibrated with 50 mM Na+/K+ phosphate buffer, pH 7.4), and then reconstituted to the initial hemoglobin (Hb) concentration by ultrafiltering excess solvent. Aliquots of the hemolyzed RBC preparation were then spiked with stock solutions of GSH, GSSG and HbSSG to the desired final concentrations, followed by addition of 30 mM NEM (final concentration). After 1-min incubation, the samples were analyzed for GSH, GSSG and HbSSG content. Sample preparation and analysis were performed on 3 separate days.
Plasma was dialyzed (30 kDa cut-off membrane tubes) against excess volume of 50 mM Na+/K+ phosphate buffer, pH 7.4 with 5 mM GSH, at room temperature for 10 h. The plasma was then dialyzed against the same buffer but without GSH, for 24 hours with fresh buffer exchanges every 8 h. Finally, the plasma was stabilized with citrate buffer (10:1 v:v) and then spiked with stock solutions of Cys, CySS, or CySS-Alb to the desired final concentrations. The precision of the method is expressed as the relative standard deviation (RSD) and the accuracy is expressed as relative error (RE) [(mean observed concentration − spiked concentration)/(spiked concentration)] × 100%.
2.5 Measurement of GSH, GSSG and thiolated proteins in whole blood, RBCs and platelets
Analysis of GSH and GSSG in whole blood, RBCs and platelets was carried out in the clear supernatants obtained by acidifying the samples with TCA followed by centrifugation as described above. In samples without NEM, both the alkylating agent and TCA were added just after thawing. The GSH-NEM conjugate was measured by HPLC with UV/Vis detector [2], whereas GSSG was measured by the GSH recycling method with some modifications [11] after NEM extraction with dichloromethane.
For the analysis of GSSP in RBCs, hemolyzed erythrocytes (see above) were centrifuged at 20,000×g for 10 min to pellet membranes. Analysis of GSSP was carried out in supernatants and pellets by incubating samples with dithiothreitol (DTT) and then the released GSH was labeled by reaction with mBrB. HPLC analysis was performed on the clear supernatants after deproteination [5,12]. Platelet GSSP were measured by the same method [13]. Further technical details are given in the supplemental section.
2.6 Measurement of thiols, disulfides and thiolated proteins in plasma
Protein thiols were measured by colorimetric reaction with Ellman’s reagent (DTNB) [14] with some modifications. Briefly, 0.05 ml plasma samples, that had been treated with stabilizing solution (see above), were mixed with 0.8 ml of 200 mM Na2HPO4 and then spiked with 10 µl of 20 mM DTNB while continuously recording the colorimetric reaction on a spectrophotometer at 410 nm, until achievement of the absorbance plateau.
LMM-SH analysis was performed by labeling with the fluorescent reagent mBrB and separation by HPLC [15]. Low molecular mass disulfides (LMM-SS) and S-thiolated protein (RSSP) analysis was carried out on NEM pre-treated samples by labeling thiols with the fluorescent reagent mBrB after reduction of disulfide bond with DTT [15]. Further technical details are given in the supplemental section.
All measurements were carried out with an Agilent series 1100 HPLC equipped with a 4.6 mm × 150 mm, 5 µm Zorbax Eclipse XDB C18 column (Agilent Technologies, Cernusco sul Naviglio, MI, Italy).
2.7 Preparation of HbSSG and CySS-Alb standard solutions
HbSSG and CySS-Alb standard solutions were obtained by mixing different proportion of completely reduced protein and completely S-thiolated protein. Completely reduced Hb and completely reduced albumin (Alb) were prepared by incubating the proteins with GSH, followed by removal of the excess of GSH by dialysis. The absence of S-thiolation was verified by HPLC as described above. The presence of only one –SH group per Alb molecule and the absence of GSH in the completely reduced Alb preparation were verified by means of the DTNB assay for thiols. Completely S-glutathionylated Hb was obtained by reacting the protein with GSSG. Completely S-cysteinylated Alb was obtained by reacting it with CySS. The efficiency of S-thiolation was confirmed by the disappearance of free –SH groups evidenced by spectrophotometric titration with DTNB. Further technical details are given in the supplemental section.
The concentration of HbSSG and CySS-Alb was determined by HPLC as described above.
2.8 Hemoglobin and protein content
Hb concentration was measured in an aliquot of haemolyzed RBCs by the cyanomethemoglobin method [16].
2.9 Statistics
Data are expressed as mean±SD. Differences between means were evaluated using Student’s t test. A value of p < 0.05 was considered statistically significant.
3. Results
3.1 Stabilization of thiols and disulfides in whole blood, RBCs, platelets and plasma
In whole blood, RBCs and platelets, thiols and disulfides were stabilized by adding NEM to whole blood before any further pre-analytical procedure [11]. Conversely, in plasma, thiols were initially stabilized by lowering the pH to about 5 with the addition of citrate buffer according to Williams et al. [17]. In fact, it is known that plasma thiols, if untreated, are quite unstable, even if stored at −80°C ([17] and Supplemental Figure 1). We observed, however, that the original procedure [17] stabilizes thiols only for a few days, while a modified procedure that combines acidification with further sample dilution extends thiol stability for months. As shown in Supplemental Figure 1, the original treatment with citrate buffer is only suitable for thiol measurement in fresh plasma or only after a storage of a few days since Cys concentration decreased significantly after one week storage at −80°. Similar observations were also made for the other plasma LMM-SH (data not shown). Conversely, the modified stabilizing procedure maintained unaltered plasma thiol levels in samples that were stored for more than six months.
