Abstract
Telephone genetic counseling (TC) for hereditary breast/ovarian cancer risk has been associated with positive outcomes in high risk women. However, little is known about how patients perceive TC. As part of a randomized trial of TC versus usual care (UC; in-person genetic counseling), we compared high risk women’s perceptions of: (1) overall satisfaction with genetic counseling; (2) convenience; (3) attentiveness during the session; (4) counselor effectiveness in providing support; and (5) counselor ability to recognize emotional responses during the session. Among the 554 participants (TC, N=272; UC, N=282), delivery mode was not associated with self-reported satisfaction. However, TC participants found counseling significantly more convenient than UC participants (OR = 4.78, 95% CI = 3.32, 6.89) while also perceiving lower levels of support (OR=0.56, 95% CI=0.40–0.80) and emotional recognition (OR = 0.53, 95% CI = 0.37–0.76). In exploratory analyses, we found that non-Hispanic white participants reported higher counselor support in UC than in TC (69.4% vs. 52.8%; OR = 3.06, 95% CI = 1.39–6.74), while minority women perceived less support in UC vs. TC (58.3% vs. 38.7%; OR = 0.80, 95% CI = 0.39–1.65). We discuss potential research and practice implications of these findings which may further improve the effectiveness and utilization of TC.
Keywords: BRCA1/BRCA2, Genetic counseling, Patient satisfaction, Telephone counseling
Introduction
Comprehensive genetic counseling for predisposition to hereditary breast/ovarian cancer is recommended for high-risk women (NCCN, 2015; USPSTF, 2013). Due to the limited number of cancer genetic counselors in the United States and their geographic concentration in urban areas, traditional genetic counseling by such providers has been inaccessible for some women (Kinney et al., 2014; Lynch et al., 2014; McDonald, Lamb, Grillo, Lucas, & Miesfeldt, 2014; National Society of Genetic Counselors, 2014a). Possibly in response to this and other logistical barriers (e.g., reimbursement, timeliness of scheduling), more non-geneticist physicians have been independently ordering BRCA1/2 testing, which may or may not be accompanied by appropriate pre- and post-test genetic counseling (Bellcross et al., 2011; Cragun et al., 2015; Vadaparampil, Scherr, Cragun, Malo, & Pal, 2015).
An increasingly common alternative approach to service delivery is for credentialed genetics professionals to deliver pre- and/or post-test genetic counseling by telephone (Baumanis, Evans, Callanan, & Susswein, 2009; Bradbury et al., 2011; Peshkin et al., 2008; Wham et al., 2010). In fact, a USA-based company has successfully contracted with Aetna and Cigna, two large managed health care companies, to provide telephone genetic counseling services for hereditary cancer risk as a covered benefit for subscribers (Sutphen et al., 2010; GenomeWeb, 2013). Furthermore, recent data indicate that about 30 percent of cancer genetics patients receive telephone genetic counseling (National Society of Genetic Counselors, 2014b).
In a recent randomized-controlled non-inferiority trial, we documented that telephone genetic counseling (TC) led to outcomes that were noninferior to in-person genetic counseling (usual care; UC) on standard psychosocial and decision making outcomes (Schwartz et al., 2014). However, this report did not evaluate participant perceptions of the content and delivery of TC versus UC. The assessment of such patient-reported quality of care measures in genetic counseling is becoming an increasingly key indicator of the potential benefits and value of such services (Biesecker et al., 2013; DeMarco, Peshkin, Mars, & Tercyak, 2004; Elliott, Chodirker, Bocangel, & Mhanni, 2014; McAllister & Dearing, 2015). Although TC and UC yield comparable psychosocial and decision making outcomes (Kinney et al., 2014; Schwartz et al., 2014), the current analysis focuses on participants’ views of these alternate approaches to pre-test genetic counseling. We evaluated participant reports of satisfaction and perceptions of four key genetic counseling components: 1) convenience; 2) ability to maintain attention during the session; 3) genetic counselor effectiveness in providing support; and 4) genetic counselor ability to recognize emotional responses during the session. Understanding these dimensions of patient satisfaction can inform the content and delivery of TC to make it more effective as its clinical use continues to increase.
Methods
Participants
From 2005–2012, women who contacted the clinical genetic counseling programs at the Lombardi Comprehensive Cancer Center (Washington, DC), Icahn School of Medicine at Mount Sinai [formerly Mount Sinai School of Medicine] (New York, NY), University of Vermont Cancer Center (Burlington, VT), and Dana Farber Cancer Institute (Boston, MA) were recruited for participation in a parallel group randomized noninferiority trial comparing standard in-person genetic counseling (UC) to TC for BRCA1/2-associated hereditary breast/ovarian cancer (Schwartz et al., 2014). All participating sites received approval from their institutional review board for this study.
Eligible participants were English-speaking women, ages 21–85, at high risk of carrying a BRCA1/2 mutation who did not have newly diagnosed (< 4 weeks) or metastatic cancer, and lived within a defined catchment area of one of the study sites. We defined high risk as women with a diagnosis of breast or ovarian cancer who met specified qualitative criteria (e.g., diagnosed with breast cancer < 50 years and a first degree relative or paternal second degree relative with breast cancer < 50 or ovarian cancer) or who had a ≥ 10% probability of testing positive for a BRCA1/2 mutation based on any predictive model in the CancerGene platform (University of Texas Southwestern Medical Center, 2015). Unaffected women were eligible if they had a documented BRCA1/2 mutation in a biological relative.
As previously reported (Schwartz et al., 2014), of 1,033 eligible women, 669 (64.8%) completed a baseline interview and agreed to randomization. For the present analysis, participants must have also completed an initial (pre-test) genetic counseling session and a telephone interview approximately two weeks after their genetic counseling session but before genetic testing results were received. The final sample for this analysis consisted of 554 women randomized to UC (N=282) or TC (N= 272).
Procedures
Procedures for the larger randomized controlled trial are described in our prior reports (Butrick et al., 2015; Schwartz et al., 2014). Briefly, eligible participants provided verbal consent prior to completing a baseline telephone interview to collect information about demographic, personal and family cancer history, knowledge levels, and psychosocial characteristics. They were then randomized in blocks of four participants stratified by study site via computer-generated random number to either UC or TC. We employed this randomization approach to ensure equal numbers of participants in each treatment arm at each site and across the study as a whole.
