Abstract
Background
In high tuberculosis (TB) burden countries a significant proportion of the latent TB reservoir is established by the age five. There are critical knowledge gaps in our understanding of the age-specific prevalence of TB infection and the influence of HIV exposure on TB infection in the first five years of life among HIV-uninfected children in sub-Saharan Africa.
Methods
We measured TB infection with the Quantiferon Gold-in-Tube (QFT) and tuberculin skin tests (TST) in 447 children ≤60 months and their 284 HIV-infected and uninfected mothers in rural Uganda.
Results
The overall prevalence of TB infection in children ≤60 months by TST was 24% (95% CI 19.9-27.9). The prevalence of TST positivity was highest among children in their first year of life (36%, 95% CI: 26.0-45.9), and declined with age to 19% at 36-60 months of age, chi-square test for trend p=0.014. In contrast, 4% (95% CI: 1.9-5.87%) of children had a positive QFT, and there was no trend detected with age, p=0.576. QFT positivity was detected as early as 5 months. HIV-exposed uninfected (HEU) children had significantly higher odds of TB infection by QFT (OR 21.2, p=0.008, 95% CI: 2.2-204.7) or positive TST or QFT (OR 2.4, p=0.020, 95%CI: 1.2-5.1) compared to HIV unexposed uninfected (HUU) children, adjusting for age, BCG vaccination, and a positive maternal TST or QFT.
Conclusions
An appreciable prevalence of TB infection was detected in early childhood. HIV-exposed uninfected children (HEU) have a higher risk for TB infection compared to children born to HIV uninfected mothers.
Keywords: Childhood TB infection, Latent TB infection, HIV exposed children
The World Health Organization estimates that a third of the world's population has latent tuberculosis (TB) infection (LTBI)1 and a significant portion of this latent TB reservoir is established in childhood.2–5 By the age of five years, tuberculin skin test (TST) positivity ranges from 12%-20%4–7 in South Africa and 12% in Kampala, Uganda.8 Despite evidence of a high prevalence of TB infection by the age five years, data characterizing the age specific prevalence in this age group are sparse.
The dual epidemic of HIV and TB is a leading cause of morbidity and mortality among children and adults in sub-Saharan Africa, but there are little data on the association between TB and perinatal HIV exposure among HIV-uninfected children. Despite being born free of HIV infection, HIV-exposed uninfected (HEU) children remain at increased risk of morbidity and mortality compared to HIV-unexposed uninfected children (HUU).9–11 This relationship is thought to be mediated by (1) direct immunologic effects associated with perinatal HIV exposure12 and (2) indirect factors, including poverty and increased exposure to maternal infections.9 Studies in South Africa and Kenya suggest a high prevalence of household TB exposure,13,14 active TB disease,15 and TB infection16 in HIV-exposed infants. To our knowledge there are no studies that examine this association beyond 1 year of age or compare the risk of TB infection to a concurrent sample of HUU children.
To address these knowledge gaps in the epidemiology of TB in early childhood, we assessed the age-specific prevalence of TB infection in children under age five years and the association between HIV exposure and TB. We used both TST and Quantiferon Gold-in-Tube Assay (QFT-IT) (Cellestis, Carnegie, Australia) to measure latent TB in children, due to concerns about false positive TST in children who have received bacille Calmette-Guérin vaccination (BCG).
METHODS
Study Design
We conducted a cross-sectional study of HIV-infected and HIV-uninfected mothers and their children ages 0-60 months. Mothers and children were recruited from the PROMOTE cohorts in Tororo, Uganda. In brief, the PROMOTE studies were designed to assess HIV and malaria outcomes in mothers and their children; the design of the PROMOTE trials (NCT00993031, NCT00948896) have been described previously.17,18 Tororo is a rural district in Eastern Uganda with an adult TB incidence of 400/100,000 with 60% of incident cases occurring in adults with HIV infection.19 Routine BCG vaccination is given at birth, and national BCG coverage in Uganda is 94%.20 Children and mothers presenting for routine visits to the study clinic were consecutively approached for study enrollment from February to August 2013. Enrollment continued until 300 mothers were recruited with the aim to recruit 150 HIV-infected and 150 HIV-uninfected mothers. This sample size achieves a power at a 0.05 significance (alpha, two-sided) to detect an odds ratio of 2, assuming TST positivity of at least 13% in HUU.8 One child per mother was already enrolled in PROMOTE, but all mothers’ children age five and under were eligible. Mothers and children with a positive symptom screen or TB contact were assessed by study clinicians and referred to the local TB clinic for active TB evaluation. Active TB was not an exclusion criterion, as our study was not designed to exclude active disease and our primary outcome measure, TB infection, could include active and LTBI. One mother and child had a clinical diagnosis of active TB. All children participating in the PROMOTE cohorts were HIV-uninfected.