3.2 Analysis of thiols and disulfides in whole blood, RBCs, platelets and plasma and validation of the procedure
The procedure summarized in Scheme 1 allows to measure either immediately or after prolonged storage the thiols and disulfides of whole blood, RBCs and platelets in 3 ml of blood. NEM-treated blood was used to measure both intracellular thiols and disulfides and plasma disulfides. Instead, citrate-treated plasma was used to measure plasma thiols that, under these conditions, were greatly stabilized. Data obtained by applying this procedure in 5 healthy donors are shown in Tables 1 and 2. Recovery, precision and accuracy were tested on hemolyzed RBC preparations spiked with three concentrations of GSH, GSSG and HbSSG, and on dialyzed plasma preparations spiked with three concentrations of Cys, CySS and CySS-Alb. As shown in Supplemental Table 1, recovery of the added reagents was not different from 100%. Intra- and inter-day precision (RSD, %) was well below 5% for the added reagents, while intra- and inter-day accuracy (RE, %) respectively ranged from −1.35% to 2.34% and from −2.84% to 1.63%.
Table 1. Basal levels of thiols and disulfides in human whole blood, red blood cells and platelets.
Human blood collected from healthy donors was treated with N-ethylmaleimide and then the blood components were separated for analyses of glutathione (GSH), glutathione disulfide (GSSG), cytoplasmic glutahionylayed proteins (GSSP) and GSSP in red blood cell membranes.
| Sample | GSH nmol/mg Hb or nmol/109 plt |
GSSG pmol/mg Hb or nmol/109 plt |
GSSP pmol/mg Hb or nmol/109 plt |
Membrane GSSP pmol/mg protein |
|---|---|---|---|---|
| Whole Blood | 8.53±0.76 | 11.0±0.8 | 9.77±1.02 | |
| RBCs | 8.48±0.69 | 11.7±0.9 | 10.5±1.00 | 0.844±0.023 |
| Platelets | 13.4 ±2.64 | 0.23±0.04 | 0.046±0.07 |
Data are the mean±SD; n= 5.
Table 2. Basal levels of thiols and disulfides in human plasma.
Human blood collected from healthy donors and treated with N-ethylmaleimide was used to measure low molecular mass disulfides (LMM-SS) and S-thiolated proteins (RSSP). For low molecular mass thiol (LMM-SH) and protein thiol (P-SH) analysis blood diluted with citrate/H2O was used.
| Sample | Cys | CysGly | Hcys | GSH | P-SH |
|---|---|---|---|---|---|
| LMM-SH/P-SH | 12.2±0.6 | 1.25±0.39 | 0.221±0.010 | 1.86±0.11 | 384±23 |
| LMM-SS | 62.4±3.2 | 4.86±0.68 | 0.912±0.073 | 1.58±0.36 | - |
| RSSP | 128±21 | 11.0±0.8 | 4.66±0.75 | 2.61±0.12 | - |
Data are the mean±SD and are expressed as µM; n= 5.
3.3 Study of stability in blood
-Whole blood
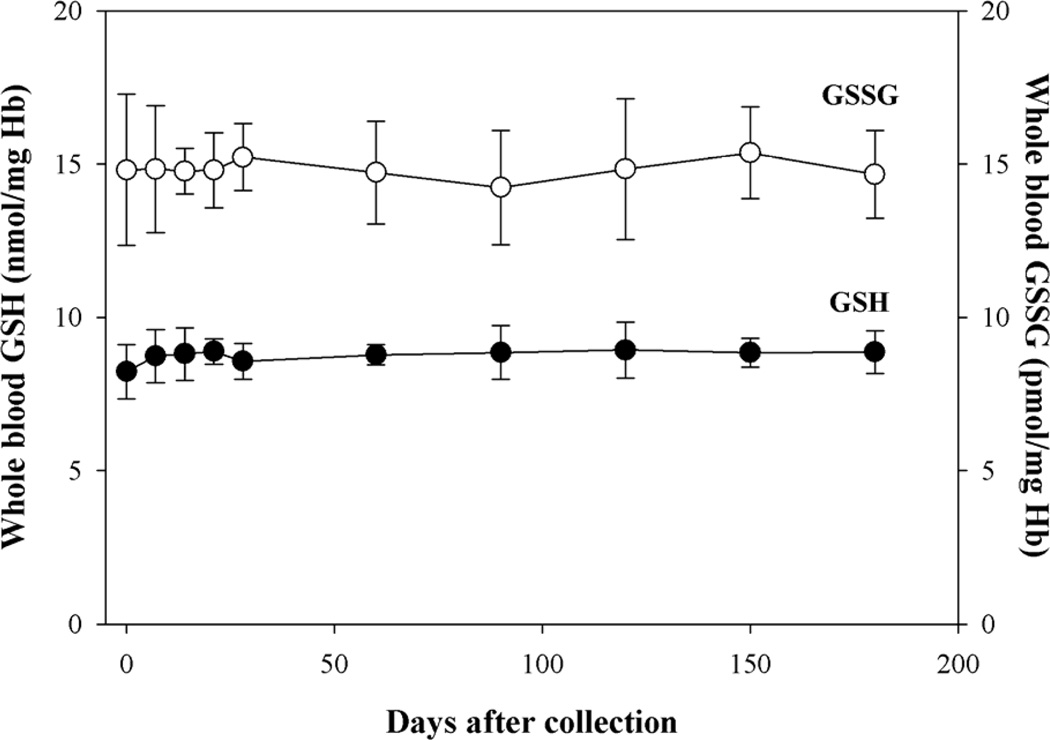
As shown in Figure 1, GSH and GSSG levels were stable for 6 months in whole blood samples from healthy adults that were stored at −80°C and were tested weekly for one month and monthly thereafter for five months. Some of the samples showed a slight decrease in GSH levels after the initial 6 months (not shown), but this change was not further explored since usually there is not any evident practical need to analyze experimental samples more than six months after collection.
