Abstract
Chemical and enzymatic hydrolysis provoke a cascade of events that undermine methacrylate-based adhesives and the bond formed at the tooth/composite interface. Infiltration of noxious agents, e.g. enzymes, bacteria, and so forth, into the spaces created by the defective bond will ultimately lead to failure of the composite restoration. This paper reports a novel, synthetic resin that provides enhanced hydrolytic stability as a result of intrinsic reinforcement of the polymer network. The behavior of this novel resin, which contains γ-methacryloxyproyl trimethoxysilane (MPS) as its Si-based compound, is reminiscent of self-strengthening properties found in nature. The efforts in this paper are focused on two essential aspects: the visible-light irradiation induced (photoacid-induced) sol-gel reaction and the mechanism leading to intrinsic self-strengthening. The FTIR band at 2840 cm−1 corresponding to CH3 symmetric stretch in −Si−O−CH3 was used to evaluate the sol-gel reaction. Results from the real-time FTIR indicated that the newly developed resin showed a limited sol-gel reaction (<5%) during visible-light irradiation, but after 48h dark storage, the reaction was over 65%. The condensation of methoxysilane mainly occurred under wet conditions. The storage moduli and glass transition temperature of the copolymers increased in wet conditions with the increasing MPS content. The cumulative amounts of leached species decreased significantly when the MPS-containing adhesive was used. The results suggest that the polymethacrylate-based network, which formed first as a result of free radical initiated polymerization, retarded the photoacid-induced sol-gel reaction. The sol-gel reaction provided a persistent, intrinsic reinforcement of the polymer network in both neutral and acidic conditions. This behavior led to enhanced mechanical properties of the dental adhesives under conditions that simulate the wet, oral environment.
Keywords: self-strengthening, dental adhesive, photoacid-induced sol-gel reaction, dynamic mechanical property, HPLC
1. INTRODUCTION
The structure of methacrylate-based dental adhesives suggests a general mechanism for their chemical and enzymatic degradation. In the oral environment, water can gradually penetrate the resin, which promotes the chemical hydrolysis of ester bonds in polymethacrylate-based materials [1–4]. This reaction may be relatively slow at neutral pH, but changes in pH, caused by diet or cariogenic bacteria, may lead to transient acid or base catalysis [5]. The carboxylate and alcohol degradation products of ester hydrolysis are more hydrophilic than the parent ester, further enhancing the local ingress of water. After years of exposure to salivary fluids, local domains of the polymethacrylate networks become sufficiently degraded and/or hydrophilic to permit access by esterases, which accelerate ester bond hydrolysis [3, 6]. In general, the ester bonds within the polymethacrylate-based network are vulnerable to two forms of hydrolytic attack: (1) chemical hydrolysis catalyzed by acids or bases and (2) enzymatic hydrolysis catalyzed by salivary enzymes (particularly esterases) [7].
Deterioration of the adhesive is difficult to detect and even more difficult to repair. Degradation of the dental adhesive and concomitant failure of the bond formed between the adhesive and dentin leads to gaps at the composite/tooth interface. These gaps are penetrated by bacteria, bacterial enzymes, oral fluids, and food. Such activity leads to decay, hypersensitivity, pulpal inflammation and composite restoration failure [6].
Several strategies to enhance the hydrolytic stability of dental adhesives or to improve the integrity of the adhesive/dentin (a/d) interfacial bond have been proposed. One strategy is to change the monomer structure, which especially increases the hydrophobicity of the monomers by introducing either a urethane group [8–10], branched methacrylate linkage [11], or ethoxylated BisGMA (BisEMA) [12]. These strategies temporarily depress water sorption, but the materials will generally reach saturation within 7–60 days [13]. Another strategy involves enhancing the monomer conversion in the hybrid layer by improving the compatibility between the photoinitiator and the hydrophilic-rich phase of adhesive [14, 15], or increasing the light-curing time of the adhesive[1, 16]. Since the gel effect and vitrification phenomena occur early in the light-irradiation process, the degree of conversion (DC) of C=C double bonds cannot reach 100% [17, 18]. A third strategy is to add an effective inhibitor (such as zinc or zinc-chelators) of dentin matrix metalloproteinnases (MMPs) in the adhesive formulation or use a biomimetic remineralization technique [4, 19–21]. The MMP-inhibition methods have shown promise [22], but these methods can lead to detrimental changes in the material, e.g. decreased monomer/polymer conversion [23]. Biomimetic remineralization offers protection of the exposed collagen, but this technique does not prevent water sorption and hydrolysis of the adhesive [4].
Si-based materials are used in many aspects of daily life and show a beneficial role in in vivo applications. Silicate gels can be synthesized by hydrolyzing tetrafunctional alkoxide precursors which employ an acid or base as a catalyst [24–26]. The hydrolysis reaction replaces the alkoxide group (OR) with a hydroxyl group (OH). The subsequent condensation reaction, involving silanol groups, produces a silicon oxygen bond (Si−O−Si) plus the by-products alcohol (ROH) or water. At the functional group level, three reactions are generally used to describe the sol-gel process [25]:
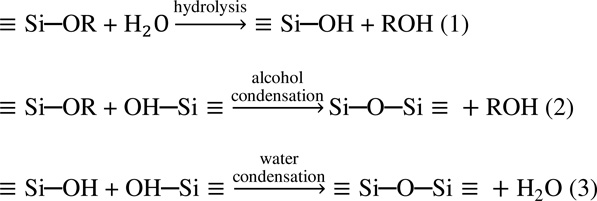 |
Currently, organosilanes are mainly used as a coupling agent to connect the organic and inorganic components in dental composites [27–30]. A common method to apply silane agents into the restorative materials is to use them pre-hydrolyzed in a solvent mixture consisting of water or ethanol. The shelf life of the pre-hydrolyzed silane solutions is relatively short, which limits its application [30].
In 1978, Fox et al. first reported that the onium salt photolysis was efficient at promoting the hydrolysis-condensation of alkoxylsilyl precursors [31]. Since the pioneering work of Crivello, onium salts have been widely used as photoinitiators to generate photoacids and initiate the cationic photopolymerization [32]. The photoacid-induced sol-gel reaction has been used in a few studies to prepare nanocomposite films [32–37]. Under acidic conditions, it is likely that an alkoxide group is protonated in a rapid first step, and then the hydrolysis occurs. Photoacids generated by photolysis of onium salts have been shown to catalyze the sol-gel reaction of alkoxides. Moreover, the addition of water was not necessary since diffusion of moisture from the air into the film was sufficient for the hydrolysis reaction [36]. The photoacid-induced sol-gel reaction opens new opportunities for the application of organosilane agents in dentistry.
We propose the development of a dental adhesive system that utilizes the sol-gel reaction in conjunction with free radical polymerization to provide a polymer with enhanced hydrolytic stability and intrinsic self-strengthening properties. The sol-gel reaction of organosilane agents and the free radical photopolymerization of methacrylate are carried out simultaneously, and both reactions are induced by visible light irradiation. To our knowledge, this “one-pot” in situ bulk polymerization approach has not been previously employed in dental adhesives. It is postulated that the visible-light induced free radical photopolymerization will be followed by a sol-gel process; these processes will lead to polysiloxane chains strictly interconnected within the polymethacrylate matrix. The first objective is to evaluate the validity of the photoacid-induced sol-gel reaction in the dental adhesive system with visible-light irradiation. The second objective is to assess the proposed intrinsic self-strengthening mechanism by monitoring the mechanical properties of the hybrid copolymers under wet conditions. The overall research hypotheses of this study were: (1) the photoacid-induced sol-gel reaction cannot occur without the addition of iodonium salt, (2) water is necessary for the hydrolysis of photoacid-induced sol-gel reaction, (3) the mechanical properties of silane-containing dental adhesive will show self-strengthening behavior under wet conditions.
2. MATERIALS & METHODS
2.1 Materials
2,2-Bis[4-(2-hydroxy-3-methacryloxypropoxy) phenyl]propane (BisGMA) and 2-hydroxyethyl methacrylate (HEMA) were obtained from Sigma-Aldrich (St. Louis, MO) and used as received without further purification as monomers in dental adhesives. γ-methacryloxypropyltrimethoxysilane (MPS) was used as received from MP Biomedicals, Santa Ana, CA. Camphoroquinone (CQ), ethyl-4-(dimethylamino) benzoate (EDMAB), and lactic acid (LA) were obtained from Sigma-Aldrich (St. Louis, MO). p-octyloxy-phenylphenyl iodonium hexafluoroantimonate (OPPIH) was purchased from Gelest Inc., (Morrisville, PA). All other chemicals were reagent grade and used without further purification.
2.2 Preparation of adhesive formulations
Neat methacrylate monomer mixtures were made by mixing 45 wt% HEMA and 55 wt% BisGMA [14, 38]. CQ (1.0 wt%), EDMAB(1.0 wt%), and OPPIH (2.0 wt%) were used as photoinitiator (PI) systems with respect to the total amount of monomers [39]. The chemical structures of monomers and PI are shown in Scheme 1. The composition of the neat resin and the experimental formulations are listed in Table 1. Mixtures of monomers/PI were prepared in brown glass vials under amber light. The preparation of adhesive formulations and their polymer beams have been reported [38]. Briefly, the mixtures containing the monomers/PI were mixed overnight at 23±2 °C to promote complete dissolution and formation of a homogeneous solution. The prepared resins were injected into a glass-tubing mold (Fiber Optic Center, Inc., part no.: ST8100, New Bedford, MA) and light-cured for 40 s at 23±2 °C with an LED light curing unit (LED Curebox, 100 mW/cm2 irradiance, Proto-tech, Portland, OR). The polymerized samples were stored in the dark at 23±2 °C for at least 48 h. The resultant rectangular beam specimens of cross section 1 × 1 mm and length 15 mm were used for dynamic mechanical analysis.
Scheme 1.
Chemical structures of components used in the formulations.
Table 1.
Degree of Conversion and Maximum Polymerization Rate of Formulations
| Run | HEMA/BisGMA (wt%) a |
MPS (wt%) |
DC (%) |
(1/s) |
|
|---|---|---|---|---|---|
| Two- component PI system (CQ/EDMAB) |
C0-2PI | 100 | - | 62.6 (0.1) | 8.8(0.4) |
| E1-2PI | 95 | 5 | 67.1 b (0.5) | 8.2(1.0) | |
| E2-2PI | 90 | 10 | 67.5 b (0.2) | 7.1(0.9) | |
| E3-2PI | 50 | 50 | 53.7 b (2.2) | 2.2(0.2) | |
| MPS-2PI | - | 100 | 18.1 b (1.8) | 1.3(0.2) | |
| Three- component PI system (CQ/EDMAB /OPPIH) |
C0-3PI | 100 | - | 66.9 (0.4) | 24.1(1.0) |
| E1-3PI | 95 | 5 | 69.5 c (0.2) | 14.4(2.2) | |
| E2-3PI | 90 | 10 | 72.0 c (0.1) | 8.2(1.0) | |
| E3-3PI | 50 | 50 | 69.4 c (1.3) | 2.4(0.2) | |
| MPS-3PI | - | 100 | 30.4 c (3.2) | 1.1(0.0) | |
| 1 wt% water containing |
C0w-3PI | 100 | - | 67.9 (1.0) | 21.5(0.7) |
| E1w-3PI | 95 | 5 | 68.6 d (0.4) | 18.7(0.8) | |
| E2w-3PI | 90 | 10 | 70.0 d (1.4) | 14.7(0.8) | |
| E3w-3PI | 50 | 50 | 72.3 d (2.7) | 2.6(0.2) | |
the resin was mixed HEMA/BisGMA in the ratio of 45/55 (w/w).
significantly (p<0.05) different from the control (C0-2PI).
significantly (p<0.05) different from the control (C0-3PI).
significantly (p<0.05) different from the control (C0w-3PI). The value in the ( ) is the standard deviation.
