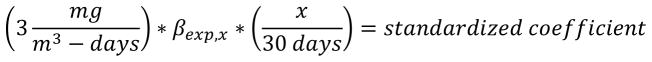Abstract
Purpose
We sought to understand the time course between exposure to manganese (Mn) and uptake into the blood, to allow a more meaningful interpretation of exposure-biomarker data, and determine the utility of blood as a biomarker of Mn exposure.
Methods
Welder trainees were monitored over the course of a five quarter training program. Each quarter, trainees gave eight blood samples and had personal air monitoring four times. A mixed model was fit to obtain estimates of airborne exposure by welding type (fixed effect), adjusted for subject (random effect). Considering weekends and days absent as zero exposure, estimated exposures were summed over various exposure windows, and related to measured blood manganese (MnB) using a mixed model.
Results
A relationship consistent with zero was found between MnB and modeled one or seven day exposure. After 30 days of preceding exposure, a one mg/m3-days increase in air Mn is associated with a 0.57 ng/mL increase in MnB (95% CI: −0.04, 1.19). Considering a 90 day exposure window and a cumulative exposure window a one mg/m3-days increase in air Mn is associated with a 0.26 (95% CI: 0.005, 0.51) and 0.09 (95% CI: 0.006, 0.17) ng/mL increase in MnB, respectively.
Conclusions
From this analysis, MnB may begin to act as a biomarker of Mn exposure over longer time periods, or at higher levels of exposure. This novel study design allowed investigation of how MnB relates to different time windows of exposure, representing the most robust Mn exposure assessment in the biomarker literature.
Keywords: manganese, welding, exposure assessment, biomarker of exposure, exposure windows, exposure modelling
INTRODUCTION
Exposure to manganese (Mn) has been implicated in manganism, a parkinsonian syndrome overlapping clinically with Parkinson’s Disease (PD), making Mn exposure assessment of interest to many public health professions (Antonini et al. 2006b; Aschner 2000; Calne et al. 1994). One such tool for exposure assessment is biomonitoring—as this is often cheaper, easier, individual-specific, and may better estimate body dose as compared to traditional air monitoring techniques. Commonly, body fluids such as urine (Ellingsen et al. 2006; Järvisalo et al. 1992; Roels et al. 1987), whole blood (Ellingsen et al. 2006; Pesch et al. 2012; Smith et al. 2007), plasma (Bowler et al. 2007; Grass et al. 2010), or serum (Chia et al. 1993; Myers et al. 2003) are used as biomarkers of exposure to Mn, but hair (Bader et al. 1999; Rodrigues et al. 2008), toenails (Laohaudomchok et al. 2011; Sriram et al. 2012), and MRI (Baker et al. 2015a; Criswell et al. 2012) have also been explored in the literature as exposure biomarkers for Mn.
Ingested Mn is tightly regulated in the body (with the majority being eliminated via feces), and ambient environmental exposures are generally low. Thus, it is inhalation in occupational environments where excessive exposure to Mn usually occurs (Aschner et al. 2005). Welding shops are one such occupational environment, with welders being exposed to eight hour mean personal breathing zone concentrations of Mn ranging from 0.04 mg/m3 to over 2.0 mg/m3 based on the welding type, degree of enclosure, and use of ventilation and respiratory protection (Hobson et al. 2011). While Mn in welding fume deposited in the nasopharyngeal airways can reach the brain through direct olfactory transport, Mn also enters the circulatory system via the lung and crosses the blood brain barrier to reach the target organ (Antonini et al. 2006b; Tjalve and Henriksson 1999). However, timing of uptake from the lung into the blood is dependent on particle size distribution, solubility of the Mn compounds, concentration, and individual factors (Berlinger et al. 2008). Given the neurologic health effects associated with Mn in welding fume, it is reasonable to assume that after uptake, inhaled Mn is circulating in the blood prior to either elimination or crossing the blood brain barrier and reaching the target organ, making blood Mn (MnB) seem an attractive biomarker of Mn exposure (Aschner et al. 2007; Criswell et al. 2011; Racette et al. 2005).
As a result, many studies have explored blood as a biomarker of Mn inhalation exposure, yet there is no clear consensus as to its utility and limitations. In previously published work, MnB levels appear to be fairly tightly regulated within an individual, and there is substantial between-individual variation, which could make interpretation of the relationship between blood Mn and Mn exposure challenging with a common cross-sectional study design (Baker et al. 2015b). It has been largely cross-sectional study designs that dominate the literature relating MnB and inhalation exposure. We reviewed the existing body of literature for articles relating airborne Mn and MnB. We weighted the 29 studies included in the review by study size, and fit a segmented regression model. Our results showed that there appeared to be a positive linear relationship between MnB and airborne Mn when Mn exposure exceeded approximately 10μg/m3 (Baker et al. 2014). However, 26 of the 29 studies included in this review were cross-sectional, relating a single MnB and air measurement that might not have been taken at pharmacologically relevant (or even concurrent) time periods.