Figure 1. Stability of GSH and GSSG in whole blood.
Blood was treated with NEM immediately after the collection. After 1 min, aliquots of sample were stored at −80°C. At the indicated times samples were deproteinized by addition of TCA and then GSH and GSSG were measured on the clear supernatant by HPLC and GSH recycling method, respectively. n= 5.
-RBCs and platelets
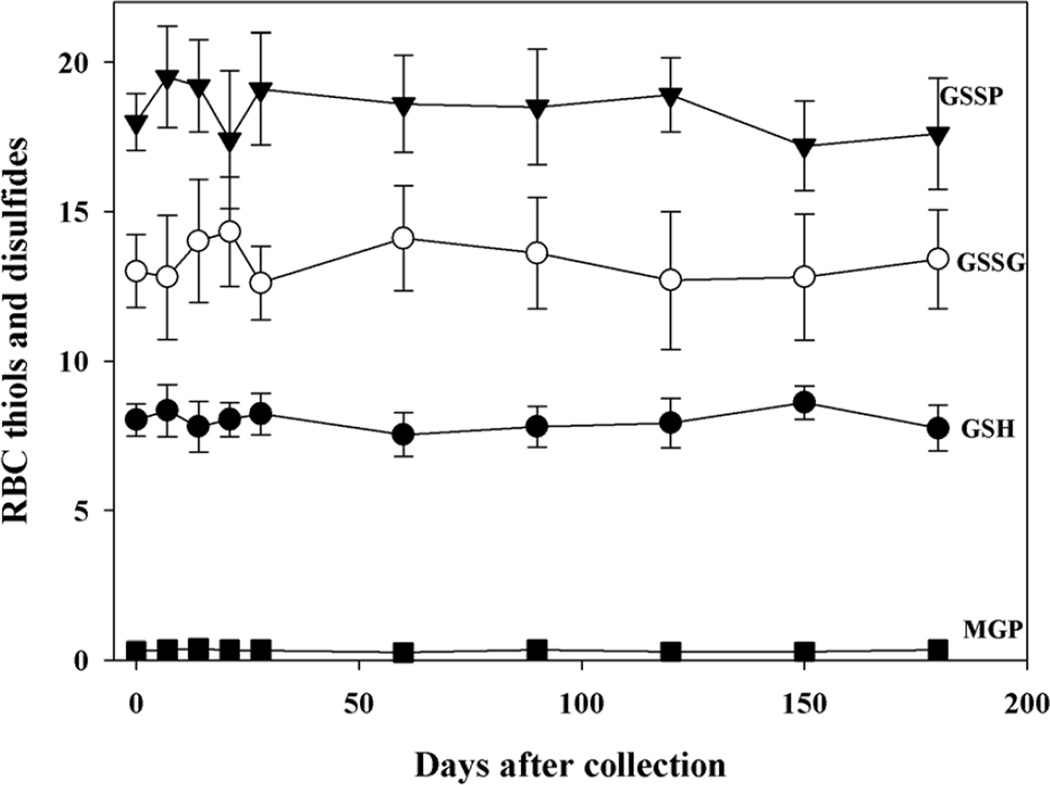
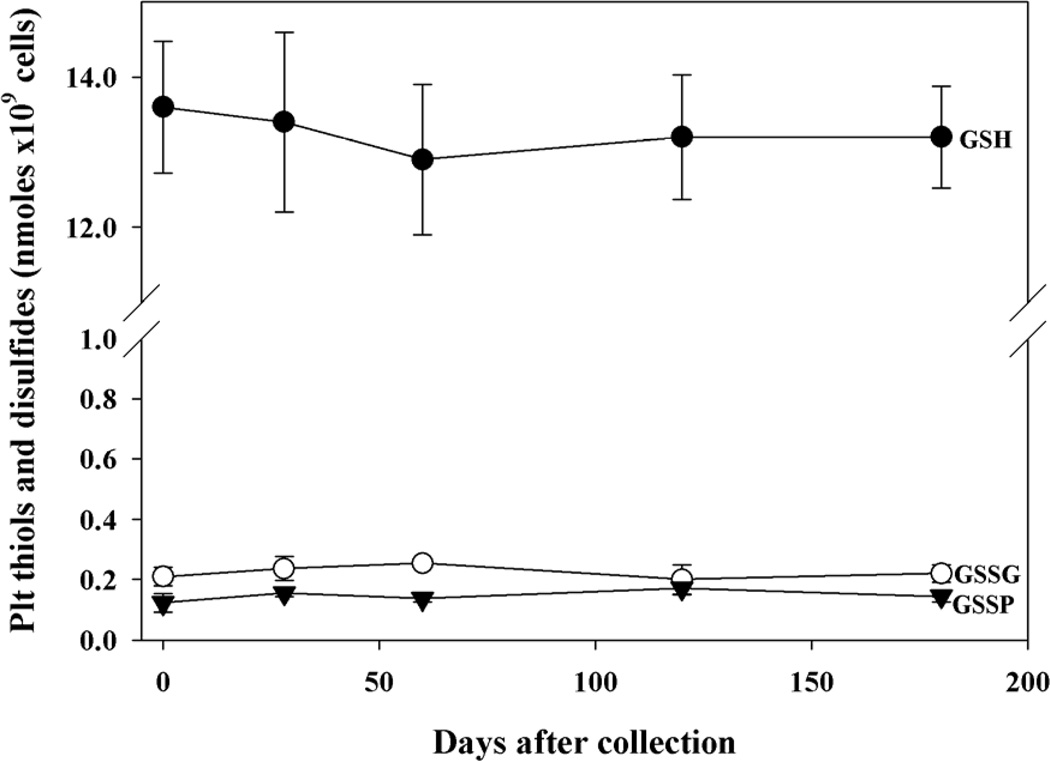
GSH and GSSG levels were stable in RBCs stored for at least six months at −80°C (Fig. 2) and, as expected, the values were almost identical to those measured in the whole blood. S-glutathionylated protein levels were measured in both RBC cytoplasm and membranes and they were very low but within the detectable range and stable over 6 months in both cell compartments. In analogy to RBCs, also the GSH, GSSG and GSSP levels of platelets were efficiently stabilized for at least 6 months by NEM pre-treatment (Fig. 3).
Figure 2. Stability of thiols and disulfides in red blood cells.
Blood was treated with NEM immediately after the collection. After 1 min, it was divided into 0.5 ml aliquots, washed by saline twice, and stored at −80°C. At the indicated times aliquots of samples were deproteinized by addition