Abstract
Background
Information on antiretroviral therapy (ART) use in HIV-infected children with severe malnutrition (SM) is lacking. We investigated long-term ART outcomes in this population.
Methods
Children enrolled in the TREAT Asia Pediatric HIV Observational Database who had SM (weight-for-height or BMI-for-age z-score <−3) at ART initiation were analyzed. Generalized estimating equations were used to investigate poor weight recovery (weight-for-age z-score <−3) and poor CD4% recovery (CD4% <25), and competing risk regression was used to analyze mortality and toxicity-associated treatment modification.
Results
Three hundred fifty five (11.9%) of 2993 children starting ART had SM. Their median weight-for-age z-score increased from −5.6 at ART initiation to −2.3 after 36 months. Not using cotrimoxazole prophylaxis at baseline was associated with poor weight recovery (OR 2.49 vs. using, 95%CI 1.66-3.74, p<0.001). Median CD4% increased from 3.0 at ART initiation to 27.2 after 36 months, and 56 (15.3%) children died during follow-up. More profound SM was associated with poor CD4% recovery (OR 1.78 for z-score <−4.5 vs. −3.5 to <−3.0, 95%CI 1.08-2.92, p=0.023) and mortality (HR 2.57 for z-score <−4.5 vs. −3.5 to <−3.0, 95%CI 1.24-5.33, p=0.011). Twenty two toxicity-associated ART modifications occurred at a rate of 2.4 per 100 patient-years and rates did not differ by malnutrition severity.
Discussion
Cotrimoxazole prophylaxis is important for the recovery of weight-for-age in severely malnourished children starting ART. The extent of SM does not impede weight-for-age recovery or antiretroviral tolerability but CD4% response is compromised in children with a very low weight-for-height/BMI-for-age z-score which may contribute to their high rate of mortality.
Keywords: Severe malnutrition, Antiretroviral therapy, Children, Asia
BACKGROUND
The Joint United Nations Children’s Fund – World Health Organization (WHO) – World Bank Child Malnutrition Database estimates that 14.5% of children in South-East Asia are malnourished, of whom 5.2% are severely malnourished.1 It is also estimated that there are 210,000 HIV-infected persons aged <15 years living in the Asia Pacific area.2 Although food insecurity is more common in resource-limited parts of the region, the association between HIV infection and adverse nutritional outcomes has been reported in both resource-rich and resource-limited settings.3
In children aged 6 to 60 months, severe malnutrition (SM) can be defined as a weight-for-height z-score <−3 or a mid-upper arm circumference less than 115 mm, and in children aged 61 months to 14 years, a BMI-for-age z-score <−3.4 The optimal management of HIV in children with SM is not well defined. Expert opinion suggests that SM should be stabilized and ART initiated as soon as possible following stabilization.3, 5 Yet information on the effectiveness and safety of antiretroviral drugs in this setting is lacking,3, 6, 7 and HIV-infected children with SM have a high risk of early mortality after starting ART.3, 8-11
The aims of this study were to describe the prevalence and predictors of SM in HIV-infected children starting ART in Asia, and to investigate how the extent of SM at ART initiation impacts treatment response.
METHODS
The study population consisted of HIV-infected patients enrolled in the TREAT Asia Pediatric HIV Observational Database (TApHOD). This cohort contributes to the International Epidemiologic Databases to Evaluate AIDS global consortium and has been described previously.12 Recruitment started in 2008. Up to March 2014, TApHOD included data from 5511 children that had ever received care from one of 16 pediatric clinics in Cambodia (n=1), India (n=1), Indonesia (n=2), Malaysia (n=4), Thailand (n=5), or Vietnam (n=3). These sites are predominantly public or university-based pediatric HIV referral clinics. A 2010 survey of ten TApHOD sites found that only 30% provide food supplementation for malnourished patients,13 however, nutritional aid (milk/formula, rice, or cash) is usually provided to children in need through local governments in the region. The same survey also indicated that 90% of sites provide nutritional counselling to patients and their caregivers, 80% provide micronutrients for patients, and 60% routinely initiate cotrimoxazole prophylaxis in HIV-exposed infants (unpublished results). TApHOD ethics approval is obtained at the sites, TREAT Asia/amfAR (coordinating center), and the Kirby Institute (data management and statistical analysis center). Patient consent is deferred to the individual participating sites and their institutional review boards. This means that some TApHOD sites require informed consent and others do not. Children aged 6 months to 14 years that initiated ART (defined as ≥3 antiretrovirals) on or after January 1, 2003 and had a height and weight measurement within 3 months of starting ART were included in this analysis. Those aged <6 months were excluded because this group requires specialized clinical intervention in the context of SM.14 Baseline was the date of ART initiation. The database included information up to March 31, 2014.
The window period for baseline CD4% and hemoglobin was within 3 months of ART initiation. For baseline viral load it was between 6 months before to 1 day after ART initiation. The measurement taken closest to baseline was used. Children were considered hepatitis B coinfected if they had any record of a positive hepatitis B surface antigen test, and hepatitis C coinfected if they had any record of a positive hepatitis C antibody test. Prior tuberculosis diagnosis was defined as any diagnosis (as determined by the treating clinic) of tuberculosis prior to or at ART start. Children were considered to be on cotrimoxazole prophylaxis if they were using cotrimoxazole at ART initiation or if they started within 3 months of initiating ART.
