Abstract
Background
HIV diagnosis, the first step in HIV care and treatment engagement, may be inhibited by substance use among female sex workers (FSW). We assessed the relationship between alcohol and marijuana use and lack of HIV infection awareness among HIV-infected FSW in Lilongwe, Malawi.
Methods
From July to September, 2014, 200 FSW aged ≥18 years were enrolled using venue-based sampling to examine substance use, HIV testing history, and serostatus ascertained by HIV rapid test. We used Poisson regression with robust variance estimates to estimate the associations of alcohol and marijuana use and lack of HIV infection awareness.
Results
Of the 138 HIV-infected FSW, 20% were unaware of their HIV infection, with 70% not testing within 6 months prior. According to the Alcohol Use Disorder Identification Tests (AUDIT), 55% of FSW unaware of their HIV infection reported hazardous, harmful, or dependent alcohol consumption. We observed a dose-response relationship between alcohol use and lack of HIV infection awareness, with alcohol dependency significantly associated with lack of HIV infection awareness (adjusted prevalence ratio: 3.0, 95% CI: 1.3, 6.8). Current marijuana use was uncommon (26%) among unaware HIV-infected FSW and weakly associated with lack of HIV infection awareness adjusted prevalence ratio: 1.1, 95% CI: 0.5, 2.5).
Conclusion
Increased levels of alcohol use is associated with lack of HIV infection awareness among HIV-infected FSW in Malawi. Frequent, consistent HIV testing integrated with alcohol reduction strategies could improve the health and infection awareness of substance-using FSW.
Keywords: alcohol, marijuana, HIV testing, sub-Saharan Africa, sex work
INTRODUCTION
Female sex workers (FSW) have been a key focus for HIV prevention efforts for over three decades, but their risk for acquiring HIV remains disproportionately high.1–6 The global prevalence of HIV among FSW is approximately 12%2 and even higher in sub-Saharan Africa, at 37%.2,7,8 In Malawi, the HIV prevalence among FSW is about 70%, the highest reported globally.2,9 Within high HIV prevalence settings, targeting HIV-infected FSW who are unaware of their infection status is imperative for the success of test and treatment strategies that provide immediate antiretroviral therapy for HIV-infected persons to improve health outcomes and reduce HIV transmission at the population level.10–12
FSW must first be aware of their HIV infection to prevent ongoing transmission and successfully engage in HIV care and treatment. While, the World Health Organization (WHO) recommends that high-risk persons undergo HIV testing at least every 12 months, FSW would highly benefit from more frequent testing at least every 6 months.13 Yet, many FSW do not access HIV testing and counseling, often because of individual, social, and structural barriers, such as the fear a positive result,14,15 lack of personal motivation,16,17 insufficient support during testing process,16,18,19 limited access or distance to HIV testing services,16 and provider stigma and discrimination.15,18 Many FSW become aware of their HIV infection as a result of opt out testing during pregnancy.14,20,21 FSW commonly undergo voluntary HIV testing for frequent illness or deterioration of health.14 To improve uptake of HIV care and treatment and prevent ongoing transmission, the barriers to HIV testing for FSW in sub-Saharan Africa must be delineated.
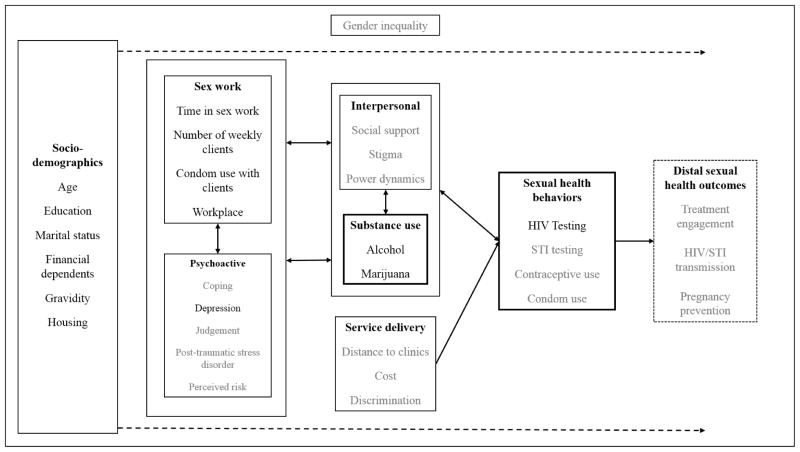
Substance use among FSW may further exacerbate known barriers to HIV testing and subsequently HIV infection awareness. FSW often engage in alcohol use to facilitate soliciting sex and cope with stressors associated with sex work.22,23 Our conceptual framework draws on the self-regulation and coping theories, in which women engage in substance use to help cope with negative life events and dysregulate emotional states brought on by sex work which in turn may influence health seeking behaviors (Figure 1).24–26 Substance use also adversely affects these health seeking behaviors by impairing cognitive functions and judgment, likely resulting in delayed HIV infection awareness.27–30 These factors of substance use and heath-seeking behaviors are all placed within the larger context of gender norms and inequality within sub-Saharan Africa.31–34 Understanding the context of sex work, psychoactive factors, and interpersonal factors are necessary to examine the relationship of substance use and HIV status awareness among FSW.
Figure 1. Conceptual framework.
This multidimensional conceptual model focuses on the potential impact of alcohol and marijuana use on engagement in sexual health behaviors, specifically HIV testing. The model depicts the influence of individual, interpersonal, and structural factors on each other, while simultaneously affecting alcohol and marijuana use among female sex workers. Additionally, gender inequality permeates all of these factors and influences how they relate to each other. The factors in grey are unmeasured, while the factors in black were measured as part of this study.