2.3 Water miscibility of adhesive formulations
About 0.5 g of each neat resin was weighed into a brown vial and water was added in increments of ~0.005 g until the mixture was visually observed to be turbid. The percentage of water in the mixture was noted (w1). The mixture was then back-titrated using the neat resin until the turbidity disappeared, and the percentage of water in the mixture was noted (w2). The water miscibility (Wwm, %) of the liquid formulation was calculated as the average of w1 and w2. Three specimens of each formulation were measured.
2.4 Real-time conversion, maximum polymerization rate and sol-gel reaction analyzed by FTIR
The degree of conversion (DC) and polymerization behavior were determined by FTIR [40]. Real-time, in situ monitoring of the photopolymerization behavior of the adhesive formulations was performed using an infrared spectrometer (Spectrum 400 Fourier transform infrared spectrophotometer, Perkin-Elmer, Waltham, MA) at a resolution of 4 cm−1. One drop of adhesive solution was placed on the diamond crystal top plate of an attenuated total reflectance (ATR) accessory (PIKE Technologies Gladi-ATR, Madison, WI) and covered with a mylar film to prevent exposure to oxygen and moisture in the air. Exposure of the adhesive resin to the commercial visible-light-polymerization unit (Spectrum® 800, Dentsply, Milford, DE), at an intensity of 550 mW/cm2, was initiated after 50 infrared spectra had been recorded. The light exposure time was 40 s. Real-time IR spectra were continuously recorded for 600 s after light activation began. A time-based spectrum collector (Spectrum TimeBase, Perkin-Elmer) was used for continuous and automatic collection of spectra during polymerization.
Full and intimate contact of the sample with the ATR crystal is essential to achieve high quality results. These characteristics were achieved by dispensing and photopolymerizing the adhesive solution directly on the crystal. During polymerization the solution transitions from a liquid to a solid. For processing the spectra of these samples, ATR correction was used with the contact factor at zero, since the penetration depth should be proportional to the wavelength. A minimum of three measurements (n=3) were carried out for each adhesive formulation.
Methacrylic double bond conversion was monitored by the band ratio profile-1637 cm−1 (C=C)/1608 cm−1 (phenyl) [14]. In the neat MPS formulation, the band at 2840 cm−1 (corresponding to CH3 symmetric stretch in −Si−O−CH3) was used to evaluate the hydrolysis conversion. In the hybrid formulation, the degree of hydrolysis was monitored by the band ratio profile-2840 cm−1 (vsym(−Si−O−CH3))/1608 cm−1(phenyl). The average of the last 50 values of the time-based spectra is reported as the DC value. The maximum polymerization rate () was determined using the maximum slope of the linear region of the DC vs. time plots [38].
2.5 Dynamic mechanical analysis (DMA)
In dynamic mechanical tests, a sinusoidal stress is applied and the resultant strain is measured to obtain the storage and loss moduli. The storage modulus (E') represents the amount of energy recovered during cyclic loading, and this value is proportional to the elasticity of a viscoelastic solid. The loss modulus (E") represents viscous dissipation in the cyclic process. The ratio of loss modulus (E") to storage modulus (E') is referred to as the mechanical damping, or tan δ. The dynamic mechanical analyses of polymethacrylate-based dental adhesives have been described [41].
DMA tests were performed on rectangular beam specimens using a TA instruments Q800 DMA (TA Instruments, New Castle, USA) with a three-point bending clamp. A minimum of three specimens were tested for each formulation. The following testing parameters were used: displacement amplitude of 15 µm, frequency of 1 Hz, and preload force of 0.01 N [42]. In addition, temperature was ramped at the rate of 3 °C/min from 20 to 200 °C. The glass transition temperature (Tg) is determined as the position of the maximum value on the tanδ versus temperature plot. For wet testing, specimens were submerged in water or 1mM lactic acid (LA) at 37 °C for 1 or 8 weeks and tests were performed using the three-point submersion clamp[38]. The test temperature was varied from 10 to 75 °C with a ramping rate of 1.5 °C/min. The Tg in wet condition is determined as the position of the maximum on the derivative storage modulus versus temperature plot.
2.6 Leachable study by High-Performance Liquid Chromatography (HPLC)
Round disk samples (4 mm diameter and 1 mm thickness) were used for the leachable study. For specimen preparation, liquid resin was injected directly into a Tzero® Hermetic Lid (P/N: 900797.901 TA Instruments Waters LLC, New Castle, DE). The lid was filled with resin, covered with a mylar film and polymerization was initiated by 40 s exposure in a LED light curing unit (LED Curebox, 100 mW/cm2 irradiance, Proto-tech, Portland, OR). The polymerized samples were stored in the dark at 23±2 °C for at least 48 h before testing. The polymerized disks were submerged in 1 mL ethanol (HPLC grade) at 23±2 °C for 1 to 56 days. The storage solutions were collected at various time intervals, i.e. every 24 h for the first 7 days and every 72 hours after 7 days, and analyzed for concentration of leachate. Fresh ethanol was added to the disk samples after each collection.
The analysis was made using high performance liquid chromatography (HPLC) on a system (Shimadzur® LC-2010C HT, software EZstart, version 7.4 SP2) equipped with a 250×4.6 mm column packed with 5µm C-18 silica (Luna®, Phenomenex Inc., Torrance, CA). The mobile phase was acetonitrile/water (70/30, v/v). The system was operated under the following conditions: 0.5 mL/min flow rate; detection at 208 nm, 20 µL sampling loop; 40 °C temperature. The column was calibrated with known concentrations of the following compounds: BisGMA, HEMA, MPS, and EDMAB, at concentrations of 5, 10, 20, 50, 100 and 250 mg/L in ethanol. The calibration curves with the linear fittings of BisGMA (5–250 mg/L, R2=0.999), HEMA (5–500 mg/L, R2=0.999), MPS (5–250 mg/L, R2=0.999), and EDMAB (5–100 mg/L, R2=0.997) were used to calculate the concentration of these species in the extracts. The limit of quantitation (LOQ) was expressed as LOQ= 10σ/S (σ: the standard deviation of the response, S: the slope of the calibration curve). The slope S was estimated from the calibration curve of the analyte. The estimate of σ may be carried out based on the calibration curve. The limits of quantification were 12 ug/mL for BisGMA, 19 ug/mL for HEMA, 14 ug/mL for EDMAB, 5 ug/mL for MPS, respectively. The concentration was based on the intensity of the chromatographic peaks at the corresponding retention times. The HPLC analysis was performed using the extract of 4 samples from each formulation.
2.7 Statistical analysis
The results were analyzed statistically using one-way analysis of variance (ANOVA) together with Tukey’s test at α = 0.05 (Microcal Origin Version 8.0, Microcal Software Inc., Northampton, MA) to identify significant differences in the means.
3. RESULTS
The water miscibility of the adhesive formulation decreased with the increase of MPS concentration. The Wwm of the control, 5, and 10 wt% MPS containing formulations are 10.53±0.03 wt%, 9.80±0.08 wt%, and 8.96±0.50 wt%, respectively.
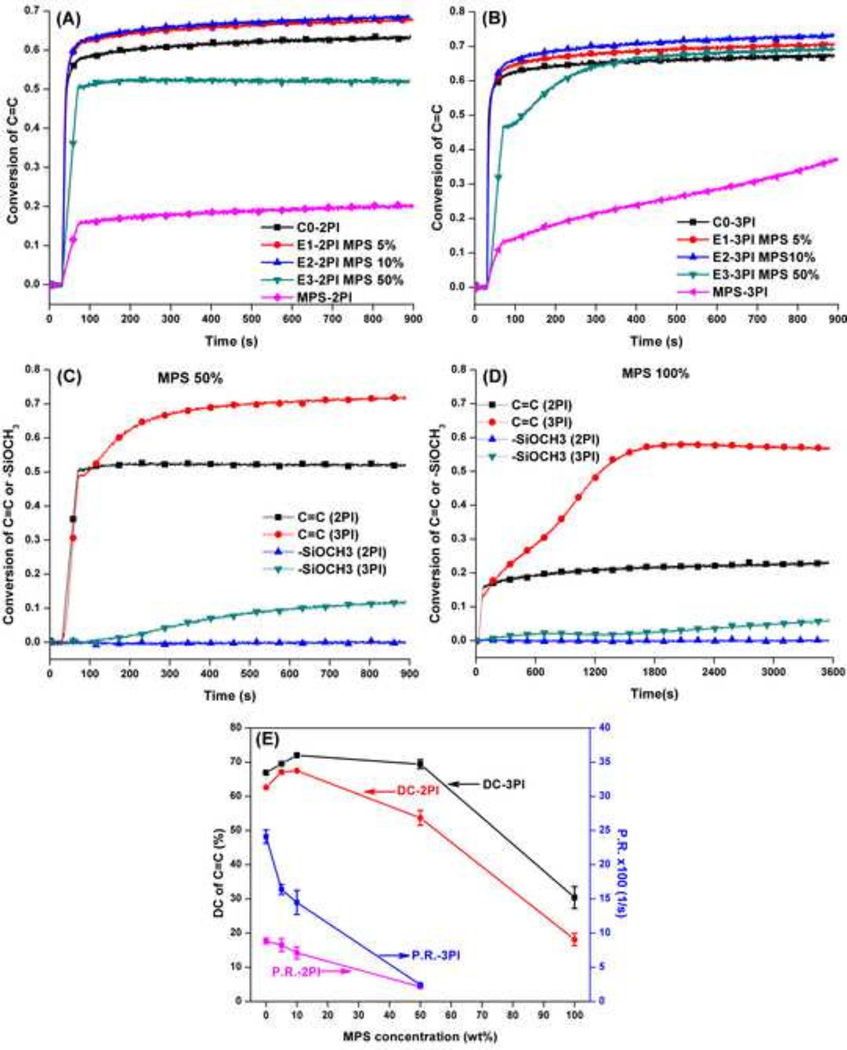
Real-time photopolymerization kinetic behavior of the methacrylate-based formulations is shown in Figure 1. The experimental formulations that utilize the two-component PI system and contain low concentrations of MPS, i.e. 5 and 10 wt %, exhibit similar DC of the C=C double bond, about 67 % at 600 s. The DC values decrease significantly to 53 and 18%, respectively, when MPS concentration reaches 50 and 100 wt%. In the three-component PI system, the DC values are significantly higher (p≤0.05) than the control (C0-3PI) and remain similar (about 70%) with the increase of MPS concentration, the exception being 100 wt% MPS. The maximum polymerization rate () decreases with the increase of MPS content. The DC and of water-containing formulations are listed in Table 1. With the addition of water, the DC of the control (C0w-3PI) increased slightly and the decreased slightly compared to that of the C0-3PI. At the same time, the DC of the water-containing formulation remained comparable with that of the corresponding neat resin formulation and the increased with the addition of water, the exception being the formulation with 50 wt% MPS. In the two-component PI systems, the hydrolysis conversion curves plateau with termination of the light-irradiation. In the three-component PI systems, the curves increase gradually after light-irradiation is terminated.
