Abstract
A genetically informed cross-lagged model was applied to twin data to explore etiological links between autistic-like traits and affective problems in early childhood. The sample comprised 310 same-sex twin pairs (143 monozygotic and 167 dizygotic; 53% male). Autistic-like traits and affective problems were assessed at ages 2 and 3 using parent ratings. Both constructs were related within and across age (r = .30−.53) and showed moderate stability (r = .45−.54). Autistic-like traits and affective problems showed genetic and environmental influences at both ages. Whereas at age 2, the covariance between autistic-like traits and affective problems was entirely due to environmental influences (shared and nonshared), at age 3, genetic factors also contributed to the covariance between constructs. The stability paths, but not the cross-lagged paths, were significant, indicating that there is stability in both autistic-like traits and affective problems but they do not mutually influence each other across age. Stability effects were due to genetic, shared, and nonshared environmental influences. Substantial novel genetic and nonshared environmental influences emerge at age 3 and suggest change in the etiology of these constructs over time. During early childhood, autistic-like traits tend to occur alongside affective problems and partly overlapping genetic and environmental influences explain this association.
Keywords: autistic-like traits, affective problems, twins, genetic
Autism spectrum disorders (ASD) are neurodevelopmental conditions that typically begin in early childhood (American Psychiatric Association, 2013). There is considerable interest in understanding the causes of co-occurrence in psychopathology generally (Smoller et al., 2013), including in ASD. ASD are known to co-occur with other forms of psychopathology at rates higher than chance (Simonoff et al., 2008).
Affective problems are characteristics that define major depressive disorder and dysthymic disorder and that are also present to varying degrees in the general population (Achenbach & Rescorla, 2000; Pickles et al., 2001). In terms of the co-occurrence of affective problems and ASD, while some studies do not find elevated levels of depression in children with ASD, elevated levels of dysthymic disorder are present (Simonoff et al., 2008), and older children and adults with ASD have elevated levels of depression (Kim, Szatmari, Bryson, Streiner, & Wilson, 2000; Lainhart & Folstein, 1994; Lake, Perry, & Lunsky, 2014).
Autistic traits are characteristics that define ASD and which are present in the general population (Ronald & Hoekstra, 2011). A strong genetic link has been shown between typical variation in autistic traits in the general population and autistic traits in more extreme groups (including the top 1%) (Lundstrom et al., 2012; Robinson et al., 2011; Ronald et al., 2006), as well as between autistic traits and diagnosed ASD (Colvert et al., 2015). Likewise, research supports the model that affective problems are on a continuum with affective disorders such as major depression (Pickles et al., 2001). Thus, support exists for the proposition that understanding the causes of autistic traits and affective problems in the general population may provide insights for understanding the causes of clinically recognized ASD and depression (Plomin, Haworth, & Davis, 2009).
Twin studies from middle childhood onwards demonstrate that autistic traits are moderately to highly heritable and show modest nonshared environmental influences (i.e., environmental influences that are unique to each family member) (Holmboe et al., 2014; for reviews see Posthuma & Polderman, 2013; Ronald & Hoekstra, 2011). Molecular genetics research concurs with twin study findings. A measured heritability study, which employs data from single nucleotide polymorphisms (SNPs) to estimate variance explained by measured genetic variants (Yang, Lee, Goddard, & Visscher, 2011), found that autistic traits are influenced by common genetic variations in middle childhood to adolescence (St Pourcain et al., 2014).
Affective problems, such as depression, are also genetically influenced. Twin studies find modest heritability, shared and nonshared environmental influences, and evidence that the etiological architecture changes from childhood to adulthood (Lau & Eley, 2006; Nivard et al., 2014; Waszczuk, Zavos, Gregory, & Eley, 2014). A measured heritability study concurs with twin study results in showing that major depression is influenced by common genetic variation in adults (Lee et al., 2013).
Far less is known about the etiology of autistic traits and affective problems in preschoolers, as compared to older ages. Research on 2-year-olds from the same sample as used here reported that autistic traits were moderately heritable, and showed modest genetic overlap with traits of Attention Deficit Hyperactivity Disorder (ADHD; Ronald, Edelson, Asherson, & Saudino, 2010). A second study on an independent sample of twins aged between 2 and 3 years also reported modest heritability of autism screening items (Stilp, Gernsbacher, Schweigert, Arneson, & Goldsmith, 2010). In terms of twin studies of affective problems in preschoolers, a large study showed that anxiety and depression in 3-year-olds was 76% heritable, with the remaining variance explained by nonshared environment (Boomsma., Van Beijsterveld, & Hudziak, 2005). There have not been any studies exploring the degree to which affective problems and autistic traits in preschoolers share genetic and environmental influences. A genetically-sensitive study on affective problems and autistic traits in preschoolers could provide clues about the early developmental and etiological processes that lead these types of traits to co-occur.