Weight and height measurements were converted into age- and sex-standardized z-scores. Height-for-age and weight-for-height z-scores for children <61 months were calculated using the WHO 2006 child growth standards and macros (ages 6 months-5 years).15 Height-for-age and BMI-for-age z-scores for children ≥61 months old were calculated using the WHO 2007 child growth standards and macros (ages 5-19 years).16 Weight-for-age z-scores were calculated using the WHO child growth standards and macros for 1977.17 The 1977 standards were used because the WHO 2007 weight-for-age standards are only applicable to children ≤10 years old,16 and a previous TApHOD analysis found the 1977 and 2007 standards give similar results.18
Endpoints
Children were considered to have SM when their baseline weight-for-height z-score was <−3 if aged 6 to 60 months or their BMI-for-age z-score was <−3 if aged 61 months to 14 years.4 Information on edematous SM diagnoses and mid-upper arm circumference was not available. In children with SM at baseline, clinical endpoints evaluated whilst on ART were: poor weight recovery (weight-for-age z-score <−3); severe anemia (hemoglobin <7.5g/dl); poor CD4% recovery (CD4% <25); toxicity-associated treatment modification (a change of initial ART associated with an adverse event); loss-to-follow-up (not seen at clinic for >12 months with no documentation of transfer); and death. Weight-for-age was preferred over weight-for-height/BMI-for-age as the measurement of weight recovery so as to avoid follow-up measurements being comprised of weight-for-height for some patients, BMI-for-age for others, and a mixture of both for the remainder.
Statistical analysis
Logistic regression conditional upon country was used to evaluate predictors of SM at ART initiation. Longitudinal analyses were performed in children with SM at baseline on an intention-to-treat basis. Proportions of children with poor weight recovery, anemia, and poor CD4% recovery were evaluated at 6±3-month intervals up to 3 years of follow-up. If, for any given time interval, multiple values were recorded for a patient, the value closest to the 6-monthly time point was used. Generalized estimating equations adjusted for time on ART and country were used to investigate predictors of poor weight recovery and poor CD4% recovery. Kaplan-Meier curves and competing risk regression adjusted for country were used to analyze predictors of toxicity-associated treatment modification, loss-to-follow-up, and mortality. Time-to-toxicity-associated treatment modification was censored at the last clinic visit and competing events were ART modification unrelated to an adverse event, loss-to-follow-up and death. Time-to-loss-to-follow-up was censored at the last clinic visit and death was a competing event. Time-to-mortality was censored at the last clinic visit and loss-to-follow-up was a competing event. Follow-up in all time-to-event analyses was left-censored. Predictors were considered for the multivariate model if one or more categories exhibited a univariate p-value <0.15 and retained in the multivariate model if one or more categories exhibited an adjusted p-value <0.05. Patients with missing data were included in all analysis, but hazard and odds ratios for missing categories are not reported.
Stata software version 13.1 was used for all statistical analysis.
RESULTS
Severe malnutrition at antiretroviral therapy initiation
Of 4105 children that started ART aged 6 months to 14 years, 2993 (72.9%) had baseline height and weight data available. Three hundred fifty five of these (11.9%) met our definition of SM. The prevalences of SM at treatment initiation were 13.5% between 2003-2006, 12.1% between 2007-10, and 8.2% between 2011-14. Cotrimoxazole prophylaxis was being used at ART initiation by 63.0% of children between 2003-06, 64.8% between 2007-2010, and 65.4% between 2011-13. See Table 1, which describes the baseline characteristics for children starting ART and those with SM at ART initiation.
Table 1.
Baseline characteristics
| All children starting ART (n=2993) |
Children with SM starting ART (n=355) |
|
|---|---|---|
|
| ||
| Age in years, median (IQR) | 5.7 (3.2 - 8.6) | 6.7 (3.0 - 9.6) |
| Male | 1502 (50.2) | 206 (58.0) |
| WHO category | ||
| 1 or 2 | 1502 (50.2) | 61 (17.2) |
| 3 | 986 (32.9) | 152 (42.8) |
| 4 | 505 (16.9) | 142 (40.0) |
|
Weight-for-height or BMI-for-age z-
score, median (IQR) |
−1.0 (−2.0 to −0.1) | −3.8 (−4.5 to −3.3) |
| Weight-for-age z-score, median (IQR) | −2.6 (−3.9 to −1.3) | −5.6 (−7.1 to −4.4) |
| Height-for-age z-score, median (IQR) | −2.4 (−3.4 to −1.5) | −3.1 (−3.9 to −2.1) |
| CD4%, median (IQR) | 9 (3 - 16) | 3 (1 - 10) |
| Missing | 283 (9.5) | 47 (13.2) |
| Viral copies/mL, median (IQR) | 186,867 (75,000 - 533,505) | 281,767 (114,00 - 750,000) |
| Missing | 2110 (70.5) | 257 (72.4) |
| Hemoglobin in g/dl, median (IQR) | 10.5 (9.4 - 11.6) | 9.6 (8.2 - 10.7) |
| Missing | 433 (14.5) | 53 (14.9) |
| HBsAg positive, n(%tested) | 96 (4.6) | 8 (3.7) |
|
Hepatitis C antibody positive,
n(%tested) |
36 (3.0) | 1 (0.7) |
| Prior tuberculosis diagnosis | 504 (16.8) | 107 (30.1) |
| Cotrimoxazole prophylaxis | 1923 (64.3) | 266 (74.9) |
| Initial ART regimen | ||
| AZT or ABC + 3TC/FTC + NNRTI | 1228 (41.0) | 111 (31.3) |
| d4T + 3TC/FTC + NNRTI | 1577 (52.7) | 222 (62.5) |
| PI-based | 118 (3.9) | 11 (3.1) |
| Other^ | 70 (2.3) | 11 (3.1) |
| Year of ART initiation | ||
| 2003 to 2006 | 1095 (36.6) | 148 (41.7) |
| 2007 to 2010 | 1323 (44.2) | 160 (45.1) |
| 2011 to 2014 | 575 (19.2) | 47 (13.2) |
| Orphan status | ||
| Both parents alive | 911 (30.4) | 107 (30.1) |
| Single parent alive | 661 (22.1) | 76 (21.4) |
| Neither parent alive | 682 (22.8) | 69 (19.4) |
| Unknown | 739 (24.7) | 103 (29.0) |
| Primary care giver | ||
| Parent | 1297 (43.3) | 151 (42.5) |
| Grandparent | 386 (12.9) | 40 (11.3) |
| Relative | 316 (10.6) | 30 (8.5) |
| Foster care | 43 (1.4) | 2 (0.6) |
| Unknown | 951 (31.8) | 132 (37.2) |
Values are n(%total) unless otherwise specified.