Study Measures
On enrollment all mothers completed a questionnaire on household demographics, history of household TB exposures, and a standardized TB symptom screen for adults21 and their children.22 Height and weight were measured for all children and malnutrition was defined per WHO criteria.23 All mothers and children participating in the PROMOTE trial completed HIV testing, and children were defined as HIV-uninfected once they had a negative HIV-1 DNA PCR 6 weeks after cessation of breastfeeding.
Quantiferon Gold-In-Tube Assays (Cellestis, Carnegie, Australia)
Blood samples were incubated and processed within six hours of collection. Following incubation all samples were stored in a −20°C freezer until the assays were run in batch at an on-site laboratory according to the manufacturer's instructions.24 The person performing the assay was blind to TST results and health-related information.
Tuberculin Skin Tests
To negate any possibility of the TST inducing a false positive IGRA response, all TSTs were placed after phlebotomy for the QFT. As recommended by the manufacturer, 5 tuberculin units of Aplisol (PPD-S) tuberculin were injected intradermally into the volar aspect of the forearm. The largest transverse diameter of induration was measured by a trained research assistant using the “ball-point” technique 48 to 72 hours after TST placement25 At the study onset Tubersol was used for 20 TSTs, but we switched to Aplisol tuberculin due to a global Tubersol shortage. Tubersol and Aplisol have similar sensitivities and specificities.26
Data and Statistical Analysis
Data were double entered into a Microsoft Access database and statistical analysis was performed using STATA software, version 12 (StataCorp, College Station, TX). Comparisons between demographics of children born to HIV-infected and HIV-uninfected mothers were performed using Pearson chi-square and the Wilcoxon rank sum tests as appropriate. Concordance between TST and QFT was assessed with the kappa statistic.27
The primary outcome of the study was TB infection in children. Given lack of a gold standard test for TB infection we defined it as (1) Positive TB test: positive TST or QFT or (2) Positive QFT. For analyses, unless otherwise specified, a positive TST was defined as ≥10mm for children and HIV-negative adults. A cut off of ≥5mm was used for HIV-infected mothers and severely malnourished children (weight for height Z-score <-3). We also present data using an alternative TST cut-off of ≥15mm, and age-specific cut-offs proposed by Chan et al.28 (positive TST defined as ≥21mm from 0-1 years, ≥18mm from age 2-3 years, and ≥13mm from 4-5 years). A positive QFT was defined per the manufacturer's guidelines.24 We used logistic regression models with robust standard errors to account for clustering by family to obtain point estimates and 95% confidence intervals (CI).
To assess the relationship between HIV exposure and TB infection, we restricted our analysis to the 281 children who had a documented HIV-1 DNA PCR after cessation of breastfeeding, which was done as part of the PROMOTE trials. The primary exposure variable of interest was perinatal HIV exposure. Bivariate logistic regression was used to generate odds ratios for covariates of interest and for the primary outcome. Multivariate models were then constructed to assess relationships between the primary outcome, perinatal HIV exposure and covariates associated with TB infection. Covariates were included in the model if the p-value was less than 0.10. Age, presence of a BCG scar, and maternal TB infection (QFT and/or TST positivity) were included a priori in multivariate logistic regression models, given previously established associations between these variables and TB infection.29,30 Reported household TB exposure was not included in the model given as it is on the causal pathway between HIV exposure and TB infection. Interactions between HIV exposure and age were explored but were not significant. Socioeconomic status was assessed by performing principal component analysis of questions regarding household possessions, as previously described.31
ETHICS
This study was approved by the Makerere University School of Medicine Research and Ethics Committee, Uganda National Council for Science and Technology, and the University of California, San Francisco Committee on Human Research. Written informed consent for the study was obtained from mothers.
RESULTS
Participant Characteristics
We enrolled 151 HIV-uninfected and 149 HIV-infected women and their 476 children ≤60 months. See Figure, Supplementary Digital Content 1 for the study flow diagram. There were no significant differences in age, maternal HIV status, poverty, or TB exposure between those who completed all study measurements and those who did not.
Study characteristics are presented in Table, Supplementary Digital Content 2. Children ranged in age from 1.7 to 59.7 months, with a median age of 34.3 months (IQR 12.2-36.5). Children born to HIV-infected mothers were younger, had a higher proportion of wasting, and lived in households that had a higher proportion of adults with a self-reported history TB. 71% of the HIV-infected women reported taking antiretroviral therapy (ART). Gender, household crowding, and proportion of children with a BCG vaccination scar were similar between groups.