Family studies show that affective disorders occur at elevated levels in relatives of individuals with ASD (Piven et al., 1991; Piven et al., 1990) and information garnered about the onset of these affective disorders implies that the increased risk is not solely during the period after the birth of the child with ASD (e.g., Bolton, Pickles, Murphy, & Rutter, 1998). That is, affective disorders appear to run in families of individuals with ASD because of shared familial effects (i.e., shared genes and/or environments), not because affective disorders are a consequence of having a relative with ASD. Mothers of children with ASD are known to take selective serotonin reuptake inhibitors (SSRIs) during pregnancy more commonly than mothers of children without ASD (Man et al., 2015). SSRI use is not equivalent to affective problems but might be considered a proxy. Although the mechanism of the association between maternal prenatal SSRI use and ASD is not known, this elevated use of SSRIs would suggest that depression and ASD to some extent run in the same families.
To our knowledge, only one twin study has explored the degree to which autistic traits and depression share genetic and environmental influences (Lundstrom et al., 2011). In this study, on adults in the Swedish population, traits of depression and autistic traits correlated approximately .3, and just over half of this covariation was explained by genetic influences, and just under half by nonshared environmental influences. Risk of depression increased monotonically with increasing scores on autistic traits. Bivariate genomic-relatedness-matrix restricted maximum likelihood (GREML) approaches that use measured genetic similarity (via SNPs) to predict phenotypic similarity in large groups of unrelated individuals suggest low genetic overlap of common genetic variants between ASD and major depressive disorder (Lee et al., 2013) but this result might be hampered by low power in the analyses.
In terms of causal explanations for two forms of psychopathology co-occurring, one potential reason is correlated risk factors, where some of the same genetic and environmental risk factors influence both phenotypes. This has been shown to be the case for ASD and ADHD (Ronald, Simonoff, Kuntsi, Asherson, & Plomin, 2008). Another mechanism that can explain co-occurrence is phenotypic causality, where one form of psychopathology leads onto another. For example, in middle childhood it has been shown that autistic traits phenotypically influence later anxiety-related behaviors (Hallett, Ronald, Rijsdijk, & Happé, 2010). These two explanations will be tested here in relation to the co-occurrence of autistic traits and affective problems in early childhood.
The aims of the present study were first to investigate the degree to which autistic traits and affective problems share genetic and environmental influences during the preschool years. Our hypothesis, based on results from studies on children and adults, was that autistic traits and affective problems would correlate significantly and show modest genetic overlap with one another in 2- and 3-year-olds. The second aim was to investigate, using a longitudinal cross-lagged model, whether across developmental time from age 2 to age 3, autistic traits impact later affective problems, and vice-versa, over and above their existing association at age 2 and any shared genetic and environmental influences between them.
Methods
Participants
The Boston University Twin Project (BUTP) sample was recruited from the Massachusetts Registry of Vital Records. Twins with birth weights less than 1,750 grams or with gestational ages less than 34 weeks were not included in the study. The sample comprised 310 same-sex twin pairs (143 MZ and 167 DZ) at age 2, of which 304 twin pairs (137 MZ and 167 DZ) were assessed at age 3. There were approximately equal numbers of males and females (53% male). Ethnicity of the sample was generally representative of the state of Massachusetts (85.4% Caucasian, 3.2% Black, 2% Asian, 7.3% Mixed, 2.2% Other). Socioeconomic status according to the Hollingshead (1975) Four Factor Index ranged from low to upper middle class (range= 20.5-66; M= 50.9, SD= 14.1). Zygosity was determined using DNA analyses obtained through cheek swab samples. In cases where DNA was not available (n=3), zygosity was determined using parent responses on physical similarity questionnaires, which have been shown to be more than 95% accurate when compared to DNA markers (Price, Freeman, Craig, Ebersole, & Plomin, 2000). All procedures were approved by the Boston University Institutional Review Board.