Other combinations mainly comprised of less commonly used NNRTI-based regimens or triple nucleoside reverse transcriptase inhibitor regimens.
Abbreviations: ART=antiretroviral therapy; SM=severe malnutrition; IQR=interquartile range; WHO=World Health Organization; HBsAg=hepatitis B surface antigen; AZT=zidovudine; ABC=abacavir; 3TC/FTC=lamivudine/emtricitabine; NNRTI=non-nucleoside reverse transcriptase inhibitor; d4T=stavudine; PI=protease inhibitor.
Age 6 to 12 months (odds ratio [OR] 4.23 vs. age 13 to 60 months, 95% confidence interval [95%CI] 2.58-6.93, p<0.001), age 11 to 14 years (OR 1.70 vs. age 13 to 60 months, 95%CI 1.16-2.50, p=0.006), CD4% <5 (OR 4.86 vs. CD4% >15, 95%CI 3.26-7.25, p<0.001), prior tuberculosis diagnosis (OR 1.71 vs. no prior diagnosis, 95%CI 1.29-2.27, p<0.001), and male sex (OR 1.39 vs. female, 95%CI 1.10-1.77, p=0.006) were associated with significantly higher odds of SM at ART initiation. Later year of ART initiation (OR 0.55 for 2011-2014 vs. 2003-2006, 95%CI 0.36-0.84, p=0.005) was associated with lower odds of SM at ART initiation.
Long-term treatment outcomes in children with severe malnutrition
Total follow-up time on ART in children with SM at baseline was 1707.1 years. Median follow-up duration was 5.0 years. Total and median follow-up time on the first ART regimen was 929.1 and 1.8 years, respectively.
Weight recovery
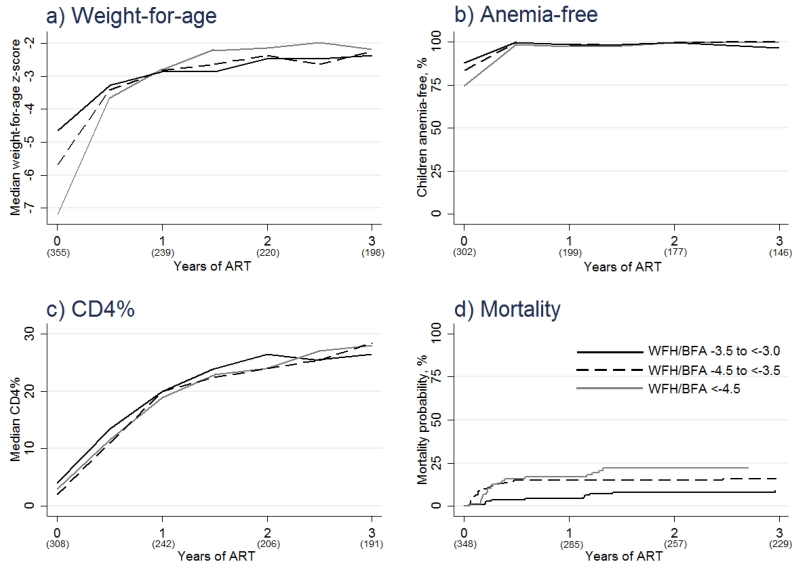
Overall median weight-for-age z-score increased on ART from −5.6 at baseline to −3.4 after 6 months, −2.8 after 12 months, −2.4 after 24 months, and −2.3 after 36 months. Median weight-for-age z-score over time, stratified by baseline weight-for-height/BMI-for-age category, is shown in Figure 1a. Table 2 shows that age 61 months to 14 years (OR 2.44 vs. age 6 to 60 months, 95%CI 1.61-3.70, p<0.001), not using cotrimoxazole prophylaxis (OR 2.49 vs. using cotrimoxazole prophylaxis, 95%CI 1.66-3.74, p<0.001), and any prior tuberculosis diagnosis (OR 1.56 vs. no prior diagnosis, 95%CI 1.05-2.33, p=0.029) were significantly predictive of a follow-up weight-for-age z-score <−3. When baseline weight-for-height/BMI-for-age was added to the final model, no association or trend was seen.
Figure 1. Treatment response in children with severe malnutrition at treatment initiation.
Values in parentheses represent number of test results available in a)-c), and overall number of children at risk in d). Abbreviations: ART=antiretroviral therapy; WFH/BFA=weight-for-height/BMI-for-age z-score.
Table 2.