After randomization, participants were scheduled for their genetic counseling session. Participants randomized to usual care (UC) received standard in-person BRCA1/2 genetic counseling and in-person result disclosure delivered by a trained genetic counselor (Peshkin et al., 2008). During the session, the genetic counselor used a standardized visual aid booklet to communicate concepts and general cancer risks associated with BRCA1/2 mutations. UC participants could provide blood for DNA testing at the conclusion of the pre-test counseling session, or could opt to provide DNA at a later time.
Women randomized to telephone counseling (TC) were mailed the standardized visual aid booklet for use during the scheduled TC session. Participants in TC completed both the genetic counseling and disclosure sessions via telephone. These sessions were delivered by a trained genetic counselor with content that was comparable to UC (Peshkin et al., 2008). TC participants could provide blood for DNA at the study site, or use a kit provided by the study take to a physician’s office or a local lab.
Two-weeks after completing the initial genetic counseling session, but before the disclosure of genetic testing results, we conducted a follow-up telephone interview to assess participants’ perception and satisfaction with the pre-test counseling.
Instrumentation
Sociodemographics/Medical History
We assessed sociodemographics, family and personal cancer history. We used personal and family cancer history to calculate participants’ a priori risk of carrying a BRCA1 or BRCA2 mutation using the BRCAPRO model (Berry et al., 2002; University of Texas Southwestern Medical Center, 2015) or by pedigree analysis for relatives of positives.
BRCA1/2 Knowledge
We administered the 27-item Breast Cancer Genetic Counseling Knowledge scale (Erblich et al., 2005). The total score was the number of correct responses (Cronbach’s alpha=0.77).
Numeracy
We used a 3-item measure to assess understanding of numerical concepts and probability (Schwartz et al., 1997). The total score was the number of correct responses (range 0–3).
Decisional Conflict regarding BRCA1/2 testing
We administered the 10-item version of the Decisional-Conflict Scale (DCS) (O’Connor, 1993). Items were scored on a weighted 3-point scale [Yes (0)/Unsure (2)/No (4)] with higher scores indicating greater decisional conflict Cronbach’s alpha was 0.84.
Distress
We measured cancer-specific distress with the Impact of Event Scale (IES) Cronbach’s alpha was 0.88 (Horowitz, Wilner, & Alvarez, 1979) and general perceived stress with the 4-item version of the Perceived Stress Scale (PSS) (Cohen, Kamarck, & Mermelstein, 1983) Cronbach’s alpha was 0.68.
Quality of Life
We administered the SF-12 Mental Component Summary (MCS) and Physical Component Summary (PCS) (Ware, Jr., Kosinski, & Keller, 1996). Higher scores reflect better quality of life. Due to complex scoring procedures we relied on published SF-12 internal consistency data (Chronbach’s alpha >0.82 and 0.75, for the PCS and MCS scales, respectively (Busija et al., 2011).
Outcomes
At the 2-week follow-up we assessed patient perceptions of their pre-test genetic counseling session using the following single item face-valid measures: 1) Satisfaction with the genetic counseling session; 2) Convenience of the genetic counseling process; 3) Ability to maintain attention during the session; 4) Counselor provision of emotional support; and 5) Counselor ability to recognize participant emotions. Responses used a 4-point Likert scale except for participant attention, which was a 3-point scale. For example, the satisfaction item was as follows: “Overall, how satisfied have you been with the education and counseling you have received through our program so far? Not at all satisfied; a little bit satisfied; somewhat satisfied; not at all satisfied.” Because responses on these measures were highly skewed, with the vast majority of patients endorsing either the highest or next to highest response, we dichotomized responses as high (highest ranking response) vs. low (all other responses). We assessed preference for TC vs. UC using a 3-point response scale (in-person, telephone, no preference). For each participant we determined whether this preference was concordant with the modality actually received. For example, TC participants who reported that they preferred TC or had no preference were concordant and those who reported that they preferred UC were discordant.
Data Analysis
We used t-tests and Chi-square tests to identify bivariate associations with patient reported perceptions of pre-test genetic counseling. To identify independent predictors of these perceptions, we used a logistic regression approach with backward variable elimination. All logistic models included randomization group along with all baseline demographic, psychosocial and clinical variables with p<0.10 bivariate associations with the specific outcome. In exploratory analyses, we tested the following variables as potential moderators of the association between randomization group and each of our outcomes: race/ethnicity, knowledge, numeracy, proband status, distress, and mutation risk. We tested each moderator separately by adding the main effect term (if not already in the model) along with the randomization group by moderator interaction term to the final multivariate model for each outcome.
Results
Sample Characteristics
Of the 669 women randomized to the trial, 554 completed both a baseline and 2-week follow-up interview, as well as a genetic counseling session prior to receiving their test result. Sample characteristics stratified by randomization group are displayed in Table 1. There were no differences between the UC and TC groups on any of the sociodemographic or clinical variables.
Table 1.
Sample Characteristics of Women Completing Randomization
| Characteristic | UC (n=282) | TC (n=272) |
|---|---|---|
| Age | ||
|
| ||
| Mean (SD) | 48.7 (13.9) | 48.2 (13.3) |
|
| ||
| BRCA1/2 probability | ||
|
| ||
| Mean (SD) | 25.5 (24.3) | 23.6 (22.1) |
|
| ||
| Education | ||
|
| ||
| College or more, N (%) | 226 (80.1%) | 223 (82.0%) |
| < College, N (%) | 56 (19.9%) | 49 (18.0%) |
|
| ||
| Employment | ||
|
| ||
| Full time, N (%) | 154 (54.6%) | 160 (58.8%) |
| < Full time, N (%) | 128 (45.4%) | 112 (41.2%) |
|
| ||
| Race* | ||
|
| ||
| White, N (%) | 248 (88.9%) | 231 (86.5%) |
| Nonwhite, N (%) | 31 (11.1%) | 36 (13.5%) |
|
| ||
| Marital status | ||
|
| ||
| Married/partner, N (%) | 179 (63.5%) | 167 (61.4%) |
| Single/widowed/divorced, N (%) | 103 (36.5%) | 105 (38.6%) |
|
| ||
| Proband status | ||
|
| ||
| Proband, N (%) | 169 (59.9%) | 162 (59.6%) |
| Relative of carrier, N (%) | 113 (40.1%) | 110 (40.4%) |
Missing data, N=3 (UC), N=5 (TC)
Satisfaction
As shown in Table 2a, the TC and UC groups did not differ on self-reported satisfaction with genetic counseling, with 83.1% of the TC group and 86.8% of the UC group reporting that they were very satisfied with their counseling (X2 (df=1, N=552) = 1.48, p=0.22). Bivariate predictors of higher satisfaction were: being a relative of a BRCA1/2 mutation carrier (X2 (df=1, n=552) = 3.3, p=0.068) and being non-Hispanic white (X2 (df=1, n=544) = 8.3, p=0.004).