Maternal TB infection rates differed by test and by HIV status. A higher proportion of HIV-infected mothers compared to HIV-uninfected mothers had a positive TST or QFT (59% vs. 42%, p<0.001), but there was no difference in positive QFTs (40% vs. 40%, p=0.96). Concordance between QFT and TST among mothers was moderate, kappa=0.512. Agreement between TST and QFT was 76.4%, and a higher proportion of HIV-infected women compared to HIV-uninfected women had a positive QFT with a negative TST, 14% vs. 9% p<0.001.
Age-Specific Prevalence of TB Infection in Children
The overall proportion of positive TSTs was 23.9% (95% CI 19.9-27.9). A positive TST (≥10mm) was detected as early as 2.6 months. When stratified by age group, the highest prevalence of TST positivity was among children in their first year of life (36%, 95% CI: 26.0-45.9) and TST positivity declined with age, chi-square test for trend: p=0.014 (Figure 1A). Applying alternative criteria for TST positivity resulted in a lower proportion of positive TSTs: 8.9% (95% CI: 6.2-11.6) using ≥15mm and 3.0% (95% CI: 1.4-4.6) using the Chan et al.28 age-specific cut-offs (Figure 2).
Figure 1. Proportion of positive Tuberculin Skin Tests (TST) and Quantiferon Gold In Tube Tests (QFT), stratified by age and mother's HIV status.
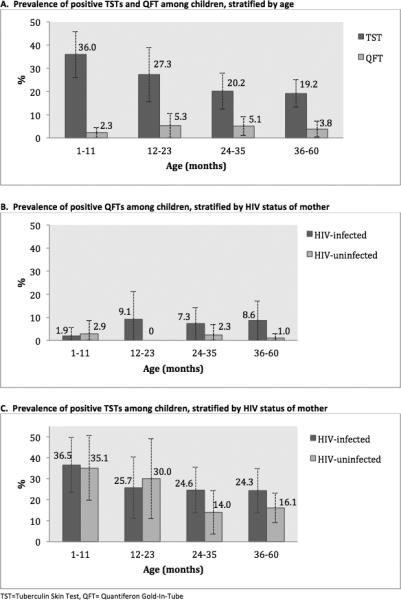
A. Proportion of positive TST and QFTs among children ≤60 months, stratified by age-category. There is a negative trend in TST positivity with increasing age, chi-square test for trend p=0.014. The trend of QFT positivity and age was not statistically significant, chi-square test for trend p=0.576. B. Proportion of positive QFTs, stratified by a HIV-status of mother. Children had a higher proportion of positive QFTs for all age-categories, though this was not statistically significant for any age-categories. C. Proportion of positive TSTs, stratified by age and mother's HIV-status. Children born to HIV-infected mothers had a higher prevalence of TST positivity in the 24-35 month age-category (p=0.013) and the 36-60 month age group (p=0.001) compared to the children born to HIV-uninfected mothers in the same age-group. A positive TST is defined as induration ≥10mm.
Figure 2. Quantiferon Gold-In-Tube (QFT) and Tuberculin Skin (TST) positivity among children ≤60 months, using different definitions for TST cut offs for positivity.
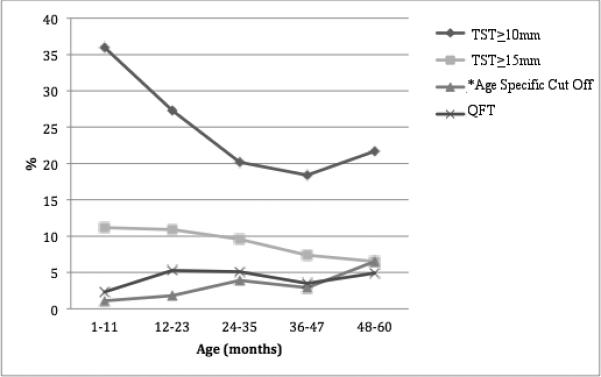
*age-specific cut-off proposed by Chan et al.28 (positive TST defined as ≥21mm from 0-1 years, ≥18mm from age 2-3 years, and ≥13mm from 4-5 years).
A positive QFT was detected as early as 5.0 months. The overall prevalence of positive QFTs was 3.9% (95% CI: 1.9-5.8); 7.2% of children had an indeterminate QFT (97% were failed negative controls and 3% were failed positive controls). We did not detect a trend in QFT-positivity by age-category (p=0.634) (Figure 1B).