Generalized estimating equation models showing baseline predictors of poor weight recovery (weight-for-age z-score <−3) in children initiating antiretroviral therapy with severe malnutrition
| Number of patients |
Univariate OR (95%CI) |
p | p overall |
Multivariate OR (95%CI) |
p | p overall |
|
|---|---|---|---|---|---|---|---|
| Age | |||||||
| 6 to 60 months | 134 | 1.00 | 1.00 | ||||
| 61 months to 14 years | 221 | 2.44 (1.62 - 3.69) | <0.001 | 2.44 (1.61 - 3.70) | <0.001 | ||
|
Weight-for-height or
BMI-for-age z-score * |
|||||||
| −3.5 to <−3.0 | 120 | 1.00 | 1.00 | ||||
| −4.5 to <−3.5 | 145 | 1.02 (0.69 - 1.51) | 0.936 | 1.09 (0.73 - 1.63) | 0.665 | ||
| <−4.5 | 90 | 0.80 (0.51 - 1.26) | 0.341 | 0.351~ | 0.97 (0.61 - 1.53) | 0.885 | 0.961~ |
|
Prior tuberculosis
diagnosis |
|||||||
| No | 248 | 1.00 | 1.00 | ||||
| Yes | 107 | 1.65 (1.11 - 2.45) | 0.013 | 1.56 (1.05 - 2.33) | 0.029 | ||
|
Cotrimoxazole
prophylaxis |
|||||||
| Yes | 266 | 1.00 | 1.00 | ||||
| No | 89 | 2.42 (1.62 - 3.60) | <0.001 | 2.49 (1.66 - 3.74) | <0.001 |
Multivariate OR adjusted for variables included in the final model (age, prior tuberculosis, cotrimoxazole prophylaxis).
Overall p for trend;
Abbreviations: OR=odds ratio; 95%CI=95% confidence interval.
Severe anaemia
At ART start, 82.8% of children had a haemoglobin level above that defined as severely anaemic. Amongst children with a weight-for-height/BMI-for-age z-score −3.5 to <−3.0, −4.5 to <−3.5, and <−4.5 the baseline proportions free of severe anaemia were 88.1%, 83.6%, and 74.7%, respectively. After 6 months of ART, the overall proportion increased to 99.5% and remained above 98% up to, and including, month 36 of ART (Figure 1b).
CD4% recovery
Overall median CD4% increased on ART from 3.0 at baseline to 12.0 after 6 months, 20.0 after 12 months, 25.0 after 24 months, and 27.2 after 36 months. Median CD4% over time, stratified by baseline weight-for-height/BMI-for-age category, is shown in Figure 1c. The final model for poor CD4% recovery included age (OR 2.93 for 61 months to 14 years vs. 6 to 60 months, 95%CI 1.85-4.65, p<0.001), sex (OR 1.55 for male vs. female, 95%CI 1.07-2.25, p=0.020), baseline weight-for-height/BMI-for-age z-score (OR 1.78 for <−4.5 vs. −3.5 to <−3.0, 95%CI 1.08-2.92, p=0.023), and baseline CD4% (OR 2.91 for <5 vs. ≥5, 95%CI 1.92-4.42, p<0.001) (Table 3).
Table 3.
Generalized estimating equation models showing baseline predictors of poor CD4% recovery (CD4% <25) in children initiating antiretroviral therapy with severe malnutrition
| Number of patients |
Univariate OR (95%CI) |
p | p overall |
Multivariate OR (95%CI) |
p | p overall |
|
|---|---|---|---|---|---|---|---|
| Age | |||||||
| 6 to 60 months | 128 | 1.00 | 1.00 | ||||
| 61 months to 14 years | 212 | 3.15 (2.01 - 4.92) | <0.001 | 2.93 (1.85 - 4.65) | <0.001 | ||
| Sex | |||||||
| Female | 141 | 1.00 | 1.00 | ||||
| Male | 199 | 1.53 (1.06 - 2.21) | 0.022 | 1.55 (1.07 - 2.25) | 0.020 | ||
|
Weight-for-height or
BMI-for-age z-score |
|||||||
| −3.5 to <−3.0 | 117 | 1.00 | 1.00 | ||||
| −4.5 to <−3.5 | 138 | 1.22 (0.80 - 1.87) | 0.350 | 1.16 (0.76 - 1.79) | 0.490 | ||
| <−4.5 | 85 | 1.56 (0.96 - 2.52) | 0.074 | 0.074~ | 1.78 (1.08 - 2.92) | 0.023 | 0.028~ |
| CD4% | |||||||
| ≥5 | 128 | 1.00 | 1.00 | ||||
| <5 | 180 | 3.71 (2.48 - 5.57) | <0.001 | 2.91 (1.92 - 4.42) | <0.001 | ||
| Unknown | 32 | - | - |
Overall p for trend;
Abbreviations: OR=odds ratio; 95%CI=95% confidence interval.
Toxicity-associated treatment modification
There were 152 treatment modifications (due to any cause) to initial ART which occurred at a rate of 16.4 (95%CI 14.0-19.2) events per 100 patient-years. High rates of treatment modification were evident in children initiating ART with stavudine (20.6 [95%CI 17.2-24.6] events per 100 patient-years) and in those with a baseline CD4% ≥5 (18.3 [95%CI 14.3-23.5] events per 100 patient-years). Twenty two modifications were associated with toxicity (2.4 events per 100 patient-years, 95%CI 1.6-3.6). The median time to toxicity-associated treatment modification was 10.6 (IQR 2.3-22.5) months and the most commonly reported toxicities were anemia (n=7) and lipodystrophy/d4T-related toxicity (n=6). Having grandparents as the primary care provider was the only significant predictor of toxicity-associated treatment modification (hazard ratio [HR] 3.98 vs. parents, 95%CI 1.51-10.48, p=0.005). Rates of toxicity-associated modification in children with baseline weight-for-height/BMI-for-age z-score −3.5 to <−3.0, −4.5 to <−3.5, and <−4.5 were 2.8 (95%CI 1.5-5.3), 1.4 (95%CI 0.6-3.3), and 3.4 (95%CI 1.6-7.1) events per 100 patient-years, respectively.