The pharmacokinetics of Mn in humans exposed to welding fume is not well established, with the current body of toxicology literature focusing on the pharmacokinetics related to primate or rodent exposure to compounds of Mn such as manganese chloride, manganese phosphate, or manganese sulfate. These chemicals differ from the Mn compounds known to predominate in a welding setting (Antonini et al. 2006b; Dorman et al. 2001; Vitarella et al. 2000; Zheng et al. 2000). To explore the timing between Mn exposure, uptake, and health effects in an occupational setting, Antonini et al. developed a pharmacokinetic model using animals exposed to generated welding fume in a controlled chamber (2006a). In contrast, the current manuscript aims to explore the timing between exposure to Mn and possible changes in blood Mn in an occupational setting by utilizing longitudinal blood and air measurements from welder trainees. With repeat exposure measurements in a well-characterized cohort, we are able to determine subject specific estimates of exposure for each day the trainee was enrolled in our study, and then sum these estimates over different time windows of exposure for comparison with longitudinal MnB measurements. With repeat blood measurements for each subject, this manuscript also aims to examine the variance components exhibited in MnB. Understanding the time course between exposure to Mn and uptake into the blood will allow a more meaningful interpretation of exposure-biomarker data, and help to determine the utility of blood as a biomarker for Mn exposure.
METHODS
Occupational setting and study population
The study setting for this manuscript is a longitudinal inception cohort of 56 student welders with no prior occupational exposure to Mn, who were enrolled in a welding training program at a technical college in Washington state. Subjects were enrolled throughout the duration of the study, with data collection occurring from April 2011 to June 2013. Enrolled welding trainees were monitored for airborne exposure and MnB over the course of their training program. Details of the study design are described elsewhere (Baker et al. 2014). All study protocols were reviewed and approved by the University of Washington Institutional Review Board and subjects provided written informed consent.
The welding training program consisted of five academic quarters, with standard academic breaks in between, where students progressed through different welding processes in the order: oxyacetylene, shielded metal arc welding (SMAW), flux core arc welding dual shield (FCAW-DS), flux core arc welding inner shield (FCAW-IS), gas metal arc welding (GMAW), and gas tungsten arc welding (GTAW). The number of days spent on each process could vary between subjects. Students also intermittently did other metalworking tasks (i.e. cutting and/or grinding) which we characterized as a separate work category if that is what the welder reported doing for the majority of the day. Trainees were in the facility Monday through Friday from about 8 AM to 2 PM, but time spent welding was interspersed with classroom instruction. Each welding booth in the facility had its own adjustable exhaust ventilation hood, and students were given the choice of whether or not to buy and wear respiratory equipment, which was not provided. There was no formal respiratory protection training or fit testing related to the traineeship.
Each quarter the subject was enrolled in the study a nurse drew 6 mL blood samples at the following times: the morning and afternoon of the first Monday and Friday of the quarter, and morning and afternoon on the last Monday and Friday of the quarter, for a potential eight blood samples each academic quarter. On each day that a subject provided blood samples, they were also fitted with a personal air pump for the duration of activity at the school to assess airborne exposure to Mn. At the end of each sampling day, subjects completed an exposure questionnaire to determine the type of welding done that day, the time spent welding, the use of any respiratory protection, and to assess any metalworking done outside of class time.
Blood Mn sampling and analysis
On mornings and afternoons of sampling days, 6 mL of whole blood was collected in plastic Vacutainer evacuated tubes containing 10.8 mg K2EDTA anticoagulant (BD, Franklin Lakes, NJ) and transported on ice to the American Industrial Hygiene Association (AIHA) Accredited University of Washington Environmental Health Laboratory (UW EHL) within two hours of collection. One mL of whole blood was pipetted into a 15 mL Corning CentriStar polypropylene centrifuge tube (Corning, NY), stored at 4 degrees C until multi-element analysis of whole blood using an Agilent 7500 CE ICP-MS via microwave assisted acid digestion (Bocca et al. 2003). Over the course of the 26 month study, blood was submitted for analysis in 18 batches, but samples were randomly assigned to batches with regard to time of day, time of week, and time of quarter. The limit of detection (LOD) for Mn ranged from 0.1 – 2.0 ng/mL and is based on three times the standard deviation of the blanks. A total of 1170 blood samples were analyzed. No blood samples fell below the LOD for Mn. The slight variability in LOD is due to changing levels of blank contamination in the UW EHL over the 26 month time period that samples were submitted for analysis. Between batches, the percent recovery for Mn as compared to the control material (ClinChek Level 1 Whole Blood Control, lyophilized for trace elements (Lot 038), Recipe Chemicals, Munich, Germany) ranged between 91% and 134% for all 18 batches with an overall average accuracy of 110% ± 12%. The average within batch variation in the control material was 6.8% ± 6.1%.
Airborne Mn exposure assessment
On each sampling day (four days each academic quarter) the subject was enrolled in the study, they were fitted with a personal air pump (SKC AirChek XR4000, Eighty Four, PA, USA) with an attached pre-weighed 37mm 0.8μm pore mixed cellulose ester filter housed inside a closed face polystyrene cassette and hung on their collar outside the welding garments and helmet. The pumps were calibrated to 2.0 L/min, and this flow rate was checked at the beginning and end of each full-day sampling period. One sample had a ± 10% change in pump flow rate and was excluded. Filters were analyzed gravimetrically for total particulate mass, digested in 10mL of a 1:1 mixture of concentrated nitric acid and deionized water using open vessel microwave assisted digestion, and analyzed at UW EHL for trace metals using an Agilent 7500 CE ICP-MS in He collision mode to eliminate polyatomic interferences. Samples were analyzed using the modified United States Environmental Protection Agency (US EPA) method 6020a Revision 1 (U.S. Environmental Protection Agency 1998). Each day of sampling included two field blanks, which were handled in the same manner as the samples deployed in the field. Over the course of the 26 month study, 622 air samples were submitted for metals analysis in six batches, and were randomized with regard to time of week and time of quarter. Between batches, assay accuracy based on the spike recovery samples ranged between 88.0% and 110% with an overall average accuracy of 99.4 ± 7.2% for the six batches. The average within batch variation in the spiked samples was 8.2% ± 9.7%. Considering differences in pump flow rate and duration of sampling between the subjects, the maximum LOD for Mn was 0.13 μg/m3 μg, based on three times the standard deviation of the blanks deployed in the field. No air samples fell below the LOD for Mn.