of TCA and GSH and GSSG were measured on the clear supernatant by HPLC and GSH recycling method, respectively. The rest of sample was used for HPLC analysis of cytoplasmic GSSP and for membrane S-glutathionylated proteins (MGP) n= 5. Values of GSH are expressed as nmoles/mg Hb; values of GSSG, GSSP, MGP are expressed as pmoles/mg Hb.
Figure 3. Stability of thiols and disulfides in platelets.
Blood was treated with NEM immediately after the collection. After 1 minute, PRP was separated, divided into 0.5 ml aliquots and then deprived of plasma. At the indicated times sample was treated with TCA. GSH and GSSG were measured on the clear supernatant by HPLC and GSH recycling method, respectively. The protein pellet was used for measurement of GSSP by HPLC. n= 5.
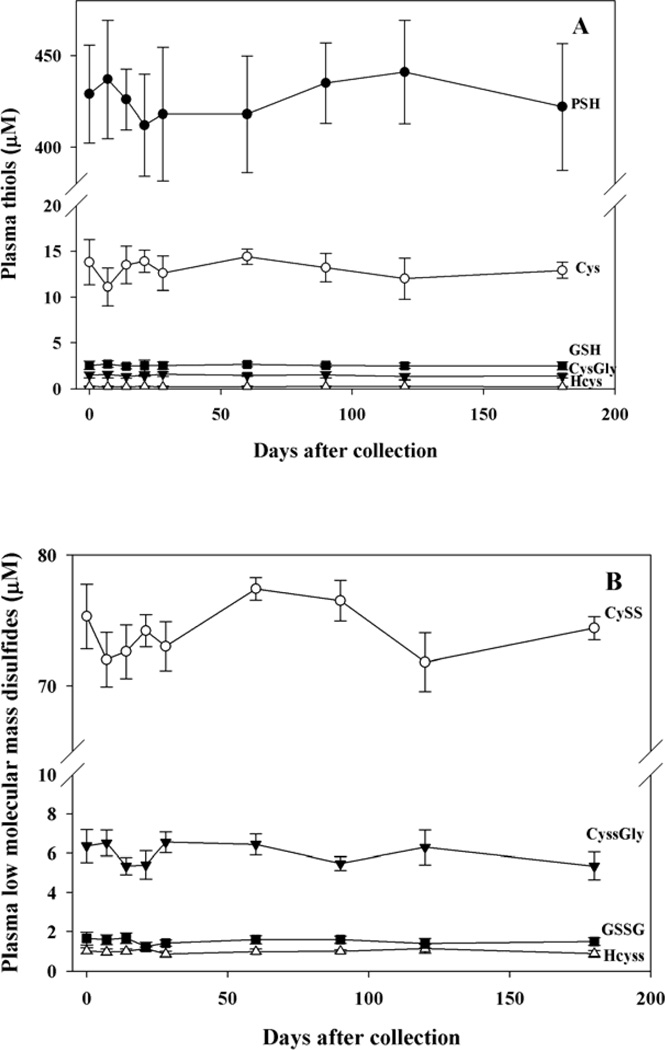
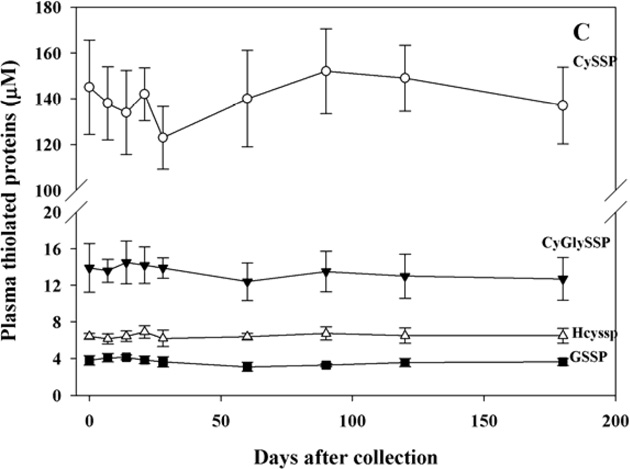
-Plasma
Plasma thiols and disulfides were measured after addition of citrate buffer to stabilize the thiols or of NEM to stabilize the disulfides (see Methods). Also in this case, the analyses were carried out weekly for one month and monthly thereafter for five months in samples stored at −80°C and all tested thiol and disulfide compounds were very stable over this time period (Fig. 4, panels A,B, and C).