Table 2a.
Bivariate Predictors of Patient Perceptions of Genetic Counseling
| Variable | Satisfaction % high |
Convenience % high |
Attention % high |
Support % high |
Emotions % high |
Concordant % Concordant |
|---|---|---|---|---|---|---|
| Randomization | ||||||
| UC | 86.8% | 35.0%*** | 95.0%* | 66.0%** | 68.8%** | 84.2% |
| TC | 83.1% | 72.4% | 89.7% | 52.9% | 55.5% | 80.9% |
| Education | ||||||
| < College Grad | 84.8% | 46.7% | 89.5% | 60.0% | 61.0% | 78.1% |
| College Grad + | 85.0% | 55.0% | 93.1% | 59.5% | 62.6% | 83.6% |
| Proband Status | ||||||
| Proband | 82.7%+ | 54.1% | 91.5% | 60.7% | 62.5% | 84.5% |
| Relative | 88.3% | 52.5% | 93.7% | 57.9% | 61.9% | 79.8% |
| Marital Status | ||||||
| Unmarried | 83.2% | 51.0% | 92.3% | 59.1% | 60.1% | 82.7% |
| Married | 86.1% | 54.9% | 92.4% | 59.8% | 63.6% | 82.5% |
| Employment | ||||||
| < Full Time | 85.4% | 54.4% | 91.6% | 61.7% | 65.4% | 82.4% |
| Full Time | 84.7% | 52.7% | 93.0% | 58.0% | 59.9% | 82.7% |
| Race | ||||||
| Non-Latino White | 86.6%*** | 54.3% | 92.9% | 61.4%+ | 63.7%+ | 82.4% |
| Other | 73.1% | 46.3% | 88.1% | 49.3% | 52.2% | 83.6% |
p<.10,
p<.05,
p<.01,
p<.001
The following variable was missing data: Race/Ethnicity (n=8)
To identify independent predictors of genetic counseling satisfaction we included randomization arm along with the significant bivariate predictors of satisfaction (proband status and race/ethnicity) in the initial step of a logistic regression with backward variable elimination. Randomization group was not associated with overall satisfaction. The only independent predictor of genetic counseling satisfaction was race/ethnicity. Non-Hispanic white participants reported higher satisfaction compared to minority participants (OR=2.20, 95% CI: 1.20–4.03).
Convenience
As shown in Table 2a, 72.4% of women in the TC arm rated the genetic counseling and testing process as extremely convenient compared to 35% of UC participants (X2 (df=1, N=552) = 77.7, p<0.0001). As noted in Table 2b, additional bivariate predictors of greater convenience were: lower BRCA1/2 carrier probability (t (548) = −2.12, p=0.035), lower perceived stress (t (550) = −1.81, p=0.071), lower numeracy (t (550) = 2.02, p = 0.044) and higher physical functioning (t (550) = 2.00, p = 0.046).
Table 2b.
Bivariate Predictors of Patient Perceptions of Genetic Counseling
| Variable | Satisfaction | Convenience | Attention | Support | Emotions | Concordant | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (n=83) |
High (n=469) |
Low (n=257) |
High (n=295) |
Low (n=42) |
High (n=510) |
Low (n=224) |
High (n=330) |
Low (n=209) |
High (n=345) |
No (n=96) |
Yes (n=455) |
|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Age | 48.8 (14.3) | 48.4 (13.5) | 47.6 (13.7) | 49.2 (13.6) | 45.6 (12.8) | 48.7 (13.7) | 47.0 (12.8) | 49.4 (14.1)* | 47.7 (13.2) | 48.9 (13.8) | 47.6 (14.3) | 48.6 (13.5) |
| BRCA Probability | 24.4 (23.8) | 24.7 (23.2) | 26.9 (23.8) | 22.7 (22.8)* | 18.6 (19.6) | 25.1 (23.5)+ | 24.6 (22.4) | 24.6 (23.9) | 25.3 (23.6) | 24.1 (23.1) | 31.3 (24.8) | 23.2 (22.8)** |
| Knowledge | 16.9 (4.6) | 17.3 (4.7) | 17.5 (4.5) | 16.9 (4.8) | 16.9 (5.0) | 17.2 (4.6) | 17.3 (4.6) | 17.1 (4.8) | 17.2 (4.6) | 17.2 (4.7) | 17.2 (4.4) | 17.2 (4.7) |
| Numeracy | 1.5 (1.0) | 1.5 (.96) | 1.6 (1.0) | 1.4 (.91)* | 1.4 (.96) | 1.5 (.96) | 1.5 (.97) | 1.5 (.96) | 1.5 (.97) | 1.5 (.96) | 1.4 (.93) | 1.5 (.97) |
| Impact of Event | 21.9 (13.5) | 21.2 (15.4) | 20.8 (15.3) | 21.8 (15.0) | 25.2 (16.4) | 21.0 (15.0)+ | 23.0 (15.1) | 20.2 (15.0)* | 22.6 (14.4) | 20.6 (15.5) | 19.8 (14.7) | 21.7 (15.2) |
| Perceived Stress | 4.7 (2.2) | 4.3 (2.5) | 4.6 (2.5) | 4.2 (2.4)+ | 4.8 (2.3) | 4.4 (2.4) | 4.8 (2.4) | 4.1 (2.5)*** | 4.8 (2.3) | 4.1 (2.5)** | 4.3 (2.5) | 4.4 (2.4) |
| Physical Function | 50.8 (8.3) | 50.5 (9.0) | 49.7 (9.2) | 51.2 (8.6)* | 50.7 (9.2) | 50.5 (8.9) | 49.5 (9.0) | 51.2 (8.8)* | 49.5 (9.2) | 51.1 (8.7)* | 50.2 (8.6) | 50.6 (9.0) |
| Mental Function | 48.6 (10.0) | 49.2 (10.5) | 49.4 (10.2) | 48.8 (10.5) | 47.8 (11.4) | 49.2 (10.3) | 47.8 (10.6) | 49.9 (10.2)* | 48.2 (10.7) | 49.6 (10.2) | 50.4 (10.7) | 48.8 (10.3) |
| Decision Conflict | 34.4 (26.5) | 29.5 (25.0) | 31.9 (26.3) | 28.8 (24.4) | 30.6 (26.9) | 30.2 (25.2) | 32.7 (26.0) | 28.5 (24.7)+ | 31.8 (25.4) | 29.2 (25.2) | 28.3 (25.5) | 30.8 (25.2) |
p<.10,
p<.05,
p<.01,
p<.001
The following variables were missing data: BRCA1/2 Probability (n=2); Knowledge (n=1)
In the multivariate model, TC participants were more likely to rate genetic counseling as highly convenient compared to UC participants (OR = 4.78, 95% CI = 3.32–6.89). Lower objective mutation risk (OR (0.5 SD Change) = 0.91, 95% CI = 0.82–0.99) and higher physical functioning (OR (0.5 SD Change) = 1.10, 95% CI = 1.01–1.21) were also independently associated with higher convenience.