Statistical analysis
Measured Mn air concentrations were normalized to 8-hr TWA Mn concentrations, and as the measured data were determined to be lognormal, the exposure samples were natural log-transformed. A mixed model was fit to obtain estimates of exposure by welding type (fixed effect), adjusted for subject (random effect) on the log-transformed data. While enrolled in the welding program, attendance and progress records were used to determine days each subject was present and welding, and what type of welding the subject was doing each day. Thus, on each day a subject was present and welding, estimated subject-specific exposure based on the type of welding done that day was calculated. Weekends, vacation days, and days the subject was absent were coded as zero-exposure days. Thus, for each subject we generated a personal calendar of estimated Mn airborne exposure for each day they were enrolled in the welding program, based on attendance and type of welding. A total of 18,833 day- and subject-specific exposure estimates were generated, including zero-exposure days. Results of the models on the ln-scale were used to calculate the subject- and day-specific arithmetic mean exposure by using maximum likelihood estimates, incorporating the within-subject variance.
For each blood sampling day, we summed preceding exposures over various exposure windows: one day, seven day, 30 day, 90 day, and cumulative, which was a summation of all exposures from entry into the welding program until the day of the blood sample. All exposure windows are in units of mg/m3-days. The average number of exposed days represented by the cumulative exposure measure is 197 ± 142, ranging from 1 day (the minimum number of days of exposure on a subject’s first blood sample) to 805 days. About 25% of cumulative exposure periods included in this mixed model represent time periods of less than 90 days. For afternoon blood samples, the same day estimated exposure counted in the exposure windows; for morning blood samples the previous day was counted as the first day of previous exposure.
The associations between predicted exposures (for all five exposure windows) and measured MnB were assessed on the native scale using a longitudinal linear mixed-effects model including subject as a random effect. Several covariates were considered in the mixed models, including use of respiratory protection (based on self-reported percentage the subject used a respirator during sampling weeks), age at baseline, and smoking status, but none were found to affect the slope parameter of interest. Thus, reported models associating MnB and estimated exposure only include subject as a random effect with no additional fixed effect covariates. All presented models use only afternoon blood values in relation to preceding windows of exposure. Using morning blood values or the change in blood value over the course of a day did not affect the coefficients of association, and we wished to avoid making redundant models by modeling morning blood, afternoon blood, and the change in blood values over the course of a day in relation to preceding windows of exposure.
To directly compare the coefficients of association relating MnB to airborne Mn exposure over the five exposure windows despite each representing different durations of exposure, all point estimates from the linear mixed-effects models were normalized to a 30-day average exposure value (see equation 1). We standardized the effects to 3 mg/m3-days based on the American Conference of Industrial Hygienists (ACGIH) recommended 8-hr time weighted average threshold limit value (TWA TLV) for inhalable Mn of 0.1 mg/m3 over a 30 day exposure window, by multiplying 0.1 mg/m3 by 30 days in a month. Each estimated coefficient of association between MnB and air exposure for the various exposure windows was normalized to the value of 3 mg Mn/m3 over 30 days (for cumulative exposure we used the mean number of days representing a cumulative exposure, x=197) as:
Equation 1.
Calculating 30 day normalized exposure for the exposure windows
x = length of the exposure window
βexp,x = coefficient of association between MnB and air exposure for the exposure window represented by x
RESULTS
Of the inception cohort, 52 of the 56 subjects were male (93%). The average subject was 28 years of age at baseline, provided 21 blood samples (SD: 5.9, range: 1, 26) while enrolled in our study, and had 11 air samples (SD: 11.4, range: 4, 48). Only 25% of subjects (n=14) used a respirator more than 90% of the time.
Measured and predicted Mn exposures, along with blood values, are summarized by welding type in Table 1. The number of predicted exposure values for each welding type is fewer than the number of measured exposure values because only one predicted exposure value was generated for each welder for each welding type they completed while enrolled in our study. Thus, welders who did not complete a welding type do not have an estimated exposure value for that welding type. The arithmetic mean predicted values are similar to the measured exposure values, though geometric mean values were more different. The measured exposure mean values are the means of all collected exposure values, whereas the predicted mean values are means of the individual mean levels. Thus, the within-subject variation has already been incorporated into the geometric mean value for the predicted exposure values, so the predicted values have higher geometric mean and lower GSDs relative to the measured exposure values.
Table 1.