Concordance of TST and QFT positivity in children was poor, κ=0.046, 2% of children had a TST+/QFT+ result, 72% had a TST−/QFT− results, 24% had a TST +/QFT−, and 2% had a TST−/QFT+ result. Comparing induration size to QFT positivity, 43% of positive QFTs occurred among children whose TST induration ≥ 15mm, and the remaining children with a positive QFT had a TST induration of 0mm. Concordance was poor when a 15mm cut off was used for TST positivity (κ=0.209), 2% of children had a TST+/QFT+ result, 89% had a TST−/QFT− results, 8% had a TST+/QFT−, and 2% had a TST−/QFT+.
Association between HIV exposure and TB Infection
Children born to HIV-infected mothers had a higher prevalence of positive QFTs compared to those who were born to HIV-uninfected mothers (6.4% vs. 1.5%, p= 0.01) (Figure 1B). We saw a trend towards a higher proportion of positive TSTs in children born to HIV-infected mothers compared to those born to HIV-uninfected mothers (27.2% vs. 20.6%, p=0.11) (Figure 1C).
Bivariate and multivariate analyses are shown in Table 1. In multivariate models we found HEU children had 21-fold higher odds of a positive QFT compared to HUU (OR 21.2, p=0.008, 95% CI:2.2-204.7), adjusting for age and maternal TB infection (Table 1A). When TB infection is defined as any positive TB test (TST≥10mm or positive QFT), HEU have a 2-fold increased odds of TB infection (OR 2.4, p=0.020, 95%CI: 1.2-5.1) compared to HUU, adjusting for age, maternal TB infection, and BCG scar (Table 1B). Maternal TB infection is an independent risk factor for childhood TB infection as measured by a positive TB test or QFT in adjusted models, and presence of BCG scar is only an independent risk factor for a positive TB test. The increased risk of TB infection among HEU compared to HUU children persists when TB infection is defined as a positive TST per the age-specific cut-offs proposed by Chan et al.28 (OR: 12.3, 95% CI: 2.1-72.1), adjusting for age, maternal TB infection, and BCG vaccination.
Table 1.
Association Between HIV exposure and Tuberculosis (TB) Infection Among Children ≤60 months
| Covariates | Outcome-Positive Quantiferon | Outcome-Any Positive TB Test (TST or QFT)* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjustedb | |||||||||
| OR | p-value | 95% CI | OR | p-value | 95% CI | OR | p-value | 95% CI | OR | p-value | 95% CI | |
| HIV | 9.5 | 0.034 | 1.2-76.8 | 21.2 | 0.008 | 2.2-204.7 | 2.4 | 0.003 | 1.4-4.3 | 2.4 | 0.02 | 1.2-5.1 |
| Age Category (months) | ||||||||||||
| 0-11 | 1 | 1 | 1 | 1 | ||||||||
| 12-35 | 2.4 | 0.43 | 0.3-20.4 | 3.6 | 0.244 | 0.4-32.1 | 0.5 | 0.113 | 0.2-1.1 | 0.5 | 0.103 | 1.1-5.1 |
| 36-60 | 1.4 | 0.78 | 0.1-13.8 | 6.4 | 0.136 | 0.6-73.0 | 0.4 | 0.028 | 0.2-0.9 | 0.5 | 0.186 | 0.2-1.2 |
| Education | ||||||||||||
| None | 1 | 1 | ||||||||||
| Primary school | 0.6 | 0.506 | 0.1-2.9 | 0.7 | 0.439 | 0.3-1.6 | ||||||
| Secondary and beyond | 0.4 | 0.402 | 0.0-4.1 | 0.8 | 0.681 | 0.3-2.2 | ||||||
| Wealth index | ||||||||||||
| Low | 1 | 1 | ||||||||||
| Medium | 0.5 | 0.407 | 0.1-2.6 | 1.2 | 0.52 | 0.6-2.2 | ||||||
| High | 0.8 | 0.836 | 0.2-4.3 | 0.7 | 0.45 | 0.3-1.7 | ||||||
| Household crowding | 0.6 | 0.477 | 0.3-2.4 | 0.8 | 0.344 | 0.4-1.3 | ||||||
| Malnutrition** | ||||||||||||
| Wasting | 2.3 | 0.429 | 0.3-19.7 | 1.2 | 0.484 | 0.7-2.2 | ||||||
| Stunting | 1.8 | 0.112 | 0.9-4.1 | 1 | 0.955 | 0.7-1.4 | ||||||
| Underweight | 1.9 | 0.144 | 0.8-4.1 | 1.1 | 0.601 | 0.7-1.7 | ||||||
| Mother with positive TB test | 4 | 0.082 | 0.8-19.5 | 5.2 | 0.045 | 1.0-26.0 | 1.9 | 0.027 | 1.1-3.4 | 2.3 | 0.01 | 1.2-4.2 |
| BCG Scar | 0.8 | 0.813 | 0.6-4.1 | 1.7 | 0.193 | 0.8-3.7 | 2.4 | 0.042 | 1.0-5.5 | |||
OR= Odds ratio; CI=Confidence interval; BCG= bacille Calmette-Guerin vaccination; TST=Tuberculin Skin Test; QFT= Quantiferon Gold-In-Tube
A positive TB test was defined as a positive TST or QFT. A positive TST is defined as induration ≥10mm for children and HIV-uninfected mothers, and ≥5mm for HIV-infected mothers.