Measures
DSM-Oriented Scales on the Child Behavior Checklist for Ages 1½-5
Autistic-like traits and affective problems were assessed with DSM-Oriented Scales on the Child Behavior Checklist for Ages 1½-5 (CBCL; Achenbach & Rescorla, 2000). Parents report on 99 problem items on a scale of 0 (not true) to 2 (very true or often true) based on the child’s behavior in the preceding 2 months. Although the CBCL includes clinical cutoffs for screening purposes, these scales were used as dimensional measures of autistic-like traits and affective problems. Studies have shown significant associations between DSM diagnoses and CBCL scores (Achenbach & Rescorla, 2000).
Autistic-Like Traits
Autistic-like traits were assessed with the CBCL Pervasive Developmental Problems DSM-Oriented Scale. This scale includes 13 items (e.g., “can’t stand things being out of place”; “rocks head, body”) and is consistent with DSM diagnostic criteria for Autistic Disorders. Previous research has found this subscale to be a sensitive and useful screening device for Autistic Disorders in toddlers, both in comparison with typically-developing children and those with other psychiatric conditions (Muratori et al., 2011; Narzisi et al., 2013) In the current sample, internal consistency as indicated by Cronbach’s alpha was .64 and .69 for ages 2 and 3, respectively.
Affective Problems
Parents rated Affective Problems on the CBCL Affective Problems DSM-Oriented Scale. This scale includes 10 items (e.g., “sad”; “doesn’t eat well”) relating to Major Depressive Disorder and Dysthymic Disorder (Achenbach & Rescorla, 2000). Internal consistency for affective problems as indicated by Cronbach’s alpha was .55 at age 2 and .58 at age 3.
Statistical Approach
Data Transformations
Both autistic-like traits and affective problems were positively skewed and were log transformed to correct for skewedness at both ages. Because twin covariances can be inflated by variance due to sex, all scores were residualized for sex effects (see McGue & Bouchard, 1984). These residualized scores were used in all behavioral genetic analyses in the present study.
Twin Design
The twin method involves comparing MZ twins, who share 100% of their genes with DZ twins who share, on average, 50% of their segregating genes. Additive genetic influence (A) is suggested when similarity on a trait varies with genetic relatedness (i.e., MZ cotwin resemblances exceed DZ resemblances). Shared environments (C) are family-wide experiences that act to make twins more similar than would be predicted by genes alone. Nonshared environmental influences (E) are effects that are unique to the individual and make individuals within a family different. Based on twin methodology, structural equation models can be used to estimate the contributions of A, C, and E to autistic-like traits and affective problems and to the covariation between the constructs.
Cross-Lagged Analysis
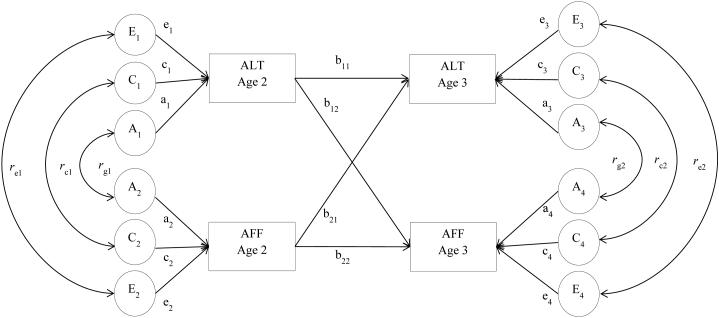
A genetically informed cross-lagged model (Burt, McGue, Krueger, & Iacono, 2005) was used to provide information about the genetic and environmental sources of variance at each age and the covariation between traits within and across ages, as well as the stability of each trait (Figure 1). This model constrains all cross-age associations to function as phenotypic partial regression coefficients (i.e., b11, b22, b12, b21). The paths leading from the phenotype at age 2 to the same phenotype at age 3 (b11 and b22) index the stability of the phenotype while controlling for any prior association with the second phenotype. The cross-lagged paths (b12 and b21) reflect the effects of each trait at Time 1 on the other trait at Time 2 and allow us to partial out the independent impact of one trait on another across age above and beyond their preexisting relation and the stability of each construct. For example, path b12 indicates whether autistic-like traits at age 2 impact affective problems at age 3 above and beyond their association at age 2 and the stability of autistic-like traits. Variances of autistic-like traits and affective problems at age 2 were decomposed into their genetic, shared environmental, and nonshared environmental path estimates. The path estimates a1, c1, e1 and a2, c2, e2 indicate the relative influences of the latent variables A1, C1, and E1 and A2, C2, and E2 on each phenotype at age 2 and the square of these path values represent genetic and environmental variances. The genetic correlation at age 2 (rg1) indicates the extent to which the genetic effects on autistic-like traits correlate with the genetic effects on affective problems independent of the heritability of each. The genetic factors that influence two measures can covary perfectly although the genetic effects on each measure may contribute only marginally to the phenotypic variance. Therefore, rg1 can be 1.0 when the heritability of each measure is modest. Conversely, two measures may be highly heritable, but if there is no genetic overlap, the genetic correlation would be zero. A similar logic applies to the shared environmental correlation (rc1) and nonshared environmental correlation (re1). The effects at age 3 are residual effects that are independent of age 2. Thus, a3, c3, e3 and a4, c4, e4 estimate the relative influences of the latent variables A3, C3, and E3 and A4, C4, and E4 on the phenotypes at age 3 that are independent of the age 2 effects, and thus reflect change. The genetic and environmental correlations at age 3 (rg2, rc2, re2) index genetic and environmental overlap of these novel effects across the phenotypes.
