Abstract
Background
STromal Interaction Molecule 1 (STIM1) is a dynamic calcium signal transducer implicated in hypertrophic growth of cardiac myocytes. STIM1 is thought to act as an initiator of cardiac hypertrophic response at the level of the sarcolemma but the pathways underpinning this effect have not been examined.
Methods and Results
To determine the mechanistic role of STIM1 in cardiac hypertrophy and during the transition to heart failure, we manipulated STIM1 expression in mice cardiac myocytes using in vivo gene delivery of specific short hairpin RNAs. In three different models, we found that Stim1 silencing prevents the development of pressure-overload induced hypertrophy but also reverses pre-established cardiac hypertrophy. Reduction in STIM1 expression promoted a rapid transition to heart failure. We further showed that Stim1 silencing resulted in enhanced activity of the anti-hypertrophic and pro-apoptotic GSK-3β molecule. Pharmacological inhibition of GSK-3 was sufficient to reverse the cardiac phenotype observed after Stim1 silencing. At the level of ventricular myocytes, Stim1 silencing or inhibition abrogated the capacity for phosphorylation of AktS473, a hydrophic motif of Akt that is directly phosphorylated by mTORC2. We found that Stim1 silencing directly impaired mTORC2 kinase activity, which was supported by a direct interaction between STIM1 and Rictor, a specific component of mTORC2 complex.
Conclusions
These data support a model whereby STIM1 is critical to deactivate a key negative regulator of cardiac hypertrophy. In cardiac myocytes, STIM1 acts by tuning Akt kinase activity through activation of mTOR complex 2 (mTORC2), which further results in repression of GSK-3β activity.
Keywords: Heart failure, Calcium, Gene therapy, STIM1, mTORC2
Cardiac hypertrophy develops in reaction to increased mechanical load or to neurohormonal stimulation as occurs in cardiac dysfunction. Cardiac hypertrophy is a compensatory response that reduces wall stress by increasing wall thickness. Although it represents an initial salutary adaptation to stress, chronic hypertrophic remodeling involves maladaptive changes in cardiac function over the long term.
Because of the limited proliferative capacity of adult cardiomyocytes (CM)1, growth of the cardiac wall is mainly sustained by an increase in CM size. A number of intra-cellular signal-transduction circuits have been implicated in the hypertrophic remodeling of adult CMs 2, including calcineurin-nuclear factor of activated T cells (NFAT) and the Akt/protein kinase B (PKB)-mammalian target of rapamycin (mTOR). These signaling circuits directly induce hypertrophic growth by altering gene expression in the nucleus with activation of a defined set of pro-hypertrophic transcription factors. However, the upstream events at the level of sarcolemma that initiate the cardiac hypertrophic response remain largely unknown.
Among different candidates, it has been observed that local alterations in Ca2+ homeostasis can be an early trigger of intracellular signaling events leading to CM growth 3. In recent years, stromal interaction molecule 1 (STIM1) has been reported as a regulator of CM growth in vitro and in vivo4-6. STIM1 was initially shown to act as an endoplasmic reticulum (ER) sensor and to mediate store-operated Ca2+ entry (SOCE), a major mechanism of Ca2+ entry in nearly all non-excitable cells 7. In these cells, a decrease in ER Ca2+ content triggers STIM1 oligomerization and subsequent interaction with some plasma membrane channels including the highly Ca2+-selective Orai1 channel 8. In this model, the STIM1-Orai1 coupling drives ER-plasma membrane junctions, mediate Ca2+ entry and communication among the ER lumen, the cytoplasm and the extracellular space and activate Ca2+-regulatory proteins 8. Recent information has however shown that STIM1 is a dynamic signal transducer that can interact with a greater diversity of proteins depending on the pathophysiological conditions 8.
In cardiac myocytes, a cell type where large fluctuations of sarcoplasmic reticulum (SR) and cytosolic Ca2+ occur with each heart beat, STIM1 not only controls SOCE but also a spontaneous Ca2+ current that occurs independently of Ca2+ store depletion 4. This spontaneous current is marginal in healthy adult CM but strengthens during the hypertrophic remodeling of CM4. It has been proposed that an interaction between STIM1 and two members of the Orai family, i.e. Orai1 and Orai3 channels, occurs in response to hypertrophic stimulation and sustain the emergence of this alternative mechanism of Ca2+ entry9. A similar phenomenon has been reported in proliferative smooth muscle cells where STIM1 controls heteromultimers of Orai1 and Orai3 that are regulated by leucotrienes C49-11. Interactions with less Ca2+ selective channels, such as TRPCs, have also been suggested and STIM1 overexpression leads to dysregulated calcium transients5, 12. All together, these data indicate that STIM1 activates in response to stress and can interact with a diversity of plasma membrane channels that will however mediate highly localized Ca2+ signals.
Importantly, Stim1 silencing has been associated with a reduction in agonist-triggered hypertrophic responses of neonatal and adult cardiomyocytes in vitro4, 13-15, as well as in the adult heart in vivo 4. In addition, cardiac-specific deletion of Stim1 triggers ER stress and progressively results in left ventricular dilation under physiological conditions16,6. There is thus evidence that STIM1 is involved in cardiac homeostasis and is required for cardiomyocyte hypertrophy but the pathways underpinning this effect are unknown.
METHODS
All procedures and animal care were approved by our institutional research committee and conformed to the animal care guideline in Mount Sinai School of Medicine in accordance to the following approved protocol #LA08-01064.
Animal experiments
Mice underwent TAC using a supraclavicular construction model as previously described 17. TAC or sham surgery was performed in 8-week-old male C57Bl/6J mice (body weight 18–22 g). Mice were anesthetized with intraperitoneal injection of Ketamine/Xylazine (respectively 100mg/kg and 10mg/kg) and placed on a ventilator. A longitudinal cut of 2–3 mm was made in the proximal portion of the sternum, allowing visualization of the aortic arch. The transverse aortic arch was ligated between the innominate and left common carotid arteries with an overlying 27-gauge needle. The needle was immediately removed leaving a discrete region of constriction. The sham group underwent a similar procedure without ligation. Burprenorphine (Buprenex) was used an analgesic before and 2 days after surgery (0.05mg/kg, IP).
TDZD-8 (GSK3β inhibitor, Sigma Aldrich) was dissolved in DMSO:PBS (1:10) to a concentration of 1.5mg/mL. Daily intraperitoneal injections were performed during the last two weeks of experimental protocol.
Cardiac echography
Echocardiography was performed under sedation by intraperitoneal ketamine up to 75mg/kg. Sedation was optimized by giving the lowest dose of ketamine needed to (1) restrain the animal and prevent motion artifact (2) maintain the heart rate in the range of 550-650 beats/minute. Ketamine was chosen based on our previous experience and considering that alternative agents had either a long duration of action (pentobarbital), potentially unsafe for heart failure animals, or a bradycardic effect (isoflurane, ketamine/xylazine) as demonstrated elsewhere18. The chest was shaved. Short-axis parasternal views of the left ventricule (LV) at the mid-papillary level were obtained using a Vivid i echocardiography apparatus with a 13MHz linear array probe (General Electric, New York, NY). M-mode measurements of the size of the LV walls and cavities were obtained by 2D guidance from the short-axis view of the LV. Three different measurements in diastole (d) and systole (s) were averaged per animal to estimate LV wall thicknesses (spetum and posterior wall) and LV internal diameter (LVID). Fraction shortening (FS) is a way of measuring LV performance. It measures and ratios the change in the diameter of the left ventricle between the contracted and relaxed states with the following formula: FS = (LVIDd – LVIDs) / LVIDd.