Loss-to-follow-up
Loss-to-follow-up occurred in 63 children at an overall rate of 3.7 (95%CI 2.9-4.7) events per 100 patient-years. We did not identify any significant predictors of loss-to-follow-up. In those with baseline weight-for-height/BMI-for-age z-score −3.5 to <−3.0, −4.5 to <−3.5, and <−4.5 rates were 3.6 (95%CI 2.4-5.4), 4.0 (95%CI 2.6-5.9), and 3.5 (95%CI 2.1-5.7) events per 100 patient-years, respectively.
Survival
Fifty six deaths (15.8% of children with SM) occurred during follow-up. Median time to death was 3.0 months. Figure 1d shows that, whilst the rate of mortality after 36 months of ART was greatest for children with a weight-for-height/BMI-for-age z-score <−4.5, there was a delay in the onset of this heightened risk. In a model adjusted for baseline CD4%, lower baseline weight-for-height/BMI-for-age z-score (HR 2.57 for <−4.5 vs. −3.5 to <−3.0, 95%CI 1.24-5.33, p=0.011) significantly predicted mortality (Table 4).
Table 4.
Competing risk regression models showing baseline predictors of mortality in children initiating antiretroviral therapy with severe malnutrition
| Deaths | Patient years follow-up |
Rate per 100 patient-years (95%CI) |
Univariate HR (95%CI) |
p | p overall |
Multivariate HR (95%CI) |
p | p overall |
|
|---|---|---|---|---|---|---|---|---|---|
| Overall | 56 | 1707.1 | 3.28 (2.52 - 4.26) | ||||||
|
|
|||||||||
| Age* | |||||||||
| 6 to 60 months | 22 | 622.5 | 3.53 (2.33 - 5.37) | 1.00 | 1.00 | ||||
| 61 months to 14 years | 34 | 1084.6 | 3.13 (2.24 - 4.39) | 1.05 (0.56 - 1.97) | 0.878 | 0.94 (0.47 - 1.85) | 0.851 | ||
| Sex* | |||||||||
| Female | 33 | 959.8 | 3.44 (2.44 - 4.84) | 1.00 | 1.00 | ||||
| Male | 23 | 747.4 | 3.08 (2.05 - 4.63) | 1.13 (0.66 - 1.92) | 0.661 | 1.12 (0.65 - 1.92) | 0.675 | ||
|
Weight-for-height or BMI-for-age
z-score |
|||||||||
| −3.5 to <−3.0 | 11 | 644.3 | 1.71 (0.95 - 3.08) | 1.00 | 1.00 | ||||
| −4.5 to <−3.5 | 24 | 629.2 | 3.81 (2.56 - 5.69) | 2.21 (1.06 - 4.62) | 0.035 | 2.07 (0.98 - 4.35) | 0.055 | ||
| <−4.5 | 21 | 433.7 | 4.84 (3.16 - 7.43) | 2.68 (1.29 - 5.56) | 0.008 | 0.005~ | 2.57 (1.24 - 5.33) | 0.011 | 0.009 ~ |
| CD4% ◇ | |||||||||
| ≥5 | 11 | 629.7 | 1.75 (0.97 - 3.15) | 1.00 | 1.00 | ||||
| <5 | 33 | 857.0 | 3.85 (2.74 - 5.42) | 2.00 (1.01 - 3.96) | 0.045 | 1.85 (0.94 - 3.64) | 0.077 | ||
| Unknown | 12 | 220.5 | 5.44 (3.09 - 9.58) | - | - | ||||
| Hemoglobin* | |||||||||
| No grade 3/4 anemia | 33 | 1248.5 | 2.64 (1.88 - 3.72) | 1.00 | 1.00 | ||||
| Grade 3/4 anemia | 12 | 246.1 | 4.88 (2.77 - 8.58) | 1.77 (0.91 - 3.43) | 0.090 | 1.61 (0.83 - 3.13) | 0.157 | ||
| Unknown | 11 | 212.5 | 5.18 (2.87 - 9.35) | - | - | ||||
| Cotrimoxazole prophylaxis* | |||||||||
| Yes | 15 | 425.1 | 3.53 (2.13 - 5.85) | 1.00 | 1.00 | ||||
| No | 41 | 1282.0 | 3.20 (2.35 - 4.34) | 1.10 (0.57 - 2.15) | 0.769 | 1.38 (0.71 - 2.68) | 0.345 | ||
| Initial ART regimen* | |||||||||
| AZT or ABC + 3TC/FTC + NNRTI | 17 | 492.1 | 3.45 (2.15 - 5.56) | 1.00 | 1.00 | ||||
| d4T + 3TC/FTC + NNRTI | 34 | 1162.7 | 2.92 (2.09 - 4.09) | 1.74 (0.91 - 3.31) | 0.091 | 1.65 (0.85 - 3.19) | 0.135 | ||
| PI-based or other^ | 5 | 52.3 | 9.56 (3.98 - 22.97) | 1.91 (0.67 - 5.47) | 0.228 | 0.209# | 2.61 (0.86 - 7.91) | 0.091 | 0.171# |
| Orphan status* | |||||||||
| Both parents alive | 18 | 447.3 | 4.02 (2.54 - 6.39) | 1.00 | 1.00 | ||||
| Single parent alive | 15 | 337.8 | 4.44 (2.68 - 7.37) | 0.96 (0.47 - 1.96) | 0.902 | 0.92 (0.45 - 1.91) | 0.834 | ||
| Neither parent alive | 10 | 364.9 | 2.74 (1.47 - 5.09) | 0.65 (0.29 - 1.45) | 0.292 | 0.510# | 0.54 (0.22 - 1.29) | 0.167 | 0.311# |
| Unknown | 13 | 557.2 | 2.33 (1.35 - 4.02) | - | - | ||||
Multivariate HR adjusted for variables included in the final model (weight-for-height or BMI-for-age z-score, CD4%).