Measured and predicted Mn exposure values (μg/m3) and blood values (ng/mL) by welding type
|
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measured (μg/m3) | Predicted (μg/m3)*** | All Blood (ng/mL) | ||||||||||
|
|
|
|
||||||||||
| Welding Type | n* | AM | GM | GSD | n** | AM | GM | GSD | n* | AM | GM | GSD |
|
|
|
|
||||||||||
| Oxyacetylene | 80 | 5.2 | 4.2 | 2.0 | 56 | 6.4 | 6.4 | 1.2 | 147 | 8.0 | 7.4 | 1.4 |
| SMAW | 315 | 34.7 | 22.8 | 3.0 | 55 | 36.8 | 36.0 | 1.2 | 547 | 8.2 | 7.5 | 1.5 |
| FCAW (Inner shield) | 32 | 34.5 | 23.6 | 3.0 | 37 | 35.7 | 34.9 | 1.2 | 92 | 9.1 | 8.4 | 1.5 |
| FCAW (Dual shield) | 75 | 40.7 | 25.5 | 3.6 | 43 | 46.1 | 44.9 | 1.3 | 141 | 9.3 | 8.8 | 1.4 |
| GMAW | 62 | 28.6 | 21.0 | 2.3 | 34 | 33.7 | 33.0 | 1.2 | 117 | 9.4 | 8.8 | 1.4 |
| GTAW | 36 | 5.5 | 4.0 | 2.3 | 29 | 6.4 | 6.3 | 1.2 | 104 | 9.6 | 8.8 | 1.5 |
| Cut/Grind | 22 | 13.1 | 5.6 | 3.7 | 21 | 8.3 | 8.0 | 1.3 | 22 | 8.6 | 8.1 | 1.4 |
| Total | 622 | 28.6 | 15.9 | 3.5 | 275 | 26.2 | 19.2 | 2.4 | 1170 | 8.6 | 8.0 | 1.5 |
|
|
|
|||||||||||
number of collected samples
number of subjects who did this type of welding
A mixed model was fit using ln-transformed exposure data to obtain predicted levels of airborne exposure by welding type (fixed effect), adjusted for subject (random effect). Arithmetic mean exposures were then calculated by using maximum likelihood estimates, incorporating the within-subject variance.
AM=arithmetic mean, GM=geometric mean, GSD=geometric standard deviation
Arithmetic mean MnB was highest during GTAW welding (9.6 ± 3.8 ng/mL) which is the last module welders complete in the program, but has some of the lowest measured Mn exposures (arithmetic mean: 5.5 ± 4.4 μg/m3). Arithmetic mean MnB was lowest during oxyacetylene welding (8.0 ± 3.0 μg/m3) which is the first welding module welders complete in the program, and would include most baseline MnB measurements. Oxyacetylene welding also represents the lowest measured airborne Mn exposure (arithmetic mean: 5.2 ± 3.1 μg/m3). The welding type with the highest measured Mn exposure was FCAW-DS, with arithmetic mean exposures of 40.7 ± 32.4 μg/m3. Only 3.4% of samples (n=21) exceed the ACGIH inhalable 8-hr TWA TLV of 0.1 mg/m3, and all measured exposures were below the Occupational Safety and Health Administration permissible exposure limit (OSHA PEL) for Mn in total inhalable dust of 5.0 mg/m3 (ceiling). Table 2 shows the predicted exposure estimates that were used in the longitudinal linear mixed models for all the time points considered in this analysis.
Table 2.
Predicted Mn exposure estimates used in the longitudinal linear mixed models, for all time windows considered
| Predicted Mn exposure values (μg/m3-days) | |||||
|---|---|---|---|---|---|
|
| |||||
| Exposure window | n observations | n subjects | AM | SD | Range |
| 1 day | 570 | 56 | 30.9 | 15.4 | 4.14, 68.9 |
| 7 day | 570 | 56 | 118.4 | 74.8 | 4.70, 324.1 |
| 30 day | 570 | 56 | 422.3 | 296.4 | 4.85, 1340.9 |
| 90 day | 570 | 56 | 1243.1 | 772.2 | 4.85, 3354.3 |
| Cumulative | 570 | 56 | 2980.0 | 2339.2 | 4.85, 10514.1 |
AM = arithmetic mean
Table 3 shows descriptive statistics for MnB over the various time points, stratified by morning and afternoon samples. For paired samples, MnB tends to decrease over the course of a day, even when stratifying by time of week and time of quarter. A paired t-test found all but one of these decreases to be significantly different from zero. Despite these apparent decreases, using morning blood values or the change in blood value over the course of a day did not meaningfully affect the coefficients of association in any of the mixed models considered here. A linear mixed model including subject as a random effect was used to estimate differences in MnB over the course of a week and quarter, stratified by time of day. The results are also reported in Table 3. MnB tended to decrease over the course of a week for both morning and afternoon samples (though only significantly different from zero in afternoon samples), and didn’t show a change different from zero over the course of a quarter for either morning or afternoon samples.
Table 3.