Among FSW in the region, substance use, including alcohol, marijuana, and opioids, is high.22,23,35,36 FSW who injected drugs are likely to have previously received an HIV test, in settings where injecting drug use is common.37,38 However in Malawi, alcohol and marijuana are the most commonly used substances.22,23,35 In Malawi, nearly 45% of FSW report consuming four to five bottles of alcoholic beverages per day, putting them at serious risk for alcohol dependency.14,39,40 Estimates of marijuana use among FSW in Malawi are lacking, but marijuana is widely available and inexpensive in this setting.41–43 Concurrent use of alcohol and marijuana use is also common among FSW in sub-Saharan Africa.44,45 FSW in South Africa who used both substances were more likely to have received an HIV test in their lifetime.45
To date, the association between alcohol and marijuana use and HIV testing, particularly among FSW in sub-Saharan Africa, has rarely been examined.45,46 In this study, we examined associations between alcohol and marijuana use and lack of awareness of HIV infection among FSW in Lilongwe, Malawi. We also describe the frequency and history of HIV testing among HIV-infected FSW.
METHODS
Study setting and participants
This cross-sectional evaluation was conducted in Lilongwe, the central region of the Republic of Malawi. The study population included FSW, defined per the Family Planning Association of Malawi as someone “who had received money in exchange for sex either regularly or occasionally up to 12 months prior to the survey”,14 who were at least 18 years of age and able to speak English or Chichewa, the predominant local language.
Study design
We designed and implemented this study through a collaboration among the University of North Carolina-Chapel Hill, UNC Project-Malawi, and Theatre for a Change (TfaC), a non-governmental organization (NGO) in Malawi with far-reaching relationships with local stakeholders including sex workers, chiefs, police, other NGOs, United Nations agencies, and government ministries. Since 2007, TfaC has developed programs focused on empowering women and girls, including FSW, in their sexual reproductive health within Lilongwe and several other districts in Malawi. We employed a cross-sectional design to systematically recruit FSW using venue-based sampling, a strategy with documented success in identifying hidden populations, such as FSW.47–51 We recruited FSW using a mobile van and an outreach team comprising a peer FSW, HIV testing counselors, interviewers, a study nurse, and a driver. The mobile van was operated by TfaC and in addition to contributing to study specific activities, TfaC staff provided group sexual risk reduction and family planning counseling at the venue after study activities were completed. Prior to recruitment, stakeholder meetings with FSW community leaders known as “Queen Mothers” were held in conjunction with TfaC and the UNC Project-Malawi community advisory board to navigate the legal and social networks involved with sex work.
FSW were recruited for participation from July to September 2014 from a purposive sample of venues in Lilongwe that were previously identified through community mapping by TfaC as known locations where women solicited sex. A total of 23 different venues were included in the venue sub-sample: 13 were bars, six were guesthouses or lodges, two were bottle shops, and two were bottle shops and guesthouses. In 2011, the Family Planning Association of Malawi conducted a nationally representative survey among FSW. This study found nearly 40% were between 20–24 years of age, approximately 60% attended primary school, and 98% were born in Malawi, comparable to our final sample of FSW.14
Data collection
All consenting FSW completed a behavioral survey soliciting detailed information on alcohol and marijuana use, HIV testing history, sociodemographic information, number of pregnancies, depression, and sex work factors (length of time in sex work, number of weekly clients, condom use, and location of soliciting sex). Trained field workers administered the survey. The survey was translated from English to Chichewa and back-translated. The survey was available in both languages to all participants.
HIV serostatus was assessed by trained HIV testing counselors for all participating FSW. Per Malawian National HIV Testing and Counseling (HTC) guidelines, FSW received serial HIV-antibody rapid tests, Determine HIV-1/2 and Uni-Gold rapid HIV-antibody. Pre-and post-HIV test counseling, clinic referral, and risk reduction counseling were administered, and both male and female condoms were offered.
Substance use assessments
Alcohol use was assessed using the WHO’s Alcohol Use Disorders Identification Test (AUDIT).52–54 The AUDIT is an internationally validated screening tool that measures alcohol use behaviors, including heavy drinking, and alcohol use disorder symptoms.55,56 The AUDIT comprises 10 questions across three specific domains: hazardous drinking, harmful drinking, and alcohol dependence symptoms.52 Hazardous (or risky) alcohol use is an alcohol consumption pattern that does not represent a current disorder but increases the risk of harmful consequences to the person or others. Harmful alcohol use is an alcohol consumption pattern that leads to adverse physical and mental health outcomes. Alcohol dependence is a condition in which a person experiences a strong desire to drink and difficulty controlling alcohol use. Each of the 10 questions has a set of responses with a corresponding score of 0 to 4. Scores from each question are added, resulting in one composite score ranging from 0 to 40, with higher scores correlated to having an alcohol use disorder. In this study, following WHO’s recommendations for implementing the AUDIT among women, an AUDIT score of 0 to 6 was considered indicative of abstinence or non-hazardous drinking, 7 to 15 of hazardous drinking, 16 to 19 of harmful drinking, and ≥20 of possible alcohol dependency.52
Marijuana use was measured in terms of reported lifetime marijuana use and number of days using marijuana within the past 30 days. Current marijuana use was defined as at least one day of marijuana use within the prior 30 days. The recall period of 30 days was selected based on previous substance use evaluations in the region.57–60 The recall period was also piloted for acceptability.
Use of other substances, including mandrax, methamphetamine, heroin, cocaine or crack, and khat, were also evaluated for lifetime and current use, however, none were reported.
HIV infection awareness assessment
To measure HIV infection awareness, we asked FSW to report the results of their last HIV test. FSW who were HIV-seropositive and self-reported that their last HIV test was negative, they never received their results from an HIV test, or that they had never previously tested for HIV were considered unaware of their HIV infection.61
Covariates
Drawing from the scientific literature we identified a set of potential confounders to explore in our analysis of the associations between alcohol and marijuana use with lack of HIV infection awareness (Figure 1). The assessments for these covariates were field tested, back translated, and piloted for acceptability. Covariates examined in the models, included age (18–24, 25–29, ≥30 years), education (never attended school/only primary school, any secondary school/more school), marital status (never married, cohabitating/married, separated/divorced/widowed), housing (private house, bottle shop/bar, hotel/guesthouse), gravidity (no previous pregnancies, any previous pregnancies), financial dependents (no dependents, any dependents), probable depression measured by the Patient Health Questionnaire-9 (PHQ9 <10, PHQ9 ≥10),62–64 and treatment for an STI in the prior 12 months (no, yes). We also examined years in sex work (<1, 1.0–1.9, 2.0–2.9, ≥3.0 years), location for soliciting paying sexual partners (bar/bottle shop, other), weekly number of clients (<10, 10–19, 20–29, ≥30 clients), condom use during vaginal sex with clients in prior 7 days (consistent use, inconsistent use), history of a client that demanded not using a condom during vaginal sex (never, ever), alcohol use prior to last vaginal sex with client (no, yes), and any marijuana use prior to last vaginal sex with client (no, yes). Categorization of the covariates was based on interpretability and replicability of study findings.