Despite not reaching significance, CD4% was retained in the final model due to its well-established importance in predicting mortality.
Overall p for trend.
Overall p for heterogeneity.
Other combinations mainly comprised of less commonly used NNRTI-based regimens or triple nucleoside reverse transcriptase inhibitor regimens;
Abbreviations: HR=hazard ratio; 95%CI=95% confidence interval; ART=antiretroviral therapy; AZT=zidovudine; ABC=abacavir; 3TC/FTC=lamivudine/emtricitabine; NNRTI=non-nucleoside reverse transcriptase inhibitor; d4T=stavudine; PI=protease inhibitor.
DISCUSSION
HIV-infection is associated with altered glucose and lipid metabolism, raised basal metabolic rate (especially when an opportunistic infection is present), multiple micronutrient deficiencies, high rates of diarrhea and malabsorption, and frequent coinfections.19 This may explain why SM prevalence in children starting ART (11.9%) far exceeded that currently estimated for the general pediatric population of South-East Asia (5.2%).1 It may also be why children with greater exposure to the damaging effects of HIV, indicated by a lower CD4% and older age (almost all children in TApHOD were vertically infected), and those with a prior diagnosis of tuberculosis, had a high probability of starting ART with SM. Children aged 6 to 12 months were probably more likely to be severely malnourished because of the tendency for perinatal infection to progress rapidly in early infancy 20 and the high rate of malnutrition that occurs in the first 1-2 years of life.21 Why males presented for ART with SM more frequently than females is uncertain but suggests boys may be more sensitive to the nutritional depletion induced by HIV, are more often subject to suboptimal pre-ART care, or are more likely to survive to ART initiation when malnourished. Encouragingly, SM prevalence at ART initiation declined with time which suggests rates of early HIV testing and the quality of nutritional support may be improving in Asia for children with signs of malnourishment.
Children with SM showed very good recovery of important nutritional status surrogates (weight-for-age, hemoglobin) after starting ART, consistent with earlier work from South Africa that found weight-for-age z-scores normalize in moderate and severely underweight, HIV-infected children using ART for 24 months.9 Those of older age, not using cotrimoxazole prophylaxis, and with a prior tuberculosis diagnosis were less likely to recover weight-for-age. In both males and females, growth rate slows beyond 24 months of age.22, 23 Also, children infected for many years are most likely to have been exposed to chronic inflammation and coinfection which might impair weight recovery. Tuberculosis coinfected children are more likely to struggle acquiring sufficient nutrition compared with HIV mono-infected children, even with adequate support, as their energy requirements can be as much as 20-30% greater during tuberculosis infection and recovery.4 This may also be compounded by the capacity for tuberculosis to impair appetite.4 To the best of our knowledge, this is the first study to indicate that cotrimoxazole prophylaxis may enhance weight recovery in malnourished children using ART. This is an important addition to the work by Prendergast et al (2011) which found cotrimoxazole prophylaxis slows the decline in weight- and height-for-age of HIV-infected children not using ART 24, and more recent studies which report on the growth promoting effects of antibiotics in malnourished children.25, 26 The mechanisms by which cotrimoxazole and other antibiotics affect weight are unclear but may include the treatment and prevention of infection, reduction of inflammatory responses resulting in less nutrient diversion and less cytokine-mediated impairment of growth, reduction in enteropathy, and alterations in gut flora.27
Naidoo et al (2010)9 reported that, amongst HIV-infected children using ART for 24 months, mean CD4% increased from 10.53 to 27.52 in those of normal baseline weight, from 9.98 to 29.30 in those with a baseline weight-for-age z-score −3 to <−2, and from 5.69 to 27.10 in those with a baseline weight-for-age z-score <−3. These results led the authors to conclude that malnutrition does not adversely affect immunological response to pediatric HIV treatment. However, after adjustment for baseline CD4%, age, and gender (well established predictors of CD4 response in children with normal nutritional status 28-30), we found more pronounced SM was significantly associated with a follow-up CD4% <25. The mechanism by which very severe malnutrition impairs CD4 response to ART requires further investigation although current literature suggests it would most likely be due to a higher risk of coinfection in this subgroup rather than a direct effect of malnutrition.31
Recent estimates on the risk of death for HIV-uninfected children with SM undergoing renutrition range from 10.4 – 14.2%.32, 33 In comparison, we found the risk of mortality was 15.8% for HIV-infected, severely malnourished children receiving ART and an earlier study reported a risk of 14.3% in severely underweight children receiving HIV treatment.9 The proximity of these figures suggests that malnutrition is the key driver of mortality in severely malnourished children with appropriately managed HIV. Indeed, this is supported by our finding that severely malnourished children with the lowest weight-for-height/BMI-for-age had the greatest risk of death.
Malnutrition leading to poor CD4% recovery may explain the high risk of death we observed amongst the most severely malnourished children starting ART. Importantly, it could not be explained by a lower rate of ART toxicity or loss-to-follow-up amongst those with a higher weight-for-height/BMI-for-age z-score. Others have reported that HIV-infected children with SM are at risk of developing an immune reconstitution-like reaction characterized by edematous malnutrition and associated with a high risk of hospitalization and death.34-36 Future studies should investigate whether such reactions are more common, more severe, or occur more rapidly in children with the most profound SM.