Fig 1. A path diagram of the cross-lagged model shown for one twin.
Latent factors appear in circles (i.e., A for genetic factors, C for shared environments, E for nonshared environments), while observed (measured) variables appear in rectangles. Latent factors at age 3 represent residual variances. Standardized path estimates for the latent factors are indicated by lowercase letters (e.g., a1, c1, e1). Cross-age stability is represented by b11 and b22, while b12 and b21 indicate cross-lagged effects. Double-headed arrows linking two latent factors represent genetic, shared environmental, or nonshared environmental correlations (i.e., rg1, rc1, re1, rg2, rc2, re2).
To understand the extent to which autistic-like traits and affective problems influence each other over time, the genetic and environmental variances in autistic-like traits and affective problems at age 3 can be further portioned into four different effects. That is, the total genetic variance in autistic-like traits at age 3 can be decomposed into: (1) stability effects: genetic influences specific to autistic-like traits at age 2 that are transmitted to age 3 (calculation; b112 × a12); (2) cross-lagged effects: genetic influences specific to affective problems at age 2 transmitted to autistic-like traits at age 3 (calculation; b212 × a 22); (3) common effects from age 2: the genetic effects common to autistic-like traits and affective problems at age 2 (calculation; 2×[b11 × a1 × rg1 × a2 × b21]); and (4) residual effects: unique genetic effects on autistic-like traits at age 3 (calculation; a32) (Larsson, Viding, Rijsdijk, & Plomin, 2008). The age 3 variance for affective problems is similarly decomposed.
Raw maximum likelihood estimation was used to account for missing data in Mx structural equation modeling software (Neale, Boker, Xie, & Maes, 2006). Goodness of model fit was assessed using likelihood-ratio chi-square (χ2) tests, calculated as the difference between the −2 log likelihood (−2LL) of the full model and that of a saturated model (i.e., a model in which the variance–covariance structure is not estimated and all variances and covariances for MZ and DZ twins are estimated). The difference in −2LL is asymptotically distributed as χ2 with degrees of freedom (df), which reflects the difference in the amount of estimated parameters between the full model and a saturated model. A reduced model, in which all nonsignificant parameters were dropped, was compared to the full model in which all parameters were estimated. The relative fit of the reduced model was determined by the chi-square difference (Δχ2) between the full model and the reduced model and corresponding change in degrees of freedom (Δdf). A nonsignificant change in chi-square between the full and reduced model indicates that the nonsignificant parameters can be dropped from the model without a significant decrement in overall model fit. Akaike’s Information Criterion (AIC; AIC= χ2 – 2*Δdf) values were also computed, with lower AIC values indicating better fit of the model to the observed data.
Results
Descriptive Statistics
Table 1 lists the means and standard deviations (SD) for autistic-like traits and affective problems at age 2 and age 3 by sex. Mean differences were assessed using linear mixed effects models fitted using the SAS PROC MIXED procedure to account for the nested structure of the data. Family was the group effect and individual twins represented repeated observations. For autistic-like traits and affective problems, the main effects for time, gender and the interaction between time and gender were nonsignificant.
Table 1.