Cardiac hemodynamic analysis
Hemodynamic measurements were performed using a 1.2 Fr pressure-volume conductance catheter (Scisense). Pressure-volume loop analysis was performed as previously described19. Mice were injected intraperitoneally with urethane (1g/kg), etomidate (10mg/kg) and morphine (1mg/kg) and mechanically ventilated with 7μL/g stroke volume at 125 respirations min−1. The chest was opened to expose the heart for an apical stab approach. To determine absolute ventricular volumes via admittance technology, myocardial and blood conductance were obtained before pressure-volume catheter placement in the left ventricle. The inferior vena cava was transiently occluded to reduce ventricular pre-load to obtain load-independent pressure-volume relationships. Hemodynamic measurements were acquired and analyzed using IOX software (EMKAtech).
rAAV vector production and purification
Recombinant AAV9.shStim1 and AAV9.shLuc were produced using the two-plasmids protocol as previously described 20 with some modifications. shRNA sequences were previously described 4, 21. HEK-293T cells (ATCC) were grown in triple flasks for 24 h (DMEM, 10% fetal bovine serum) before adding the linear polyethylenimine and the 2 plasmids. 72 hours after transfection (DMEM, 2% fetal bovine serum), the viruses were purified and concentrated from benzonase-treated crude cell lysates over an iodixanol density gradient (Optiprep, Greiner Bio-One Inc.). Finally, viruses were formulated into lactated Ringer’s solution (Baxter Healthcare Corporation) using dialysis membranes Spectra/Por2 MWCO 12-14 KDa, 10 mm flat width, 0.32 ml/cm dialysis membranes (Spectrum Labs), titrated by PCR and by Coomassie stained gels and then stored at −80 °C. For cardiotropic expression, Wild Type male mice received 1E+11 viral genome of AAV9.shStim1 or AAV9.shLuc or PBS by tail vein injection. AAV9.shStim1 was built according to shRNA sequence that was previously described and validated 4. To avoid liver toxicity, a novel AAV9.shLuc was re-developed using an anti-luciferase sequence that was previously described and validated21.
Cardiac myocyte isolation and treatment
Cardiomyocytes were isolated from C57Bl/6 mouse hearts. Male mice (25-32 g), infected with AAV9.shSTIM1 or control, were used. In brief, after heparin (50 U) was injected, animals were anaesthetized with intraperitoneal injection of ketamine (100mg/g). The heart was quickly removed from the chest and the aorta was retrogradely perfused at 37 °C for 3 min with calcium-free Tyrode buffer (137 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES [pH 7.4], 10 mM 2, 3-butanedione monoxime, and 5 mM taurine) oxygenated with 100% O2. The enzymatic digestion was initiated by adding collagenase type B (300 U/ml; Worthington) and hyaluronidase (0.1 mg/ml; Worthington) to the perfusion solution. When the heart became swollen after 10 min of digestion, the left ventricle was quickly removed, cut into several chunks, and further digested in a shaker (60-70 rpm) for 10 min at 37 °C in the same enzyme solution. The cell suspension was filtered through a cell strainer (100 μm pore size; BD Falcon) and gently centrifuged at 500 rpm for 1 min. The pellet containing myocyte fraction was plated on lamimin-coated dish (Life Science). The supernatant containing non-myocyte fraction was centrifuged at 14000 rpm for 10min and the pellet was collected. For in vitro hypertrophic experiments, cardiac myocytes were starved overnight. The next morning, cells were treated for 48 hours with Angiotensin II (100nM) +/− YM-58483 (1μM). After 48 hours, cells were lysed using RIPA buffer.
Western blots and antibodies
Western blots were performed using 10%, 4-20% or 10-20% tris-glycine gels (Invitrogen) regarding the size of the studied proteins. Used primary antibodies were GAPDH (Millipore), STIM1 (Sigma Aldrich), SERCA2a (home made), phospho and total GSK 3 β, Akt (total, S473, T308), mTOR, Rictor, mSN1, mLTS8, Foxo3 (S253, total), PRAS40 (T246, total) and Caspase3 (all from Cell Signaling). Secondary antibodies were appropriate HRP-linked antibodies (Sigma Aldrich).
Phospho-Kinase assay
HEK cells (ATCC) were respectively cultured in DMEM 10% Fetal Bovine Serum, 1% Penicillin/Streptomycin (Gibco). Proteome Profiler (R&D Systems®) was performed on HEK cells according to manufacturer instructions. HEK cells were used at 90% confluence. Briefly, selected capture antibodies have been spotted in duplicate on nitrocellulose membranes. Cell lysate samples are diluted and mixed with a cocktail of biotinylated detection antibodies. The sample/antibody mixture is then incubated with the array. Any protein/detection antibody complex present is bound by its cognate immobilized capture antibody on the membrane. Streptavidin-Horseradish Peroxidase and chemiluminescent detection reagents are added, and a signal is produced in proportion to the amount of cytokine bound. Chemiluminescence is detected in the same manner as a Western blot.
Immuno-Precipitation
Freshly isolated adult mouse cardiomyocytes were lysed using RIPA buffer (Boston Bio Products) with Protease and Phosphatase inhibitor (Roche). 300μg of proteins were diluted in a final volume of 600μl of lysis buffer and incubated 2 hours with rotation at 4°C with either Rictor (1.5μg) or STIM1 (5μg) antibody. Control immuno-precipitation was performed by using rabbit IgG (5μg, Abcam). In the mean time, 10mg of Protein A-sepharose beads (Sigma) were washed with PBS and saturated with PBS-BSA 1mg/ml. Cell lysates with primary antibody were mixed with beads and incubated with rotation at 4°C over-night. Beads containing immuno-precipitates were washed 3 times with ice-cold PBS. Proteins were released from beads by adding 25μl of Laemmli buffer and by agitation for 1 hour at 37°C at 1200 rpm. Supernatant was removed from the protein A–sepharose, and was analyzed by immunoblotting for Rictor and STIM1.
mTORC2 Kinase assay
For functional mTORC2 kinase assay experiments22, 23, freshly isolated adult mouse cardiomyocytes were lysed using CHAPS lysis buffer [40 mM HEPES, pH 7.4, 120 mM NaCl, 2 mM EDTA, 0.3% CHAPS, 10 mM pyrophosphate, 10 mM glycerophosphate, 50 mM NaF, Protein inhibitor cocktail (Sigma) at 1:100]. Rictor antibody (1.5μg) was added to the cleared cellular lysates (1 mL Lysis buffer) and incubated with rotation at 4°C for 60 min. After another 1 hour of incubation with 60 μl of 50% slurry of protein G–agarose (Calbiochem), immuno-precipitates captured by protein G–agarose were washed 3 times with CHAPS wash buffer (CHAPS lysis buffer without Protein Inhibitor Cocktail) and once with kinase wash buffer [25 mM Hepes (pH 7.4), 20 mM KCl]. For in vitro mTORC2 kinase reactions, immunoprecipitates were incubated in a final volume of 20 μl at 37°C for 30 min in mTORC2 kinase buffer [25mM HEPES (pH 7.4), 100 mM potassium acetate, 1 mM MgCl2] containing 250 ng of inactive His-Akt1 (Millipore) and 500 μM ATP (Sigma). The reaction was stopped by the addition of 20 μl of Laemmli buffer. Samples were boiled 5 min. After a quick spin, the supernatant was removed from the protein G–agarose, and was analyzed by immunoblotting for phospho-Ser473 Akt and total Akt.
Histological analysis
At the moment of euthanasia, hearts were stopped in diastole by injecting 1M KCl solution in the left ventricle and then prepared in Tissue-Tek OCT compound (Sakura Fine technical) and sectioned into 8μm slices (Microm HM560 Cryo-star, Thermo Scientific).
Double immunolabeling on cryostat sections allowed the identification of endothelial cells with Caveolin 1 antibody (Santa Cruz) and cardiomyocytes by vinculin antibody (Sigma-Aldrich). For each animal, 3 sections minimum were taken at different levels of myocardium were processed. Left ventricular fields in which cross sections of capillaries and cardiomyocytes were clearly detectable (subendocardial area) were recorded using an Olympus IX71 microscope equipped with an Olympus DP72 camera. A minimum of 6 fields/section was recorded at 20x magnification (corresponding of a minimum of 1000 cells measured). The cross section area of cardiomyocyte was measured using Image J software (NIH) by a masked observer. For interstitial fibrosis quantification, heart sections were stained using Masson’s Trichrome staining kit (Sigma Aldrich).
Interstitial fibrosis staining and quantification
Briefly, slides were fixed in Bouin solution (Sigma Aldrich) 15 minutes at 56 degrees Celsius. After washes in tap water (15 minutes) and deionized water, slides were stained with Hematoxylin10 minutes (Fisher Scientific). After 5 minutes wash in tap water, slides were dipped in a mix solution of phosphotungstic acid and phosphomolybdic acid solutions (2.5%) for 5 minutes, then in Aniline solution 5 minutes and finally in Acetic acid solution (1%) 2 minutes. Slides were washed in deionized water and then dehydrated by Ethanol 95% and 100% bath 2 minutes each. After 2 baths of xylene, slides were mounted with Cyotseal 60 (Thermo Scientific). 20x magnification pictures were taken using software Spot v 3.5.7.1 (Diagnostics Instruments, Inc) and fibrosis was quantified using Image J software (NIH). At least 10 fields per animal were quantified and at least 4 animals per group.