Statistical analysis
Sociodemographic characteristics, sexual history, HIV testing history, and substance use were summarized using frequencies and proportions for categorical variables and medians and interquartile ranges (IQR) for continuous variables.
We conducted analyses for the associations of alcohol and marijuana use, separately, with the primary outcome of lack of HIV infection awareness. We also used a product interaction term to assess the association between alcohol and marijuana use together and lack of HIV infection awareness. However, this interaction term was not significant and not presented in the results. We explored the association between alcohol use and time since most recent HIV testing (≤6 months vs. >6 months) among FSW unaware of their HIV infection. We used Poisson regression with robust variance estimates to estimate bivariable and, when sample size and number of events permitted, multivariable prevalence ratios (PR) with 95% confidence intervals (CI). 65,66
Potential confounding variables were assessed one-by-one, and retained in final adjusted multivariable models if removal resulted in a >10% change in the prevalence ratio estimate. Interactions were only considered for variables of interest that met positivity assumptions and were retained in the final adjusted multivariable models if they had public health relevance and reached statistical significance at alpha=0.10. Collinearity was evaluated in the model using Pearson’s correlation coefficient; none of the covariates showed significant correlation (p-value ≥0.5).
All analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC, USA).
Ethics
The research protocol, survey, and consent forms were reviewed and approved by the Institutional Review Board at the University of North Carolina and the Malawi Ministry of Health and Population National Health Sciences Research Committee (HSRC). All participants provided written informed consent prior to completing study activities. All study related activities were conducted in a safe and private location.
RESULTS
Among 200 FSW, 138 (69%) had confirmed HIV infection (Table 1). About half (41%) of those HIV-infected were 18 to 24 years old. Most (66%) never attended school or received only primary school. Most (85%) were separated, divorced, or widowed; approximately 60% lived in a bar or bottle shop. Nearly all (92%) HIV-infected FSW reported at least one previous pregnancy. A quarter (24%) of HIV-infected FSW reported receiving treatment for an STI in the prior 12 months. The median time exchanging sex for money was 3 years (interquartile range: 1–6) and the median number of clients per week was 21 (IQR: 10–35). About two-thirds (64%) reported ever having a client who demanded not using a condom during vaginal sex.
Table 1.
Characteristics of HIV-infected female sex workers in Lilongwe, Malawi, July–September 2014, n=138
| n | (%) | |
|---|---|---|
| Age (years) | ||
| 18–24 | 57 | (41) |
| 25–29 | 44 | (32) |
| ≥30 | 37 | (27) |
| Education | ||
| Never attend or only primary school | 91 | (66) |
| Any secondary or more school | 47 | (34) |
| Marital status | ||
| Never married | 15 | (11) |
| Married (legal or traditional) or co-habitating | 6 | (4) |
| Separated, divorced, or widowed | 117 | (85) |
| Housing | ||
| Private house | 17 | (12) |
| Bar or bottle shop | 80 | (58) |
| Guesthouse or hotel | 41 | (30) |
| Number of pregnancies | ||
| 0 | 11 | (8) |
| ≥1 | 127 | (92) |
| Number of financial dependents | ||
| 0 | 6 | (4) |
| ≥1 | 132 | (96) |
| Depression | ||
| No probable depression | 125 | (91) |
| Probable depression | 13 | (9) |
| Treated for an STI in prior 12 months | ||
| No | 105 | (76) |
| Yes | 33 | (24) |
| Duration of sex work (years) | ||
| <1.0 | 15 | (11) |
| 1.0–1.9 | 24 | (17) |
| 2.0–2.9 | 22 | (16) |
| ≥3.0 | 77 | (56) |
| Location for soliciting clients | ||
| Bar or bottle shop | 124 | (90) |
| Other | 14 | (10) |
| Number of clients per weeka | ||
| <10 | 27 | (20) |
| 10–19 | 33 | (24) |
| 20–29 | 33 | (24) |
| ≥30 | 44 | (32) |
| Condom use with client in past 7 days | ||
| Inconsistent | 36 | (26) |
| Consistent | 102 | (74) |
| Ever had a client demand not using a condom during vaginal sex | ||
| No | 50 | (36) |
| Yes | 88 | (64) |
| Alcohol use prior to last vaginal sex with client | ||
| No | 97 | (70) |
| Yes | 41 | (30) |
| Drug use prior to last vaginal sex with client | ||
| No | 129 | (93) |
| Yes | 9 | (7) |
Missing data due to not knowing or refused to answer: number of clients in past 7 days: n=1
Alcohol use was relatively common before engaging in sex work. Nearly a third (30%) reported alcohol use prior to last vaginal sex act with a client, with less than 10% reporting any marijuana use prior to last vaginal sex act with a client.
HIV testing and infection awareness
Of those HIV-infected, 111 (80%, 95% CI: 73, 87%) were aware of their HIV infection (Table 2). Among the 20% who were unaware of being HIV-infected, 20 (74%) had tested negative previously, 5 (19%) had never been tested, and 2 (7%) had been tested but had not received results. More than half (52%) of HIV-infected FSW who were unaware of being HIV-infected had not been tested within the last 12 months; 26% reported testing within 6 months prior. About three-fourths (77%) of those who had previously tested received their last HIV test at a governmental health center.