Figure 1.
Real-time conversion of C=C double bond of control and experimental adhesive formulations with different PI, (A) two-component PI (CQ/EDMAB), (B) three-component PI (CQ/EDMAB/OPPIH), and conversion of C=C or methoxysilyl moiety (-SiOCH3) at higher MPS concentration (C) MPS 50 wt% (E3-2PI and E3-3PI), (D) MPS 100 wt% (MPS-2PI and MPS-3PI), and (E) DC and vs. MPS content. The adhesives were light-cured for 40 s at 25 °C using a commercial visible light lamp (Spectrum® 800, Dentsply, Milford, DE. Intensity is 550 mW/cm2).
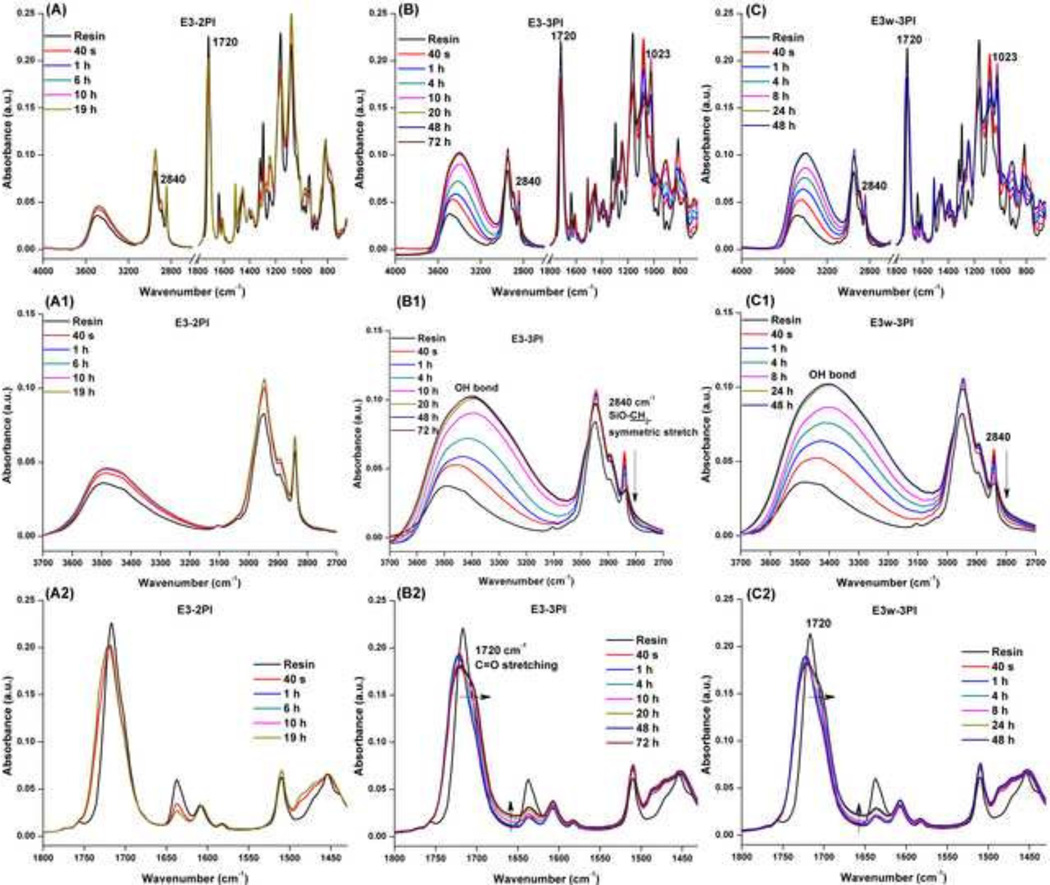
Figure 2 shows the characteristic peaks of FTIR spectra of three adhesive formulations before and after visible-light irradiation. The intensity of methoxy (−SiOCH3, 2840 cm−1) [43] in the two-component PI system remained the same even after 19 h, which indicated that the sol-gel reaction is not occurring (Figure 2A). With the addition of iodonium salt (OPPIH, E3-3PI), the intensity of methoxy decreased gradually and the maximum peak position moved from 2840 to 2836 cm−1. At the same time, the intensity of the band at 3450 cm−1 (OH stretching mode) increased. In the water-containing formulation (E3w-3PI, 1 wt% water), the characteristic peak showed the same trend with that of the E3-3PI formulation (Figure 2C).These results indicated that the hydrolysis step of the sol-gel reaction occurred with storage time. The degree of hydrolysis of E3-2PI, E3-3PI, and E3w-3PI, calculated from the band ratio, were ~0 (19 H), 67% (48H) and 69% (48H), respectively (see SI Figure 2). With the addition of water into the formulation, the hydrolysis reaction was faster than that of the neat resin during the first 24 h, but the final values at 48 h were similar. To verify the condensation step of the sol-gel reaction of formulations E3-3PI and E3w-3PI, a characteristic peak at 1023 cm−1 assigned to the Si−O−Si antisymmetric stretch mode was identified and it was noted that this spectral feature increased with time (Figure 2B & 2C). The gradual broadening of the peak at 1720 cm−1 (C=O stretching) and elevated baseline at 1637 cm−1 was attributed to increasing water concentration in the copolymers. These results are evidence that the condensation reaction between silanol-silanol or silanol-hydroxyl groups occurred with storage time and these reactions produced water.
Figure 2.
FTIR characteristic peaks of E3-2PI (A, A1, A2), E3-3PI (B, B1, B2), E3w-3PI (C, C1, C2) formulations before and after visible-light irradiation.
It is noted that E1 and E2 formulations (e.g., containing 5% MPS and 10% MPS, respectively) were not reported by analyzing their FTIR spectra. This is because the contribution from the signal of vsym(−SiOCH3) of these formulations was weak and overlapped with that vsym(−CH2) of methacrylate in the FTIR spectra, when the MPS concentration was lower than 50 wt% (unpublished data). These two formulations were evaluated for the mechanical properties.
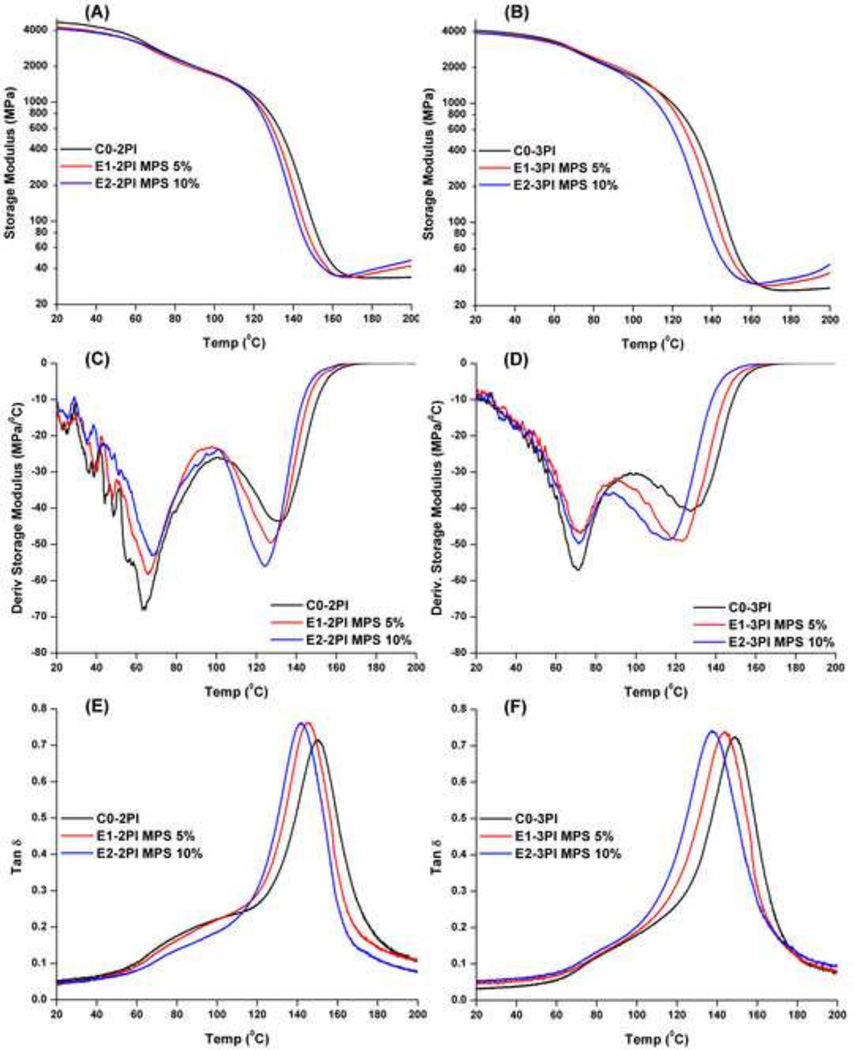
The dynamic mechanical properties of methacrylate copolymer specimens in the dry condition at various temperatures are shown in Figure 3 and Table 2. With the increase of MPS content from 5 to 10 wt%, whether in the two-component or three-component PI, the storage modulus values at 25 or 37 °C were lower than that of the control. The Tg values of the polymer samples decreased from about 150 °C for the control to 145 and 141 °C for 5 and 10 wt% MPS, respectively.
Figure 3.
Representative storage modulus (A and B), derivative storage modulus (C and D), and tan δ (E and F) vs. temperature curves of the controls and experimental adhesive copolymers in dry conditions using two-component (CQ/EDMAB) and three-component (CQ/EDMAB/OPPIH) PI.
Table 2.
DMA Results of Two/Three-component PI Dentin Adhesive in Dry Condition
| Run | Storage Modulus (MPa) | Rubbery Modulus (MPa) |
Tga(°C) | |
|---|---|---|---|---|
| 25 °C | 37 °C | |||
| C0-2PI | 4503 (85) | 4251 (93) | 32.3 (1.1) | 149.8 (0.6) |
| E1-2PI | 4230*(88) | 4060 (99) | 34.6*(0.9) | 145.8*(0.9) |
| E2-2PI | 4065*(73) | 3872*(84) | 34.8*(0.3) | 141.5*(0.3) |
| C0-3PI | 4074 (54) | 3909 (65) | 27.6 (0.2) | 149.4 (0.5) |
| E1-3PI | 3863 (80) | 3724 (64) | 29.6# (0.6) | 145.0#(1.1) |
| E2-3PI | 3680#(136) | 3543#(132) | 29.7# (1.0) | 139.1#(1.4) |
Tg is obtained from the maximum value on the tanδ vs. temperature profile.
significantly (p<0.05) different from control C0-2PI and C0-3PI, respectively.