Mn Blood descriptives over various time points, stratified by time of day
| Morning | Afternoon | Afternoon - Morning* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | AM | SD | 95% CI | n | AM | SD | 95% CI | n | AM | SD | 95% CI | |
|
|
|
|
||||||||||
| Day | 600 | 8.84 | 3.84 | 570 | 8.38 | 3.19 | 557 | −0.45 | 0.13 | (−0.70, −0.19) | ||
| Week | ||||||||||||
| Begin | 315 | 8.95 | 3.79 | 301 | 8.51 | 3.30 | 299 | −0.38 | 0.19 | (−0.76, −0.02) | ||
| End | 285 | 8.72 | 3.90 | 269 | 8.23 | 3.06 | 258 | −0.53 | 0.19 | (−0.90, −0.16) | ||
| End - Begin** | 600 | −0.25 | 0.24 | (−0.71, 0.21) | 570 | −0.33 | 0.16 | (−0.65, −0.01) | ||||
| Quarter | ||||||||||||
| Begin | 335 | 8.91 | 3.81 | 313 | 8.29 | 3.14 | 309 | −0.64 | 0.20 | (−1.04, −0.24) | ||
| End | 265 | 8.75 | 3.88 | 257 | 8.49 | 3.25 | 248 | −0.22 | 0.16 | (−0.53, 0.08) | ||
| End - Begin** | 600 | −0.18 | 0.24 | (−0.64, 0.29) | 570 | 0.21 | 0.17 | (−0.12, 0.53) | ||||
AM = arithmetic mean
Paired t-test to assess difference in blood Mn over the course of a day (Afternoon - Morning) stratified by time of week and time of quarter
Linear mixed model including subject as a random effect to estimate paired difference in blood Mn over the course of a week and quarter, stratified by time of day
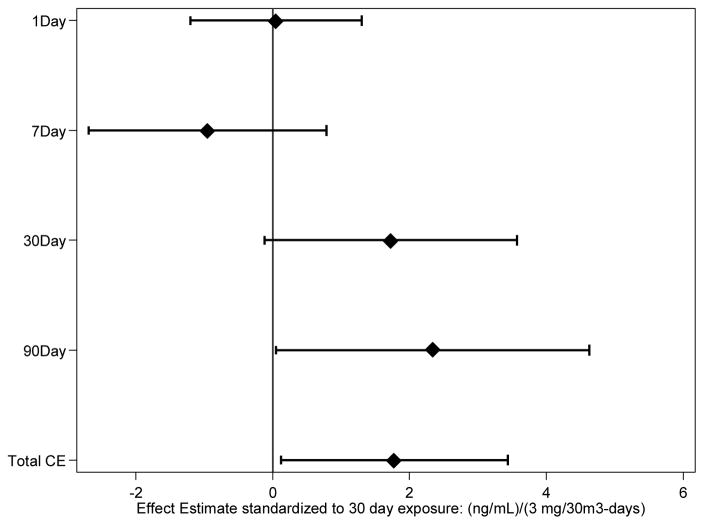
Table 4A shows the estimated coefficients of association between predicted exposure over the various time windows and MnB measured in the afternoon. Table 4B shows these coefficients standardized to a 30-day exposure period. Afternoon MnB showed no relationship with that day’s exposure, or the preceding seven or 30 days of exposure. However, when considering the preceding 90 days exposure, a slight relationship emerges, with a one mg/m3-days increase in air Mn being associated with, on average, a 0.26 ng/mL increase in MnB (SE: 0.13, 95% CI: 0.005, 0.514). Similarly, when considering all preceding exposures (cumulative exposure) a one mg/m3-days increase in air Mn is associated with, on average, a 0.09 ng/mL increase in MnB (SE: 0.04, 95% CI: 0.005, 0.174). Standardized to a 30-day exposure period, a 3 mg/m3 increase in 90 day Mn exposure is associated with a 2.34 ng/mL average increase in MnB (SE: 1.17, 95% CI: 0.049, 4.63), and a 3 mg/m3 increase in cumulative exposure is associated with an average increase in MnB of 1.77 ng/mL (SE: 0.85, 95% CI: 0.125, 3.44). Figure 1 demonstrates the increasing relationship between MnB and exposure over longer time periods or higher levels of cumulative exposure when considering these standardized coefficients.
Table 4.
A & B. Linear mixed model associations between exposure windows and MnB
| A.
| |||||||
|---|---|---|---|---|---|---|---|
| Fixed Effects (ng/mL)/mg/m3-days | Coefficient (SE) | CI | Within Var (SE) | % | Between Var (SE) | % | Total Var |
| 1 day exposure window | 0.48 (6.37) | −12.02, 12.98 | 3.7 (0.23) | 29% | 8.9 (1.2) | 71% | 12.6 |
| 7 day exposure window | −1.36 (1.26) | −3.84, 1.12 | 3.7 (0.23) | 39% | 5.9 (1.2) | 61% | 9.6 |
| 30 day exposure window | 0.57 (0.31) | −0.04, 1.19 | 3.7 (0.23) | 39% | 5.8 (1.2) | 61% | 9.5 |
| 90 day exposure window | 0.26 (0.13) | 0.005, 0.51 | 3.7 (0.23) | 39% | 5.7 (1.2) | 61% | 9.4 |
| Cumulative exposure | 0.09 (0.04) | 0.006, 0.17 | 3.7 (0.23) | 39% | 5.7 (1.2) | 61% | 9.4 |
| B.
| ||
|---|---|---|
| Adjusted to 30 day exposure period (ng/mL)/(3 mg/m3-days) | Coefficient (SE) | CI |
| 1 day exposure window | 0.05 (0.64) | −1.20, 1.30 |
| 7 day exposure window | −0.95 (0.89) | −2.69, 0.79 |
| 30 day exposure window | 1.71 (0.94) | −0.12, 3.57 |
| 90 day exposure window | 2.34 (1.17) | 0.05, 4.63 |
| Cumulative exposure | 1.77 (0.85) | 0.13, 3.44 |
Figure 1.