Table 2.
Frequency and history of HIV testing by HIV infection awareness among HIV-infected female sex workers in Lilongwe, Malawi, July–September 2014
| All (n=138) | Unaware of HIV infection (n=27) | Aware of HIV infection (n=111) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| median | (IQR) | median | (IQR) | median | (IQR) | |
|
|
||||||
| Number of lifetime HIV tests | 2 | (2–4) | 2 | (1–4) | 2 | (2–4) |
|
|
||||||
| n | (%) | n | (%) | n | (%) | |
|
|
||||||
| Time since last HIV test (months) | ||||||
| ≤ 6 | 42 | (30) | 7 | (26) | 35 | (32) |
| 6.1–11.9 | 26 | (19) | 6 | (22) | 20 | (18) |
| ≥ 12.0 | 70 | (51) | 14 | (52) | 56 | (50) |
| Location of most recent HIV testa | ||||||
| Health center, governmental | 95 | (71) | 17 | (77) | 78 | (70) |
| Private clinic | 13 | (10) | 1 | (5) | 12 | (11) |
| HIV/AIDS comprehensive care and treatment clinic | 7 | (5) | 0 | (0) | 7 | (6) |
| Other | 16 | (12) | 4 | (18) | 12 | (11) |
IQR: interquartile range
Missing due to not knowing most recent HIV test location, n=2; or not previously testing, n=5
Substance use
According to the AUDIT scale, about half (45%) of all HIV-infected FSW were abstinent or non-hazardous drinkers, 28% were hazardous drinkers, 12% were harmful drinkers, and 15% were alcohol dependent (Table 3). FSW who were unaware of their HIV infection were more likely to be harmful drinkers (19%) or dependent drinkers (26%) when compared to FSW who were aware of their HIV infection (11% and 12%, respectively).
Table 3.
Associations of alcohol and marijuana use with lack of HIV infection awareness (n=138)
| Substance use | Unaware of HIV infection (n=27) | Aware of HIV infection (n=111) | Unaware of HIV infection | |||
|---|---|---|---|---|---|---|
|
| ||||||
| n | (%) | n | (%) | Unadjusted PR (95% CI) | Adjusted PR (95% CI) | |
|
|
||||||
| Alcohol use (AUDIT) | ||||||
| Non-hazardous drinking (score 0–6) | 9 | (33) | 53 | (47) | 1.00 | 1.00 |
| Hazardous drinking (score 7–15) | 6 | (22) | 33 | (30) | 1.1 (0.34, 2.8) | 1.2 (0.5, 3.4)b |
| Harmful drinking (score 16–19) | 5 | (19) | 12 | (11) | 2.0 (0.8, 5.3) | 2.7 (1.0, 7.6)b |
| Alcohol dependence (score ≥20) | 7 | (26) | 13 | (12) | 2.4 (1.0, 5.6) | 3.0 (1.3, 6.8)b |
| Marijuana use | ||||||
| No current marijuana use | 20 | (74) | 85 | (77) | 1.00 | 1.00 |
| Current marijuana usea | 7 | (26) | 26 | (23) | 1.1 (0.5, 2.4) | 1.1 (0.5, 2.5)c |
AUDIT= Alcohol Use Disorder Identification Test; PR=prevalence ratio; CI=confidence interval
Current use defined as reported use in past 30 days
Adjusted for duration in sex work (years), alcohol use prior to last vaginal sex with client, and number of clients per week
Adjusted for marital status and number of clients per week
The proportion of FSW reporting current marijuana use was similar among FSW unaware (23%) and aware of their HIV infection (26%). The proportion of both harmful and dependent alcohol drinking and current marijuana use was also comparable among FSW who were unaware (11%) and aware (8%) of their HIV infection.
Alcohol use and HIV infection awareness
Among HIV-infected FSW, a dose-response relationship was observed between alcohol use and lack of HIV infection awareness, with a statistically significant association between alcohol dependence and lack of HIV infection awareness (Table 3). In multivariable analysis adjusting for duration in sex work, alcohol use prior to last vaginal sex with a client, and number of clients per week, FSW who were hazardous drinkers (adjusted PR: 1.2, 95% CI: 0.5, 3.4) or harmful drinkers (adjusted PR: 2.7, 95% CI: 1.0, 7.6) were more likely to be unaware of their HIV infection when compared to abstinent or non-hazardous drinkers. Also, FSW who were dependent drinkers were 3.0 (95% CI: 1.3, 6.8) times as likely to be unaware of their HIV infection compared to FSW who were abstinent or non-hazardous drinkers.
Upon exploring the relationship between alcohol use and time since last HIV test among FSW unaware of their HIV infection, FSW who were harmful drinkers or alcohol dependent were more likely to have not received an HIV test within the 6 months prior. In bivariable analysis, given the small number of events, FSW who were harmful drinkers or alcohol dependent were 1.4 (95% CI: 0.6, 3.1) times as likely to most recently receive an HIV test more than the prior 6 months than FSW who were nonhazardous or hazardous drinkers (results not shown).
Marijuana use and HIV infection awareness
Comparing current to non-current marijuana use, the prevalence ratio of lack of HIV infection awareness in multivariable analysis was 1.1 (95% CI: 0.5, 2.5), adjusting for marital status and number of clients per week (Table 3).
DISCUSSION
In this population of HIV-infected FSW in Lilongwe, most HIV-infected FSW were aware of their HIV infection. Among those that were unaware of their HIV infection, over two-thirds had not been tested within the last 6 months prior to our study. Most HIV-infected FSW reported at least hazardous and harmful alcohol consumption; many FSW were alcohol dependent. We observed a dose-response relationship between increased levels of alcohol use and lack of HIV infection awareness, with FSW who were dependent drinkers being significantly more likely to be unaware of their HIV infection compared to FSW with non-hazardous alcohol consumption patterns. Marijuana use was relatively uncommon and weakly associated with lack of HIV infection awareness.