Means (standard deviations) of measures of autistic-like traits and affective problems at age 2 and age 3 by gender.
| Measures | Males | Females | Gender F (df) |
Time F (df) |
Time x Gender F (df) |
|---|---|---|---|---|---|
|
|
|
|
|||
| Autistic-like traits |
|||||
| Age 2 | 2.64 (2.48) | 2.48 (2.25) | |||
| Age 3 | 3.03 (2.96) | 2.75 (2.27) | .00 (315)
p=.56 |
.15 (300)
p=.70 |
.09 (301)
p=.95 |
| Affective problems |
|||||
| Age 2 | 1.54 (1.78) | 1.40 (1.63) | |||
| Age 3 | 1.57 (1.79) | 1.62 (1.84) | .29 (315)
p=.59 |
.12 (303)
p=.73 |
.66 (304)
p=.42 |
a Means presented for the non-transformed variables for ease of interpretation
Phenotypic Correlations
There were moderate age-specific relations among autistic-like traits and affective problems at age 2 (r=.47, p<.001, df=309) and at age 3 (r=.53, p<.001, df=294). There was also moderate stability in autistic-like traits (r=.54, p<.001, df=291) and affective problems (r=.45, p<.001, df=294) across age. The cross-age cross-construct correlation between autistic-like traits at age 2 and affective problems at age 3 was modest (r=.30, p<.001, df=292) and a similar correlation was obtained for affective problems at age 2 and autistic-like traits at age 3 (r=.31, p<.001, df=293).
Intraclass and Cross Correlations
Table 2 presents the intraclass correlations and cross-trait cross-twin correlations for autistic-like traits and affective problems at ages 2 and 3 by zygosity. For both measures at both ages, the intraclass correlations for MZ twins exceeded those of DZ twins suggesting that genetic influences contribute to individual differences in these traits. In all instances, the DZ intraclass correlations were greater than half of the MZ correlations, which suggested that shared environmental influences also impacted these traits. With one exception, the cross-age cross-trait correlation between autistic-like traits at age 2 and affective problems at age 3, the cross correlations showed a similar pattern of results. The genetic and environmental influences on these phenotypes can be tested by more powerful multivariate genetic model-fitting analyses.
Table 2.
Twin intraclass and cross correlations.
|
Age 2
|
Age 3
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Autistic-like
traits |
Affective
problems |
Autistic-like
traits |
Affective
problems |
|||||
| MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | |
| Age 2 | ||||||||
| Autistic-like traits |
.58 | .38 | - | - | - | - | - | - |
| Affective problems |
.31 | .34 | .65 | .40 | - | - | - | - |
| Age 3 | ||||||||
| Autistic-like traits |
.46 | .27 | .27 | .22 | .70 | .39 | - | - |
| Affective problems |
.22 | .25 | .28 | .30 | .45 | .30 | .73 | .40 |
Note. MZ monozygotic twins, DZ dizygotic twins. Intraclass correlations are bolded. All correlations are significant at p<.05.
Model-Fitting Analyses
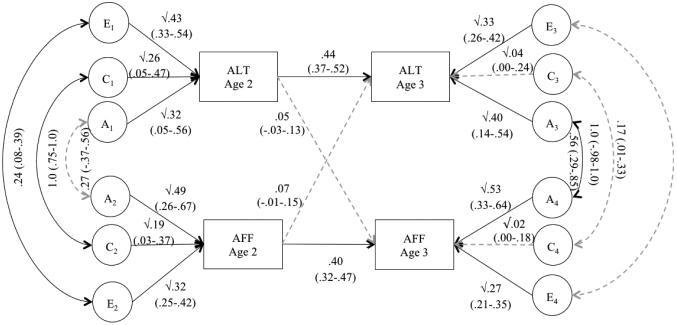
Figure 2 presents the path estimates from the full cross-lagged model. The genetic correlation at age 2, the residual shared environmental effects on both autistic-like traits and affective problems at age 3 and the shared environmental correlation connecting the constructs at age 3 were nonsignificant. The cross-lagged paths (i.e., the path from autistic-like traits at age 2 to affective problems at age 3 and the path from affective problems at age 2 and autistic-like traits) were also nonsignificant in the full model and dropping these paths did not result in significantly worse model fit (see Table 3). The nonshared environmental correlation at age 3 was significant in the full model but became nonsignificant after all nonsignificant paths from the full model were dropped. It is likely, therefore, that the significance of this path in the full model is due to chance and interpretations of this path should be made cautiously. All other paths were significant in the reduced model. Results from the full model are reported to avoid artificial inflation of parameter estimates (Figure 2 and Table 4).