Attention
As shown in Table 2a, 95% of UC participants reported no difficulty maintaining attention during the session compared to 89.7% of TC participants (X2 (df=1, n=552) = 5.50, p=0.019). As noted in Table 2b, Additional bivariate predictors of attentiveness were: lower cancer specific distress (t (550) = −1.75, p=0.081) and higher BRCA1/2 carrier probability (t (548) = 1.73, p=0.084).
In the final multivariate model, only randomization group predicted attention with TC participants reporting lower attentiveness during the session (OR=0.46, 95% CI=0.24–0.90).
Support
As shown in Table 2a, 66% of women in the UC arm reported that their counselor was extremely effective at providing support compared to 52.9% of those in the TC arm (X2 (df=1, n= 554) = 9.74, p=0.002). Additional bivariate predictors of ratings of counselor supportiveness as shown in Tables 2a and 2b were: being of non-Hispanic White race/ethnicity (X2 (df =1, n=546) = 3.60, p=0.058), older age (t (552) = 2.07, p = 0.039), lower cancer specific distress (t (552) = −2.15, p=0.032), lower perceived stress (t (552) = −3.36, p<0.001), lower decisional conflict (t (551) = −1.95, p=0.051), higher physical (t (552) = 2.28, p=0.023) and mental quality of life (t (552) = 2.38, p=0.018).
In the final multivariate model, women randomized to TC were less likely to report high counselor support compared to those in UC (OR=0.56, 95% CI=0.40–0.80). Additional independent predictors of perceived counselor supportiveness were: physical quality of life (OR (0.5 SD Change) = 1.10, 95% CI=1.01–1.20) and lower perceived stress (OR (0.5 SD Change) = 0.86, 95% CI = 0.78–0.94).
Emotion
As displayed in Table 2a, 68.8% of UC participants reported that their counselor was extremely effective at recognizing their emotions compared to 55.5% of those in TC (X2 (df=1, n=554) = 10.39, p=0.001). Other bivariate predictors of ability to recognize emotions were being of non-Hispanic White race/ethnicity (X2 (df=1, n=546) = 3.27, p=0.07), lower perceived stress (t (552) = −3.29, p=0.001) and higher physical quality of life (t (552) = 2.15, p=0.032), as shown in Tables 2a and 2b.
In the multivariate model, participants randomized to TC were less likely than UC participants to report high counselor emotional recognition (OR = 0.53, 95% CI = 0.37–0.76). Additional independent predictors of emotional recognition were lower perceived stress (OR (0.5 SD Change) = 0.86, 95% CI = 0.79–0.95) and higher physical quality of life (OR (0.5 SD Change) = 1.09, 95% CI = 1.00–1.19).
Preference for Telephone vs. In-Person Genetic Counseling
As displayed in Table 2a, 80.9% of TC participants reported that they preferred TC or had no preference compared to 84.2% of UC participants who reported that they preferred UC or had no preference (X2 (df=1, n=551) = 1.07, p=0.300). The only significant bivariate or multivariate predictor of concordance between preference and assigned intervention arm was lower BRCA1/2 carrier probability (t (547) = −3.08, p = 0.002; OR = 0.85, 95% CI = 0.77–0.95) (Table 2b).
Exploratory Moderator Analyses
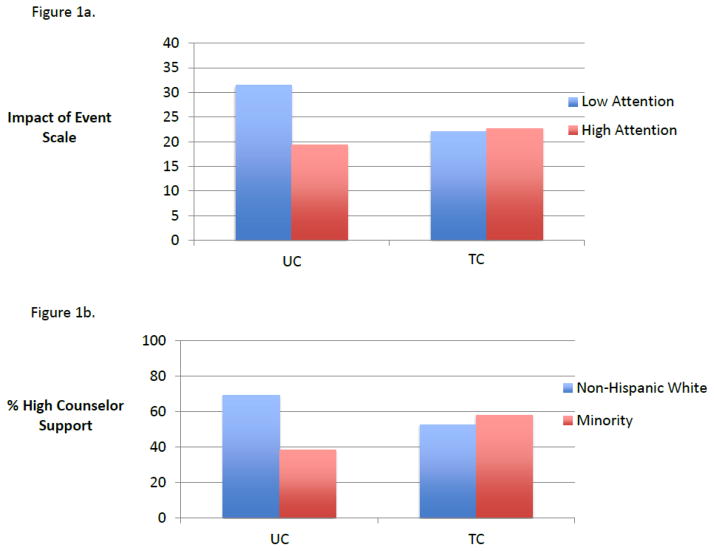
In exploratory analyses, only two variables significantly moderated the associations between randomization group and patient outcomes. Cancer-specific distress moderated the association between group assignment and self-reported attention (group by attention interaction X2 (df=1, n=552) = 5.10, p=0.024). As displayed in Figure 1, higher cancer distress was associated with greater difficulty maintaining attention in UC (OR = 0.71, 95% CI = 0.56–0.91) but not within TC (OR = 1.02, 95% CI = 0.84–1.25). Race/ethnicity moderated the association between group and perceived counselor supportiveness (X2 (df=1, n=546) = 6.02, p=0.014). As displayed in Figure 1, non-Hispanic white participants reported higher counselor support in UC compared to TC (69.4% vs. 52.8%; OR = 3.11, 95% CI = 1.39, 6.74) but the opposite, albeit non-significant, trend was exhibited among minority participants who reported less support in UC vs. TC (58.3% vs. 38.7%; OR = 0.80, 95% CI = 0.39–1.65).