Statistical analyses
Time-course experiments were analyzed using a two-way repeated-measure ANOVA, followed by a Bonferroni post hoc test. Other quantitative data were analyzed using a one-way ANOVA followed by a Bonferroni post hoc test. Continuous variables were compared between two groups using a Student t test with Welch correction. Exact tests were used for experiments with n<5. All analyzes were performed using GraphPad Prism 6. Pvalues of 0.05 were considered significant. Data are presented as means ± SEM. Densitometry of Western blots, quantification of cardiac fibrosis, and quantification of cell size were performed using NIH ImageJ software.
RESULTS
Stim1 silencing leads to progressive cardiac dilation and dysfunction under physiological conditions
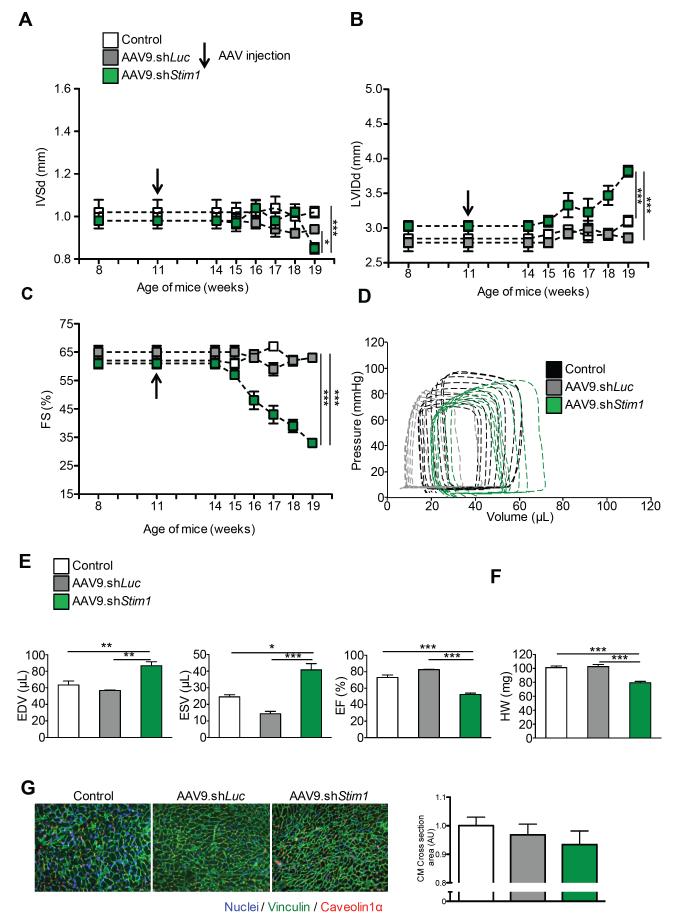
We first developed recombinant cardiotropic adeno-associated viruses of serotype 9 (AAV9) encoding shRNA directed against Stim1 under control of the U6 promoter (Supplemental figure 1A). We used AAV9 because it shows strong tropism for the heart, especially for cardiomyocytes, after a single tail vein injection4, 24. We found that cardiac STIM1 expression is reduced by 75% three weeks after administration of AAV9.shStim1 as compared to controls (Supplemental figure 1B-C). In this first set of experiments, C57/Bl6J mice were injected with AAV9.shStim1 or an AAV9 encoding a shRNA directed against luciferase or a single PBS injection as negative controls. There were no statistically significant differences in left ventricular wall thickness, volumes, and function between the two control groups over the 8-weeks follow-up period (Figures 1A-E). In contrast, we found that five weeks after infection mice treated with AAV9.shStim1 started to present significant differences with a progressive decline in fractional shortening and a mild increase in left ventricular diastolic volume, indicative of the development of a mild cardiac dilation and dysfunction (Figures 1A-E). At the time of sacrifice (8 weeks after injection), AAV9.shStim1 treated animals presented with a slight decrease in heart weights (Figure 1F) and a non-significant trend for smaller cardiomyocyte size (Figure 1G).
Figure 1.
Stim1 silencing under physiological conditions. Time-course analysis of A, inter ventricular septum thickness (IVSd), B, left ventricular internal diastolic diameter (LVIDd) and C, fractional shortening (FS) of wild-type mice treated with AAV9.shStim1 (green), AAV9.shLuciferase (gray) or PBS (white). Mice were followed up to 8 weeks after infection. d = in diastole. D, Representative Pressure-Volume loops in wild-type mice treated with AAV9.shStim1 (green), AAV9.shLuciferase (gray) or PBS (black). E, Hemodynamic parameters (end diastolic volumes (EDV); end systolic volumes (ESV); ejection fraction (EF)) assessed 8 weeks after AAV injection. F, Heart weights. G, Cardiomyocyte areas in the three groups. Immunofluorescence analysis was performed on left ventricular sections (8μm) using an antibody against Vinculin (green) and Caveolin1α (red). Nuclei were stained with DAPI. Images were taken at 20X magnification. N=5-10 animals per group for all experiments, * p<0.05, ** p<0.01, *** p<0.001
STIM1 is critical for the development of adaptive cardiac hypertrophy
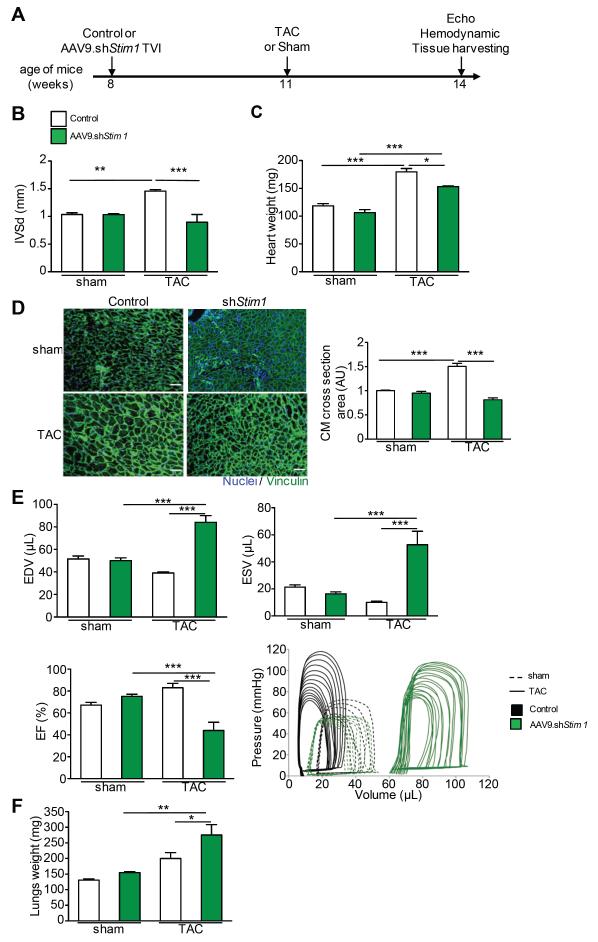
We then assessed in vivo the contribution of STIM1 in the development of pressure-overload cardiac hypertrophy (LVH model). In this experiment, mice were first infected with AAV9.shStim1 or control (1.10e11 drp) and were subjected to TAC or Sham surgery three weeks later (Figure 2A). This timing was based on our results showing a reduction in STIM1 expression by 75% in AAV9.shStim1 treated animals as compared to controls at that time point (Supplemental figure 1B-C). The animals were then followed up for three more weeks before sacrifice (Figure 2A). Reduced cardiac STIM1 expression, as achieved after infection with AAV9.shStim1, blunted the development of left ventricular hypertrophy in TAC-treated mice as identified by echocardiographic data on left ventricular wall thickness and measures of heart weights (Figure 2B-C). Concordantly, TAC-treated mice that received AAV9.shStim1 had cardiomyocyte sizes almost identical to the one observed in both Sham control groups (Figure 2D). However, TAC-treated mice that had received AAV9.shStim1 developed left ventricular dilation and decreased systolic function as assessed by echocardiography (not shown) and hemodynamics recordings (Figure 2E) as well as a significant increase in lung weights (Figure 2F), indicative of the development of pulmonary edema. Importantly, at that time point, all of these parameters were unchanged in sham-treated mice that received AAV9.shStim1 (Figure 2B-F), suggesting that the role of Stim1 is exacerbated in response to hypertrophic stimulation.