wasting=weight for height z-score<-2, stunting= height for age z-score<-2; underweight= weight for age z-score <-2
Adjusted for age-category and mother with a positive TB test
Adjusted for age
DISCUSSION
In this cross-sectional study of Ugandan children, we found an appreciable prevalence of positive TSTs and QFTs in the first five years of life, and we detected QFT positivity as early as 5 months. Our data support prior findings of a high prevalence of TB infection among HIV-exposed infants in sub-Saharan Africa.9,14,16 Additionally, these data suggest that an increased risk of TB infection among HIV-exposed children exists for the first five years of life, independent of BCG vaccination and maternal TB infection.
We found a large difference between TST and QFT positivity. We hypothesize that much of this difference can be attributed to false positive TSTs from BCG vaccination, the effect of which wanes with age. Our age-specific data on TST positivity were consistent with age-specific trends from a national TST survey in Taiwan.28 In this survey Chan et al. found the highest prevalence of TST positivity in the first year of life (approximately 60%) with a nadir at 6-7 years of age, which they attributed to a waning BCG effect. In Gambia, 22% of BCG vaccinated infants had a positive TST at 4.5 months compared to 1% of unvaccinated infants, suggesting a high proportion of TST positivity in the first year of life is attributable to BCG vaccination.32 A non-linear trend of TB infection in children under age five and survivorship bias inherent in prevalence surveys are alternative explanations of the negative association of TST positivity and age.
Interferon Gamma Release Assays (IGRA) do not cross-react to BCG vaccination, and thus have been proposed as a more specific means to measure TB infection in prevalence surveys. To our knowledge the only two studies that examine the prevalence of TB infection with an IGRA among children five and under from the general population in East Africa are a retrospective study in Kenya, which found 11% of HIV-exposed to have a positive T-SPOT.TB16 and a prospective study of five year olds in Entebbe, Uganda, in which 10% of children were found to have a positive T-SPOT.TB.33 These estimates are higher than our estimate for QFT positivity among our HEU children, and we suspect this is due to differences in the sample: the Entebbe cohort was older and urban and the Kenyan cohort included HIV-infected children, different test characteristics of the T-SPOT.TB compared to the QFT,34 and the majority of mothers were not receiving antiretroviral therapy (ART).
The lower prevalence of QFT positivity compared to TST positivity in our study could also be partially attributable to false negative QFTs. False-negative IGRAs could be due to age-inappropriate interferon gamma cut-offs, as young children are thought to produce lower amounts of IFN-ϒ in response to antigens.35,36 Additionally, younger children may preferentially exhibit a T helper-2 response to TB infection which is detected with the TST but not the IGRA.37 Longitudinal studies are necessary to determine the most accurate test for detecting TB infection in this early age group.
Revised age-specific cut-offs for diagnosing LTBI with TSTs may better account for the BCG effect in early childhood. IGRAs may be a more specific method for detecting TB infection, but they are more expensive, require more laboratory infrastructure, and may have a higher probability of false negatives compared to TST, thereby making it a difficult test to implement on a wider-scale in resource limited settings. In this study, when using TST cut-offs ≥15 mm or age-specific cut-offs such as those proposed by Chan et al., we found a TST prevalence closer to that found by the QFT. Applying optimized age-specific cut-offs in high-TB burden settings in East Africa may improve estimates of the prevalence of childhood TB infection in future TST surveys.
This study identified HEU children as a high-risk population for TB infection, with higher odds of QFT and TST test positivity compared to HUU children. Increased TB exposure in the home may underlie this association, as a higher number of household TB contacts were reported in the households of HIV-infected mothers in our study and in other studies from sub-Saharan Africa. HEU, compared to HUU, may have a higher risk of acquiring TB infection from their mothers. HIV-infected mothers are at a high risk of TB reactivation or developing active disease if infected, especially in the peripartum period.38,39 Studies on HIV-infected women in PMTCT cohorts in India30 and Kenya29 show a high rate of TB disease in the post-partum period, 5 and 3.5 per 100 person years, respectively. In these cohorts TST and IGRA were both predictive of maternal TB infection as well as infant death or childhood TB disease. Our study complements these findings, as we found a positive maternal TST or IGRA independently predicted TB infection in children.