Table 3 shows the DMA results of neat resin and water-containing formulations in wet conditions (water or 1 mM LA solution). The Tg values and storage moduli of water-containing samples (C0w/E1w/E2w-3PI) at 25 or 37 °C are similar with the neat resin (C0/E1/E2-3PI). The storage moduli at 70 °C of water-containing samples are significantly higher (p≤0.05) than those of the neat resin. The storage moduli and Tg values of the water-containing samples are comparable whether stored in water or 1 mM LA solution for 1 week.
Table 3.
DMA Results of Neat Resin and Water-containing Dentin Adhesive in Wet Condition a
| Storage medium | Run | Storage modulus (MPa) | Tgb (°C) | ||
|---|---|---|---|---|---|
| 25 °C | 37 °C | 70 °C | |||
| Water | C0-3PI | 2401(35) | 1903(28) | 208.3 (0.3) | 58.7 (0.3) |
| E1-3PI | 2469(31) | 2023(33) | 359.4*(12.2) | 61.7*(0.2) | |
| E2-3PI | 2334(112) | 1961(112) | 557.1*(23.4) | 66.0*(0.6) | |
| Water | C0w-3PI | 2553(14) | 2090(8) | 297.4 (6.7) | 59.8 (0.3) |
| E1w-3PI | 2450(49) | 2044(37) | 416.2#(3.3) | 62.6#(0.2) | |
| E2w-3PI | 2387(105) | 2030(89) | 602.5#(28.3) | 66.6#(0.5) | |
| LA (1mM) | C0w-3PI | 2490(23) | 2054(23) | 310.3 (7.5) | 60.4 (0.4) |
| E1w-3PI | 2423(13) | 2027(13) | 413.0&(12.9) | 63.2&(0.2) | |
| E2w-3PI | 2383(55) | 2027(56) | 637.3&(20.6) | 67.6&(0.2) | |
the copolymer beams were stored in water or LA (1 mM) for 1week at 37 °C.
Tg is obtained from the position of maximum on the derivative storage modulus versus temperature plot.
significantly (p<0.05) different from control C0-3PI and C0w-3PI, respectively.
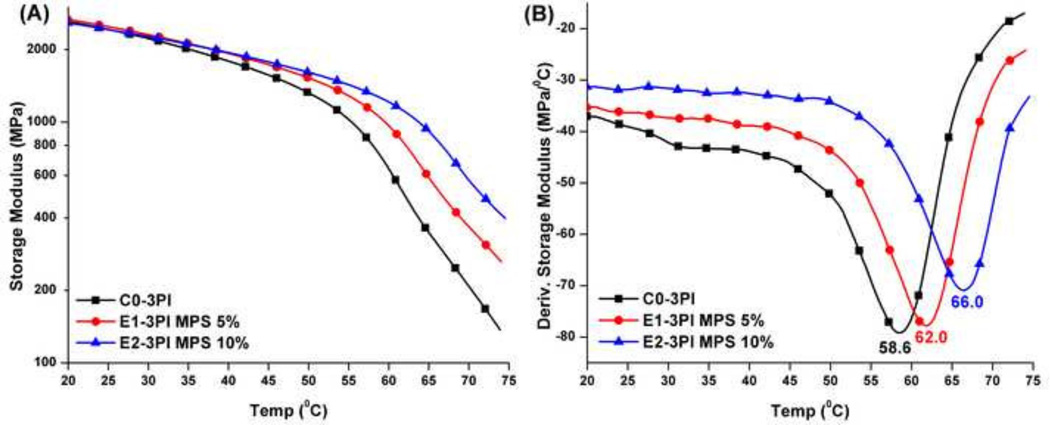
The mechanical properties of adhesive copolymers using three-component PI system in wet condition are shown in Figure 4. With the increase of MPS content from 5 to 10 wt%, the storage moduli at 37 °C are comparable with that of the control (C0-3PI). The storage moduli at 70 °C and Tg values of the experimental formulations with the 3PI system are significantly higher than those of the control (C0-3PI, p≤0.05, see Table 4). The storage moduli of the two-component PI systems are lower than that of three-component PI systems when the specimens were stored in water or LA solution for 1 week (Plots of two-component PI systems see SI Figure 3).
Figure 4.
Representative storage modulus (A) and derivative storage modulus (B) vs. temperature curves of the controls and experimental adhesive copolymers in wet conditions (stored in water for 1 week) using three-component PI.
Table 4.
DMA Results of Two/Three-component PI Dentin Adhesive in Wet Conditions
| Time | Run | Storage modulus (MPa) | Tg (°C) | ΔTg (°C) | ||
|---|---|---|---|---|---|---|
| 25 °C | 37 °C | 70 °C | ||||
| 1week (water) |
C0-2PI | 2337 (86) | 1852(97) | 151.4 (20.2) | 58.7 (0.9) | - |
| E1-2PI | 2143*(95) | 1717(73) | 183.5 (11.2) | 61.6*(0.2) | 2.9 | |
| E2-2PI | 2228 (30) | 1839(32) | 323.9*(12.3) | 65.2*(0.1) | 6.5 | |
| 1week (water) |
C0-3PI | 2406(26) | 1928(48) | 204.2 (7.1) | 58.6 (0.3) | - |
| E1-3PI | 2469(31) | 1986(67) | 349.0*(14.1) | 62.0*(0.5) | 3.4 | |
| E2-3PI | 2314(100) | 1950(94) | 557.1*(23.4) | 66.0*(0.6) | 7.4 | |
| 1 week (LA solution) |
C0-3PI | 2466(31) | 1993(31) | 231.4 (25.0) | 59.1 (0.3) | - |
| E1-3PI | 2353(57) | 1940(37) | 341.8*(11.0) | 62.4*(0.4) | 3.3 | |
| E2-3PI | 2360(18) | 1977(11) | 524.5*(11.6) | 66.3*(0.2) | 7.2 | |
| 8 weeks (water) |
C0-3PI | 2365(27) | 1908(9) | 215.8(24.5) | 64.6(0.6) | - |
| E1-3PI | 2371(51) | 1982(47) | 519.2(68.0) | 69.0(0.5) | 4.4 | |
| E2-3PI | 2326(44) | 1993(31) | 847.9(16.0) | >75.0 | >10.4 | |
| 8 weeks (LA solution) |
C0-3PI | 2441(70) | 1979(40) | 229.0 (12.6) | 64.0 (0.4) | - |
| E1-3PI | 2369(117) | 1990(103) | 490.7*(42.0) | 68.4*(0.3) | 4.2 | |
| E2-3PI | 2417(72) | 2070(45) | 876.4*(12.6) | 73.5*(0.6) | 9.5 | |
significantly (p<0.05) different from control C0-3PI. ΔTg =Tg(experimental)-Tg(control).
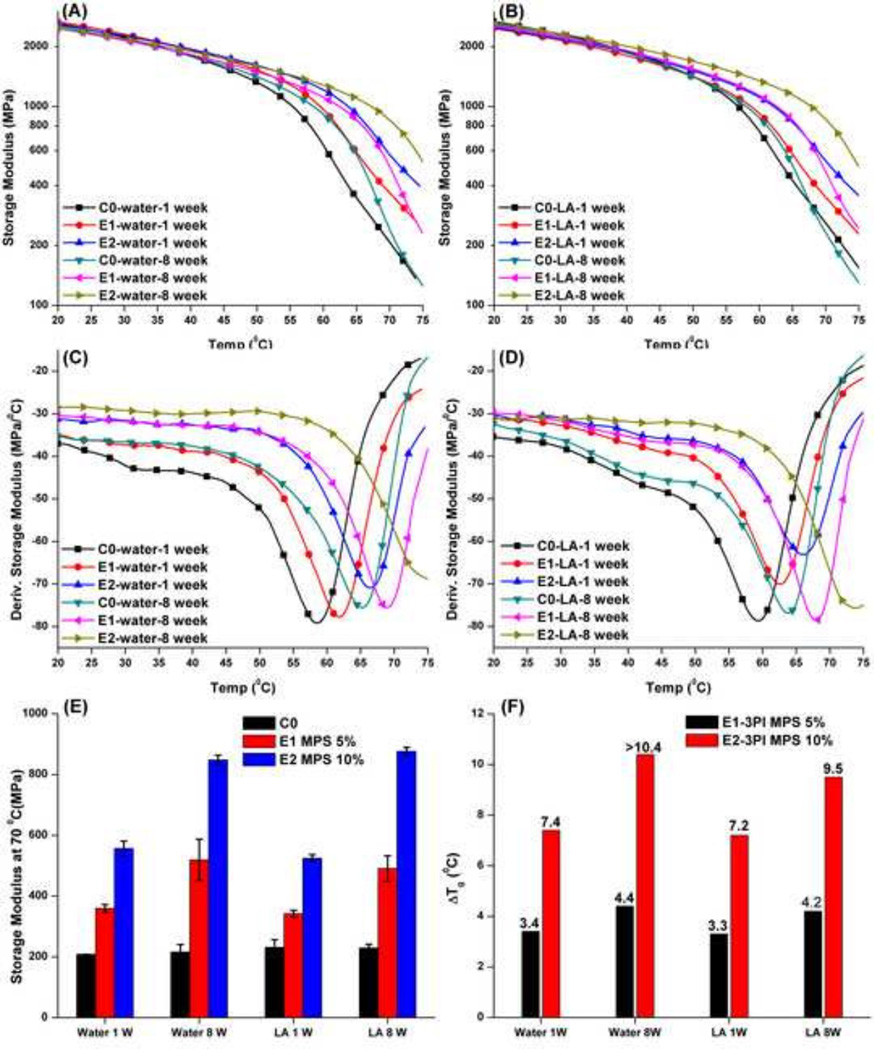
Figure 5 shows the mechanical properties of the control and experimental copolymers stored in water or 1 mM LA for 1 and 8 weeks. With the increase in storage time from 1 to 8 weeks in water or LA solution, the storage moduli of control (C0-3PI) remain similar at about 200 MPa at 70 °C. The Tg values of the control formulations showed about 5–6 °C increase after 8 weeks. Stored in water or LA solution, the storage moduli at 25 or 37 °C for the experimental formulations are similar to those of the control (Table 4 and Figure 5E). The storage moduli and Tg values of the experimental formulations do not become significantly different, whether stored in water or LA solution. The storage moduli at 70 °C for the experimental formulations are significantly higher than those of the control.
Figure 5.
Representative storage modulus (A and C) and derivative storage modulus (B and D) vs. temperature curves, the storage moduli at 70 °C bar figure (E), and Tg difference (F) between the control (C0-3PI) and experimental adhesive copolymers (E1/E2-3PI) stored in water or 1 mM LA solution for 1 and 8 weeks.