Figure 2.
Stim1 silencing before TAC prevented the establishment of cardiac hypertrophy. A, Schematic timeline to study Stim1 silencing effect on TAC-induced left ventricular hypertrophy (LVH). B, Cardiac walls thickness assessed by echocardiography in sham vs. TAC animals treated with AAV9.shStim1 (green), or control (white). IVS=Interventricular Septum, d=in diastole. C, Heart weights. D, Left, immunofluorescence analysis of left ventricular sections (8μm) using an antibody against Vinculin (green). Nuclei were stained with DAPI. Images were taken at 20X magnification. Right, quantification of cardiomyocyte area in the four groups. E, Cardiac function assessed by hemodynamic measurements. Top left, End Diastolic volume (EDV). Top right, End Systolic Volume (ESV). Bottom left, Ejection Fraction (EF) and bottom right, characteristic pressure-volume loops. AAV9.shStim1 treated groups are in green, controls are in white for the bar graphs and in black for the PV loops. F, Lung weights. N≥8 per groups. * p<0.05, ** p<0.01, *** p<0.001.
In another set of experiments, focusing on the transition from cardiac hypertrophy to heart failure (HF model), we first subjected mice to pressure-overload or sham before randomly assigned mice to AAV9.shStim1 or control administration three weeks later (Figure 3A). During the first three weeks following surgery, mice developed cardiac hypertrophy that was similar among TAC-treated groups at the time of AAV administration (Supplemental Figure 2A-C). Three weeks after randomization, echocardiography was performed on a weekly basis and final evaluation was performed five weeks after AAV9 administration (Figure 3A). Whereas TAC-treated animals showed similar echocardiographic data three weeks after injection, a significant decrease in left ventricular ejection fraction as well as a marked increase in left ventricular diameter was observed in TAC-treated animals that received AAV9.shStim1 as compared to controls (Figure 3B). The progressive development of left ventricular dilation and systolic dysfunction started three weeks after AAV9.shStim1 administration in line with the time needed to achieve a significant reduction in cardiac STIM1 expression (Supplemental Figure 1C). Hemodynamic recordings confirmed the significant increase in left ventricular volumes and the significant decrease in contractility parameters in TAC-AAV9.shStim1 treated animals as compared to controls, an effect that was not seen in sham-AAV9.shStim1 treated animals (Figure 3C). In marked contrast to TAC-control group, TAC-AAV9.shStim1 treated animals presented a significant reduction in hypertrophic markers as assessed by the significant reduction in wall thickness (Figure 3D), heart weights (Figure 3E) and in cardiomyocyte area (Figure 3F). By the end of this experiment (i.e., 8 weeks after TAC and 5 weeks after AAV9 administration), TAC-AAV9.shStim1 treated animals presented with cardiomyocyte size that was even smaller than the one measured in sham animals thus indicating cellular atrophy. These intriguing results were observed despite persistent increased afterload in all TAC-treated groups (Figure 3C). TAC-AAV9.shStim1 treated animals also presented with an increase in lung weights, in line with the rapid worsening of heart failure (Figure 3E).
Figure 3.
Stim1 silencing after TAC reverted established cardiac hypertrophy. A, Schematic timeline to study Stim1 silencing effect on TAC-induced heart failure (HF). B, Time-course of Fractional Shortening (left) and Left Ventricular Internal Diameter (right) assessed by echocardiography. d=in diastole. Sham (dotted line) vs. TAC (straight line) animals treated with AAV9.shStim1 (green) or control (white). C, Cardiac function and volumes assessed by hemodynamic measurements. Top left, End Diastolic volume (EDV). Top right, End Systolic Volume (ESV). Bottom left, Ejection Fraction (EF) and bottom right, representative pressure-volume loops in the four groups of animals. D, Time-course of Inter Ventricular Septum thickness in diastole (IVSd) assessed by echocardiography in the four groups of animals. Sham (dotted line) vs. TAC (straight line) animals treated with AAV9.shStim1 (green) or control (white). E, Heart and lung weights at the time of sacrifice. F, Left, immunofluorescence analysis of left ventricular sections (8μm) using an antibody against Vinculin (green) and Caveolin1α (red). Nuclei were stained with DAPI. Images were taken at 20X magnification. Right, quantification of cardiomyocyte area. N≥6 per groups. * p<0.05, ** p<0.01, *** p<0.0001.
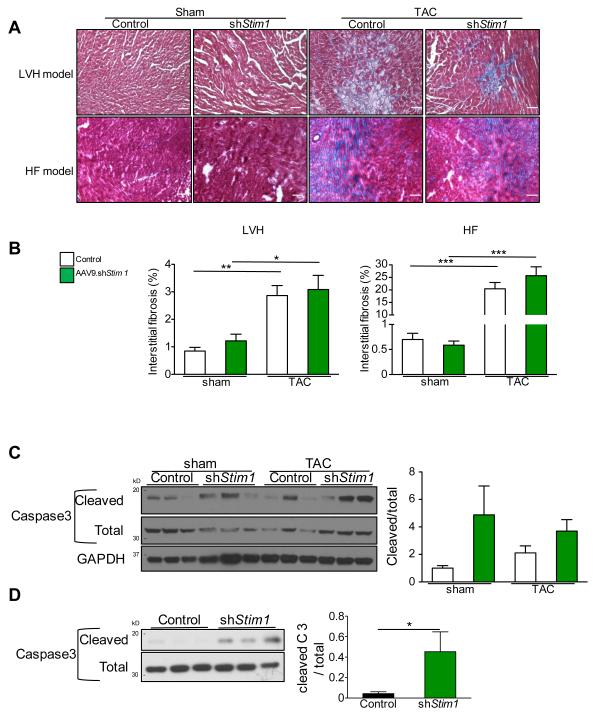
Overall, these results indicate that STIM1 is critical for the development but also the persistence of adaptive cardiac hypertrophy. Reduced STIM1 expression promotes cardiac dilation with a rapid transition to heart failure in a context of pressure-overload. We performed further histological and biochemical analyses in both LVH and HF models. We found that, in both control and shStim1 groups, pressure-overload was associated with a significant development of interstitial fibrosis as compared to Sham animals (Figure 4A-B). Whereas there were no statistically significant differences between sham-control vs. sham-AAV9.shStim1 treated animals, there was a trend for higher fibrosis levels in TAC-AAV9.shStim1 treated animals that however did not reach significance (Figure 4B). We also observed a non-significant trend for higher activation of apoptosis as assessed by an increase in cleavage of the pro-apoptotic protease caspase-3 in total hearts extracts from AAV9.shStim1-treated animals (Figure 4C). Importantly, a statistically significant difference was observed in cardiac myocytes isolated from the heart of wild-type mice treated with AAV9.shStim1 as compared to control (Figure 4D).
Figure 4.
Influence of Stim1 silencing on the establishment of cardiac interstitial fibrosis and on the activation of Caspase 3. A, Characteristic images of Masson’s trichrome staining of left ventricular sections of sham and TAC control vs. shStim1 treated animals in both LVH and HF models. B, Quantification of interstitial fibrosis from mice of LVH model (left) and from mice of HF model (Right). C, Left, Western blot analysis on whole cardiac tissue of total and cleaved Caspase 3 from mice of the HF model. Right, quantification of Western blots. D, Left, Western blot analysis on isolated cardiac myocytes of total and cleaved Caspase 3 from wild-type mice treated with AAV9.shStim1 or control. Right, quantification of Western blots. n≥6 animals per group for fibrosis measurements and n=3 animals per group for caspase3 Western Blot, * p<0.05, ** p<0.01, *** p<0.001.