The proportion of HIV-infected FSW in our Lilongwe sample who were unaware of their HIV infection was lower than previous estimates among FSW in sub-Saharan Africa. 14,61,67 FSW within our sample may have previously participated in TfaC sexual and reproductive health promotion programs, and therefore may have been more likely than the overall FSW population to be aware of their HIV infection. Furthermore, the large majority of our FSW reported previous pregnancies and may have undergone HIV testing as part of routine antenatal care programs.68
FSW unaware of their HIV infection were testing infrequently, with most having no reported test within the last 6 months. Most of the FSW in our study who were unaware of their HIV infection had not tested within the WHO recommended HIV testing period.13 Strategies that increase the frequency of testing, such as venue-based HIV testing, must be implemented to reach FSW and improve HIV infection awareness.
Notably, a sizeable proportion (26%) of those HIV-infected and unaware of their HIV infection had previously tested negative within the six months prior to our study. It is possible that this proportion of FSW had recently acquired HIV and therefore had less opportunity to become aware of their HIV infection. Although research staff were trained extensively in non-judgmental interviewing techniques and ensuring confidentiality, it is also possible FSW provided social desirable responses and did not disclose the positive results of their last HIV test. Given these likely recent HIV infections, FSW in Malawi should be encouraged to receive HIV testing and counseling more frequently than every 12 months to prevent ongoing transmission and access timely HIV care and treatment. HIV-infected FSW who do not receive timely diagnoses will not optimally engage in HIV care and treatment, and may continue to unknowingly transmit HIV through high-risk sexual behaviors.
Alcohol use among HIV-infected FSW in Lilongwe was prevalent, possibly due to the high proportion of HIV-infected FSW in our sample who reported living and working in bars or bottle shops where alcohol is readily available. In other sub-Saharan African settings, FSW who worked at bars or other alcohol venues were more likely to consume alcohol or binge drink when compared to FSW not working in alcohol-serving venues.22,40,69 The prevalence of alcohol use may have been lower if more non-venue-based FSW had been included in our sample. But our prevalence is consistent with previous high prevalence estimates of alcohol use among FSW recruited within the larger community and clinic settings in sub-Saharan Africa.22,23,70,71 To reduce the prevalence of alcohol use, FSW in Lilongwe should be reached using a venue-based approach, particularly within bars or bottle shops, for alcohol risk reduction efforts.
We found in this population that increased alcohol consumption was associated with lack of HIV infection awareness. As part of our analysis, we also explored defining lack of HIV infection awareness as FSW who were HIV-seropositive and self-reported that their last HIV test was more than 6 months prior and was negative. When using this more conservative definition of lack of HIV infection awareness to account for FSW who reported testing within the previous 6 months, the dose-response relationship remained (Supplement 1). In Kenya, FSW with harmful alcohol drinking (AUDIT score 16–19) were more likely to self-report never having been tested HIV, compared to hazardous alcohol drinkers (AUDIT score 7–15).46 However, this study was restricted to FSW who drank regularly with hazardous or harmful drinking (AUDIT scores ranging from 7 to 19). Our results suggest a statistically significant relationship between alcohol dependence and lack of HIV infection awareness among HIV-infected FSW in Lilongwe and a clear dose-response relationship between increased alcohol use and lack of HIV infection awareness.
Alcohol use and lack of HIV infection awareness may be due to alcohol use influencing sexual risk behaviors leading to increased recent HIV infections and therefore, less time to obtain HIV testing.6,22,40,70,72 However, among those that were unaware of their HIV infection, we found FSW who were harmful drinkers or alcohol dependent were more likely to have not received an HIV test within the 6 months prior. Therefore, FSW who are harmful drinkers or alcohol dependent may prioritize drinking, which plausibly has an adverse influence on health-seeking behaviors.73,74
Gender norms and inequality likely also plays important role in our observed relationship between alcohol use and lack of HIV infection awareness. The social and economic burden on women, particularly in sub-Saharan Africa, can lead or keep women engaged in sex work.31,44,75 Once engaged in sex work, alcohol is used as a means to cope with negative sex work related events or facilitate sexual client relationships.24,25,44 Furthermore, women in this region are generally less likely to access HIV testing services due to fear of disclosing their HIV sero-status.34 More comprehensive evaluations among FSW are needed to further understand the gender dynamics and the effect on health-seeking behaviors, such as HIV testing.
Caution must be used in drawing causal interpretations from cross-sectional data, but alcohol use likely predates lack of awareness of HIV infection for FSW within our sample of unaware HIV-infected FSW. Per our study procedures, alcohol use was ascertained prior to determination of HIV serostatus among those reporting previously never testing or HIV-negative.
Among HIV-infected FSW in Lilongwe, current marijuana was uncommon and not associated with HIV infection awareness.23,76 FSW in South Africa report marijuana as the most commonly used substance after alcohol, with approximately 70% reporting marijuana use.23,76 FSW in our sample may have been more likely to use alcohol than marijuana since alcohol may be more readily available in the alcohol-serving venues used for recruitment. Marijuana use was not associated with HIV infection awareness. A qualitative study among street-based FSW using marijuana found that most FSW reported receiving an HIV test at some point but some reported never testing for HIV.76 Severe marijuana use, which was not examined within our study, may more directly affect HIV infection awareness. Future research with improved marijuana use assessments are needed to examine the frequency, duration, potency, and severity of marijuana use.
These findings emphasize the importance of improving HIV testing uptake to increase infection awareness as a first step in HIV care and treatment engagement for HIV-infected substance-using FSW in Malawi. Targeted strategies including frequent, alcohol serving venue-based HIV testing and counseling should be considered to better reach alcohol-using FSW for timelier HIV diagnoses and infection awareness. Alcohol serving venue-based alcohol reduction strategies may also have the potential to mitigate alcohol use and expand HIV infection awareness among highly sexually active FSW in Malawi.