Figure 4. Stability of thiols and disulfides in plasma.
Blood was treated with citrate buffer, plasma was then separated, treated again with citrate buffer, diluted with H2O and stored at −80°C for thiol analyses. Disulfides were measured on plasma-NEM obtained by RBC purification and stored at −80°C. At the indicated times protein thiols were measured by colorimetric conjugation with DTNB, LMM-SH (panel A), LMM-SS (panel B) and RSSP (panel C) were measured by HPLC analysis with fluorometric detection. n= 5.
In all these samples, the addition of NEM or dilution with citrate/H2O protected thiols from oxidation for months, while omission of these pre-treatments resulted in a significant oxidation, mostly in plasma and whole blood (see Supplemental Figure 1 and Supplemental Table 2).
4. Discussion
The main information resulting from this manuscript are:
a new protocol for simultaneous and accurate measurement of thiols and disulfides in stored RBCs, platelets, plasma and whole blood is proposed;
all physiological thiols and disulfides including LMM-SH, P-SH, LMM-SS and RSSP, can be measured in the above blood fractions starting from ~3 ml blood samples;
thiols and disulfides are stable for at least six months in samples that are maintained at ultra-low temperature and, under these conditions, they can be moved between laboratories without sample decay or compromising the analyses.
Accurate measurement of thiols and disulfides is complicated by ex vivo oxidation of thiols, a phenomenon that can markedly influence the thiol redox balance. This problem tends to occur during blood manipulation, perhaps because of presence of iron in hemoglobin [3]. We and others have previously demonstrated that artifactual oxidation of thiols can be prevented by adding NEM to the blood collection tubes either before or soon after sample collection [3, 18–21]. The alkylating agent NEM is particularly well suited for this purpose because it addresses two opposite phenomena that influence the thiol-disulfide equilibrium during sample handling, i.e. (a) the artifactual oxidation of thiols primarily as the consequence of blood acidification and (b) the regeneration of GSH from GSSG due to persistent ex vivo catalytic activity of GSSG reductase. Indeed, while NEM prevents thiol oxidation by rapidly permeating cell membranes and chelating –SH groups, it also stops endogenous regeneration of GSH by blocking the GSSG reductase activity [3,22]. Following the testing of a variety of experimental conditions in several blood fractions (not shown) we have concluded that addition of a 0.1 volume of a 310 mM NEM solution is the most effective measure for preventing both artificial degradation and loss of GSH and rise in GSSG and GSSP concentration. After 1-min incubation with NEM, samples can be used for appropriate measurements or stored at ultra-low temperature for months without any alteration (Figs 1,2, and 3). As mentioned in the introduction, cellular and extracellular compartments have very different thiol distribution and redox state. Additionally, the extracellular compartment is a pro-oxidant milieu, which demands the use of different analytical strategies, in particular for thiol analysis [23,24].