Stim1 silencing is associated with increased GSK3 beta activity
Although alternative mechanisms of action have been described 25, STIM1 primarily functions as a dynamic calcium coordinator of cellular calcium signals though interactions with highly calcium-selective plasma membrane channels 8. Therefore, we asked whether the inappropriate reversal of pre-established cardiac hypertrophy after Stim1 silencing might be linked to increases in the activity of some calcium-dependent anti-hypertrophic molecules. Among different candidates, we detected significant changes in GSK-3β phosphorylation levels following Stim1 silencing (Figure 5A). GSK-3β is constitutively active in un-stimulated cells and GSK-3β is expressed in the mammalian heart where it negatively regulates cardiac hypertrophy. GSK-3β is inhibited through specific phosphorylation of Serine 926. In line with previous reports27, we found that GSK-3β activity is reduced in hearts from TAC-control animals as compared to sham as indicated by a significant increase in the inactive (i.e., phosphorylated) GSK-3β levels (Figure 5A). Stim1 silencing was associated with a significant reduction in GSK-3β phosphorylation levels in both Sham and TAC-treated animals (Figure 5A), indicating a higher level of activity. We then sought to determine if the anti-hypertrophic effects observed after Stim1 silencing were caused by enhanced GSK-3β activity. We thus tested the ability of a pharmacological inhibitor of GSK-3β (TDZD8) to restore hypertrophic responsiveness in AAV9.shStim1 treated animals. We induced pressure-overload heart failure in mice as described in HF model (Figure 3A). However, six weeks after TAC surgery and three weeks after AAV9.shStim1 administration, we randomly assigned animals to receive the GSK-3β inhibitor (TDZD8 10mg/kg daily) or vehicle (DMSO:PBS, 1:10) through intra-peritoneal injections during two weeks (Figure 5B). We documented that treatment with TDZD8 effectively inhibited cardiac GSK-3β in vivo by examining the ratio of phospho-GSK-3β to total GSK-3β in total heart extracts. Treatment with TDZD8 resulted in a significant increase of GSK-3β phosphorylation as compared to treatment with vehicle (Figure 5C). We observed that TDZD8 treatment restored the ability of TAC-AAV9.shStim1 to maintain adaptive cardiac hypertrophy as assessed by echocardiographic (Figure 5D) and morphometric (Figure 5E) analyses. Heart weights were significantly higher in TAC-AAV9.shStim1 treated animals that received TDZD8 (Figure 5F). In addition, TDZD8 blunts the development of cardiac dilation and reduces left ventricular dysfunction after Stim1 silencing (Figure 5D). These results indicate that STIM1 sustains hypertrophic response through the repression of GSK-3β activity.
Figure 5.
GSK-3β inhibition blocked Stim1 silencing pro-atrophic effect. A, Left, Western blot analysis on whole cardiac tissue of GSK3β (total and phosphorylated form of GSK3β at Ser9) and STIM1 from the four groups in the TAC-induced HF model. Right, quantification of phosphorylated form of GSK3β at Ser9 on total. B, Schematic timeline to study GSK3β inhibition effect in the context of Stim1 silencing in a model of TAC-induced heart failure. Vehicle or GSK3β inhibitor (TDZD8) were administered daily via intra-peritoneal administration starting 3 weeks after AAV9.shStim1 injection. C, Left, Western blot analysis on whole cardiac tissue of STIM1 and GSK3β after vehicle or TDZD8 treatment. Right, quantification of Western blots. D, Time-course of Inter Ventricular Septum (IVS) thickness (left), Left Ventricular Internal Diameter (LVID) in diastole (middle) and Fractional Shortening (right) assessed by serial echocardiography in AAV9.shStim1-treated mice receiving vehicle (green) or GSK3β inhibitor (red) E, Left, immunofluorescence analysis of left ventricular sections (8μm) using an antibody against Vinculin (green). Images were taken at 20X magnification. Right, quantification of cardiomyocyte area. F, Heart weights. d=in diastole. n≥3 per groups. * p<0.05, ** p<0.01, *** p<0.001.
STIM1/ORAI-dependent modulation of Akt and GSK3 activities
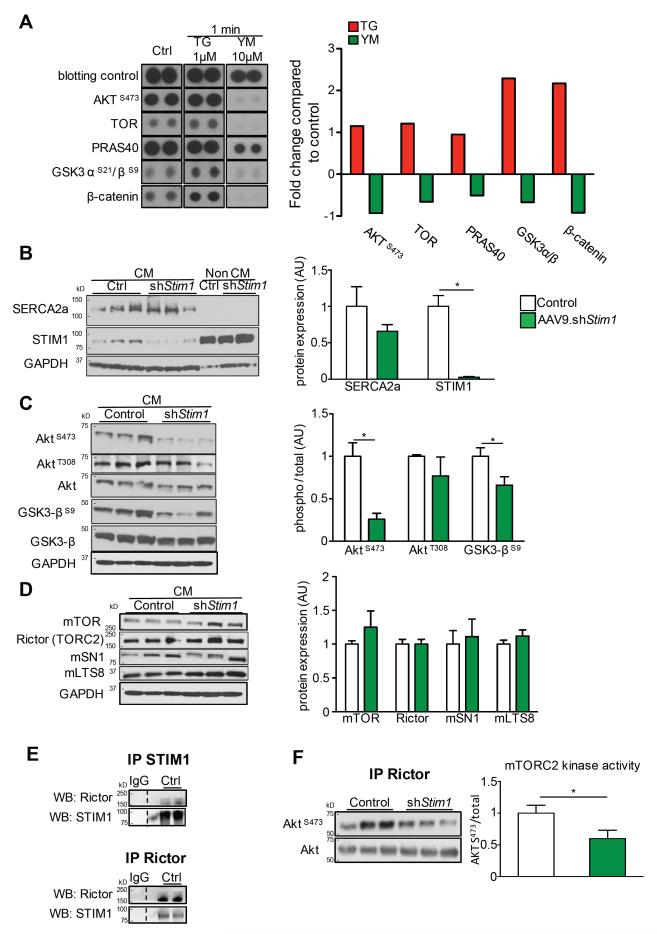
GSK-3β is one of the most studied downstream targets of Akt and the reduced phosphorylation of GSK-3βS9 after Stim1 silencing suggested a reduction in Akt activity. Concordantly, a significant increase in Akt phosphorylation has been shown to be essential for cardiac hypertrophy after TAC in animals28. To analyze the STIM1/Orai-induced phosphorylation events, we first used a human phospho-antibody array in HEK293 cells (Human phospho-kinase array) and analyze site-specific phosphorylation of 45 preselected kinases known to be involved in important signaling pathways (listed in supplemental table 1). In an initial approach, we applied 1 μM of Thapsigargin (TG), a classical activator of STIM1/Orai-mediated SOCE in non-excitable cells. We found that TG application resulted in notable changes in proteins of the Akt pathway (Figure 6A). We observed an important increase in the phosphorylation of GSK3 and β-catenin phosphorylation as well as AktS473, mTOR, and PRAS40 (Figure 6A). We then applied 10 μM of YM-58483, a potent STIM1/Orai pharmacological inhibitor, to HEK293 cells. Reciprocally, we found an important reduction in the phosphorylation of GSK3, β-catenin, mTOR and PRAS40 phosphorylation in the minute following YM-58483 application. The phosphorylation of AktS473 was also particularly reduced (Figure 6A). Phosphorylation of AktT308 was minimal under basal conditions and was not affected by TG or YM-58483 application. These data strongly suggest that STIM1 is required for Akt activity and signaling.
Figure 6.
STIM1/ORAI dependent calcium entry regulates Akt and GSK3β phosphorylation in HEK cells and cardiac myocytes. A, Human phospho-kinase Assay (R&D Systems®) in HEK cells. Typical patterns of five main targets (AktS473, TOR, PRAS40, GSK3 αS21/βS9, β-catenin) and blotting control in basal conditions (left panel), in response to the STIM1 activator Thapsigargin (middle panel) and to the STIM1/Orai blocker YM-58483. Right, quantification of typical changes in phosphorylation of the selected 5 targets compared to control condition. B, Left, Western blot analysis of SERCA2a and STIM1 on isolated cardiac myocytes and non cardiac myocytes fractions from control and AAV9.shStim1 treated mice. Right, quantification of Western blots. C, Left, Western blot analysis of phosphorylation of AktS473, AktT308, GSK3βS9 on isolated cardiac myocytes from control and AAV9.shStim1 infected mice. Right, quantification of Western blots. D, Left, Western blot analysis of mTOR, Rictor, mSN1 and mLTS8 on isolated cardiac myocytes from control and AAV9.shStim1 infected mice. Right, quantification of Western blots. E, STIM1 (Top) or Rictor (Bottom) were immuno-precipitated from isolated mouse cardiac myocytes. Immunoprecipitates with control IgG were used as control. Representative immunoblots of STIM1 and Rictor in both co-immunoprecipitates (as indicated) are shown. F, mTORC2 in vitro kinase assay was performed on control and shStim1-treated cardiac myocytes using immunopurified mTORC2 (Rictor) and recombinant kinase-dead Akt as a substrate. Left, Western blot analysis of AktS473 and total Akt, Right, quantification of Western blot. n=3 per groups for Western blot, n=3 in duplicate for mTORC2 kinase assay. * p<0.05, ** p<0.01, *** p<0.001.