Fig 2.
Best-Fitting Model
Table 3.
Model fitting results for autistic-like traits and affective problems at age 2 and age 3.
| Fit of model compared to
saturated modela |
Difference in fit of
modelsb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | −2LL | df | χ 2 | Δ df | p | AIC | Δ χ 2 | Δ df | p |
| Saturated | 11010.859 | 2338 | |||||||
| Full | 11097.563 | 2388 | 86.704 | 50 | <.001 | −13.296 | |||
| Reduced | 11107.291 | 2395 | 96.432 | 57 | <.001 | −17.568 | 9.728 | 7 | .205 |
Note. −2LL= log likelihood statistic; df= degrees of freedom; χ 2= chi-square fit statistic; AIC= Akaike’s information criterion.
Overall fit of the model determined by the difference in -2LL of the model and that of a saturated model.
Relative fit of the model determined by the χ 2 difference (Δχ2) between full model and reduced model, in which all nonsignificant paths were dropped.
Table 4.
Proportion of the total genetic, shared environmental, and nonshared environmental variation in autistic-like traits and affective problems at age 3.
| Autistic-Like Traits at age 3 | Total Variance1 |
A | C | E |
|---|---|---|---|---|
| Total ACE variance due to: | 1.000 | .471 | .104 | .441 |
| Autistic-like traits at age 2
(cross-age stability effects) |
.196 |
.063
(13.4%) |
.050
(48.1%) |
.083
(18.8%) |
| Affective problems at age 2
(cross-lagged effects) |
.025 | .002 (.42%) |
.0009 (.87%) |
.023 (5.2%) |
| Common effects at age 2 | .026 | .007 (1.5%) |
.014 (13.5%) |
.005 (1.1%) |
| Specific effects at age 3 | .769 |
.399
(84.7%) |
.040 (38.4%) |
.330
(74.8%) |
|
| ||||
| Affective Problems at age 3 |
Total Variance |
A | C | E |
|
| ||||
| Total ACE variance due to: | 1.000 | .613 | .056 | .405 |
| Affective problems at age 2
(cross-age stability effects) |
.156 |
.076
(12.4%) |
.030
(53.6%) |
.050
(12.3%) |
| Autistic-like traits at age 2
(cross-lagged effects) |
.067 | .0009 (.15%) |
.0007 (1.3%) |
.066 (16.3%) |
| Common effects at age 2 | .028 | .004 (.65%) |
.009 (16.1%) |
.015 (3.7%) |
| Specific effects at age 3 | .823 |
.533
(86.9%) |
.016 (28.6%) |
.274
(67.7%) |
Note. A=Genetic influences; C=Shared environmental influences; E= Nonshared environmental influences. Percentages of genetic, shared environmental, and nonshared environmental influences, respectively, due to four sources are provided in parentheses. Significant estimates from the reduced model are bolded.
Genetic and Environmental Influences on Autistic-Like Traits and Affective Problems in Early Childhood
The genetic and environmental contributions to autistic-like traits and affective problems at age 2 are the squared path values of the latent variables A1, C1, E1 and A2, C2, E2 in Figure 2. These values represent the percentage of variance due to genetic, shared environmental, and nonshared environmental effects, respectively. For autistic-like traits, genetic factors explained 32% of the variance, and shared environmental and nonshared environmental influences contributed 26% and 43% of the variance, respectively. For affective problems, genetic factors explained 49% of the variance, shared environmental influences explained 19% of the variance, and nonshared environmental factors accounted for 32% of the variance.
The paths on the right side of the model at age 3 represent novel genetic and environmental effects that are independent of the effects at age 2. Total genetic, shared environmental and nonshared environmental variances on autistic-like traits and affective problems are presented in Table 4. For autistic-like traits at age 3, genetic factors accounted for approximately 47% of the variance, shared environmental influences 10%, and nonshared environmental influences 44%. For affective problems, genetic influences accounted for approximately 61% of the variance, shared environmental factors 6%, and nonshared environmental influences 41%.