Effect estimate of exposure windows
Variance components are also presented in Table 4A, and it can be seen that for all exposure windows considered the majority of variance is between subjects. This was similar to what was found in our previous work on a more restricted sample of subjects from this cohort (Baker et al. 2015b).
DISCUSSION
The longitudinal study design presented here allowed investigation of relationships between measured MnB and modeled inhalation exposures occurring over different time windows, the first time such work has been presented in the literature. With this analysis, we found that when considering the preceding 30 days of exposure, a one mg/m3-days increase in air Mn is associated with, on average, a 0.57 ng/mL increase in MnB (95% CI: −0.04, 1.19), but this estimate is still consistent with a zero slope. However, when considering a 90 day preceding exposure window, and a cumulative exposure window, a one mg/m3-days increase in air Mn is associated with a 0.26 (95% CI: 0.005, 0.51) and 0.09 (95% CI: 0.006, 0.17) ng/mL increase in MnB, respectively, both results that were found to be significantly different from a zero slope.
Point estimates for all the exposure windows (one day, seven day, 30 day, 90 day, cumulative) were normalized to a 3mg/m3-days exposure. This exposure was based on 30 days of exposure at the ACGIH recommended 8-hr TWA TLV for inhalable Mn of 0.1 mg/m3. Upon normalizing the point estimates, an increasing trend emerged when looking at the relationship between afternoon MnB and summed exposures in the preceding seven, 30, and 90 day exposure windows. Though the coefficients for one, seven, and 30 day exposure windows were consistent with a zero slope, when considering the 90 day exposure window a 3 mg/m3-days increase in exposure over a 30 day period was consistent with a 2.34 ng/mL increase in MnB (SE: 1.17, 95% CI: 0.049, 4.63). Cumulative exposure also maintained a significant increase in MnB, with a 3 mg/m3-days increase in exposure over a 30 day window being consistent with a 1.77 ng/mL increase in MnB (SE: 0.85, 95% CI: 0.13, 3.44).
This relationship between MnB and Mn air exposure at higher accumulated levels of exposure (which would occur after a longer time of exposure) is consistent with what was presented in our previous review relating measures of MnB and Mn exposure with mostly cross-sectional data from the literature. In that meta-analysis, when weighting by study size and fitting a segmented regression, the relationship between MnB and Mn exposure became apparent after a certain threshold of exposure was reached, at about 10 μg/m3 (Baker et al. 2014). Similarly, Pesch et al. (2012) found that measured MnB seemed to relate to measured respirable Mn in air at around 50–100 μg/m3, below which no obvious correlation was present. However, both our review and the work by Pesch et al. found associations between MnB and exposure at lower levels of exposure than we found in this study, as we didn’t see an apparent relationship until after 30 days of accumulated exposure, and the mean value used in our model for a 30 day accumulated exposure window was about 420 μg/m3-days. However, models presented in this paper are specific to accumulated exposure and duration of exposure, whereas our review paper and the work by Pesch et al. are specific to daily exposure estimates. As such, neither our review nor the work by Pesch et al. took into account the temporal aspects between air exposure and blood uptake, or used a cumulative measure of exposure for different time periods, which was done in this study, thanks to our ability to quantify temporal resolution with our longitudinal study design. Also, as subjects in our study were welding trainees with no previous occupational exposure to Mn, the delay before seeing a relationship between blood Mn and predicted airborne Mn could also be due to the time necessary for the blood Mn to reach a steady state in the trainees. With career welders or in persons with long term, consistent exposure to Mn, the relationship between blood Mn and air Mn could manifest sooner, or stronger.
Creating exposure windows based on subject-specific estimates and their personal calendar of exposure is a powerful method to extrapolate the timing between exposure to Mn and its potential uptake into blood. This method could only be utilized because of the longitudinal measurements collected on the subjects, and the detailed information available on their daily tasks and attendance. The mixed model fit allowed creation of unique exposure estimates for each enrolled subject, and for each type of welding they did in the program. The exposure assessment done for this study represents the most robust exposure assessment for any study relating MnB and inhalation exposure to Mn. Using a mixed model including subject as a random effect means the data presented here is essentially the average of all individual trends observed in these data. Therefore, even though the models aren’t predicting on an individual basis, they are controlling for inter-subject variation, thus making this substantially different from a repeated-measures design that does not control for within subject trends.
When looking at the variance components related to the relationship between afternoon MnB and seven day, 30 day, 90 day, and cumulative exposure time windows the majority of variance (61%) was found to be between individuals. Even more of the total variance (71%) was between individuals when assessing the relationship between afternoon MnB and the preceding one day of exposure. This supports our previous work, where the majority of variance in MnB levels in a subset of the apprentice welders was between individuals (94%) and only about 6% of the total variance was within an individual (Baker et al. 2015b). Thus, in that subcohort, Mn seemed to be fairly tightly regulated in the blood, and the high degree of between-subject variation in MnB was due to differences in individual biochemistry and baseline MnB, and not due to exposure duration or accumulation. However, this previous work only looked at a subset of the total welding cohort presented in this analysis (n=9 subjects), and analyses were restricted to only their first academic quarter of study in the apprentice training program, a time period of approximately 70 days with relatively low levels of exposure. Thus, it is logical that in this current study, which looks at some longer durations of exposure, there would be more variation within a subject as the tight regulation of Mn in the blood may be overcome due to increased exposure duration. Compared to the previous work, in this study there was an increase in total variance, and in particular, a greater proportion of the total variance was within a subject. This increase in within-subject variance, coupled with the relationship we began to see between MnB and modeled exposure in longer exposure windows, drives the conclusion that MnB may serve as a biomarker of exposure to Mn, but only over longer time periods, such that the tight regulatory control of Mn in the blood is perturbed. As shown in previous studies (Apostoli et al. 2000; Bader et al. 1999), due to its high inter-individual variance, MnB is a biomarker that should be used to differentiate groups by exposure status, rather than for the characterization of the exposure of individuals.