Supplementary Material
Acknowledgments
This work was supported by the NIH Research Training Grant (R25 TW009340) funded by the Fogarty International Center, the NIH Office of the Director Office of AIDS Research, ORWH, NCI, and NHLBI, the NIAID T32 training grant (T32 AI0700), the UNC Center for AIDS Research, an NIH funded program (P30 AI50410), and the National Institutes of Health (KL2 TR001109). We gratefully acknowledge the outreach team for their dedication, interviewing skills, knowledge, and commitment to this work. We would like to thank Sarah MacLean for her review of the literature and manuscript. We are also grateful to the study participants who courageously shared their time, thoughts, and stories to this research.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Impact of an innovative approach to prevent mother-to-child transmission of HIV--Malawi, July 2011–September 2012. MMWR. Morbidity and mortality weekly report. 2013;62(8):148–151. [PMC free article] [PubMed] [Google Scholar]
- 2.Baral S, Beyrer C, Muessig K, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(7):538–549. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- 3.Matovu JK, Ssebadduka BN. Sexual risk behaviours, condom use and sexually transmitted infection treatment-seeking behaviours among female sex workers and truck drivers in Uganda. Int J STD AIDS. 2012;23(4):267–273. doi: 10.1258/ijsa.2011.011313. [DOI] [PubMed] [Google Scholar]
- 4.Scorgie F, Chersich MF, Ntaganira I, Gerbase A, Lule F, Lo YR. Socio-demographic characteristics and behavioral risk factors of female sex workers in sub-saharan Africa: a systematic review. AIDS Behav. 2012;16(4):920–933. doi: 10.1007/s10461-011-9985-z. [DOI] [PubMed] [Google Scholar]
- 5.Vuylsteke B, Semde G, Sika L, et al. HIV and STI prevalence among female sex workers in Cote d’Ivoire: why targeted prevention programs should be continued and strengthened. PLoS One. 2012;7(3):e32627. doi: 10.1371/journal.pone.0032627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zachariah R, Spielmann MP, Harries AD, Nkhoma W, Chantulo A, Arendt V. Sexually transmitted infections and sexual behaviour among commercial sex workers in a rural district of Malawi. Int J STD AIDS. 2003;14(3):185–188. doi: 10.1258/095646203762869197. [DOI] [PubMed] [Google Scholar]
- 7.Chersich MF, Luchters S, Ntaganira I, et al. Priority interventions to reduce HIV transmission in sex work settings in sub-Saharan Africa and delivery of these services. J Int AIDS Soc. 2013;16:17980. doi: 10.7448/IAS.16.1.17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahmanesh M, Patel V, Mabey D, Cowan F. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: a systematic review. Tropical Medicine & International Health. 2008;13(5):659–679. doi: 10.1111/j.1365-3156.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 9.Lancaster KE, Powers KA, Lungu T, et al. The HIV Care Continuum among Female Sex Workers: A Key Population in Lilongwe, Malawi. PLoS One. 2016;11(1):e0147662. doi: 10.1371/journal.pone.0147662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Prevention and treatment of HIV and other sexually transmitted infections for sex workers in low- and middle- income countries: Recommendations for a public health approach. Geneva, Switzerland: 2012. [PubMed] [Google Scholar]
- 14.Family Planning Association of Malawi. Counting the uncatchables: Report of the situation analysis of the magnitude, behavioral patterns, contributing factors, current interventions and impact of sex work in HIV prevention in Malawi. Lilongwe, Malawi: 2011. [Google Scholar]
- 15.Munoz J, Adedimeji A, Alawode O. ‘They bring AIDS to us and say we give it to them’: Socio-structural context of female sex workers’ vulnerability to HIV infection in Ibadan, Nigeria. SAHARA J: journal of Social Aspects of HIV/AIDS Research Alliance/SAHARA, Human Sciences Research Council. 2010;7(2):52–61. doi: 10.1080/17290376.2010.9724957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dugas M, Bedard E, Batona G, et al. Outreach strategies for the promotion of HIV testing and care: closing the gap between health services and female sex workers in Benin. J Acquir Immune Defic Syndr. 2015;68(Suppl 2):S198–205. doi: 10.1097/QAI.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 17.Batona G, Gagnon MP, Simonyan DA, Guedou FA, Alary M. Understanding the intention to undergo regular HIV testing among female sex workers in Benin: a key issue for entry into HIV care. J Acquir Immune Defic Syndr. 2015;68(Suppl 2):S206–212. doi: 10.1097/QAI.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 18.Scorgie F, Nakato D, Harper E, et al. ‘We are despised in the hospitals’: sex workers’ experiences of accessing health care in four African countries. Culture, health & sexuality. 2013;15(4):450–465. doi: 10.1080/13691058.2012.763187. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Li B, Pan J, et al. Factors associated with utilization of a free HIV VCT clinic by female sex workers in Jinan City, Northern China. AIDS Behav. 2011;15(4):702–710. doi: 10.1007/s10461-010-9703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papworth E, Schwartz S, Ky-Zerbo O, et al. Mothers who sell sex: a potential paradigm for integrated HIV, sexual, and reproductive health interventions among women at high risk of HIV in Burkina Faso. J Acquir Immune Defic Syndr. 2015;68(Suppl 2):S154–161. doi: 10.1097/QAI.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz S, Papworth E, Thiam-Niangoin M, et al. An urgent need for integration of family planning services into HIV care: the high burden of unplanned pregnancy, termination of pregnancy, and limited contraception use among female sex workers in Cote d’Ivoire. J Acquir Immune Defic Syndr. 2015;68(Suppl 2):S91–98. doi: 10.1097/QAI.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 22.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wechsberg W, Wu L, Zule W, et al. Substance abuse, treatment needs and access among female sex workers and non-sex workers in Pretoria, South Africa. Substance abuse treatment, prevention, and policy. 2009;4 doi: 10.1186/1747-597X-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akers RL, Krohn MD, Lanza-Kaduce L, Radosevich M. Social learning and deviant behavior: a specific test of a general theory. Am Sociol Rev. 1979;44(4):636–655. [PubMed] [Google Scholar]