STIM1 is required for mTORC2 signaling in cardiac myocytes
After identifying STIM1 as a modulator of Akt phosphorylation on a human cellular model, we further examined whether this mechanism also translates into mice cardiac myocytes. Wild-type mice were treated with AAV9.shStim1 or control and cardiac myocytes were isolated three weeks later. This strategy resulted in a significant reduction by 90% of STIM1 in isolated cardiac myocytes (characterized by Serca2a expression) whereas STIM1 expression was not altered in non-cardiac myocytes cells (Figure 6B). Concordant with our phospho-kinase array data, we found that Stim1 silencing was associated with a strong reduction in the phosphorylation of Ser473 but not of Thr308 in Akt (Figure 6C). Ser473 in the hydrophic motif of Akt is directly phosphorylated by mTORC2 29. Our later results thus suggest an important reduction of mTORC2 activity following Stim1 silencing. The phosphorylation of both S473 and T308 sites is required to support full activation of Akt and the phosphorylation profile of main Akt substrates (GSK3, PRAS40, Foxo3) was examined. We observed that Stim1 silencing was mainly associated with a significant reduction in phosphorylation of Ser9 in GSK-3β while there were no significant changes in the phosphorylation levels of Foxo3S253 or PRAS40T246 (Supplemental Figure 3).
Because mTORC2 is a multiprotein complex composed of several proteins that are required for its activity, we first assessed the expression of mTORC2 main components (mTor, Rictor, mSN1, mLST8) in isolated cardiac myocytes and did not find any influence of Stim1 silencing on mTORC2 integrity (Figure 6D). We then asked whether STIM1 directly influences mTORC2 activity. We found that STIM1 directly interacts with the mTORC2 complex through a direct physical interaction between STIM1 and Rictor as revealed by specific co-immunoprecipitation experiments (Figure 6E). We then specifically tested mTORC2 activity according to STIM1 expression using an in vitro mTORC2 kinase assay23. We purified mTORC2 complexes from wild-type and Stim1-deficient cardiac myocytes by immunoprecipitating Rictor. We then incubated these isolated complexes with kinase-dead Akt as a substrate, thus avoiding Akt auto-phosphorylation. Akt-pS473 was then measured to directly reflect mTORC2 activity23, 29. We found that Stim1 silencing resulted in a significant reduction in mTORC2 kinase activity toward Akt (Figure 6F). These findings indicate that STIM1 is required for mTORC2 activity and signaling in cardiac myocytes.
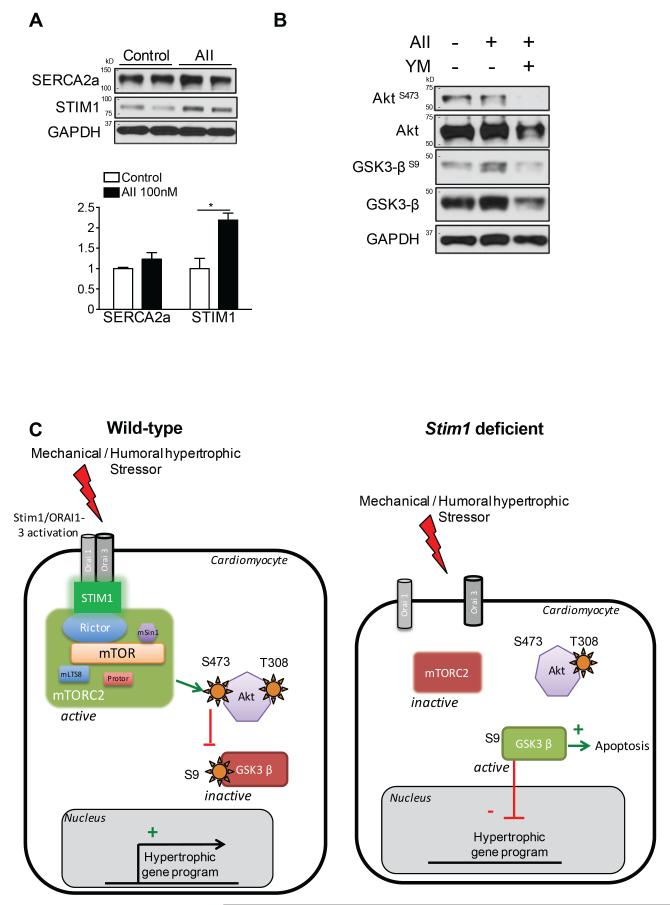
Finally, previous reports have shown that STIM1 activates in response to a mechanical or humoral hypertrophic stressor, which allows gating with plasma membrane Orai channels9. We thus sought to determine whether inhibition of STIM1/Orai complex at the plasma membrane reproduces the effects on mTORC2/Akt activation observed after Stim1 silencing. For this purpose, normal cardiac myocytes were isolated from wild-type mice and stimulated for 48h with angiotensin II or vehicle. Angiotensin II stimulation induced an increase in STIM1 expression, as well as GSK-3βS9 phosphorylation (Figure 7A-B). We applied YM-58483, a potent and selective pharmacological inhibitor of Orai channels, and found a deep decrease in AktS473 and GSK-3βS9 phosphorylation in angiotensin II treated cardiomyocytes (Figure 7B).
Figure 7.
Pharmacological STIM1/ORAI inhibition blocked AktS473 and GSK3βS9 phosphorylation in isolated cardiac myocytes. A, Left, Western blot analysis of SERCA2a and STIM1 on isolated cardiac myocytes from control mice treated 48 hours with Angiotensin II 100nM. Right, quantification of Western blots. B, Western blot analysis of phosphorylation of Akt at Ser473 and GSK3β at Ser9 on isolated cardiac myocytes from control mice treated with 48 hours with Angiotensin II 100nM +/− YM-58483 1μM. C, Schematic model of the role of STIM1 activation on mTORC2/Akt signaling during hypertrophic stimulation in wild-type (left) and Stim1-deficient (right) cardiac myocytes.
DISCUSSION
We and others have previously reported that STIM1 is a critical regulator of cardiac myocytes growth 4, 6, 13-16. These studies were mainly conducted in isolated neonatal and adult cardiomyocytes where Stim1 silencing was associated with an important reduction in agonist-triggered hypertrophic response. STIM1 was reported as an essential activator of the NFAT transcription factor, a well-known positive regulator of cardiac growth 2. This observation was in accordance with genomic screens that identified STIM1 as a critical regulator of NFAT nuclear translocation in immune cells 30. By activating, STIM1 allows Ca2+ entry that further activates the Ca2+ sensor calmodulin and calcineurin 7, 30. This signaling model has however not been reproduced in cardiac cells.
In our current study, we found that reduction in STIM1 expression not only prevented the development of cardiac hypertrophy but also resulted in an inappropriate reversal of established hypertrophy and promotion of left ventricular dilation and dysfunction. Consequently, in response to pressure overload, mice treated with AAV9.shStim1 eventually develop cardiac fibrosis in contrast with our previous observations in rats4. However, both models differ by the type of constriction (thoracic vs. abdominal) and the lack of transition to heart failure in rats. In line with our results, it was reported that Stim1 cardiac myocyte specific deletion in mice led to cardiac dilation, contractile dysfunction and wall thinning over the long-term under physiological conditions6. This supports a direct effect of STIM1 on cardiac homeostasis that is enhanced in response to stress as shown in our study.