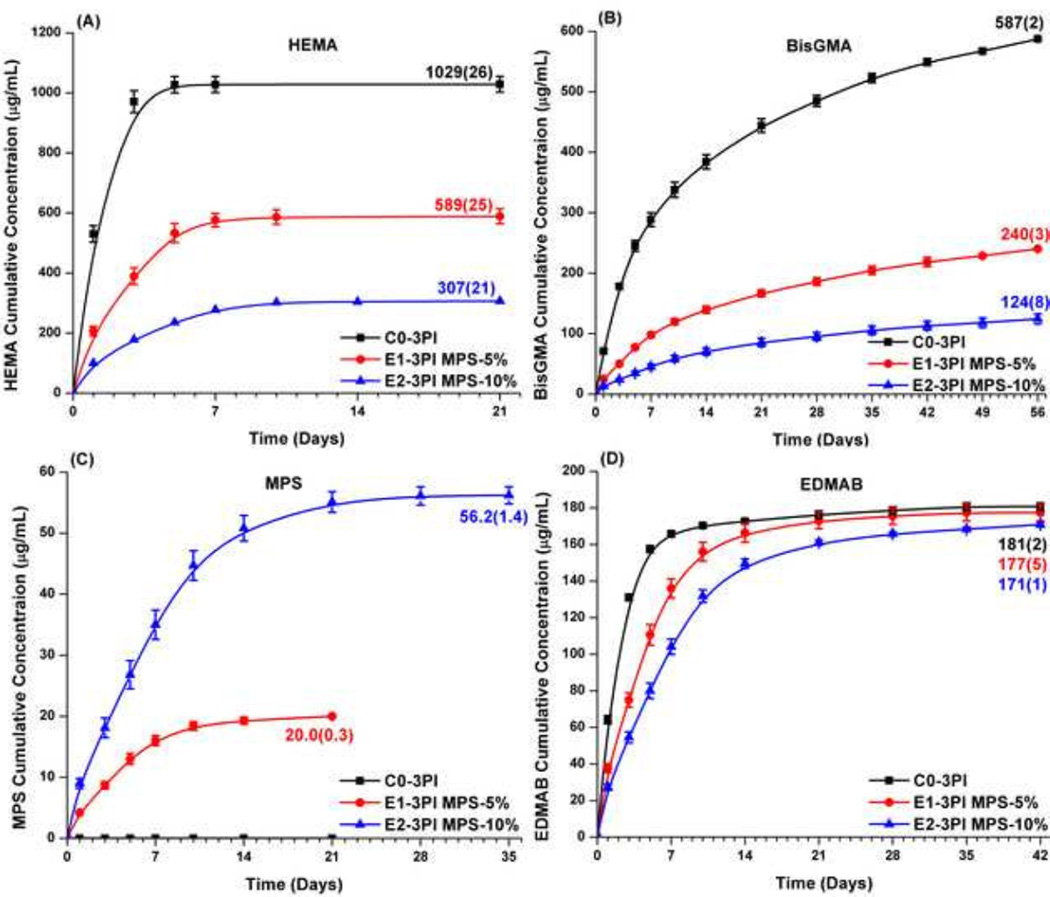
Figure 6 shows the results of cumulative leachate from the copolymers (control, E1-3PI, E2-3PI) as a function of incubation time in ethanol at 23±2 °C. With the increase of MPS concentration from 0 to 10 wt% in the formulations, the cumulative release of HEMA decreased from 1029±26 to 307±21 µg/mL and the cumulative release of BisGMA decreased from 587±2 to 124±8 µg/mL. With the increase of MPS concentration from 0 to 10 wt%, the percentage of leached HEMA decreased from 11.4 to 3.8 wt% (calculated method see SI information) and the percentage of leached BisGMA decreased from 5.3 to 1.2 wt%. The cumulative release of MPS increased from 20.0±0.3 to 56.2±1.4 µg/mL, and the percentage of leached MPS was 2.0 and 2.8 wt%, respectively. The cumulative release of EDMAB was similar regardless of the concentration of MPS in the adhesive formulation.
Figure 6.
Cumulative release from the dental adhesive copolymers as a function of incubation time in ethanol: (A) HEMA, (B) BisGMA, (C) MPS, and (D) EDMAB.
4. DISCUSSION
4.1 Role of iodonium salt
Under acidic solution, alkoxysilyl groups react with water or moisture and generate silanol groups that can go on to condense to form a polysiloxane network [44]. Photoacid-induced or photoacid-catalyzed sol-gel reaction is now recognized as a powerful synthetic approach to prepare silica-based hybrid materials [33, 45]. In photoacid-catalyzed sol-gel reactions, the onium salt is crucial to the generation of the Brønsted acid, which can catalyze the hydrolysis and condensation of alkoxysilyl moieties through formation of silanol functions and their subsequent condensation. Iodonium salts can be directly decomposed by absorption of UV light and generate photoacid species [33, 34, 36]. Iodonium salts cannot be directly decomposed by absorption of visible light. The iodonium salt can, however, be decomposed through redox reaction with the amine free radicals and the generated phenyl free radicals and Brønsted acids [46–48], the latter of which have been used to initiate the cationic polymerization of epoxy or vinyl ether monomers to prepare interpenetrating polymer network (IPN) structures. Here, the generated photoacid was utilized to catalyze the sol-gel reaction of MPS. The first step was to confirm the validity of the photoacid-induced sol-gel reaction in the visible-light induced photopolymerization of methacrylate-based dental adhesives.
The mechanism and conversion of photo-induced sol-gel reactions that utilize iodonium salts have been studied by 29Si Cross-polarization magic angle spinning (CPMAS) NMR and FTIR [33, 34, 49]. To generate Brønsted acids via photo-irradiation, onium salts can undergo a cascade of homolytic or heterolytic cleavages and yield protonic super-acids under UV-light irradiation [45]. It is well known that most of the onium salts do not absorb light significantly above 300 nm unless additional chromophores are incorporated into the salt structure. Therefore, most of the photoacid catalyzed sol-gel reactions have been engaged by UV-light sources. The reported conversion of the photoacid catalyzed sol-gel reaction lies between 60–100 %, varying with the structure of precursors, irradiation time, types and content of onium salt, film thickness, and so forth [33, 45, 49]. However, onium salts are not only decomposed by UV-light, they also act as electron acceptors in redox reaction with free radicals, so-called free radical promoted cationic polymerization (FRPCP) [48]. The overall mechanism of FRPCP involves oxidiation of the photochemically formed free radicals by the onium salt. At the same time, cationic species (Brønsted acids that can catalyze the sol-gel reaction) are generated.
In this study, iodonium salt (OPPIH) can react with an amine radical (produced from co-initiator EDMAB) and generate Brøstend acid, which is crucial to catalyze the hydrolysis of alkoxysilyl moieties. When the MPS concentration was lower than 50 wt%, the signal of νsym(−SiOCH3) was overlapping with that νsym(−CH2) of methacrylate in the FTIR spectra (unpublished data). To evaluate the efficiency of the photoacid catalyzed sol-gel reaction, 50 (E3-2PI/3PI) and 100 wt% MPS formulations were used to quantitatively characterize the reaction by monitoring the characteristic peak of −SiOCH3. In two-component PI systems, the conversion vs. time curves reached a plateau after terminating the light-irradiation (Figure 1C and D). The intensity of methoxy did not change even after 19 h storage (Figure 2A). This result indicates that the sol-gel reaction cannot occur without the addition of iodonium salt OPPIH.
In the three-component PI systems, the conversion-time curves showed an increasing trend after light-irradiation was terminated (Figure 1C and D). After 48 h storage, the intensity of methoxy at 2840 cm−1 decreased (Figure 2C) and the intensity of hydrogen bonded OH around 3400 cm−1 increased. This result indicates that the sol-gel reaction continues even after the light-irradiation has terminated. During the photoacid-induced sol-gel reaction of -Si(OCH3)3 moieties, intermediate species such as −Si(OCH3)2(OH) or −Si(OCH3)(OH)2 are products of a partial hydrolysis reaction. Due to the hydroxyl group acting in an electron-donating role, the maximum peak position of −SiOCH3 shifted from 2840 to 2836 cm−1. From Figure 2G, the degree of hydrolysis can reach about 70% after light-irradiation is terminated and the specimen is stored for 48h. These results indicate that most of the methoxysilyl moieties have been hydrolyzed and the silanol groups were generated. Due to the highly crosslinked structure of polymethacrylate-based matrix, the mobility of polymer side-chains was very limited, which retarded the condensation of silanol groups. Figure 2D shows that the intensity of polymeric OH stretching increased and the maximum peak position shifted from 3450 to 3370 cm−1, which was attributed to the formation of hydrogen bonds during the storage. When MPS content is 50 wt%, the DC of C=C for the three-component PI system is significantly higher than that of the two-component PI system, which is attributed to the production of an active phenyl radical with regeneration of the original CQ in the presence of the iodonium salt [41].
The FTIR study indicated that with visible-light irradiation: (1) photoacid catalyzed sol-gel reaction can occur with the addition of iodonium salt; (2) the hydrolysis-condensation rate was relatively low compared to the free radical polymerization; (3) and the degree of hydrolysis and condensation of MPS can reach over 65 % after 48h dark storage. In the two-component PI system, the amine free radical, formed after the hydrogen abstraction from amine by activated photosensitizer CQ*, guided the polymerization of methacrylate at a high rate. Due to the lack of photoacid catalyst, MPS can only be copolymerized with HEMA and BisGMA, and the hydrolysis of methoxysilyl moieties cannot occur. Therefore, based on these results, the first hypothesis, the photoacid-induced sol-gel reaction cannot occur without the addition of iodonium salt, is accepted.
In the three-component PI systems, the amine radicals could not only initiate the polymerization of methacrylate, but also be oxidized by OPPIH to form H+ protons, which can catalyze the hydrolysis of methoxysilyl moieties. However, the degree of hydrolysis of methoxysilyl groups was relatively low (< 5%) immediately after terminating light-irradiation. This phenomenon could be attributed to the following: (1) over 50 % of the C=C double bonds could be polymerized and a polymethacrylate-based network structure forms in just a few seconds of light-irradiation; (2) the lack of water in the neat resin formulation.
4.2 Role of water in formulations
It is well known that the proper quantity of water can promote hydrolysis and condensation in the conventional sol-gel process [25]. However, in the photoacid-catalyzed sol-gel process, it was considered that a small amount of water permeated from the ambient atmosphere (humidity 20–55%) and that this was suitable to enhance the hydrolysis rates [45, 49]. With excess water, superacids can be hydrolyzed leading to their replacement by H3O+ with lower acidity and weaker catalytic activity [33, 34, 43]. In the present study, the specimens were prepared in a glass tube, which inhibited moisture penetration from the atmosphere; therefore, 1 wt% water was added into the liquid formulation.