There were several limitations to this study. As an observational analysis the selection of patients was not random and meant that we had to adjust for confounding factors using statistical methods. Further, the estimated effect sizes for associations involving tuberculosis must be interpreted with caution given the well-known difficulty of diagnosing pediatric tuberculosis and our inability to clearly distinguish active tuberculosis from the data available. Information on mid-upper arm circumference and the presence of edema would have allowed us to expand our definition for SM and to investigate subgroups of severely malnourished children. Patient level data on nutritional supplementation and support was not available, although, a large proportion of TApHOD sites provide nutritional counselling and micronutrients, and most malnourished children in this analysis would have received either dietary supplementation from their clinic or food aid through their local government. Unfortunately, data on viral load, ART adherence, and ART dosing data was insufficient to include in our statistical analyses.
Cotrimoxazole prophylaxis enhances the recovery of weight-for-age in severely malnourished children starting ART. The extent of SM at ART initiation does not impede weight-for-age recovery or antiretroviral tolerability but CD4% recovery is impaired in children with a very low weight-for-height/BMI-for-age which may contribute to their high rate of mortality. These results highlight the importance of early SM and HIV diagnosis, and the role of cotrimoxazole in SM recovery.
Acknowledgements
The TREAT Asia Pediatric HIV Network: CV Mean, V Saphonn*, and S Sarun, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; FJ Zhang, Beijing Ditan Hospital, Capital Medical University, Beijing, China; N Kumarasamy*, S Saghayam, and E Chandrasekaran, YRGCARE Medical Centre, CART CRS, Chennai, India; DK Wati*, LPP Atmikasari, and IY Malino, Sanglah Hospital, Udayana University, Bali, Indonesia; N Kurniati*, and D Muktiarti, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; SM Fong*,‡, M Thien, M Lim, and F Daut, Hospital Likas, Kota Kinabalu, Malaysia; NK Nik Yusoff*, and P Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; KA Razali*, TJ Mohamed, NF Abdul Rahman, and NADR Mohammed, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R Nallusamy*, and KC Chan, Penang Hospital, Penang, Malaysia; T Sudjaritruk*, V Sirisanthana, L Aurpibul, and P Oberdorfer, Department of Pediatrics, Faculty of Medicine, Chiang Mai University and Research Institute for Health Sciences, Chiang Mai, Thailand; R Hansudewechakul*, S Denjanta, W Srisuk, and A Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P Lumbiganon*, P Kosalaraksa, P Tharnprisan, and T Udomphanit, Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; G Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand; T Bunupuradah*, T Puthanakit, W Prasitsuebsai, and W Chanthaweethip, HIV-NAT, the Thai Red Cross AIDS Research Centre, Bangkok, Thailand; K Chokephaibulkit*, K Lapphra, W Phongsamart, and S Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; KH Truong*,†, QT Du, and CH Nguyen, Children’s Hospital 1, Ho Chi Minh City, Vietnam; VC Do*, TM Ha, and VT An Children’s Hospital 2, Ho Chi Minh City, Vietnam; LV Nguyen*, KDT Khu, AN Pham, and LT Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam; ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; AH Sohn*, N Durier, and C Sethaputra, TREAT Asia/amfAR -- The Foundation for AIDS Research, Bangkok, Thailand; DA Cooper, MG Law*, and A Kariminia, The Kirby Institute, UNSW Australia, Sydney, Australia.
sources of funding: This work was supported by amfAR, The Foundation for AIDS Research, with funding from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), and the AIDS Life Association. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
*TApHOD Steering Committee member
† Current Steering Committee Chair;
‡ co-Chair
References
- 1.UNICEF. WHO. World Bank [6 Apr 2015];Joint child malnutrition estimates (UNICEF-WHO-WB) - Regional prevalence and numbers affected for wasting and severe wasting in 2013. Available at: http://apps.who.int/gho/data/view.wrapper.nutrition-1-5?lang=en.
- 2.UNAIDS [21 Feb 2014];HIV in Asia and the Pacific: UNAIDS report 2013. Available at: http://www.unaids.org/en/resources/documents/2013/name,89768,en.asp.
- 3.WHO [19 Feb 2014];Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach - 2010 revision. Available at: http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [PubMed]
- 4.WHO [19 Feb 2014];Guidelines for an integrated approach to the nutritional care of HIV-infected children (6 months - 14 years) Available at: http://www.who.int/nutrition/publications/hivaids/9789241597524/en/ [PubMed]
- 5.WHO. UNICEF [19 Feb 2014];Management of the child with a serious infection or severe malnutrition. Guidelines for care at the first-referral level in developing countries. Available at: http://www.who.int/maternal_child_adolescent/documents/fch_cah_00_1/en/
- 6.Musoke PM, Fergusson P. Severe malnutrition and metabolic complications of HIV-infected children in the antiretroviral era: clinical care and management in resource-limited settings. Am J Clin Nutr. 2011;94(6):1716S–1720S. doi: 10.3945/ajcn.111.018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose AM, Hall CS, Martinez-Alier N. Aetiology and management of malnutrition in HIV-positive children. Arch Dis Child. 2014 doi: 10.1136/archdischild-2012-303348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8(8):477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 9.Naidoo R, Rennert W, Lung A, et al. The influence of nutritional status on the response to HAART in HIV-infected children in South Africa. Pediatr Infect Dis J. 2010;29(6):511–513. doi: 10.1097/INF.0b013e3181d1e989. [DOI] [PubMed] [Google Scholar]
- 10.Taye B, Shiferaw S, Enquselassie F. The impact of malnutrition in survival of HIV infected children after initiation of antiretroviral treatment (ART) Ethiop Med J. 2010;48(1):1–10. [PubMed] [Google Scholar]
- 11.Callens SF, Shabani N, Lusiama J, et al. Mortality and associated factors after initiation of pediatric antiretroviral treatment in the Democratic Republic of the Congo. Pediatr Infect Dis J. 2009;28(1):35–40. doi: 10.1097/INF.0b013e318184eeb9. [DOI] [PubMed] [Google Scholar]
- 12.Kariminia A, Chokephaibulkit K, Pang J, et al. Cohort profile: the TREAT Asia pediatric HIV observational database. Int J Epidemiol. 2011;40(1):15–24. doi: 10.1093/ije/dyp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IeDEA Pediatric Working Group A survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa--the International epidemiologic Databases to Evaluate AIDS (IeDEA) J Int AIDS Soc. 2013;16:17998. doi: 10.7448/IAS.16.1.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerac M, Tehran I, Lelijveld N, et al. [14 Apr 2015];Inpatient treatment of severe acute malnutrition in infants aged <6 months. 2012 Feb; Available at: http://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infantandchildren_review9.pdf.