Figure 1.
Figure 1a. Baseline Distress Moderates the Impact of Group on Attentiveness
Figure 1b. Race/Ethnicity Moderates the Impact of Group on Perceived Counselor Support
Discussion
In this report, we explored perceptions of and satisfaction with genetic counseling among a group of high risk women randomized to receive BRCA1/2 genetic counseling by telephone or in-person. We assessed elements that may contribute to patient satisfaction such as patient perceptions of convenience, ability to maintain attention during the session, and the counselor’s ability to provide support and recognize emotions. We found that genetic counseling delivery mode was not associated with overall patient satisfaction or with preference for telephone vs. in-person counseling. This is consistent with our prior report in which we found that TC was non-inferior to in-person counseling on a standardized measure of genetic counseling satisfaction and a wide variety of psychosocial and decision making outcomes (Schwartz et al., 2014).
Although there were no differences in overall satisfaction, the TC and UC groups did differ in their perceptions of several genetic counseling components. Participants in both arms highly rated their genetic counselors’ ability to provide support and recognize emotion; however, ratings on these factors were somewhat lower among women in the TC arm. It is not surprising that in the absence of nonverbal cues signaling worry, distress, or lack of understanding, genetic counselors appeared to be more effective at providing support and recognizing emotions among UC participants. Similarly, women obtaining TC may have had more difficulty identifying counselors’ efforts at support in the absence of nonverbal cues from the counselor. Interestingly and importantly, the lower perceived supportiveness and emotional recognition within the TC arm did not adversely impact overall satisfaction. Thus, it is possible that for some counselees, the provision of very high levels of support is not integral to their needs and expectations. However, regardless of delivery mode, women with lower perceived stress were more likely to report high emotional recognition by the counselor. It is likely that such women presented with fewer indicators of emotional distress or concerns relative to women with higher stress, and thus they perceived that their needs were recognized and attended to by the counselor.
Not surprisingly, telephone counseling participants ranked their method of genetic counseling delivery as more convenient than UC participants. Because patients may inherently recognize the tradeoffs that come with telephone delivery, the slightly reduced supportiveness and attentiveness perceived by TC participants may be counterbalanced by their greatly increased perceptions of convenience. Independent of delivery mode, women with lower objective risk and those with higher physical functioning were more likely to perceive their method of genetic counseling as convenient. Perhaps lower risk women considered genetic counseling to be a less urgent medical appointment. For these women and those with higher physical functioning, the mode of genetic counseling may not matter as their lack of urgency allows them to schedule an appointment at their convenience.
Our exploratory moderator analyses suggest some interesting patterns of participant response to genetic counseling by delivery mode. Although participants randomized to UC reported greater overall attentiveness compared to TC participants, this association was moderated by pre-counseling cancer-specific distress. UC participants reported more difficulty maintaining attention if they were distressed but distress was unrelated to attentiveness for TC participants. Distressed counselees may devote more effort to emotional regulation in a face-to-face session, impeding their ability to attend to informational content (Kelly et al., 2014). In contrast, although TC participants overall reported slightly lower attentiveness, the lack of a face-to-face interaction in TC may have allowed distressed participants to focus less on regulating their emotions and maintaining their composure. Going forward it will be crucial to develop and implement strategies to enhance attentiveness throughout TC sessions, which on average lasted just over an hour (Schwartz et al., 2014). Although we employed “counseling probes” in this study, many of the affective probes were designed primarily for use after a participant already exhibited signs of inattention or anxiety (Peshkin et al., 2008). Future work could explore more proactive and nuanced strategies to assist genetic counseling providers in optimizing communication, attention and support (Patrick-Miller et al., 2014).
In other exploratory analyses, we found that race/ethnicity modified the association between group and counselor supportiveness. Non-Hispanic white participants in the UC arm reported higher counselor support than in the TC arm, whereas minority women perceived higher genetic counselor support in the TC vs. the UC group. Given the low number of minority participants in this trial, this finding must be interpreted cautiously. However, this finding is interesting in light of our previous report of lower uptake of genetic testing among minority women in the TC arm (Butrick et al., 2015). It is possible that in UC, minority patients were more likely than non-minority participants to believe that the genetic counselor may not have fully understood or addressed how genetic risk information might impact them emotionally or socially, particularly when the counselor was of a different ethnic or racial background. It is also conceivable that the genetic counselors had more difficulty interpreting non-verbal cues from minority clients, as has been reported in other settings (Levine & Ambady, 2013). Conversely, delivery by telephone may reduce differences in underlying implicit biases by genetic counselors (Schaa, Roter, Biesecker, Cooper, & Erby, 2015), the occurrence of which has been reported in other medical settings (Blair et al., 2013; Cooper et al., 2012). Of note, however, non-Hispanic white participants reported overall higher satisfaction than minority participants. It is possible that issues related to support as mentioned above influenced the degree of satisfaction among minority participants. Although these findings are intriguing, they must be considered as hypothesis generating given the low number of minority participants overall. These findings do not explain the differential uptake of testing among minorities in the TC arm. However, our data raise the question of whether greater perceived support among minority women in the TC arm suggests that such women made informed decisions not to pursue testing – one that was consistent with their own values and preferences (Halbert et al., 2012).
Study Limitations
The results of our study may not be generalizable to all individuals who seek genetic counseling and testing, including those with a more varied socioeconomic status or risk level. Similarly, all participants in this study agreed to be randomized to TC vs. UC and as such might not represent the general population of genetic counseling patients. As described in our previous reports, about 35 percent of eligible women declined study participation, of whom over one-third explicitly stated they did not want telephone counseling (Schwartz et al., 2014). Also, specific subgroups of potential high-risk individuals were not eligible for participation in the study, including newly diagnosed breast cancer patients, women with metastatic ovarian cancer, men, and unaffected probands. Second, our measures of satisfaction and related domains were fairly broad, and thus we were not able to ascertain why women ranked specific aspects higher or lower than others. Third, as a randomized clinical trial, all of our counselors utilized a standard comprehensive counseling protocol with built in quality controls. Thus, the content and quality of telephone counseling delivered outside of a trial may differ and could affect outcomes. Fourth, our findings pertain only to English-speaking counselees. Finally, our moderator analyses must be viewed as exploratory and hypothesis-generating. In particular, our intriguing findings regarding race/ethnicity must be interpreted extremely cautiously given the low sample size and homogenous grouping of minority participants.