Interestingly, measured MnB exhibited a mean decrease over the course of a day, even when stratifying by time of week and time of quarter. Generally, these decreases were statistically different from zero. This finding was contrary to what was expected to be seen a priori; it was assumed an increase in MnB would be present over the course of a day as exposure increased. Taking urine samples every four hours for a 24 hour period from welders, Jarvisalo et al. found a diurnal variation of Mn in urine, with higher urine Mn values found in the morning compared to in the afternoon or evening (Järvisalo et al. 1992). Given the burden to subjects in collecting serial blood samples in a similar manner, diurnal variability has not been established for MnB in humans, though seems a plausible hypothesis. In this study, of the 557 paired blood samples taken on a given day, 37% (n=204) exhibited an increase in MnB over the course of a day, wheras 63% (n=353) exhibited a decrease in MnB over the course of a day. Diurnal variability could explain the apparent decrease observed in MnB over the course of a day, and changes in the type of welding being done over the course of a week or quarter (for example, moving from a high exposure welding practice to a low exposure welding practice) could explain some of the decreases observed over long time periods in these data. However, other pharmacokinetic reasons may explain the changes seen over the course of a week and academic quarter (even when controlling for time of day) which would require further investigation.
The existing body of literature that has explored blood as a biomarker of Mn exposure has employed largely cross-sectional study designs, often trying to relate a single blood and exposure measurement collected on the same day (Baker et al. 2014). The results presented here show that a cross-sectional approach to assess the utility of blood as a biomarker of Mn would not be ideal, since a single blood sample is likely representing exposures accumulated over a longer preceding time period (at least 30 days) not over the sampled work shift. The longitudinal study design and subject-specific exposure modeling allowed the exploration of different time windows of exposure and changes in individual MnB levels over time, which is an improvement over the existing body of literature and can provide more insight into the biochemical properties (and time course) of inhaled Mn in welding fume.
Acknowledgments
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under award number R01ES017809. Marissa Baker was further supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under the Biostatistics, Epidemiologic, and Bioinformatics Training in Environmental Health, award number T32ES015459.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- Antonini JM, et al. Design, construction, and characterization of a novel robotic welding fume generator and inhalation exposure system for laboratory animals. J Occup Environ Hyg. 2006a;3:194–203. doi: 10.1080/15459620600584352. quiz D145 K5GUR8076517R664 [pii] [DOI] [PubMed] [Google Scholar]
- Antonini JM, Santamaria AB, Jenkins NT, Albini E, Lucchini R. Fate of manganese associated with the inhalation of welding fumes: potential neurological effects. Neurotoxicology. 2006b;27:304–310. doi: 10.1016/j.neuro.2005.09.001. S0161-813X(05)00141-5 [pii] [DOI] [PubMed] [Google Scholar]
- Apostoli P, Lucchini R, Alessio L. Are current biomarkers suitable for the assessment of manganese exposure in individual workers? American journal of industrial medicine. 2000;37:283–290. doi: 10.1002/(sici)1097-0274(200003)37:3<283::aid-ajim6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Aschner M. Manganese: brain transport and emerging research needs. Environ Health Perspect. 2000;108(Suppl 3):429–432. doi: 10.1289/ehp.00108s3429. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. S0041-008X(07)00106-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M, Dietz MC, Ihrig A, Triebig G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int Arch Occup Environ Health. 1999;72:521–527. doi: 10.1007/s004200050410. 90720521.420 [pii] [DOI] [PubMed] [Google Scholar]
- Baker MG, Criswell SR, Racette BA, Simpson CD, Sheppard L, Checkoway H, Seixas NS. Neurological outcomes associated with low-level manganese exposure in an inception cohort of asymptomatic welding trainees. Scand J Work Environ Health. 2015a;41:94–101. doi: 10.5271/sjweh.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MG, Simpson CD, Sheppard L, Stover B, Morton J, Cocker J, Seixas N. Variance components of short-term biomarkers of manganese exposure in an inception cohort of welding trainees. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements. 2015b;29:123–129. doi: 10.1016/j.jtemb.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MG, Simpson CD, Stover B, Sheppard L, Checkoway H, Racette BA, Seixas NS. Blood manganese as an exposure biomarker: state of the evidence. J Occup Environ Hyg. 2014;11:210–217. doi: 10.1080/15459624.2013.852280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlinger B, Ellingsen DG, Naray M, Zaray G, Thomassen Y. A study of the bio-accessibility of welding fumes. J Environ Monit. 2008;10:1448–1453. doi: 10.1039/b806631k. [DOI] [PubMed] [Google Scholar]