Immunologic differences between HEU and HUU may also contribute to the relationship between HIV exposure and childhood TB infection. In utero exposure to maternal infections, including HIV, is hypothesized to prime the developing immune system leading to impaired T and B cell immune responses.40–43 Several studies have found impaired BCG immunogenicity and interferon gamma response to PPD among HEU infants.43–46 Jones et al. found a relationship between impaired T-lymphocyte responses to BCG and both HIV exposure and maternal TB sensitization (positive IGRA) among South African infants at birth, but this relationship was no longer detected at 16 weeks of life. Additional data are needed to assess the duration of immunologic differences between HEU and HUU and the correlation between clinical outcomes and immunologic differences.
Our study has important strengths and limitations. We were not able to determine the accuracy of each test in this population, as there is no gold standard for LTBI. However, by using both tests we were able to provide an upper and lower estimate of TB infection in this sample, as well as highlight future research directions for estimating TB infection in this vulnerable age group. While we were able to highlight age-specific trends in TST positivity we were not powered enough to detect age-specific differences in QFT prevalence. Due to the cross-sectional nature of this study we were not able to account for survivorship bias and age-cohort effects that could have affected age-specific trends in TB infection. Advanced maternal immunosuppression has been associated with a higher risk of morbidity and mortality among HEU,47,12,29 thus it is possible that our study underestimates the risk of TB infection among HEU children given 70% of women in our study are on ART. Lastly, generalizability may be limited as the sample was recruited from women and children participating in a clinical trial on malaria and HIV.
Strategies are needed to address this appreciable prevalence of childhood TB infection and to decrease the size of the latent TB reservoir. Antenatal and PMTCT clinics are ideal for locations targeted TB-control interventions,39 as these are common settings where HIV-infected women interface with the health care system. Scaling-up proven interventions such as isoniazid preventive therapy (IPT) and TB screening among HIV-infected women are likely to benefit both mother and child. Contact tracing and IPT in children is feasible and high yield, but rarely implemented in East Africa.48,49 Overall, these data highlight the importance of targeted TB prevention efforts in early childhood, especially among young children born to HIV-infected mothers.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the children and mothers who participated in this study. We also thank Deo Ekol, Sam Balikoowa, Abel Kakuru, and the PROMOTE study staff for their tireless laboratory and research support.
FUNDING
This work was supported by the National Institutes of Allergy and Infectious Diseases [T32 AI51982(Havlir); K24 AI060530 (Havlir)] and the National Institutes of Health, University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI027763).
Footnotes
PRIOR REPORTS: Preliminary data from this study was reported as an oral abstract (abstract #94) at the Conference of Retroviruses and Opportunistic Infections, Boston, Massachusetts, February 2014.
DISCLOSURES: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.World Health Organization . Global Tuberculosis Control: WHO Report 2011. Geneva, Switzerland: 2011. [Google Scholar]
- 2.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Beyers N. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10(3):259–263. [PubMed] [Google Scholar]
- 3.Marais BJ, Graham SM, Maeurer M, Zumla A. Progress and challenges in childhood tuberculosis. Lancet Infect Dis. 2013;13(4):287–289. doi: 10.1016/S1473-3099(13)70031-8. [DOI] [PubMed] [Google Scholar]
- 4.Middelkoop K, Bekker L-G, Myer L, Dawson R, Wood R. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis. 2008;47(3):349–355. doi: 10.1086/589750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanaube K, Sismanidis C, Ayles H, et al. Annual Risk of Tuberculous Infection Using Different Methods in Communities with a High Prevalence of TB and HIV in Zambia and South Africa. PLoS ONE. 2009;4(11) doi: 10.1371/journal.pone.0007749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middelkoop K, Bekker L-G, Liang H, et al. Force of tuberculosis infection among adolescents in a high HIV and TB prevalence community: a cross-sectional observation study. BMC Infect Dis. 2011;11:156. doi: 10.1186/1471-2334-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanaube K, Hargreaves J, Fielding K, et al. Risk factors associated with positive QuantiFERON-TB Gold In-Tube and tuberculin skin tests results in Zambia and South Africa. PloS One. 2011;6(4):e18206. doi: 10.1371/journal.pone.0018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whalen CC, Zalwango S, Chiunda A, et al. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PloS One. 2011;6(2):e16137. doi: 10.1371/journal.pone.0016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotton MF, Slogrove A, Rabie H. Infections in HIV-exposed Uninfected Children With Focus on Sub-Saharan Africa. Pediatr Infect Dis J. 2014;33(10):1085–1086. doi: 10.1097/INF.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 10.Marquez C, Okiring J, Chamie G, et al. Increased Morbidity in Early Childhood Among HIV-exposed Uninfected Children in Uganda is Associated with Breastfeeding Duration. J Trop Pediatr. 2014;60(6):434–41. doi: 10.1093/tropej/fmu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landes M, van Lettow M, Chan AK, Mayuni I, Schouten EJ, Bedell RA. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PloS One. 2012;7(10):e47337. doi: 10.1371/journal.pone.0047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol. 2014;176(1):11–22. doi: 10.1111/cei.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekker A, Du Preez K, Schaaf HS, Cotton MF, Hesseling AC. High tuberculosis exposure among neonates in a high tuberculosis and human immunodeficiency virus burden setting. Int J Tuberc Lung Dis. 2012;16(8):1040–1046. doi: 10.5588/ijtld.11.0821. [DOI] [PubMed] [Google Scholar]
- 14.Cotton MF, Schaaf HS, Lottering G, et al. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis. 2008;12(2):225–227. [PubMed] [Google Scholar]
- 15.Madhi SA, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med. 2011;365(1):21–31. doi: 10.1056/NEJMoa1011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cranmer LM, Kanyugo M, Jonnalagadda SR, et al. High prevalence of tuberculosis infection in HIV-1 exposed Kenyan infants. Pediatr Infect Dis J. 2014;33(4):401–406. doi: 10.1097/INF.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterbauer B, Kapisi J, Bigira V, et al. Factors associated with malaria parasitaemia, malnutrition, and anaemia among HIV-exposed and unexposed Ugandan infants: a cross-sectional survey. Malar J. 2012;11:432. doi: 10.1186/1475-2875-11-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natureeba P, Ades V, Luwedde F, et al. Lopinavir/ritonavir versus Efavirenz-based Antiretroviral Treatment for the Prevention of Malaria among HIV-infected Pregnant Women. J Infect Dis. 2014 Jun; doi: 10.1093/infdis/jiu346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Personal Communication with Tororo Municipality-Public Health. 2011 [Google Scholar]
- 20.Uganda Bureau of Statistics and ICF International . Uganda Demographic and Health Survey 2011. Kampala, Uganda and Calverton, Maryland: 2012. [Google Scholar]
- 21.WHO . WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. WHO; [July 4, 2014]. http://www.who.int/tb/publications/2012/tb_hiv_policy_9789241503006/en/. [PubMed] [Google Scholar]
- 22.International Union Against Tuberculosis and Lung Disease (The Union) Desk-Guide for Diagnosis and Management of TB in Children 2010. Paris, France: 2010. [Google Scholar]
- 23.WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards: Length/height-for-Age, Weight-for-Age, Weight-for Length, Weight-for Heigh and Body Mass Index for Age: Methods and Development. World Health Organization; Geneva: 2006. www.who.int/childgrowth/standards/technical_report/en/index.html. [Google Scholar]
- 24.Cellestis Limited . QuantiFERON®-TB Gold In-Tube (IT) [Package Insert] Victoria, Australia: 2006. [Google Scholar]
- 25.Center for Disease Control and Prevention Mantoux tuberculin skin test facillitators guide. 2013 Nov; http://www.cdc.gov/tb/education/mantoux/pdf/mantoux.pdf.
- 26.Villarino ME, Burman W, Wang YC, et al. Comparable specificity of 2 commercial tuberculin reagents in persons at low risk for tuberculous infection. JAMA. 1999;281(2):169–171. doi: 10.1001/jama.281.2.169. [DOI] [PubMed] [Google Scholar]
- 27.Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977;(33):159–174. [PubMed] [Google Scholar]
- 28.Chan P-C, Chang L-Y, Wu Y-C, et al. Age-specific cut-offs for the tuberculin skin test to detect latent tuberculosis in BCG-vaccinated children. Int J Tuberc Lung Dis. 2008;12(12):1401–1406. [PubMed] [Google Scholar]