Practice Implications
Our findings suggest that patients perceive that telephone counseling comes with some tradeoffs. While telephone genetic counseling is decidedly more convenient than in-person counseling, it also appears to provide slightly less support to patients. However, these differences seem to counterbalance each other, yielding comparable overall satisfaction and preferences.
This study suggests several ways in which telephone delivery can be made more responsive to patient needs. Genetic counselors may need to be more cognizant of nonverbal patient cues and to proactively assess and respond to patient distress at the outset of genetic counseling, regardless of how or whether patients raise these types of concerns themselves. Routine assessment of psychosocial concerns prior to cancer genetic counseling may help genetic counselors to recognize and address these issues more effectively (Eijzenga et al., 2014).
With respect to findings related to minorities, disparities in genetic counseling and testing between non-Hispanic whites and non-white women are well documented (Hall et al., 2009; Levy et al., 2011; Sussner et al., 2011; Cukier et al., 2013; Sussner et al., 2015). Barriers include less access, financial and emotional concerns, concerns about potential discrimination, as well as competing time demands and family priorities (Adams, Christopher, Williams, & Sheppard, 2015; Forman & Hall, 2009; Mai et al., 2014; Sussner, Jandorf, Thompson, & Valdimarsdottir, 2013; Vadaparampil et al., 2010). In conjunction with systematic efforts to overcome these barriers, women’s overall satisfaction with and the perceived convenience and acceptability of telephone genetic counseling may be key components in increasing uptake of genetic counseling and testing to targeted populations through this mode of delivery. It is hoped that improved reimbursement for telephone counseling will encourage broader availability of this service (Madlensky, 2014).
Research Recommendations
Further research into patient-reported outcomes related to satisfaction with cancer genetic counseling could explore nuances of key components such as emotional recognition and support by the counselor. A structured review of audio and video recorded telephone and in-person sessions along with more detailed assessments of these domains with patients may elucidate ways that counselors can augment their attention to affective issues. In addition, it would be interesting to assess the concordance between genetic counselors’ perceptions of the session with patient-reported outcomes. It is also important to examine potential changes in patient perceptions of genetic counseling after their post-test genetic counseling session, and to determine whether specific pre-test outcomes predict post-test outcomes.
Research focused on specific patient subgroups who may be prime candidates for telephone counseling is important, including male relatives of BRCA1/2 carriers, in whom there is a low rate of testing compared to female relatives (Fehniger, Lin, Beattie, Joseph, & Kaplan, 2013); newly diagnosed breast cancer patients, who may make urgent surgical decisions based on BRCA1/2 results (Schwartz et al., 2004); and women with metastatic ovarian cancer, who may be ill and need the information for chemotherapy decisions and to provide information for relatives (Society of Gynecologic Oncology, 2014). In addition, one concern related to the prospect of population testing for BRCA1/2 (King, Levy-Lahad, & Lahad, 2014) is how to rapidly scale up the ability to provide genetic counseling. Exploring the role of telephone counseling, video conferencing, and other delivery modes, with attention to patient-reported outcomes across different at-risk populations, will be important to evaluate the cost effectiveness and benefits of such screening.
Finally, since we completed our study, the approach to pre-test genetic counseling has become substantially more complex because many women presenting for hereditary breast/ovarian cancer genetic testing are offered the option of undergoing first-line multigene panel tests that include BRCA1/2 (Bradbury et al., 2015; Tung et al., 2015). Invariably, women who pursue telephone counseling will be candidates for such expanded testing, and it will be important to assess patient-reported outcomes as genetic counseling protocols are refined to address such testing. Thus, our data may be used as a starting point for further research to elucidate and assess concrete strategies that could be implemented in clinical genetic counseling practice to maximize patient satisfaction, particularly as telephone counseling becomes more commonplace.
Acknowledgments
The authors acknowledge contributions of genetic counselors Karen Brown, Diana Moglia Tully, Alexandra Lebensohn, and Emily Dalton. In addition, we thank our research assistants for performing telephone interviews: Elizabeth Poggi, Kara-Grace Leventhal, Lisa Moss, Sarah Kelleher, Patty Vegella, Angie Tong, Lesley King, Lisa Feeley, Lauren Vanhusen, Kathy Corso and Liz Zeida. We also thank Susan Marx and Aryana Jacobs for assistance with manuscript preparation. Finally, we are grateful to all of the women who participated in the study.
Funding: The study was supported by Grants R01 CA108933 and P30 CA051008 from the National Cancer Institute and by the Jess and Mildred Fisher Center for Hereditary Cancer and Clinical Genomics Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Human Studies and Informed Consent: All procedures followed were in accordance with the ethical standards of the responsible institutional review committees of the collaborating institutions, national guidelines, and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all individual participants included in the study.
Animal Studies: No animal studies were carried out by the authors for this article.
Conflict of Interest: Ms. Peshkin, Mr. Kelly, Ms. Similuk, Ms. DeMarco, Dr. Valdimarsdottir, Ms. Forman, Ms. Rispoli Joines, Ms. Davis, Ms. McKinnon, Dr. Graves, Dr. Isaacs, Dr. Wood, and Ms. Jandorf declare that they have no conflict of interest. Ms. Nusbaum is an employee of GeneDx, but was employed at Georgetown University during all patient accrual and data collection. Dr. Hooker is an employee of NextGxDx, but was employed at Georgetown University during her participation in the study. Ms. McCormick has obtained paid compensation from Myriad Genetics. Dr. Garber has research funding from Myriad Genetics. Dr. Schwartz serves as an uncompensated member of the Scientific Advisory Board for InformedDNA (St. Petersburg, FL).