- Bocca B, Alimonti A, Forte G, Petrucci F, Pirola C, Senofonte O, Violante N. High-throughput microwave-digestion procedures to monitor neurotoxic elements in body fluids by means of inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2003;377:65–70. doi: 10.1007/s00216-003-2029-4. [DOI] [PubMed] [Google Scholar]
- Bowler RM, et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007;64:167–177. doi: 10.1136/oem.2006.028761. oem.2006.028761 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Chia SE, Foo SC, Gan SL, Jeyaratnam J, Tian CS. Neurobehavioral functions among workers exposed to manganese ore. Scand J Work Environ Health. 1993;19:264–270. doi: 10.5271/sjweh.1475. pii. [DOI] [PubMed] [Google Scholar]
- Criswell SR, et al. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup Environ Med. 2012;69:437–443. doi: 10.1136/oemed-2011-100119. oemed-2011-100119 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell SR, Perlmutter JS, Videen TO, Moerlein SM, Flores HP, Birke AM, Racette BA. Reduced uptake of [(1)(8)F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology. 2011;76:1296–1301. doi: 10.1212/WNL.0b013e3182152830. WNL.0b013e3182152830 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, James RA, Marshall MW, Parkinson CU, Wong BA. Influence of particle solubility on the delivery of inhaled manganese to the rat brain: manganese sulfate and manganese tetroxide pharmacokinetics following repeated (14-day) exposure. Toxicol Appl Pharmacol. 2001;170:79–87. doi: 10.1006/taap.2000.9088. S0041-008X(00)99088-4 [pii] [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Dubeikovskaya L, Dahl K, Chashchin M, Chashchin V, Zibarev E, Thomassen Y. Air exposure assessment and biological monitoring of manganese and other major welding fume components in welders. J Environ Monit. 2006;8:1078–1086. doi: 10.1039/b605549d. [DOI] [PubMed] [Google Scholar]
- Grass DS, et al. Airborne particulate metals in the New York City subway: a pilot study to assess the potential for health impacts. Environ Res. 2010;110:1–11. doi: 10.1016/j.envres.2009.10.006. S0013-9351(09)00196-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson A, Seixas N, Sterling D, Racette BA. Estimation of particulate mass and manganese exposure levels among welders. Ann Occup Hyg. 2011;55:113–125. doi: 10.1093/annhyg/meq069. meq069 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvisalo J, Olkinuora M, Kiilunen M, Kivistö H, Ristola P, Tossavainen A, Aitio A. Urinary and blood manganese in occupationally nonexposed populations and in manual metal arc welders of mild steel. Int Arch Occup Environ Health. 1992;63:495–501. doi: 10.1007/BF00572116. [DOI] [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, Weisskopf MG. Toenail, blood, and urine as biomarkers of manganese exposure. J Occup Environ Med. 2011;53:506–510. doi: 10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JE, et al. The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology. 2003;24:885–894. doi: 10.1016/S0161-813X(03)00081-0. S0161-813X(03)00081-0 [pii] [DOI] [PubMed] [Google Scholar]
- Pesch B, et al. Levels and predictors of airborne and internal exposure to manganese and iron among welders. J Expo Sci Environ Epidemiol. 2012;22:291–298. doi: 10.1038/jes.2012.9. jes20129 [pii] [DOI] [PubMed] [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005;64:230–235. doi: 10.1212/01.WNL.0000149511.19487.44. 64/2/230 [pii] [DOI] [PubMed] [Google Scholar]
- Rodrigues JL, Batista BL, Nunes JA, Passos CJS, Barbosa F. Evaluation of the use of human hair for biomonitoring the deficiency of essential and exposure to toxic elements. Science of the Total Environment. 2008;405:370–376. doi: 10.1016/j.scitotenv.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Roels H, Lauwerys R, Genet P, Sarhan MJ, de Fays M, Hanotiau I, Buchet JP. Relationship between external and internal parameters of exposure to manganese in workers from a manganese oxide and salt producing plant. Am J Ind Med. 1987;11:297–305. doi: 10.1002/ajim.4700110307. [DOI] [PubMed] [Google Scholar]
- Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, Lucchini R. Biomarkers of Mn exposure in humans. Am J Ind Med. 2007;50:801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- Sriram K, Lin GX, Jefferson AM, Roberts JR, Andrews RN, Kashon ML, Antonini JM. Manganese accumulation in nail clippings as a biomarker of welding fume exposure and neurotoxicity. Toxicology. 2012;291:73–82. doi: 10.1016/j.tox.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Tjalve H, Henriksson I. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–195. [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. [Accessed September 30, 2015];Method 6020A (SW-846): Inductively Coupled Plasma-Mass Spectrometry Revision 1. 1998 http://www2.epa.gov/homeland-security-research/epa-method-6020a-sw-846-inductively-coupled-plasma-mass-spectrometry.
- Vitarella D, Wong BA, Moss OR, Dorman DC. Pharmacokinetics of inhaled manganese phosphate in male Sprague–Dawley rats following subacute (14-day) exposure. Toxicology and applied pharmacology. 2000;163:279–285. doi: 10.1006/taap.1999.8874. [DOI] [PubMed] [Google Scholar]
- Zheng W, Kim H, Zhao Q. Comparative toxicokinetics of manganese chloride and methylcyclopentadienyl manganese tricarbonyl (MMT) in Sprague-Dawley rats. Toxicol Sci. 2000;54:295–301. doi: 10.1093/toxsci/54.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]