- 15. [29 Jul 2014];WHO 2006 child growth standards and macros (ages 6mth-5yrs) Available at: http://www.who.int/childgrowth/software/en/
- 16.WHO [29 Jul 2014];WHO 2007 child growth standards and macros (ages 5-19yrs) Available at: http://www.who.int/growthref/en/
- 17.WHO WHO child growth standards and macros. 1977 [Google Scholar]
- 18.Hansudewechakul R, Sirisanthana V, Kurniati N, et al. Antiretroviral therapy outcomes of HIV-infected children in the TREAT Asia pediatric HIV observational database. J Acquir Immune Defic Syndr. 2010;55(4):503–509. doi: 10.1097/QAI.0b013e3181f5379a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO [16 Jul 2014];Updates on the management of severe acute malnutrition in infants and children. Available at: http://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infantandchildren/en/ [PubMed]
- 20.Gray L, Newell ML, Thorne C, et al. Fluctuations in symptoms in human immunodeficiency virus-infected children: the first 10 years of life. Pediatrics. 2001;108(1):116–122. doi: 10.1542/peds.108.1.116. [DOI] [PubMed] [Google Scholar]
- 21.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 22.CDC [29 Jul 2014];Growth charts. Available at: http://www.cdc.gov/growthcharts/who_charts.htm.
- 23.Adopt Vietnam [29 Jul 2014];Vietnamese and SE Asian growth charts and discussion. Available at: http://www.adoptvietnam.org/adoption/growth-chart.htm#Growthcharts.
- 24.Prendergast A, Walker AS, Mulenga V, et al. Improved growth and anemia in HIV-infected African children taking cotrimoxazole prophylaxis. Clin Infect Dis. 2011;52(7):953–956. doi: 10.1093/cid/cir029. [DOI] [PubMed] [Google Scholar]
- 25.Gough EK, Moodie EE, Prendergast AJ, et al. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g2267. doi: 10.1136/bmj.g2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trehan I, Goldbach HS, LaGrone LN, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med. 2013;368(5):425–435. doi: 10.1056/NEJMoa1202851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones KD, Thitiri J, Ngari M, et al. Childhood malnutrition: toward an understanding of infections, inflammation, and antimicrobials. Food Nutr Bull. 2014;35(2 Suppl):S64–70. doi: 10.1177/15648265140352S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis J, Walker AS, Castro H, et al. Age and CD4 count at initiation of antiretroviral therapy in HIV-infected children: effects on long-term T-cell reconstitution. J Infect Dis. 2012;205(4):548–556. doi: 10.1093/infdis/jir787. [DOI] [PubMed] [Google Scholar]
- 29.Patel K, Hernan MA, Williams PL, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis. 2008;46(11):1751–1760. doi: 10.1086/587900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Intasan J, Bunupuradah T, Vonthanak S, et al. Comparison of adherence monitoring tools and correlation to virologic failure in a pediatric HIV clinical trial. AIDS Patient Care STDS. 2014;28(6):296–302. doi: 10.1089/apc.2013.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rytter MJ, Kolte L, Briend A, et al. The immune system in children with malnutrition--a systematic review. PLoS One. 2014;9(8):e105017. doi: 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fergusson P, Chinkhumba J, Grijalva-Eternod C, et al. Nutritional recovery in HIV-infected and HIV-uninfected children with severe acute malnutrition. Arch Dis Child. 2009;94(7):512–516. doi: 10.1136/adc.2008.142646. [DOI] [PubMed] [Google Scholar]
- 33.Madec Y, Germanaud D, Moya-Alvarez V, et al. HIV prevalence and impact on renutrition in children hospitalised for severe malnutrition in Niger: an argument for more systematic screening. PLoS One. 2011;6(7):e22787. doi: 10.1371/journal.pone.0022787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachou H, Tylleskar T, Downing R, et al. Severe malnutrition with and without HIV-1 infection in hospitalised children in Kampala, Uganda: differences in clinical features, haematological findings and CD4+ cell counts. Nutr J. 2006;5:27. doi: 10.1186/1475-2891-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prendergast A, Bwakura-Dangarembizi MF, Cook AD, et al. Hospitalization for severe malnutrition among HIV-infected children starting antiretroviral therapy. AIDS. 2011;25(7):951–956. doi: 10.1097/QAD.0b013e328345e56b. [DOI] [PubMed] [Google Scholar]
- 36.Wang ME, Castillo ME, Montano SM, et al. Immune reconstitution inflammatory syndrome in human immunodeficiency virus-infected children in Peru. Pediatr Infect Dis J. 2009;28(10):900–903. doi: 10.1097/INF.0b013e3181a4b7fa. [DOI] [PMC free article] [PubMed] [Google Scholar]