At the cellular level, Stim1 silencing promoted cardiac myocytes cells atrophy and apoptosis suggesting that STIM1 activation is rather critical to repress the action of some anti-hypertrophic and pro-apoptotic molecules. Of note, this phenotype was not concordant with current knowledge on Calcineurin/NFAT signaling in the heart that is usually associated with reduction of cardiac hypertrophic response but with preservation of systolic function and cardiac volumes 2, 31, 32. Conversely, accumulating evidence suggests that glycogen synthase kinase-3 (GSK-3) negatively regulates cardiac hypertrophy and that the inhibition of GSK-3 by hypertrophic stimuli is an important mechanism in the stimulation of cardiac hypertrophy 33. GSK-3 is constitutively active in cardiac myocytes where it negatively regulates hypertrophic transcriptional effectors such as GATA4, beta-catenin and NFAT. Pro-hypertrophic stimulation rapidly inactivates GSK-3 through increase in phosphorylation27. GSK-3 has two mammalian isoforms, GSK-3α and –β, which are both expressed in the heart. Even if the exact contribution of each isoforms in cardiac hypertrophy is not fully understood, studies generally show that hyperactive GSK-3 decrease hypertrophic response by reducing cardiac myocyte size but also increasing apoptosis34. Cardiac-specific GSK-3α transgenic mice develop cardiac dysfunction in response to pressure-overload34. Reciprocally, inhibition of GSK-3 during heart failure is protective35. In our study, we found that activity of GSK-3β was dramatically enhanced in the absence of STIM1. Of note, pharmacological inhibition of GSK-3 was sufficient to reverse the cardiac phenotype observed after Stim1 silencing. These data support an unanticipated model where STIM1 is critical to deactivate a key negative regulator of cardiac hypertrophy36.
We further define an intracellular pathway for the regulation of cardiac hypertrophic growth that links STIM1 activation to Akt kinase activity through regulation of mTOR complex 2 (mTORC2) activity. Akt has long been recognized as a pivotal participant in hypertrophic signaling 28 and GSK-3β is one of its direct downstream target. In line with our observation, Akt1−/− mice subjected to seven days of TAC presented LV dilation and contractile dysfunction37. Reciprocally, cardiac-specific over-expression of active Akt prevents pressure-overload induced heart failure partially by reducing apoptosis38. Multiple lines of evidence thus support a cardioprotective role for Akt activation 28 that however involves the phosphorylation of two residues for full activation29: threonine 308 in the activation loop and serine 473 in the c-terminal hydrophobic motif. One of the most surprising results of our study is that Akt phosphorylation specifically at Ser473 was largely dependent on STIM1 activity as demonstrated by the strong decrease after reduction of STIM1 expression or pharmacological inhibition of the STIM1/Orai complex. In contrast, Akt phosphorylation at Thr308, an event driven by the PI3k/PDK1 signaling, was not affected by Stim1 silencing. Even if other candidates have been proposed, phosphorylation of Ser473 is largely supported by mTORC229, 39. Growing evidence shows that mTOR pathway plays a key role in the development of cardiac hypertrophy40. Mice with inducible cardiac-specific mTOR or raptor deletion do not develop compensatory hypertrophy in response to pressure overload and rather develop massive ventricular dilation and cardiac dysfunction associated with apoptosis, autophagy, mitochondrial abnormalities, sarcomere disarray, metabolic abnormalities41, 42, a phenotype that fits with the one we observed after Stim1 silencing. Cardiac-overexpression of mTOR preserves cardiac function during pressure overload, an effect that was potentially linked to increased mTORC2 activity 40, 43. mTOR is indeed a component of both mTORC1 and C2 complexes.
Our results indicate that cardiac abnormalities that developed after Stim1 silencing are explained by an impaired upstream activation of mTORC2. We demonstrated that STIM1 is required for proper mTORC2 kinase activity toward Akt. We then found a direct interaction between STIM1 and rictor, a specific component of mTORC2 complex. These findings suggest that STIM1-mTORC2 interaction is critical to support mTORC2 activity and conveys pro-hypertrophic signaling in cardiac myocytes. The data on specific role of mTORC2 in the regulation of cardiomyocyte growth is scarce but, in addition to our study, some evidence also argues for a cardioprotective role. Rapamycin, a drug that strongly inhibits mTORC1 but to a lesser level mTORC2, blunts cardiac hypertrophy development in response to pressure overload 44 and improves cardiac function in mice with decompensated hypertrophy45. It was recently reported that cardiac-specific mTORC2 disruption through Rictor deletion leads to impaired cardiac growth and response to pressure overload, cardiac dilation and cardiac dysfunction46, features that we similarly found after Stim1 silencing.
To the best of our knowledge, a connection between STIM1 activation and mTORC2/Akt signaling was not previously established. Intriguingly, modifications in Akt Ser473 phosphorylation were observed in skeletal muscle from Stim1-deficient mice 47 and in arterial smooth muscle cells after Orai3 knockdown 48 thus asking whether the STIM1/Akt coupling is specifically observed in muscular cells. We and others have previously shown that in cardiac myocytes, as well as in smooth muscle cells, STIM1 co-immunoprecipitates with Orai1 and 3 channels under resting conditions9, 49. STIM1 might thus represent a large protein complex that can adapt to different stresses to interact with a diversity of complexes12. In response to hypertrophic or proliferative stimulus, a large recruitment of Orai3 to STIM1 and Orai1 occurs thus allowing for a store-independent entry9, 49. This contrasts with the hypothetical model in immune cells where calcium store depletion triggers STIM1 binding and activation of plasma membrane channels including Orai1 8. In addition, STIM1 can also interact with other channels, including TRPCs that have also been reported as effectors of cardiac hypertrophy50.
Altogether, these findings support a novel model (Figure 7C) whereby STIM1 activation, notably in response to hypertrophic stimulation, is critical to tune Akt kinase activity through activation of mTOR complex 2 (mTORC2), which ultimately results in repression of the anti-hypertrophic activity of GSK-3β.
Supplementary Material
Clinical Perspectives.
Cardiac hypertrophy is a compensatory response to increased mechanical load or to neurohormonal stimulation that reduces wall stress by increasing wall thickness. Although it represents an initial salutary adaptation to stress, chronic hypertrophic remodeling involves maladaptive changes in cardiac function over the long term. Different molecules and signaling circuits have been shown to regulate hypertrophic growth but the upstream events at the level of sarcolemma that initiate the cardiac hypertrophic responses remain largely unknown. Here, we provide evidence that STIM1, previously reported as a critical regulator of cardiac myocytes growth, activates in response to hypertrophic stimulation to tune Akt kinase activity though a direct regulation of mTOR complex 2 (mTORC2) activity. Growing evidence shows that mTOR pathway plays a key role in the development of cardiac hypertrophy and we report a direct interaction between STIM1 and Rictor, a specific component of mTORC2 complex. This ultimately results in repression of the anti-hypertrophic molecule GSK-3β, thus promoting cardiac growth. Reciprocally, in the absence of STIM1, the heart is unable to develop or to sustain pre-established cardiac hypertrophy which rapidly leads to left ventricular dilation and dysfunction. These mechanistic insights show a critical connection between STIM1 activation and mTORC2/Akt signaling to support cardiac hypertrophic response to stress and avoid rapid transition to heart failure.
Acknowledgements
We acknowledge the support of the NHLBI Gene Therapy Resource Program (GTRP) for the production of recombinant AAV9s.
Funding Sources: This work was supported by NIH grants RO1 HL113497 (J.S.H.), R00HL116645 (C.K.) and HL083156, HL093183, HL119046, and P20HL100396 and a National Heart, Lung, and Blood Institute Program of Excellence in Nanotechnology (18) Award, Contract HHSN268201000045C (R.J.H.). Part of the work was funded by a Leducq Foundation grant (J.S.H).
Footnotes
Disclosures: None
Author contributions: J.S.H., R.J.H. and L.B. designed the study. L.B. conducted the animal and biochemical experiments with the help of J.O., M.C, A.L, M.N., D.S.M., CK. L.B. and E.K. generated rAAV9s. J.S.H and L.B. drafted the manuscript with critical revision from R.J.H and C.P.