With the additional 1 wt% water, the DC values of control and experimental specimens were increased slightly. However, the maximum polymerization rate of the experimental samples showed a significant increase, and the rate increased about 30 and 80 % when the MPS content was 5 or 10 wt%, respectively. It is well known that the maximum polymerization rate is usually correlated to the viscosity of formulations. In the present study, the viscosity of experimental formulations decreased with increasing MPS content (unpublished data). Therefore, the contradictory results may be attributed to the autoacceleration phenomena in the free-radical polymerization reaction [50] . With the addition of 1 wt% water, the sol-gel reaction of MPS was slightly accelerated, and the hydrolysis of methoxysilyl groups increased from <5% to about 10%. It is likely that this increase promoted the formation of nano- or micro-gels during visible-light irradiation. The formation of these structures enhanced the gel effect associated with the free-radical polymerization reaction. The hydrolysis rate of the water-containing formulation (Fig. 2G) was faster than that of the neat resin (E3-3PI), which indicated that a small amount of water can promote the sol-gel reaction. Compared with the published reports of sol-gel reaction rates, [33, 43, 51, 52] our result was relatively low even with the addition of water into the formulation. The present study indicated that the polymethacrylate-based network, which formed first, retarded the photoacid-induced sol-gel reaction. Therefore, based on the FTIR results, the second hypothesis, water is necessary for the hydrolysis of photoacid-induced sol-gel reaction, is rejected.
4.3 Dynamic Mechanical Analysis
In this study, the DMA tests were carried out using both standard and submersion 3-point bending methods. It was anticipated that the results acquired with the water-submersion clamp would be more representative of the behavior of the polymer in the wet, oral environment.
The storage moduli of methacrylate polymers containing MPS in dry condition are shown in Figure 3 and Table 2. The rubbery moduli are higher than those of the control. Comparing the storage moduli of the copolymers prepared with the two-component or three-component PI, the values of the two-component were higher than those of the three-component in the dry condition. Mechanical properties have been correlated with the DC of monomers [42, 53, 54], and the DC of the three-component PI systems was higher than that of the two-component PI (see Figure 1E). These contradictory results could be attributed to the long-tail alkyl group of iodonium salt (OPPIH), which can act as a plasticizer in the copolymer. This result was further confirmed by the slightly lower glass transition temperature (Tg) of the three-component PI samples in the dry condition (Table 2).
It has been reported that the Tg of hybrid copolymers containing the alkoxylsilane component show an obvious increase based on DSC and DMA measurements [34]. This increase was attributed to the formation of silica phase during irradiation with UV-light. In the present study, with the increase of MPS content in neat resin formulation from 0 to 10 wt%, the Tg of copolymers prepared with the three-component PI decreased from 149.4 to 139.1 °C. The results indicated that the sol-gel reaction was limited under visible-light irradiation. Meanwhile, the real-time FTIR results supported this conclusion. During visible-light irradiation, amine free radicals were generated via electron/proton transfer between EDMAB and excited CQ, and engaged the polymerization of methacrylate monomers. At the same time, some amine radicals were oxidized by OPPIH, and photoacids were formed, which catalyzed the sol-gel reaction of MPS. Due to the fast rate of free radical polymerization, the transition from liquid to solid occurs within several seconds. With the formation of the polymethacrylate-based matrix, the mobility of chains and functional groups (-SiOCH3) was restrained, which could depress further polymerization and the sol-gel reaction.
The intensity of the maximum tan δ peak reflects the extent of the mobility of the copolymer chain segments. A higher peak means a longer distance between crosslink points. It is reported that the heterogeneity will increase as the crosslink agent concentration increases within a copolymer system [53]. From Figure 3, with the addition of MPS in the formulations, whether in two-component or three-component PI formulations, the tan δ peak values increased slightly, which indicated that the mobility of copolymer chain was enhanced. The mole concentrations of BisGMA were 23.7, 22.6, and 21.6 mol% in the formulation of C0, E1, and E2, respectively. With the decrease of BisGMA concentration in the formulations, the crosslink density of copolymers was reduced, and the mobility of copolymer chains was improved. At the same time, from the derivative storage modulus curves (Figure 2 C and D), two transition peaks were observed. The first transition temperature could be attributed to the side-chains or oligomer, while the second transition temperature is due to the movement of segments of the main chains (Tg) [55]. It can be found the Tg values are comparable in both two/three-component PI formulations, however, the first transition temperature of two-component PI was obviously lower than that of the three-component PI formulation. This could be due to the lower DC in the two-component PI.
The water-submersion method used in this work is expected to simulate the wet environment of the mouth. Samples can be heated to only ~75 °C for the 3-point bending water submersion clamp. The storage moduli of the controls (C0-2PI and C0-3PI) and experimental samples measured by the water-submersion method were significantly lower than those of the dry samples. A similar result has been noted in our previous work [56]. The difference is attributed to the plasticization of the copolymer in the water. Water can be attracted to the polar functional groups (such as hydroxyl and ester) of the copolymer to form hydrogen bonds and decrease the intermolecular interaction of the copolymers. Water related plasticization can be a concern in the hybrid layer where the adhesive forms a composite with water rich collagen as shown through recent in vitro studies [57]. Indeed, the simultaneous water diffusion and mechanical loading can lead to anomalously high creep strain or lower strengths. Comparison of the storage moduli at 70 °C indicates significantly higher values for the three-component PI samples versus the two-component PI. There are two reasons for the phenomenon: (1) the DC values of three-component PI systems were higher; (2) the photoacid-induced sol-gel reaction enhanced the crosslink density.
With the increase in storage time from 1 to 8 weeks in water, the Tg values of the copolymers with three-component PI system increased from 58.7 to 64.6 °C. When the samples were soaked in water, water penetrated into the network and the mobility of polymer chains was enhanced. The “trapped” free radical can further initiate the un-reacted C=C double bond and improve the crosslink density, and the Tg values shift to higher temperature. At the same time, the “trapped” strong photo-generated Brønsted acid was efficient at driving the sol-gel reaction. The difference in the glass transition temperature of the control (C0-3PI) and the experimental (E1/E2-3PI), stored in water for 1 week, was 3.3 and 7.2 °C and for 8 weeks was 4.4 and >10.4 °C. The increase in the differences in Tg indicated that the sol-gel reaction occurred in wet conditions and continued for an extended storage time. In the present study, after 8 weeks, the storage moduli of the experimental samples at 70 °C increased about 44 (E1–3PI, MPS 5%) and 52% (E2–3PI, MPS 10%), respectively.
To simulate the oral acidic environment, 1 mM lactic acid solution (LA) was used as the storage solution [58–60]. The storage moduli of the control specimens were similar after storage in LA solution for 8 weeks. These results indicated that the polymethacylate-based network was stable under the present experimental conditions. In comparison, the storage moduli of the experimental samples in LA solution (pH= 3.5/25 °C) were slightly lower than the values in water. This may be attributed to the effect of pH on the sol-gel reaction.
In an acidic environment (pH = 3.5/25 °C) the silanol species were most likely to be protonated and the hydrolysis rate of methoxysilyl groups was fast. However, the condensation rate was relatively slow compared with neutral [25], which was prone to the formation of branched structure. It is well known that the rate of the hydrolysis reaction of alkoxysilyl groups increases more efficiently than the condensation reaction under acidic conditions. The rate of condensation is lower than the rate of hydrolysis at low pH (<pH 3) and higher at neutral pH[25], which is beneficial to the intrinsic self-strengthening dental adhesive. It has been reported that acid-catalyzed gels which are richer in silanol (-SiOH) exhibit a very uniform microstructure throughout the material [61]. In the present work, when the MPS content is 50 wt% in the formulation, the increase of the hydrogen bonded OH group’s intensity with storage time supported that the rich silanol moieties are generated (see Figure 2D and F).
The mechanical properties of dental adhesive in wet conditions could be affected by the increase of water sorption. The water molecules enhance the movement of the copolymer chain segments by increasing free volume, thus increasing the flexibility of the adhesive. In the present work, water sorption values of the experimental copolymers were slightly higher than the control (unpublished data). However, whether in neutral or acidic solution, the storage moduli of the experimental copolymers (E1/E2-3PI) at 37 °C were similar with that of the control (C0-3PI), and the storage moduli of the experimental samples at 70 °C were significantly higher than that of the control (p≤0.05). Based on the results from the mechanical property measurements under wet conditions, the third hypothesis, the mechanical properties of silane-containing dental adhesive will show self-strengthening behavior under wet conditions, is accepted.
Due to the viscoelastic nature of dental adhesive copolymers, the analysis of their long-term behavior is essential and could be a first step toward making better predictions and estimates of clinic longevity. According to the time-temperature correspondence principle, events taking place at higher temperatures could be correlated to those at lower temperatures but at longer times [62, 63]. In wet conditions, the MPS-containing copolymers had higher storage moduli at high temperature (70 °C) than that of the control. This result suggests that the experimental copolymers possessed better mechanical performance in higher temperature and may have enhanced longevity at lower temperature as compared to the control. Although we have not determined the temperature shift factor as a function of temperature, the present DMA results suggest that the sol-gel reaction significantly modified the copolymer network at the molecular level. The prediction of longevity of silane-containing copolymer is important for in vivo applications and is part of an ongoing study.
4.4 HPLC analysis
Ethanol was used as the solvent to enhance the solubility of the hydrophobic chemicals, i.e., BisGMA, MPS, and EDMAB and accelerate their diffusion from the polymers. In this work, only the leachates from the three-component PI samples (e.g., C0, E1-3PI & E2-3PI) were analyzed. The HPLC results indicated considerable leaching of both HEMA and BisGMA from the control formulation. With the increase of MPS concentration, the amount of both HEMA and BisGMA that was leached from the polymer decreased significantly. This may be attributed primarily to the following: i) higher DC of C=C bond, i.e. with the increase of MPS, the DC of experimental polymer was significantly higher than that of the control (see Table 1, three-component PI); ii) the crosslink density of the network formed through the condensation reaction between the silanol/silanol or silanol/hydroxyl groups of HEMA/BisGMA inhibited leaching of these species. With the increase of MPS concentration from 0 to 10% in the adhesive formulations, the DC values are 66.9±0.4, and 72.0± 0.1, respectively. However, with the addition of 5 or 10 wt% MPS in the formulations, the cumulative amounts of leached monomers (HEMA and BisGMA) were reduced about 50 and 70%, respectively. This result suggests that the higher DC was not the major reason for the significant reduction in the amount of leached monomers. The rubbery modulus values of the experimental samples were significantly higher than the control samples (Table 2). These results suggest a relative increase in the crosslink density of the copolymers with the addition of MPS. It has been reported that covalent bonds might form by heterocondensation reaction of HEMA/BisGMA hydroxyl groups and silanols [64, 65].
In regard to the release rate of different species, HEMA release (< 10 days) was more rapid than MPS (~14–21 days) and BisGMA (> 49 days). The result showed that more hydrophobic compounds were eluted slowly from the copolymers into ethanol. With the increase of MPS contents, the cumulative concentrations of co-initiator EDMAB were similar. With the increase of incubation time, over 85 % EDMAB could be released. This is due to the fact that the EDMAB molecules were only trapped into the crosslinked network. However, the release rate was depressed. The slow release rates of experimental samples could be attributed to the higher crosslink density. These results provide valuable information for the development of dental adhesives with lower toxicity and good mechanical properties.