References
- Adams I, Christopher J, Williams KP, Sheppard VB. What Black women know and want to know about counseling and testing for BRCA1/2. Journal of Cancer Education. 2015;30(2):344–352. doi: 10.1007/s13187-014-0740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumanis L, Evans JP, Callanan N, Susswein LR. Telephoned BRCA1/2 genetic test results: prevalence, practice, and patient satisfaction. Journal of Genetic Counseling. 2009;18(5):447–463. doi: 10.1007/s10897-009-9238-8. [DOI] [PubMed] [Google Scholar]
- Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. American Journal of Preventive Medicine. 2011;40(1):61–66. doi: 10.1016/j.amepre.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Berry DA, Iversen ES, Jr, Gudbjartsson DF, Hiller EH, Garber JE, Peshkin BN, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. Journal of Clinical Oncology. 2002;20(11):2701–2712. doi: 10.1200/JCO.2002.05.121. [DOI] [PubMed] [Google Scholar]
- Biesecker BB, Erby LH, Woolford S, Adcock JY, Cohen JS, Lamb A, et al. Development and validation of the Psychological Adaptation Scale (PAS): use in six studies of adaptation to a health condition or risk. Patient Education and Counseling. 2013;93(2):248–254. doi: 10.1016/j.pec.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair IV, Steiner JF, Fairclough DL, Hanratty R, Price DW, Hirsh HK, et al. Clinicians’ implicit ethnic/racial bias and perceptions of care among Black and Latino patients. Annals of Family Medicine. 2013;11(1):43–52. doi: 10.1370/afm.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury AR, Patrick-Miller L, Fetzer D, Egleston B, Cummings SA, Forman A, et al. Genetic counselor opinions of, and experiences with telephone communication of BRCA1/2 test results. Clinical Genetics. 2011;79(2):125–131. doi: 10.1111/j.1399-0004.2010.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury AR, Patrick-Miller L, Long J, Powers J, Stopfer J, Forman A, et al. Development of a tiered and binned genetic counseling model for informed consent in the era of multiplex testing for cancer susceptibility. Genetics in Medicine. 2015;17(6):485–492. doi: 10.1038/gim.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, Osborne RH. Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL) Arthritis Care & Research (Hoboken) 2011;63(Suppl 11):S383–S412. doi: 10.1002/acr.20541. [DOI] [PubMed] [Google Scholar]
- Butrick M, Kelly S, Peshkin BN, Luta G, Nusbaum R, Hooker GW, et al. Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling. Genetics in Medicine. 2015;17(6):467–475. doi: 10.1038/gim.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cooper LA, Roter DL, Carson KA, Beach MC, Sabin JA, Greenwald AG, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. American Journal of Public Health. 2012;102(5):979–987. doi: 10.2105/AJPH.2011.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragun D, Camperlengo L, Robinson E, Caldwell M, Kim J, Phelan C, et al. Differences in BRCA counseling and testing practices based on ordering provider type. Genetics in Medicine. 2015;17(1):51–57. doi: 10.1038/gim.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukier YR, Thompson HS, Sussner K, Forman A, Jandorf L, Edwards T, Bovbjerg DH, Schwartz MD, Valdimarsdottir HB. Factors associated with psychological distress among women of African descent at high risk for BRCA mutations. Journal of Genetic Counseling. 2013;22(1):101–107. doi: 10.1007/s10897-012-9510-1. [DOI] [PubMed] [Google Scholar]
- DeMarco TA, Peshkin BN, Mars BD, Tercyak KP. Patient satisfaction with cancer genetic counseling: a psychometric analysis of the Genetic Counseling Satisfaction Scale. Journal of Genetic Counseling. 2004;13(4):293–304. doi: 10.1023/b:jogc.0000035523.96133.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijzenga W, Aaronson NK, Hahn DE, Sidharta GN, van der Kolk LE, Velthuizen ME, et al. Effect of routine assessment of specific psychosocial problems on personalized communication, counselors’ awareness, and distress levels in cancer genetic counseling practice: a randomized controlled trial. Journal of Clinical Oncology. 2014;32(27):2998–3004. doi: 10.1200/JCO.2014.55.4576. [DOI] [PubMed] [Google Scholar]
- Elliott AM, Chodirker BN, Bocangel P, Mhanni AA. Evaluation of a clinical genetics service--a quality initiative. Journal of Genetic Counseling. 2014;23(5):881–889. doi: 10.1007/s10897-014-9713-8. [DOI] [PubMed] [Google Scholar]
- Erblich J, Brown K, Kim Y, Valdimarsdottir HB, Livingston BE, Bovbjerg DH. Development and validation of a Breast Cancer Genetic Counseling Knowledge Questionnaire. Patient Education and Counseling. 2005;56(2):182–191. doi: 10.1016/j.pec.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fehniger J, Lin F, Beattie MS, Joseph G, Kaplan C. Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. Journal of Genetic Counseling. 2013;22(5):603–612. doi: 10.1007/s10897-013-9592-4. [DOI] [PubMed] [Google Scholar]
- Forman AD, Hall MJ. Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. The Breast Journal. 2009;15(Suppl 1):S56–S62. doi: 10.1111/j.1524-4741.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- GenomeWeb. InformedDNA to provide genetic counseling for Cigna members. 2013 Retrieved June 16, 2015, from https://www.genomeweb.com/clinical-genomics/informeddna-provide-genetic-counseling-cigna-members.