- 25.Shiffman S. Coping and substance use. Academic Press; 1985. [Google Scholar]
- 26.Gonzalez A, Mimiaga MJ, Israel J, Andres Bedoya C, Safren SA. Substance use predictors of poor medication adherence: the role of substance use coping among HIV-infected patients in opioid dependence treatment. AIDS Behav. 2013;17(1):168–173. doi: 10.1007/s10461-012-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chitwood DD, McBride DC, French MT, Comerford M. Health care need and utilization: a preliminary comparison of injection drug users, other illicit drug users, and nonusers. Subst Use Misuse. 1999;34(4–5):727–746. doi: 10.3109/10826089909037240. [DOI] [PubMed] [Google Scholar]
- 28.Sohler NL, Wong MD, Cunningham WE, Cabral H, Drainoni M-L, Cunningham CO. Type and pattern of illicit drug use and access to health care services for HIV-infected people. AIDS Patient Care and STDs. 2007;21(S1):S-68–S-76. doi: 10.1089/apc.2007.9985. [DOI] [PubMed] [Google Scholar]
- 29.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. American Journal of Medicine. 2003;114(7):573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 30.Simmonds L, Coomber R. Injecting drug users: a stigmatised and stigmatising population. The International journal on drug policy. 2009;20(2):121–130. doi: 10.1016/j.drugpo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Boutayeb A. The impact of HIV/AIDS on human development in African countries. BMC Public Health. 2009;9(Suppl 1):S3. doi: 10.1186/1471-2458-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig MA, Lutalo T, Zhao F, et al. Coercive sex in rural Uganda: prevalence and associated risk factors. Soc Sci Med. 2004;58(4):787–798. doi: 10.1016/s0277-9536(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 33.Jewkes RK, Levin JB, Penn-Kekana LA. Gender inequalities, intimate partner violence and HIV preventive practices: findings of a South African cross-sectional study. Soc Sci Med. 2003;56(1):125–134. doi: 10.1016/s0277-9536(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 34.MacPherson EE, Richards E, Namakhoma I, Theobald S. Gender equity and sexual and reproductive health in Eastern and Southern Africa: a critical overview of the literature. Global health action. 2014;7:23717. doi: 10.3402/gha.v7.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbonye M, Nakamanya S, Nalukenge W, King R, Vandepitte J, Seeley J. ‘It is like a tomato stall where someone can pick what he likes’: structure and practices of female sex work in Kampala, Uganda. BMC Public Health. 2013;13:741. doi: 10.1186/1471-2458-13-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambdin BH, Bruce RD, Chang O, et al. Identifying programmatic gaps: inequities in harm reduction service utilization among male and female drug users in Dar es Salaam, Tanzania. PLoS One. 2013;8(6):e67062. doi: 10.1371/journal.pone.0067062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deering KN, Montaner JS, Chettiar J, et al. Successes and gaps in uptake of regular, voluntary HIV testing for hidden street- and off-street sex workers in Vancouver, Canada. AIDS Care. 2015;27(4):499–506. doi: 10.1080/09540121.2014.978730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Brown K, Ding G, et al. Factors associated with HIV testing history and HIV-test result follow-up among female sex workers in two cities in Yunnan, China. Sex Transm Dis. 2011;38(2):89–95. doi: 10.1097/OLQ.0b013e3181f0bc5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuckit MA. Alcohol-use disorders. The Lancet. 2009;373(9662):492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- 40.Chersich MF, Luchters SM, Malonza IM, Mwarogo P, King’ola N, Temmerman M. Heavy episodic drinking among Kenyan female sex workers is associated with unsafe sex, sexual violence and sexually transmitted infections. Int J STD AIDS. 2007;18(11):764–769. doi: 10.1258/095646207782212342. [DOI] [PubMed] [Google Scholar]
- 41.Bisika T, Konyani T, Chamangwana I. Rapid Situation Assessment of Drug Abuse and HIV&AIDS in Malawi. Zomba, Malawi: Center for Social Research, University of Malawi; 2004. [Google Scholar]
- 42.United Nations Office on Drugs and Crime. Cannabis: A Short Review. Vienna, Austria: 2012. [Google Scholar]
- 43.Peltzer K. Causative and intervening factors of harmful alcohol consumption and cannabis use in Malawi. Substance Use & Misuse. 1989;24(2):79–85. doi: 10.3109/10826088909047276. [DOI] [PubMed] [Google Scholar]
- 44.Mbonye M, Nalukenge W, Nakamanya S, et al. Gender inequity in the lives of women involved in sex work in Kampala, Uganda. J Int AIDS Soc. 2012;15(Suppl 1):1–9. doi: 10.7448/IAS.15.3.17365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luseno WK, Wechsberg WM. Correlates of HIV testing among South African women with high sexual and substance-use risk behaviours. AIDS Care. 2009;21(2):178–184. doi: 10.1080/09540120802017594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bengtson AM, L’Engle K, Mwarogo P, King’ola N. Levels of alcohol use and history of HIV testing among female sex workers in Mombasa, Kenya. AIDS Care. 2014;26(12):1619–1624. doi: 10.1080/09540121.2014.938013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stueve A, O’Donnell LN, Duran R, San Doval A, Blome J. Time-space sampling in minority communities: results with young Latino men who have sex with men. Am J Public Health. 2001;91(6):922–926. doi: 10.2105/ajph.91.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston LG, Sabin K, Mai TH, Pham TH. Assessment of respondent driven sampling for recruiting female sex workers in two Vietnamese cities: reaching the unseen sex worker. J Urban Health. 2006;83(6 Suppl):i16–28. doi: 10.1007/s11524-006-9099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health Aff (Millwood) 2009;28(6):1677–1687. doi: 10.1377/hlthaff.28.6.1677. [DOI] [PubMed] [Google Scholar]