While we currently view pre-treatment with NEM as essential for the analysis of thiols and disulfides in cellular samples, we use acidification and sample dilution to prepare plasma for the analysis of thiols. Prior to now, derivatization of just collected plasma has been frequently used to prevent thiol oxidation [25], but this method is somewhat impractical because it requires completion of the analyses within the day of collection and avoidance of sample freezing. Moreover, very low plasma concentration – low micromolar - of individual thiols (Cys, CysGly, Hcys, GSH and γ–GluCys) can be precisely identified only if the analytical sensitivity is enhanced by labeling the – SH group with special probes (usually fluorescent molecules) which, however, precludes the use of NEM to protect the sample from oxidation. The addition of acid and/or the metal chelator EDTA to plasma is also frequently used to minimize sample oxidation, but these treatments only partially address the problem. Plasma dilution with Stabilyte [17] is an alternative strategy to stabilize thiols which involves lowering the pH of sample. The treatment was shown to effectively stabilize samples for several hours after blood collection at room temperature. Additionally it allows sample stabilization for 1–2 weeks at −80°C (17, Fig. 1 S). Here, we have described an alternative protocol that, by combining acidification with sample dilution, greatly increases the stability of samples over time (Fig 1S). Our pre-analytical procedure allows measurement of plasma thiols with good recovery, precision and accuracy for at least 6 months on samples that have been stored at ultra-low temperature (Table 1S and Fig. 4). The schematic summary of the suggested procedure for stabilizing thiols and disulfide in blood samples is shown in Scheme 1. Application of this procedure for analyses in blood of 5 control subjects (Tables 1 and 2) yielded values that agree with those reported by us in previous works [2,4,5,13]. Conversely, since the use of NEM as a masking agent is still often neglected, there is a discrepancy - mostly for the disulfide forms in whole blood and RBCs- between values reported by us and by some other research groups. In fact, whether thiols are not protected during sample handling by treatment with NEM, they easily oxidize and cause an artificial increase of their disulfide forms, the extent of which depends on several factors (such as the pH, the kind of acid used to deproteinize the sample, the dilution of sample). Consequently, the values found in literature for basal levels of disulfides in biosamples are frequently higher than those measured by us [11, manuscript in preparation].
The proposed procedures are suitable for long term storage of the samples and for delayed analysis of thiol redox status in almost all components in blood. Specifically, the use of NEM allows the accurate measurement of GSH and GSSG in whole blood and RBCs and reliable calculation of the GSH/GSSG ratio for assessment of redox balance. This procedure also allows the measurement of S-glutathionylated proteins, mostly HbSSG in RBCs, a parameter that is attracting growing interest not only as marker of oxidative stress but also as a signaling event capable of altering protein functionalities [5,26–28]. In plasma, acidification with citrate buffer allows delayed analysis of several LMM thiols that are present at similar or higher concentration than GSH. Also in this case, however, if the purpose is to measure the disulfides, thiol masking with NEM is needed before protein precipitation to prevent oxidation artefacts. Measurement of the LMM thiols present in plasma is significant since these are attributed effects on basic cell functions such as proliferation, differentiation and apoptosis and their concentrations and redox status vary in response to disease, environmental factors, and nutrition [7].
The pre-treatment of whole blood with NEM also allows for more accurate analysis of other oxidation products of thiols, such as S-nitrosothiols (RSNOs) as well. In fact, use of NEM in the analysis of these molecules both prevents S-transnitrosation reactions with physiological thiols and avoids artifactual increase of RSNO concentration during the acidification step, when nitrite is converted into nitrous acid, a strong nitrosating agent [29]. However, we did not include the measurement of these molecules in our protocol because of their extremely low concentration, that makes their analysis very time consuming and dependent on expensive instrumentation [29,30].
The main limitation of NEM as thiol stabilizing agent results from the ability of cells to export GSH conjugates, including GS-NEM, from the intra- to extracellular compartment. This mechanism is used by cells to eliminate toxic compounds with rates that vary depending on the chemical nature of the conjugate [31]. We have previously demonstrated that human RBCs export this conjugate [32].
The same phenomenon also occurs in platelets, where the conjugate GS-NEM is one of the substrates exported by the multidrug resistance protein 1 (MRP1/ABCC1) [33,34] and in leukocytes [35]. Therefore, intracellular GSH levels could be underestimated if a substantial amount of GS-NEM is exported from cells during tissue preparation. Whereas this problem is easily addressed for the isolation of RBC by using a 30s high speed centrifugation, it becomes more challenging when the goal is to isolate other blood components that normally require longer processing times, e.g. white cells and platelets. Here, we show that this technical problem can be circumvented for platelets by reducing the centrifugation time from the standard 20 min at 200×g to 40s at 4,200×g, which result in negligible export of the conjugate GS-NEM. We, however, were not able to develop a new method that allows addition of NEM to blood followed by rapid separation of white cells (and their sub-populations) and accurate measurement of thiols and disulfides in this cell type.
From time to time, new analytical methods are proposed to detect thiols and disulfides in biological fluids. These, however, generally devote scarce attention to the pre-analytical preparation of the samples which, in our hands, is critical for accuracy of the measurements. It is to note that the importance of selecting the most suitable conditions to store biosamples has been demonstrated recently also for other biomarkers of oxidative stress [36]. In particularly, it was observed that storage may decisively compromise the outcome of clinical studies in which malonyldialdehyde and isoprostans are measured in plasma since the measured values vary depending on the time of storage itself.
5. Conclusions
Here we describe a simple, useful procedure to stabilize thiols and disulfides in blood. Apart from the measurement of the GS-NEM conjugate with HPLC [2], which is necessary for GSH analysis in whole blood, RBCs and platelets, the rest of parameters can be measured by different analytical methods.
We think that lack of robust procedures to measure levels of thiols and disulfides in blood, has hampered significant progress in determining whether GSH/GSSG/RSSP unbalance plays a real role in the pathogenesis of disease. Our procedure offers a solution to this problem and has the advantage that it can be applied to samples stored for several months improving the reliability of the analyses carried out in long-term studies and making easier their interpretation.
Supplementary Material
Highlights.