Concurrent Overlap between Autistic-Like Traits and Affective Problems at Each Age
At age 2, the genetic correlation (rg1) was nonsignificant, suggesting that although autistic-like traits and affective problems are genetically influenced at age 2, these effects are unique to the phenotypes. The shared environmental correlation at age 2 (rc1=1.0) indicates that autistic-like traits and affective problems are influenced by the same shared environmental factors. The nonshared environmental correlation at age 2 (re1=.24) suggests that although these two constructs have some nonshared environmental influences in common, many of the effects are unique to each phenotype. At age 3, the genetic correlation (rg2) between the residual variances of autistic-like traits and affective problems was .56. This moderate-to-high correlation suggests that roughly 50% of the novel genetic effects that emerge at age 3 overlap across autistic-like traits and affective problems. Given that the genetic correlation at age 2 was nonsignificant, this suggests substantial developmental change in common genetic effects underlying the association between autistic-like traits and affective problems from age 2 to age 3.
Autistic-Like Traits and Affective Problems across Age (Stability and Cross-Lagged Effects)
The stability paths (i.e., b11, b22), but not the cross-lagged paths (b12, b21) were significant in the full model and could not be dropped without a significant decrement in fit, Δχ2=303.549, df=2, p<.001. This indicates that there is stability in both phenotypes but the phenotypes do not mutually influence each other across age. Estimates of variance due to stability effects can be obtained by squaring the partial regression coefficients for the respective paths. Stability effects from autistic-like traits at age 2 explained 19.3% of the variance in autistic-like traits at age 3 and the stability effects from affective problems from age 2 explained 16% of the variance affective problems at age 3.
Genetic and Environmental Influences over Time
Table 4 decomposes the genetic, shared environmental, and nonshared environmental effects on autistic-like traits and affective problems at age 3 into components that are transmitted from age 2 and residual effects that are specific to age 3. The total variance at age 3 is the sum of the component variances. Approximately 20% of the total variance at age 3 reflects effects that are stable from age 2. These effects contribute modestly to the total variances for genetic and nonshared environmental variances at age 3, but account for all of the significant shared environmental variance in autistic-like traits at age 3. Unique variance specific to age 3 was substantial (77%) and accounted for most of the genetic (84.7%; i.e., .399/.471×100) and nonshared environmental (74.8%; i.e., .330/.441×100) influences on this trait at age 3.
The effects on affective problems at age 3 show a similar pattern of results. Approximately 16% of the total variance at age 3 is due to stability effects. As with autistic-like traits, these effects contribute modestly to the total variances for genetic and nonshared environmental variances at age 3, but account for all of the significant shared environmental variance in affective problems. Unique variance specific to age 3 was, again, substantial (82%) and accounted for most of the genetic (86.9%; i.e., .533/.613×100) and nonshared environmental (67.7%; i.e., .274/.405×100) influences on this trait at age 3. Although no novel shared environmental effects emerge at age 3 for both autistic-like traits and affective problems, shared environmental influences persist longitudinally from age 2 through the cross-age stability paths.
Discussion
Our study shows that autistic-like traits and affective problems tend to travel together in young children, as demonstrated by their correlations ranging from .47−.53. These results from our community sample parallel those from clinical studies of older individuals diagnosed with ASD, which show that depression and dysthymic disorder co-occur at an elevated rate in individuals with ASD (e.g., Kim et al., 2000; Simonoff et al., 2008). It is also informative to be aware of the degree of stability in psychopathology across age for making predictions about individual trajectories, and the results here for each trait on its own (correlations of .54 and .45 for autistic-like traits and affective problems, respectively) are in line with existing reports on older children of considerable stability of autistic-like traits and affective problems individually across development (e.g., Holmboe et al., 2014; Nivard et al., 2014; St Pourcain et al., 2014).
Both autistic-like traits and affective problems showed moderate heritability, low shared environment, and modest nonshared environmental influence in our sample. These results mirror the existing literature: in the only other preschool twin sample that has been assessed on autism screening items, the estimates of heritability (46%), low shared environment (12%), and modest nonshared environmental influence (42%) are closely similar (within 2%) and not significantly different from the estimates derived in the present sample at age 3 (see first row of Table 4) (Stilp et al., 2010). Compared to a previous twin study of anxiety and depression in 3 year olds, which showed 76% heritability and 24% nonshared environmental influences, the etiology of affective problems in our sample at age 3 was comparable, with a lower heritability point estimate (61%).