- 50.Powers KA, Miller WC, Pilcher CD, et al. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. Aids. 2007;21(16):2237–2242. doi: 10.1097/QAD.0b013e3282f08b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peitzmeier S, Mason K, Ceesay N, et al. A cross-sectional evaluation of the prevalence and associations of HIV among female sex workers in the Gambia. Int J STD AIDS. 2014;25(4):244–252. doi: 10.1177/0956462413498858. [DOI] [PubMed] [Google Scholar]
- 52.World Health Orgainzation (WHO) International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, Switzerland: World Health Orgainzation; 2000. [Google Scholar]
- 53.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 54.Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol Clin Exp Res. 1997;21(4):613–619. [PubMed] [Google Scholar]
- 55.Woolf-King SE, Maisto SA. Alcohol use and high-risk sexual behavior in sub-Saharan Africa: a narrative review. Archives of sexual behavior. 2011;40(1):17–42. doi: 10.1007/s10508-009-9516-4. [DOI] [PubMed] [Google Scholar]
- 56.Parry CDH, Plüddemann A, Steyn K, Bradshaw D, Norman R, Laubscher R. Alcohol use in South Africa: Findings from the first demographic and health survey. Journal of studies on alcohol. 2005;66(1):91–97. doi: 10.15288/jsa.2005.66.91. [DOI] [PubMed] [Google Scholar]
- 57.Wechsberg WM, Luseno WK, Lam WK, Parry CD, Morojele NK. Substance use, sexual risk, and violence: HIV prevention intervention with sex workers in Pretoria. AIDS Behav. 2006;10(2):131–137. doi: 10.1007/s10461-005-9036-8. [DOI] [PubMed] [Google Scholar]
- 58.Pluddemann A, Flisher AJ, Mathews C, Carney T, Lombard C. Adolescent methamphetamine use and sexual risk behaviour in secondary school students in Cape Town, South Africa. Drug and alcohol review. 2008;27(6):687–692. doi: 10.1080/09595230802245253. [DOI] [PubMed] [Google Scholar]
- 59.Wechsberg WM, Luseno WK, Karg RS, et al. Alcohol, cannabis, and methamphetamine use and other risk behaviours among Black and Coloured South African women: a small randomized trial in the Western Cape. The International journal on drug policy. 2008;19(2):130–139. doi: 10.1016/j.drugpo.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meade CS, Towe SL, Watt MH, et al. Addiction and treatment experiences among active methamphetamine users recruited from a township community in Cape Town, South Africa: A mixed-methods study. Drug Alcohol Depend. 2015;152:79–86. doi: 10.1016/j.drugalcdep.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cowan FM, Mtetwa S, Davey C, et al. Engagement with HIV prevention treatment and care among female sex workers in Zimbabwe: a respondent driven sampling survey. PLoS One. 2013;8(10):e77080. doi: 10.1371/journal.pone.0077080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monahan PO, Shacham E, Reece M, et al. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. J Gen Intern Med. 2009;24(2):189–197. doi: 10.1007/s11606-008-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cholera R, Gaynes BN, Pence BW, et al. Validity of the Patient Health Questionnaire-9 to screen for depression in a high-HIV burden primary healthcare clinic in Johannesburg, South Africa. J Affect Disord. 2014;167:160–166. doi: 10.1016/j.jad.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pence BW, Gaynes BN, Atashili J, et al. Validity of an interviewer-administered patient health questionnaire-9 to screen for depression in HIV-infected patients in Cameroon. Journal of affective disorders. 2012;143(1):208–213. doi: 10.1016/j.jad.2012.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zou G. A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 66.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhana A, Luchters S, Moore L, et al. Systematic review of facility-based sexual and reproductive health services for female sex workers in Africa. Globalization and health. 2014;10:46. doi: 10.1186/1744-8603-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kasenga F, Byass P, Emmelin M, Hurtig AK. The implications of policy changes on the uptake of a PMTCT programme in rural Malawi: first three years of experience. Global health action. 2009;2 doi: 10.3402/gha.v2i0.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agha S, Chulu Nchima M. Life-circumstances, working conditions and HIV risk among street and nightclub-based sex workers in Lusaka, Zambia. Cult Health Sex. 2004;6(4):283–299. doi: 10.1080/13691050410001680474. [DOI] [PubMed] [Google Scholar]
- 70.Chersich MF, Bosire W, King’ola N, Temmerman M, Luchters S. Effects of hazardous and harmful alcohol use on HIV incidence and sexual behaviour: a cohort study of Kenyan female sex workers. Globalization and health. 2014;10:22. doi: 10.1186/1744-8603-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yadav G, Saskin R, Ngugi E, et al. Associations of sexual risk taking among Kenyan female sex workers after enrollment in an HIV-1 prevention trial. J Acquir Immune Defic Syndr. 2005;38(3):329–334. [PubMed] [Google Scholar]
- 72.Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N, Rehm J. Causal considerations on alcohol and HIV/AIDS—a systematic review. Alcohol and Alcoholism. 2010;45(2):159. doi: 10.1093/alcalc/agp091. [DOI] [PubMed] [Google Scholar]
- 73.Hahn JA, Woolf-King SE, Muyindike W. Adding fuel to the fire: alcohol’s effect on the HIV epidemic in Sub-Saharan Africa. Curr HIV/AIDS Rep. 2011;8(3):172–180. doi: 10.1007/s11904-011-0088-2. [DOI] [PubMed] [Google Scholar]
- 74.Zarkin GA, Bray JW, Babor TF, Higgins-Biddle JC. Alcohol drinking patterns and health care utilization in a managed care organization. Health Serv Res. 2004;39(3):553–570. doi: 10.1111/j.1475-6773.2004.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karim QA, Karim SS, Soldan K, Zondi M. Reducing the risk of HIV infection among South African sex workers: socioeconomic and gender barriers. Am J Public Health. 1995;85(11):1521–1525. doi: 10.2105/ajph.85.11.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parry CDH, Dewing S, Petersen P, et al. Rapid assessment of HIV risk behavior in drug using sex workers in three cities in South Africa. AIDS and Behavior. 2009;13(5):849–859. doi: 10.1007/s10461-008-9367-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.