A new procedure to stabilize thiols and disulfides in different blood fractions for months is proposed
N-ethylmaleimide was used to stabilize thiols in whole blood, RBCs, platelets and disulfides in plasma
Addition of citrate buffer followed by dilution with H2O was used to stabilize plasma thiols
The concentration of thiols and disulfides resulted to be stable for 6 months in stored samples
Only 3 ml of blood were needed to measure thiols and disulfides in the different blood fractions
Acknowledgments
This work was supported in part by grants to PF from US National Institutes of Health (NIH) National Center for Complementary and Alternative Medicine (NCCAM) (grant no. AT004490) and US Department of Veterans Affairs (Merit Review no. 1I01CX000264).
List of abbreviations
- Alb
albumin
- Cys
cysteine
- CysGly
Cysteinylglycine
- CyGlySSP
protein mixed disulfides with CysGly
- CySS
cystine
- CySS-Alb
S-cysteinylated albumin
- CySSGly
cystinylglycine
- CySSP
S-cysteinylated proteins
- DTNB
5’-dithiobis-(2-nitrobenzoic acid)
- DTT
dithiothreitol
- γ-GluCys
γ-Glutamylcysteine
- GSH
glutathione
- GSSG
glutathione disulfide
- GSSP
S-glutathionylated proteins
- Hb
hemoglobin
- HbSSG
S-glutathionylated hemoglobin
- Hcys
homocysteine
- HcySS
homocystine
- HcySSP
S-homocysteinylated proteins
- LMM-SH
low molecular mass thiols
- LMM-SS
low molecular mass disulfides
- mBrB
monobromobimane
- MGP
membrane S-glutathionylated proteins
- NEM
N-ethylmaleimide
- Plt
platelets
- PRP
platelet-rich plasma
- P-SH
protein sulfhydryl group
- RBCs
red blood cells
- RSSP
S-thiolated proteins
- -SH
sulfhydryl group
- TCA
trichloroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid. Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 2.Giustarini D, Dalle-Donne I, Milzani A, Rossi R. Detection of glutathione in whole blood after stabilization with N-ethylmaleimide. Anal. Biochem. 2011;415:81–83. doi: 10.1016/j.ab.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Rossi R, Milzani A, Dalle-Donne I, Giustarini D, Lusini L, Colombo R, Di Simplicio P. Blood glutathione disulfide: in vivo factor or in vitro artifact? Clin. Chem. 2002;48:742–753. [PubMed] [Google Scholar]
- 4.Giustarini D, Dalle-Donne I, Colombo R, Petralia S, Giampaoletti S, Milzani A, Rossi R. Protein glutathionylation in erythrocytes. Clin. Chem. 2003;49:327–330. doi: 10.1373/49.2.327. [DOI] [PubMed] [Google Scholar]
- 5.Khazim K, Giustarini D, Rossi R, Verkaik D, Cornell JE, Cunningham SE, Mohamad M, Trochta K, Lorenzo C, Folli F, Bansal S, Fanti P. Glutathione redox potential is low and glutathionylated and cysteinylated hemoglobin levels are elevated in maintenance hemodialysis patients. Trans. Res. 2013;162:16–25. doi: 10.1016/j.trsl.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giustarini D, Dalle-Donne I, Lorenzini S, Milzani A, Rossi R. Age-related influence on thiol, disulfide and protein-mixed disulfide levels in human plasma. J. Gerontol. Biol. Sci. 2006;61:1030–1038. doi: 10.1093/gerona/61.10.1030. [DOI] [PubMed] [Google Scholar]
- 7.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu. Rev. Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 8.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms and biomarkers. Crit. Rev. Clin. Lab. Sci. 2009;6:241–281. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- 10.Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R. An improved HPLC measurement for GSH and GSSG in human blood. Free Radic. Biol. Med. 2003;35:1356–1372. doi: 10.1016/j.freeradbiomed.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Giustarini D, Dalle-Donne I, Milzani A, Fanti P, Rossi R. Analysis of GSH/GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013;8:1660–1669. doi: 10.1038/nprot.2013.095. [DOI] [PubMed] [Google Scholar]
- 12.Rossi R, Giustarini D, Milzani A, Dalle-Donne I. Membrane skeletal protein S-glutathionylation and hemolysis in human red blood cells. Blood Cells Mol. Dis. 2006;37:180–187. doi: 10.1016/j.bcmd.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Rossi R, Giustarini D, Dalle-Donne I, Milzani A. Protein S-glutathionylation and platelet anti-aggregating activity of disulfiram. Biochem. Pharmacol. 2006;72:608–615. doi: 10.1016/j.bcp.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Ellman G, Lysko H. A precise method for the determination of whole blood and plasma sulfhydryl groups. Anal. Biochem. 1979;93:98–102. [PubMed] [Google Scholar]
- 15.Giustarini D, Dalle-Donne I, Milzani A, Rossi R. Low molecular mass thiols, disulfides and protein mixed disulfides in rat tissues: influence of sample manipulation, oxidative stress and ageing. Mech. Ageing. Dev. 2011;132:141–148. doi: 10.1016/j.mad.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Tentori L, Salvati AM. Hemoglobinometry in human blood. Methods Enzymol. 1981;76:707–715. doi: 10.1016/0076-6879(81)76152-4. [DOI] [PubMed] [Google Scholar]