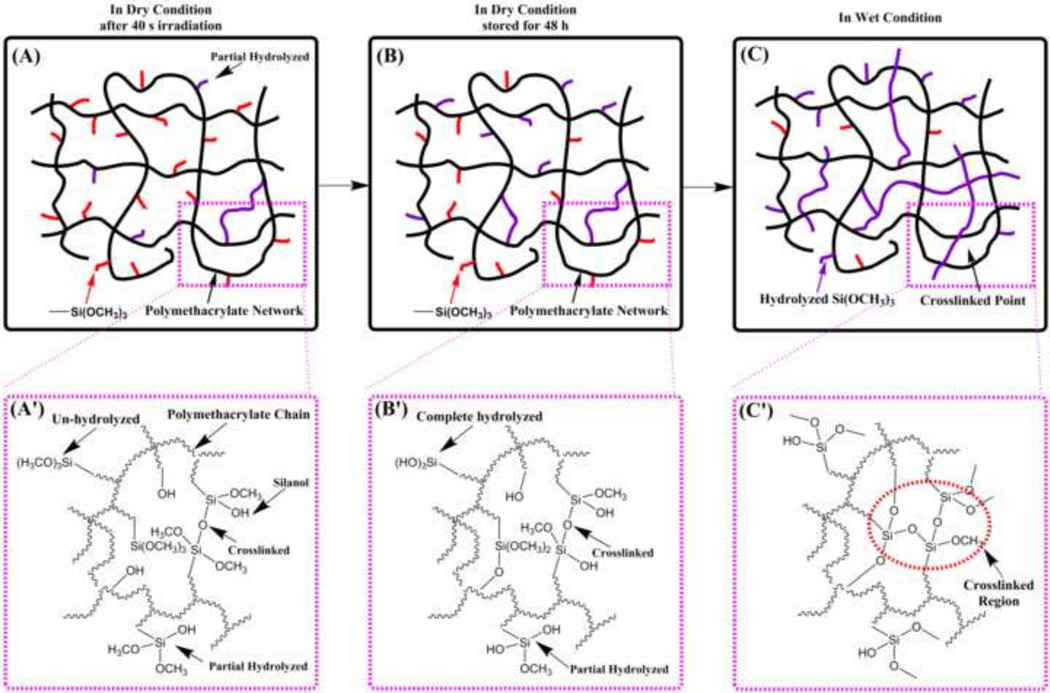
As shown in Scheme 2, based on the DMA and HPLC results, we propose a mechanism for the intrinsic self-strengthening processes after visible-light irradiation. When the liquid formulation is irradiated by visible-light, polymethacrylate-based matrix network is formed by simultaneous free radical cross-linking polymerization of methacrylate monomers (HEMA, BisGMA, and MPS) and the methoxysilyl groups shows limited hydrolysis and condensation (Scheme 2A). When the copolymer sample is stored in the dark for 48 h, the photoacid-induced sol-gel reaction continued and about 65% of methoxysilyl groups have been hydrolyzed (Scheme 2B). After the copolymer specimens are soaked in water or LA solution, the autonomous hydrolysis and condensation of methoxysilyl moieties continues to occur and new crosslinked points are formed (Scheme 2C). At the same time, the silanol groups could react with the hydroxyl groups of HEMA or BisGMA to form covalent bonds (Si−O−C), which significantly reduced the leachates in ethanol. In the case of MPS-containing formulations, the hydrolysis rate of trialkoxysilyl group is relatively slow compared with the fast free radical polymerization of the C=C bonds in the methacrylate monomers. The present intrinsic self-strengthening dental adhesive system offers the potential of prolonging the functional lifetime in a wet environment. The present autonomic sol-gel reaction provided a slow and continued reaction, which can gradually generate the Si−O−Si bond and resist hydrolytic degradation.
Scheme 2.
Proposed polymethacrylate-based matrix network structure and the intrinsic self-repair processes: (A) polymethacrylate-based network formed by free radical initiated polymerization and limited photoacid-induced sol-gel reaction in dry condition after 40 s irradiation; (B) photoacid-induced sol-gel reaction of sample stored in dry condition for 48 h; (C) self-repair via sol-gel reaction in wet environment; (A’), (B’), and (C) show the magnify of part of the network structure.
5. CONCLUSION
Through this work, we showed an intrinsic self-strengthening, Si-based (MPS) hybrid system for potential use as dental adhesive. The properties and behavior of this system are reminiscent of living organisms such as marine invertebrates. The self-strengthening dental adhesive systems were prepared through a dual curing process, which involves the free radical photopolymerization and then slow hydrolysis and condensation (sol-gel reaction) of alkoxylsilane groups. FTIR results verified that limited sol-gel reaction catalyzed by the photo-generated Brønsted acid occurred during the light irradiation (40 s), and the hydrolysis and condensation process was autonomic and carried out mainly during dark storage. The Tg values of the experimental copolymers in wet conditions increased with increasing MPS content and storage time. This behavior suggested a decrease in the mobility of the polymer segments as a result of further crosslinking in wet condition. The HPLC results indicated that the cumulative amounts of leached monomers were reduced significantly. In conclusion, photoacid-induced sol-gel reaction was limited during irradiation, and the subsequent sol-gel reaction in wet condition is a suitable method to enhance the mechanical properties of the newly developed dental adhesive copolymers.
Supplementary Material
Statement of Significance.
A self-strengthening dental adhesive system was developed through a dual curing process, which involves the free radical photopolymerization followed by slow hydrolysis and condensation (photoacid-induced sol-gel reaction) of alkoxylsilane groups. The concept of “living” photoacid-induced sol-gel reaction with visible-light irradiation was confirmed in the polymer. The sol-gel reaction was retarded by the polymethacrylate network, which was generated first; the network extended the life and retained the activity of silanol groups. The self-strengthening behavior was evaluated by monitoring the mechanical properties of the hybrid copolymers under wet conditions. The present research demonstrates the sol-gel reaction in highly crosslinked network as a potentially powerful strategy to prolong the functional lifetime of engineered biomaterials in wet environments.
Acknowledgments
This investigation was supported by Research Grant: R01 DE022054 and R01 DE025476 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
REFERENCES
- 1.Feitosa VP, Watson TF, Vitti RP, Bacchi A, Correr-Sobrinho L, Correr AB, Sinhoreti MAC, Sauro S. Prolonged Curing Time Reduces the Effects of Simulated Pulpal Pressure on the Bond Strength of One-step Self-etch Adhesives. Oper. Dent. 2013;38:545–554. doi: 10.2341/12-180-L. [DOI] [PubMed] [Google Scholar]
- 2.Salz U, Zimmermann J, Zeuner F, Mozner N. Hydrolytic stability of self-etching adhesive systems. J. Adhes. Dent. 2005;7:107–116. [PubMed] [Google Scholar]
- 3.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Tjaderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol ILS, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR, Pashley DH. Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent. Mater. 2013;29:999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakki A, Cilli R, Mondelli RFL, Kalachandra S, Pereira JC. Influence of pH environment on polymer based dental material properties. J. Dent. 2005;33:91–98. doi: 10.1016/j.jdent.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL. Adhesive/Dentin Interface: The Weak Link in the Composite Restoration. Ann. Biomed. Eng. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostoryz EL, Dharmala K, Ye Q, Wang Y, Huber J, Park JG, Snider G, Katz JL, Spencer P. Enzymatic Biodegradation of HEMA/BisGMA Adhesives Formulated With Different Water Content. J. Biomed. Mater. Res. Part B. 2009;88B:394–401. doi: 10.1002/jbm.b.31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JG, Ye Q, Topp EM, Spencer P. Enzyme-Catalyzed Hydrolysis of Dentin Adhesives Containing a New Urethane-Based Trimethacrylate Monomer. J. Biomed. Mater. Res. Part B. 2009;91B:562–571. doi: 10.1002/jbm.b.31430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finer Y, Santerre JP. Salivary esterase activity and its association with the biodegradation of dental composites. J. Dent. Res. 2004;83:22–26. doi: 10.1177/154405910408300105. [DOI] [PubMed] [Google Scholar]
- 10.Hagio M, Kawaguchi M, Motokawa W, Miyazaki K. Degradation of methacrylate monomers in human saliva. Dent. Mater. J. 2006;25:241–246. doi: 10.4012/dmj.25.241. [DOI] [PubMed] [Google Scholar]
- 11.Yourtee DM, Smith RE, Russo KA, Burmaster S, Cannon JM, Eick JD, Kostoryz EL. The stability of methacrylate biomaterials when enzyme challenged: Kinetic and systematic evaluations. J. Biomed. Mater. Res. 2001;57:522–531. doi: 10.1002/1097-4636(20011215)57:4<522::aid-jbm1198>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Sideridou I, Tserki V, Papanastasiou G, Study of water sorption. solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials. 2003;24:655–665. doi: 10.1016/s0142-9612(02)00380-0. [DOI] [PubMed] [Google Scholar]
- 13.Ferracane JL. Water sorption and solubility of experimental dental composites. Abstr. Pap. Am. Chem. Soc. 1997;214 142-POLY. [Google Scholar]
- 14.Ye Q, Park J, Topp E, Spencer P. Effect of photoinitiators on the in vitro performance of a dentin adhesive exposed to simulated oral environment. Dent. Mater. 2009;25:452–458. doi: 10.1016/j.dental.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moszner N, Hirt T. New polymer-chemical developments in clinical dental polymer materials: Enamel-dentin adhesives and restorative composites. J. Polym. Sci. Pol. Chem. 2012;50:4369–4402. [Google Scholar]
- 16.Hass V, Luque-Martinez I, Sabino NB, Loguercio AD, Reis A. Prolonged exposure times of one-step self-etch adhesives on adhesive properties and durability of dentine bonds. J Dent. 2012;40:1090–1102. doi: 10.1016/j.jdent.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Truffier-Boutry D, Demoustier-Champagne S, Devaux J, Biebuyck JJ, Mestdagh M, Larbanois P, Leloup G. A physico-chemical explanation of the post-polymerization shrinkage in dental resins. Dent. Mater. 2006;22:405–412. doi: 10.1016/j.dental.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials. 2002;23:1819–1829. doi: 10.1016/s0142-9612(01)00308-8. [DOI] [PubMed] [Google Scholar]
- 19.Toledano M, Yamauti M, Osorio E, Osorio R. Zinc-Inhibited MMP-Mediated Collagen Degradation after Different Dentine Demineralization Procedures. Caries Res. 2012;46:201–207. doi: 10.1159/000337315. [DOI] [PubMed] [Google Scholar]
- 20.Mazzoni A, Carrilho M, Papa V, Tjaderhane L, Gobbi P, Nucci C, Di Lenarda R, Mazzotti G, Tay FR, Pashley DH, Breschi L. MMP-2 assay within the hybrid layer created by a two-step etch-and-rinse adhesive: Biochemical and immunohistochemical analysis. J. Dent. 2011;39:470–477. doi: 10.1016/j.jdent.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Tezvergil-Mutluay A, Agee KA, Hoshika T, Carrilho M, Breschi L, Tjaderhane L, Nishitani Y, Carvalho RM, Looney S, Tay FR, Pashley DH. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent. Mater. 2010;26:1059–1067. doi: 10.1016/j.dental.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brackett MG, Li N, Brackett WW, Sword RJ, Qi YP, Niu LN, Pucci CR, Dib A, Pashley DH, Tay FR. The critical barrier to progress in dentine bonding with the etch-and-rinse technique. J. Dent. 2011;39:238–248. doi: 10.1016/j.jdent.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallan S, Araujo MVF, Cilli R, Prakki A. Mechanical properties and characteristics of developmental copolymers incorporating catechin or chlorhexidine. Dent. Mater. 2012;28:687–694. doi: 10.1016/j.dental.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Song LY, Ge XW, Wang MZ, Zhang ZC. Direct preparation of silica hollow spheres in a water in oil emulsion system: The effect of pH and viscosity. J. Non-Cryst. Solids. 2006;352:2230–2235. [Google Scholar]