- Halbert CH, Kessler L, Collier A, Weathers B, Stopfer J, Domchek S, et al. Low rates of African American participation in genetic counseling and testing for BRCA1/2 mutations: racial disparities or just a difference? Journal of Genetic Counseling. 2012;21(5):676–683. doi: 10.1007/s10897-012-9485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115(10):2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Ellington L, Schoenberg N, Agarwal P, Jackson T, Dickinson S, et al. Linking genetic counseling content to short-term outcomes in individuals at elevated breast cancer risk. Journal of Genetic Counseling. 2014;23(5):838–848. doi: 10.1007/s10897-014-9705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312(11):1091–1092. doi: 10.1001/jama.2014.12483. [DOI] [PubMed] [Google Scholar]
- Kinney AY, Butler KM, Schwartz MD, Mandelblatt JS, Boucher KM, Pappas LM, et al. Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. Journal of the National Cancer Institute. 2014;106(12) doi: 10.1093/jnci/dju328. pii: dju328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine CS, Ambady N. The role of non-verbal behaviour in racial disparities in health care: implications and solutions. Medical Education. 2013;47(9):867–876. doi: 10.1111/medu.12216. [DOI] [PubMed] [Google Scholar]
- Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and Hispanic women particularly at risk. Genetics in Medicine. 2011;13(4):349–355. doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HT, Snyder C, Stacey M, Olson B, Peterson SK, Buxbaum S, et al. Communication and technology in genetic counseling for familial cancer. Clinical Genetics. 2014;85(3):213–222. doi: 10.1111/cge.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlensky L. Is it time to embrace telephone genetic counseling in the oncology setting? Journal of Clinical Oncology. 2014;32(7):611–612. doi: 10.1200/JCO.2013.53.8975. [DOI] [PubMed] [Google Scholar]
- Mai PL, Vadaparampil ST, Breen N, McNeel TS, Wideroff L, Graubard BI. Awareness of cancer susceptibility genetic testing: the 2000, 2005, and 2010 National Health Interview Surveys. American Journal of Preventive Medicine. 2014;46(5):440–448. doi: 10.1016/j.amepre.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister M, Dearing A. Patient reported outcomes and patient empowerment in clinical genetics services. Clinical Genetics. 2015;88(2):114–121. doi: 10.1111/cge.12520. [DOI] [PubMed] [Google Scholar]
- McDonald E, Lamb A, Grillo B, Lucas L, Miesfeldt S. Acceptability of telemedicine and other cancer genetic counseling models of service delivery in geographically remote settings. Journal of Genetic Counseling. 2014;23(2):221–228. doi: 10.1007/s10897-013-9652-9. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast and Ovarian. V.2.2015. 2015 Retrieved September 8, 2015, from http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
- National Society of Genetic Counselors. 2014 Professional Status Survey: Executive Summary. 2014a Retrived May 12, 2015, from http://nsgc.org/p/cm/ld/fid=68.
- National Society of Genetic Counselors. 2014 Professional Status Survey: Work Environment. 2014b Retrieved May 12, 2015, from http://nsgc.org/p/do/sd/sid=2478&type=0.
- O’Connor AM. User Manual-Decisional Conflict Scale (10-item question format) Ottawa, ON, Canada: Ottawa Hospital Research Institute; 1993. 1993 (updated 2010) Retrieved May 12, 2015, from http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf. [Google Scholar]
- Patrick-Miller LJ, Egleston BL, Fetzer D, Forman A, Bealin L, Rybak C, et al. Development of a communication protocol for telephone disclosure of genetic test results for cancer predisposition. JMIR Research Protocols. 2014;3(4):e49. doi: 10.2196/resprot.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshkin BN, DeMarco TA, Graves KD, Brown K, Nusbaum RH, Moglia D, et al. Telephone genetic counseling for high-risk women undergoing BRCA1 and BRCA2 testing: rationale and development of a randomized controlled trial. Genetic Testing. 2008;12(1):37–52. doi: 10.1089/gte.2006.0525. [DOI] [PubMed] [Google Scholar]
- Schaa KL, Roter DL, Biesecker BB, Cooper LA, Erby LH. Genetic counselors’ implicit racial attitudes and their relationship to communication. Health Psychology. 2015;34(2):111–119. doi: 10.1037/hea0000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz LM, Woolshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Annals of Internal Medicine. 1997;127(11):966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Lerman C, Brogan B, Peshkin BN, Halbert CH, DeMarco T, et al. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. Journal of Clinical Oncology. 2004;22(10):1823–1829. doi: 10.1200/JCO.2004.04.086. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Valdimarsdottir HB, Peshkin BN, Mandelblatt J, Nusbaum R, Huang AT, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. Journal of Clinical Oncology. 2014;32(7):618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society of Gynecologic Oncology. SGO Clinical Practice Statement: Genetic Testing for Ovarian Cancer. 2014 Oct; Retrieved May 12, 2015, from https://www.sgo.org/clinical-practice/guidelines/genetic-testing-for-ovarian-cancer/
- Sussner KM, Edwards T, Villagra C, Rodriguez MC, Thompson HS, Jandorf L, et al. BRCA genetic counseling among at-risk Latinas in New York City: new beliefs shape new generation. Journal of Genetic Counseling. 2015;24(1):134–148. doi: 10.1007/s10897-014-9746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussner KM, Edwards TA, Thompson HS, Jandorf L, Kwate NO, Forman A, et al. Ethnic, racial and cultural identity and perceived benefits and barriers related to genetic testing for breast cancer among at-risk women of African descent in New York City. Public Health Genomics. 2011;14(6):356–370. doi: 10.1159/000325263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussner KM, Jandorf L, Thompson HS, Valdimarsdottir HB. Barriers and facilitators to BRCA genetic counseling among at-risk Latinas in New York City. Psycho-oncology. 2013;22(7):1594–1604. doi: 10.1002/pon.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphen R, Davila B, Shappell H, Holtje T, Vadaparampil S, Friedman S, et al. Real world experience with cancer genetic counseling via telephone. Familial Cancer. 2010;9(4):681–689. doi: 10.1007/s10689-010-9369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung N, Battelli C, Allen B, Kaldate R, Bhatnagar S, Bowles K, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33. doi: 10.1002/cncr.29010. [DOI] [PubMed] [Google Scholar]
- U.S.Preventive Services Task Force. Recommendation Summary. BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. 2013 Dec; Retrived May 12, 2015, from http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing.
- University of Texas Southwestern Medical Center at Dallas. CancerGene. 2015 doi: 10.1097/00001888-200009001-00107. Retrieved May 12, 2015, from http://www.utsouthwestern.edu/education/medical-school/departments/surgery/divisions/surgical-oncology/research/cancergene/index.html. [DOI] [PubMed]
- Vadaparampil ST, Quinn GP, Small BJ, McIntyre J, Loi CA, Closser Z, et al. A pilot study of hereditary breast and ovarian knowledge among a multiethnic group of Hispanic women with a personal or family history of cancer. Genetic Testing and Molecular Biomarkers. 2010;14(1):99–106. doi: 10.1089/gtmb.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadaparampil ST, Scherr CL, Cragun D, Malo TL, Pal T. Pre-test genetic counseling services for hereditary breast and ovarian cancer delivered by non-genetics professionals in the state of Florida. Clinical Genetics. 2015;87(5):473–477. doi: 10.1111/cge.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wham D, Vu T, Chan-Smutko G, Kobelka C, Urbauer D, Heald B. Assessment of clinical practices among cancer genetic counselors. Familial Cancer. 2010;9(3):459–468. doi: 10.1007/s10689-010-9326-9. [DOI] [PubMed] [Google Scholar]