References
- 1.Porrello ER, Olson EN. A neonatal blueprint for cardiac regeneration. Stem Cell Res. 2014;13:556–570. doi: 10.1016/j.scr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 3.Molkentin JD. Dichotomy of ca2+ in the heart: Contraction versus intracellular signaling. J Clin Invest. 2006;116:623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulot JS, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y, Merkle S, Cazorla O, Ouille A, Dupuis M, Hadri L, Jeong D, Muhlstedt S, Schmitt J, Braun A, Benard L, Saliba Y, Laggerbauer B, Nieswandt B, Lacampagne A, Hajjar RJ, Lompre AM, Engelhardt S. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation. 2011;124:796–805. doi: 10.1161/CIRCULATIONAHA.111.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correll RN, Goonasekera SA, van Berlo JH, Burr AR, Accornero F, Zhang H, Makarewich CA, York AJ, Sargent MA, Chen X, Houser SR, Molkentin JD. Stim1 elevation in the heart results in aberrant ca(2+) handling and cardiomyopathy. J Mol Cell Cardiol. 2015;87:38–47. doi: 10.1016/j.yjmcc.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins HE, He L, Zou L, Qu J, Zhou L, Litovsky SH, Yang Q, Young ME, Marchase RB, Chatham JC. Stromal interaction molecule 1 is essential for normal cardiac homeostasis through modulation of er and mitochondrial function. Am J Physiol Heart Circ Physiol. 2014;306:H1231–1239. doi: 10.1152/ajpheart.00075.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 8.Soboloff J, Rothberg BS, Madesh M, Gill DL. Stim proteins: Dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saliba Y, Keck M, Marchand A, Atassi F, Ouille A, Cazorla O, Trebak M, Pavoine C, Lacampagne A, Hulot JS, Fares N, Fauconnier J, Lompre AM. Emergence of orai3 activity during cardiac hypertrophy. Cardiovasc Res. 2015;105:248–259. doi: 10.1093/cvr/cvu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Cobos JC, Zhang X, Zhang W, Ruhle B, Motiani RK, Schindl R, Muik M, Spinelli AM, Bisaillon JM, Shinde AV, Fahrner M, Singer HA, Matrougui K, Barroso M, Romanin C, Trebak M. Store-independent orai1/3 channels activated by intracrine leukotriene c4: Role in neointimal hyperplasia. Circ Res. 2013;112:1013–1025. doi: 10.1161/CIRCRESAHA.111.300220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson JL, Shuttleworth TJ. Molecular basis of activation of the arachidonate-regulated ca2+ (arc) channel, a store-independent orai channel, by plasma membrane stim1. J Physiol. 2013;591:3507–3523. doi: 10.1113/jphysiol.2013.256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao G, Li T, Brochet DX, Rosenberg PB, Lederer WJ. Stim1 enhances sr ca2+ content through binding phospholamban in rat ventricular myocytes. Proc Natl Acad Sci U S A. 2015;112:E4792–4801. doi: 10.1073/pnas.1423295112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA. Stim1-dependent store-operated ca(2)(+) entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol. 2012;52:136–147. doi: 10.1016/j.yjmcc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, Sussman MA, Most P. Orai1 and stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1329–1334. doi: 10.1016/j.yjmcc.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohba T, Watanabe H, Murakami M, Sato T, Ono K, Ito H. Essential role of stim1 in the development of cardiomyocyte hypertrophy. Biochem Biophys Res Commun. 2009;389:172–176. doi: 10.1016/j.bbrc.2009.08.117. [DOI] [PubMed] [Google Scholar]
- 16.Collins HE, Zhu-Mauldin X, Marchase RB, Chatham JC. Stim1/orai1-mediated soce: Current perspectives and potential roles in cardiac function and pathology. Am J Physiol Heart Circ Physiol. 2013;305:H446–458. doi: 10.1152/ajpheart.00104.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong D, Cha H, Kim E, Kang M, Yang DK, Kim JM, Yoon PO, Oh JG, Bernecker OY, Sakata S, Le TT, Cui L, Lee YH, Kim do H, Woo SH, Liao R, Hajjar RJ, Park WJ. Picot inhibits cardiac hypertrophy and enhances ventricular function and cardiomyocyte contractility. Circ Res. 2006;99:307–314. doi: 10.1161/01.RES.0000234780.06115.2c. [DOI] [PubMed] [Google Scholar]
- 18.Stein AB, Tiwari S, Thomas P, Hunt G, Levent C, Stoddard MF, Tang XL, Bolli R, Dawn B. Effects of anesthesia on echocardiographic assessment of left ventricular structure and function in rats. Basic Res Cardiol. 2007;102:28–41. doi: 10.1007/s00395-006-0627-y. [DOI] [PubMed] [Google Scholar]
- 19.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 21.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microrna/short hairpin rna pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 22.Ikenoue T, Hong S, Inoki K. Monitoring mammalian target of rapamycin (mtor) activity. Methods Enzymol. 2009;452:165–180. doi: 10.1016/S0076-6879(08)03611-2. [DOI] [PubMed] [Google Scholar]
- 23.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mtorc2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Piras BA, O'Connor DM, French BA. Systemic delivery of shrna by aav9 provides highly efficient knockdown of ubiquitously expressed gfp in mouse heart, but not liver. PLoS One. 2013;8:e75894. doi: 10.1371/journal.pone.0075894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, stim1, reciprocally controls orai and cav1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (gsk3): Regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, De Acetis M, Vecchione C, Marino G, Altruda F, Silengo L, Tarone G, Lembo G. Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat Med. 2003;9:68–75. doi: 10.1038/nm805. [DOI] [PubMed] [Google Scholar]
- 28.Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, Gude N, Avitabile D, Alvarez R, Sundararaman B, Quijada P, Mason M, Konstandin MH, Malhowski A, Cheng Z, Khan M, McGregor M. Myocardial akt: The omnipresent nexus. Physiol Rev. 2011;91:1023–1070. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of akt/pkb by the rictor-mtor complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 30.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: Store-operated ca2+ entry and nfat activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Hill JA, Rothermel B, Yoo KD, Cabuay B, Demetroulis E, Weiss RM, Kutschke W, Bassel-Duby R, Williams RS. Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy. Preservation of systolic function. J Biol Chem. 2002;277:10251–10255. doi: 10.1074/jbc.M110722200. [DOI] [PubMed] [Google Scholar]
- 32.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in calcineurin abeta -deficient mice. Proc Natl Acad Sci U S A. 2002;99:4586–4591. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: A novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 34.Zhai P, Gao S, Holle E, Yu X, Yatani A, Wagner T, Sadoshima J. Glycogen synthase kinase-3alpha reduces cardiac growth and pressure overload-induced cardiac hypertrophy by inhibition of extracellular signal-regulated kinases. J Biol Chem. 2007;282:33181–33191. doi: 10.1074/jbc.M705133200. [DOI] [PubMed] [Google Scholar]
- 35.Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Gao S, Hong C, Yatani A, Avila J, Sadoshima J. Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circ Res. 2007;101:1164–1174. doi: 10.1161/CIRCRESAHA.107.160614. [DOI] [PubMed] [Google Scholar]
- 36.Hardt SE, Sadoshima J. Negative regulators of cardiac hypertrophy. Cardiovasc Res. 2004;63:500–509. doi: 10.1016/j.cardiores.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 37.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 38.Ceci M, Gallo P, Santonastasi M, Grimaldi S, Latronico MV, Pitisci A, Missol-Kolka E, Scimia MC, Catalucci D, Hilfiker-Kleiner D, Condorelli G. Cardiac-specific overexpression of e40k active akt prevents pressure overload-induced heart failure in mice by increasing angiogenesis and reducing apoptosis. Cell Death Differ. 2007;14:1060–1062. doi: 10.1038/sj.cdd.4402095. [DOI] [PubMed] [Google Scholar]
- 39.Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase b/akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 40.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114:549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. Mtorc1 regulates cardiac function and myocyte survival through 4e-bp1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Ruegg MA, Pedrazzini T, Brink M. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- 43.Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. Mtor attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–1266. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 45.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mtor signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 46.Sciarretta S, Zhai P, Maejima Y, Del Re DP, Nagarajan N, Yee D, Liu T, Magnuson MA, Volpe M, Frati G, Li H, Sadoshima J. Mtorc2 regulates cardiac response to stress by inhibiting mst1. Cell Rep. 2015;11:125–136. doi: 10.1016/j.celrep.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li T, Finch EA, Graham V, Zhang ZS, Ding JD, Burch J, Oh-hora M, Rosenberg P. Stim1-ca(2+) signaling is required for the hypertrophic growth of skeletal muscle in mice. Mol Cell Biol. 2012;32:3009–3017. doi: 10.1128/MCB.06599-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Zhang X, Gonzalez-Cobos JC, Stolwijk JA, Matrougui K, Trebak M. Leukotriene-c4 synthase, a critical enzyme in the activation of store-independent orai1/orai3 channels, is required for neointimal hyperplasia. J Biol Chem. 2015;290:5015–5027. doi: 10.1074/jbc.M114.625822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Zhang W, Gonzalez-Cobos JC, Jardin I, Romanin C, Matrougui K, Trebak M. Complex role of stim1 in the activation of store-independent orai1/3 channels. J Gen Physiol. 2014;143:345–359. doi: 10.1085/jgp.201311084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eder P, Molkentin JD. Trpc channels as effectors of cardiac hypertrophy. Circ Res. 2011;108:265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.