- 29.Jonnalagadda S, Lohman Payne B, Brown E, et al. Latent tuberculosis detection by interferon γ release assay during pregnancy predicts active tuberculosis and mortality in human immunodeficiency virus type 1-infected women and their children. J Infect Dis. 2010;202(12):1826–1835. doi: 10.1086/657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002-2005. Clin Infect Dis. 2007;45(2):241–249. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 31.Young S, Murray K, Mwesigwa J, et al. Maternal Nutritional Status Predicts Adverse Birth Outcomes among HIV-Infected Rural Ugandan Women Receiving Combination Antiretroviral Therapy. PLoS ONE. 2012;7(8):e41934. doi: 10.1371/journal.pone.0041934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ota MOC, Goetghebuer T, Vekemans J, et al. Dissociation between tuberculin skin test and in vitro IFN-gamma responses following neonatal BCG vaccination. J Trop Pediatr. 2006;52(2):136–140. doi: 10.1093/tropej/fmi087. [DOI] [PubMed] [Google Scholar]
- 33.Nkurunungi G, Lutangira JE, Lule SA, et al. Determining Mycobacterium tuberculosis infection among BCG-immunised Ugandan children by T-SPOT.TB and tuberculin skin testing. PloS One. 2012;7(10):e47340. doi: 10.1371/journal.pone.0047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hesseling AC, Mandalakas AM, Kirchner HL, et al. Highly discordant T cell responses in individuals with recent exposure to household tuberculosis. Thorax. 2009;64(10):840–846. doi: 10.1136/thx.2007.085340. [DOI] [PubMed] [Google Scholar]
- 35.Connell TG, Curtis N, Ranganathan SC, Buttery JP. Performance of a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax. 2006;61(7):616–620. doi: 10.1136/thx.2005.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lighter J, Rigaud M, Eduardo R, Peng C-H, Pollack H. Latent tuberculosis diagnosis in children by using the QuantiFERON-TB Gold In-Tube test. Pediatrics. 2009;123(1):30–37. doi: 10.1542/peds.2007-3618. [DOI] [PubMed] [Google Scholar]
- 37.Lewinsohn DA, Gennaro ML, Scholvinck L, Lewinsohn DM. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int J Tuberc Lung Dis. 2004;8(5):658–674. [PubMed] [Google Scholar]
- 38.Marais BJ, Gupta A, Starke JR, El Sony A. Tuberculosis in women and children. The Lancet. 2010;375(9731):2057–2059. doi: 10.1016/S0140-6736(10)60579-X. [DOI] [PubMed] [Google Scholar]
- 39.Getahun H, Sculier D, Sismanidis C, Grzemska M, Raviglione M. Prevention, Diagnosis, and Treatment of Tuberculosis in Children and Mothers: Evidence for Action for Maternal, Neonatal, and Child Health Services. J Infect Dis. 2012 Mar; doi: 10.1093/infdis/jis009. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra I, Mungai P, Wamachi A, et al. Helminth- and Bacillus Calmette-Guérin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162(11):6843–6848. [PubMed] [Google Scholar]
- 41.Malhotra I, Ouma JH, Wamachi A, et al. Influence of maternal filariasis on childhood infection and immunity to Wuchereria bancrofti in Kenya. Infect Immun. 2003;71(9):5231–5237. doi: 10.1128/IAI.71.9.5231-5237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malhotra I, Mungai P, Wamachi A, et al. Helminth- and Bacillus Calmette-Guérin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol Baltim Md 1950. 1999;162(11):6843–6848. [PubMed] [Google Scholar]
- 43.Van Rie A, Madhi SA, Heera JR, et al. Gamma interferon production in response to Mycobacterium bovis BCG and Mycobacterium tuberculosis antigens in infants born to human immunodeficiency virus-infected mothers. Clin Vaccine Immunol. 2006;13(2):246–252. doi: 10.1128/CVI.13.2.246-252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kidzeru EB, Hesseling AC, Passmore J-AS, et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS. 2014;28(10):1421–1430. doi: 10.1097/QAD.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazzola TN, da Silva MTN, Abramczuk BM, et al. Impaired Bacillus Calmette-Guérin cellular immune response in HIV-exposed, uninfected infants. AIDS. 2011;25(17):2079–2087. doi: 10.1097/QAD.0b013e32834bba0a. [DOI] [PubMed] [Google Scholar]
- 46.Jones CE, Hesseling AC, Tena-Coki NG, et al. The impact of HIV exposure and maternal Mycobacterium tuberculosis infection on infant immune responses to bacille Calmette-Guérin vaccination. AIDS. 2015;29(2):155–165. doi: 10.1097/QAD.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asbjörnsdóttir KH, Slyker JA, Weiss NS, et al. Breastfeeding is associated with decreased pneumonia incidence among HIV-exposed, uninfected Kenyan infants. AIDS. 2013;27(17):2809–15. doi: 10.1097/01.aids.0000432540.59786.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banu Rekha VV, Jagarajamma K, Wares F, Chandrasekaran V, Swaminathan S. Contact screening and chemoprophylaxis in India's Revised Tuberculosis Control Programme: a situational analysis. Int J Tuberc Lung Dis. 2009;13(12):1507–1512. [PubMed] [Google Scholar]
- 49.Jaganath D, Zalwango S, Okware B, et al. Contact investigation for active tuberculosis among child contacts in Uganda. Clin Infect Dis. 2013;57(12):1685–1692. doi: 10.1093/cid/cit645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