- 25.Brinker CJ. Hydrolysis and Condensation of Silicates - Effects on Structure. J. Non-Cryst. Solids. 1988;100:31–50. [Google Scholar]
- 26.Song LY, Ge XW, Zhang ZC. Interfacial fabrication of silica hollow particles in a reverse emulsion system. Chem Lett. 2005;34:1314–1315. [Google Scholar]
- 27.Ozcan M. The use of chairside silica coating for different dental applications: A clinical report. J. Prosthet. Dent. 2002;87:469–472. doi: 10.1067/mpr.2002.124365. [DOI] [PubMed] [Google Scholar]
- 28.Ho GW, Matinlinna JP. Insights on Ceramics as Dental Materials. Part I: Ceramic Material Types in Dentistry. Silicon. 2011;3:109–115. [Google Scholar]
- 29.Eslamian L, Ghassemi A, Amini F, Jafari A, Afrand M. Should silane coupling agents be used when bonding brackets to composite restorations? An in vitro study. Eur J Orthodont. 2009;31:266–270. doi: 10.1093/ejo/cjn106. [DOI] [PubMed] [Google Scholar]
- 30.Lung CYK, Matinlinna JP. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012;28:467–477. doi: 10.1016/j.dental.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Fox FJ, Noren RW, Krankkala GE. Catalyst for condensation of hydrolyzable silanes and storage stable compositions thereof. 4101513. (St. Paul, MN) USA: Minnesota Mining and Manufacturing Company; United States Patent. 1978
- 32.Crivello JV. The discovery and development of onium salt cationic photoinitiators. J. Polym. Sci. Pol. Chem. 1999;37:4241–4254. [Google Scholar]
- 33.Kowalewska A. Photoacid catalyzed sol-gel process. J. Mater. Chem. 2005;15:4997–5006. [Google Scholar]
- 34.Amerio E, Sangermano M, Malucelli G, Priola A, Voit B. Preparation and characterization of hybrid nanocomposite coatings by photopolymerization and sol-gel process. Polymer. 2005;46:11241–11246. [Google Scholar]
- 35.Malucelli G, Priola A, Sangermano M, Amerio E, Zini E, Fabbri E. Hybrid nanocomposites containing silica and PEO segments: preparation through dual-curing process and characterization. Polymer. 2005;46:2872–2879. [Google Scholar]
- 36.Chemtob A, Versace DL, Belon C, Croutxe-Barghorn C, Rigolet S. Concomitant Organic-Inorganic UV-Curing Catalyzed by Photoacids. Macromolecules. 2008;41:7390–7398. [Google Scholar]
- 37.Sallenave X, Dautel OJ, Wantz G, Valvin P, Lere-Porte JP, Moreau JJE. Tuning and Transcription of the Supramolecular Organization of a Fluorescent Silsesquioxane Precursor into Silica-Based Materials through Direct Photochemical Hydrolysis-Polycondensation and Micropatterning. Adv. Funct. Mater. 2009;19:404–410. [Google Scholar]
- 38.Song L, Ye Q, Ge X, Misra A, Laurence JS, Berrie CL, Spencer P. Synthesis and evaluation of novel dental monomer with branched carboxyl acid group. J. Biomed. Mater. Res. B. 2014;102:1473–1484. doi: 10.1002/jbm.b.33126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song LY, Ye Q, Ge XP, Singh V, Misra A, Laurence JS, Berrie CL, Spencer P. Development of methacrylate/silorane hybrid monomer system: relationship between photopolymerization behavior and dynamic mechanical properties. J. Biomed. Mater. Res. Part B. 2015 doi: 10.1002/jbm.b.33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge XP, Ye Q, Song LY, Misra A, Spencer P. Synthesis and evaluation of novel siloxane-methacrylate monomers used as dentin adhesives. Dent. Mater. 2014;30:1073–1087. doi: 10.1016/j.dental.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J, Ye Q, Topp EM, Misra A, Kieweg SL, Spencer P. Effect of photoinitiator system and water content on dynamic mechanical properties of a light-cured bisGMA/HEMA dental resin. J. Biomed. Mater. Res. Part A. 2010;93A:1245–1251. doi: 10.1002/jbm.a.32617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JG, Ye Q, Topp EM, Lee CH, Kostoryz EL, Misra A, Spencer P. Dynamic Mechanical Analysis and Esterase Degradation of Dentin Adhesives Containing a Branched Methacrylate. J. Biomed. Mater. Res. Part B. 2009;91B:61–70. doi: 10.1002/jbm.b.31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chemtob A, Peter M, Belon C, Dietlin C, Croutxe-Barghorn C, Vidal L, Rigolet S. Macroporous organosilica films via a template-free photoinduced sol-gel process. J. Mater. Chem. 2010;20:9104–9112. [Google Scholar]
- 44.Brinker CJ, Scherer GW. Solgel science: the physics and chemistry Sol-Gel processing. Academic Press, INC.; 1990. [Google Scholar]
- 45.De Paz H, Chemtob A, Croutxe-Barghorn C, Le Nouen D, Rigolet S. Insights into Photoinduced Sol-Gel Polymerization: An in Situ Infrared Spectroscopy Study. J. Phys. Chem. B. 2012;116:5260–5268. doi: 10.1021/jp212386e. [DOI] [PubMed] [Google Scholar]
- 46.Crivello JV. Radical-Promoted Visible Light Photoinitiated Cationic Polymerization of Epoxides. J. Macromol. Sci. Part A-Pure Appl. Chem. 2009;46:474–483. [Google Scholar]
- 47.Durmaz YY, Moszner N, Yagci Y. Visible light initiated free radical promoted cationic polymerization using acylgermane based photoinitiator in the presence of onium salts. Macromolecules. 2008;41:6714–6718. [Google Scholar]
- 48.Bi YB, Neckers DC. A visible-light initiating system for free-radical promoted cationic polymerization. Macromolecules. 1994;27:3683–3693. [Google Scholar]
- 49.Chemtob A, Belon C, Croutxe-Barghorn C, Brendle J, Soulard M, Rigolet S, Le Houerou V, Gauthier C. Bridged polysilsesquioxane films via photoinduced sol-gel chemistry. New J. Chem. 2010;34:1068–1072. [Google Scholar]
- 50.Oshaughnessy B, Yu J. Autoacceleration in Free-Radical Polymerization. Phys Rev Lett. 1994;73:1723–1726. doi: 10.1103/PhysRevLett.73.1723. [DOI] [PubMed] [Google Scholar]
- 51.Crivello JV. Design and synthesis of photoacid generating systems. J. Photopolym Sci. Technol. 2008;21:493–497. [Google Scholar]
- 52.Croutxe-Barghorn C, Belon C, Chemtob A. Polymerization of Hybrid Sol-Gel Materials Catalyzed by Photoacids Generation. J. Photopolym Sci. Technol. 2010;23:129–134. [Google Scholar]
- 53.Young JS, Kannurpatti AR, Bowman CN. Effect of comonomer concentration and functionality on photopolymerization rates, mechanical properties and heterogeneity of the polymer. Macromol. Chem. Phys. 1998;199:1043–1049. [Google Scholar]
- 54.Ferracane JL, Greener EH. The Effect of Resin Formulation on the Degree of Conversion and Mechanical-Properties of Dental Restorative Resins. J. Biomed. Mater. Res. 1986;20:121–131. doi: 10.1002/jbm.820200111. [DOI] [PubMed] [Google Scholar]
- 55.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J. Biomed. Mater. Res. Part A. 2007;80A:342–350. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh V, Misra A, Marangos O, Park J, Ye QA, Kieweg SL, Spencer P. Viscoelastic and fatigue properties of model methacrylate-based dentin adhesives. J. Biomed. Mater. Res. Part B. 2010;95B:283–290. doi: 10.1002/jbm.b.31712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh V, Misra A, Parthasarathy R, Ye Q, Spencer P. Viscoelastic properties of collagen-adhesive composites under water-saturated and dry conditions. J. Biomed. Mater. Res. Part A. 2015;103:646–657. doi: 10.1002/jbm.a.35204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su BH, Su JC, Li Y, Ran JG, Su BY. Effect of Soaking Solution with Different pH on the Solubility of Cements. Rare Metal Mat Eng. 2009;38:830–833. [Google Scholar]
- 59.Sheibaninia A, Sepasi S, Saghiri MA. The effect of an acidic food-simulating environment on the shear bond strength of self-ligating brackets with different base designs. Int J Dent. 2014;2014:689536. doi: 10.1155/2014/689536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva EM, Almeida GS, Poskus LT, Guimaraes JGA. Influence of organic acids present in the oral biofilm on the microtensile bond strength of adhesive systems to human dentin. J. Biomed. Mater. Res. Part B. 2012;100B:735–741. doi: 10.1002/jbm.b.32506. [DOI] [PubMed] [Google Scholar]
- 61.Morris CA, Rolison DR, Swider-Lyons KE, Osburn-Atkinson EJ, Merzbacher CI. Modifying nanoscale silica with itself: a method to control surface properties of silica aerogels independently of bulk structure. J. Non-Cryst. Solids. 2001;285:29–36. [Google Scholar]
- 62.Williams ML, Landel RF, Ferry JD. Mechanical Properties of Substances of High Molecular Weight .19. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-Forming Liquids. J. Am. Chem. Soc. 1955;77:3701–3707. [Google Scholar]
- 63.Aklonis JJ, MacKnight WJ. Introduction to polymer viscoelasticity. 3rd. Wiley: Chichester; Hoboken, N.J: John Wiley [distributor]; 2005. [Google Scholar]
- 64.Costantini A, Luciani G, Silvestri B, Tescione F, Branda F. Bioactive poly(2-hydroxyethylmethacrylate)/silica gel hybrid nanocomposites prepared by sol-gel process. J. Biomed. Mater. Res. Part B. 2008;86B:98–104. doi: 10.1002/jbm.b.30993. [DOI] [PubMed] [Google Scholar]
- 65.Huang SL, Chin WK, Yang WP. Viscosity, particle size distribution, and structural investigation of tetramethyloxysilane/2-hydroxyethyl methacrylate sols during the sol-gel process with acid and base catalysts. J. Polym. Sci. Polym. Phys. 2004;42:3476–3486. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.