- 17.Williams RH, Maggiore JA, Reynolds RD, Helgason CM. Novel approach for the determination of the redox status of homocysteine and other aminothiols in plasma from healthy subjects and patients with ischemic stroke. Clin. Chem. 2001;47:1021–1039. [PubMed] [Google Scholar]
- 18.Srivastava SK, Beutler E. Oxidized glutathione levels in erythrocytes of glucose-6-phosphate-dehydrogenase–deficient subjects. Lancet. 1968;2:23–24. doi: 10.1016/s0140-6736(68)92892-4. [DOI] [PubMed] [Google Scholar]
- 19.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 20.Schofield D, Mei G, Braganza JM. Some pitfalls in the measurement of blood glutathione. Clin. Sci. 1993;85:213–218. doi: 10.1042/cs0850213. [DOI] [PubMed] [Google Scholar]
- 21.Sacchetta P, Di Cola D, Federici G. Alkaline hydrolysis of N-ethylmaleimide allows a rapid assay of glutathione disulfide in biological samples. Anal. Biochem. 1986;154:205–208. doi: 10.1016/0003-2697(86)90516-6. [DOI] [PubMed] [Google Scholar]
- 22.Hansen RE, Winther JR. An introduction for analyzing thiols and disulfides: reactions, reagents, and practical considerations. Anal. Biochem. 2009;394:147–158. doi: 10.1016/j.ab.2009.07.051. [DOI] [PubMed] [Google Scholar]
- 23.Velury S, Howell S. Measurement of plasma thiols after derivatization with monobromobimane. J. Chromatogr. 1988;424:141–146. doi: 10.1016/s0378-4347(00)81085-3. [DOI] [PubMed] [Google Scholar]
- 24.Anderson ME, Meister A. Dynamic state of glutathione in blood plasma. J. Biol. Chem. 1980;255:9530–9533. [PubMed] [Google Scholar]
- 25.Mansoor MA, Svardal AM, Ueland PM. Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma. Anal. Biochem. 1994;200:218–229. doi: 10.1016/0003-2697(92)90456-h. [DOI] [PubMed] [Google Scholar]
- 26.Mieyal JJ, Chock PB. Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation. Antioxid. Redox Signal. 2012;16:471–475. doi: 10.1089/ars.2011.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehrmann DC, Rose K, Calcutt MW, Beller AB, Hill S, Rogers TJ, Steele SD, Hachey DL, Aschner JL. Glutathionylated γG and γA subunits of hemoglobin F: a novel posttranslational modification found in extremely premature infants by LC-MS and nanoLC-MS/MS. Mass Spectrom. 2014;49:178–183. doi: 10.1002/jms.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen HJ, Lin WP, Chiu SD, Fan CH. Multistage mass spectrometric analysis of human hemoglobin glutathionylation: correlation with cigarette smoking. Chem. Res. Toxicol. 2014;27:864–872. doi: 10.1021/tx5000359. [DOI] [PubMed] [Google Scholar]
- 29.Giustarini D, Milzani A, Dalle-Donne I, Rossi R. Detection of S-nitrosothiols in biological fluids: A comparison among the most widely applied methodologies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;851:124–139. doi: 10.1016/j.jchromb.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 30.Tsikas D, Schmidt M, Böhmer A, Zoerner AA, Gutzki FM, Jordan J. UPLC-MS/MS measurement of S-nitrosoglutathione (GSNO) in human plasma solves the S-nitrosothiol concentration enigma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013;927:147–157. doi: 10.1016/j.jchromb.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Ballatori N, Krance SM, Marchan R, Hammond CL. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Aspects Med. 2009;30:13–28. doi: 10.1016/j.mam.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi R, Milzani A, Dalle-Donne I, Giannerini F, Giustarini D, Lusini L, Colombo R, Di Simplicio P. Different metabolizing ability of thiol reactants in human and rat blood. J. Biol Chem. 2001;276:7004–7010. doi: 10.1074/jbc.M005156200. [DOI] [PubMed] [Google Scholar]
- 33.Jedlitschky G, Greinacher A, Kroemer HK. Transporters in human platelets: physiologic function and impact for pharmacotherapy. Blood. 2012;119:3394–3402. doi: 10.1182/blood-2011-09-336933. [DOI] [PubMed] [Google Scholar]
- 34.Deeley RG, Cole SPC. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1.) FEBS Letters. 2006;580:1103–1111. doi: 10.1016/j.febslet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Scott RB, Matin S, Hamilton SC. Glutathione, glutathione S-transferase, and transmembrane transport of glutathione conjugate in human neutrophil leukocytes. J. Lab. Clin. Med. 1990;116:674–680. [PubMed] [Google Scholar]
- 36.Tsikas D, Rothmann S, Schneider JY, Suchy MT, Trettin A, Modun D, Stuke N, Maassen N, Frölich JC. Development, validation and biomedical applications of stable-isotope dilution GC-MS and GC-MS/MS techniques for circulating malondialdehyde (MDA) after pentafluorobenzyl bromide derivatization: MDA as a biomarker of oxidative stress and its relation to 15(S)-8-iso-prostaglandin F2α and nitric oxide (NO) J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2015 doi: 10.1016/j.jchromb.2015.10.009. pii: S1570-0232(15)30228-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.