The present study also tackled the important question of why autistic-like traits and affective problems covary. Their association was caused by the presence of genetic and environmental influences that were partly overlapping for both autistic-like traits and affective problems in our study. Sources of covariance between the two domains may differ across age. At age 2 years, shared and nonshared environmental influences explained the covariance between autistic-like traits and affective problems. At age 3, new genetic effects emerged for both autistic-like traits and affective problems and these genetic effects substantially covaried across domains. That is, some of the same genes that influence autistic-like traits also influence affective problems, and this would lead them to present in combination more often than chance. Likewise, the results showed that some of the same environmental factors that influence autistic-like traits also influence affective problems and this would also increase the likelihood of their co-occurrence. The next step for research is to identify the specific genes and environments that play a role in a common causal pathway underlying both of these forms of psychopathology. However, given the differential pattern of genetic covariance across age, researchers will need to carefully consider possible age differences when searching for specific genes.
An advantage of longitudinal assessments at ages 2 and 3 years in our sample was that cross-lagged effects could be tested. The cross-lagged paths explored whether, over and above any shared genetic and environmental influences between autistic-like traits and affective problems, these two types of problems phenotypically impacted each other across early childhood from ages 2 to 3 years. Previous research, for example, has shown that autistic-like traits at age 7 contribute to the presence of internalizing traits at age 12 (Hallett et al., 2010). Our study showed that during early childhood, autistic-like traits and affective problems covary because of shared genetic and environmental factors but there was no evidence for phenotypic effects on each other across time. That is, the presence of autistic-like traits at age 2 does not in and of itself impact subsequent affective problems a year later, and vice-versa, affective problems at age 2 do not impact subsequent autistic-like traits at age 3. One reason why a phenotypic interaction was found in a previous study (Hallett et al., 2010) but not in our sample might be the difference in ages of the samples (middle childhood and early childhood, respectively). For example, older children who struggle with social communication may choose to avoid group situations and may find it difficult to express themselves. Maintaining rigid routines may become more stressful as children progress through school. It is possible that such dynamics could mean that the presence of autistic traits impact later emotional problems in older children, over and above any shared genetic and environmental causes between autistic traits and emotional problems. The lack of self-consciousness in young children, and less demanding environments relative to those in middle childhood, would make such dynamics less likely to occur. A second reason for the different findings may relate to the different measures employed: in particular the use of affective problems subscales in the present study compared to a broader emotional problems subscale in the previous study (Hallett et al., 2010). A third reason may be that different genetic and environmental influences operate on autistic-like traits and affective problems across early and middle childhood, which could affect their mutual influences. Indeed, even across one year in early childhood, there is substantial genetic and environmental change. There is likely even more etiological change across a wider age span, but this is an empirical question.
The present set of results should be considered in light of the study limitations. The twin design makes a number of assumptions such as that there is no assortative mating and that there are equal environments for MZ and DZ twins, but these assumptions have been thoroughly tested (Evans & Martin, 2000). The ACE model-fitting used here assumes that genetic effects are additive, and does not directly model gene-environment interaction or correlation effects (for more information see Rijsdijk & Sham, 2002). The limitations of the twin design can be resolved by using other research designs to address the same research question. Characteristics of the sample merit discussion. The modest sample size led to wide confidence intervals for some estimates and the sample was not powered to test for etiological sex differences. The sample did not include children at the very severe end of the autism spectrum. Eight children out of the sample of 624 individuals were diagnosed with an ASD at age 3. This prevalence is still within current prevalence estimates of ASD, taking into account relatedness between twin pairs (Baird et al., 2006), indicating that the sample was reasonably representative. Furthermore, the sample was selected not to include children who had a very low birth weight or were preterm. It is important that data from twins generalizes to singletons. There is good evidence that twins do not show different mean levels of autistic-like traits than singletons (Curran et al., 2011) or CBCL-assessed internalizing behaviors in childhood (Gau, Silberg, Erickson, & Hewitt, 1992).
It was advantageous to employ scales from the CBCL, which have been widely used and assessed for reliability and validity (Achenbach & Rescorla, 2000). Nevertheless, the reliability, as assessed by Cronbach’s alpha, for both scales was modest in the present sample, and may have led to inflated estimates of the nonshared environment term (which includes measurement error). The CBCL autistic-like traits scale has been shown to have modest specificity for detecting children with ADOS-G classification of autism, and therefore this limitation regarding the measure’s validity should be taken in account when assessing the results. Nonetheless, this subscale has been shown to be a sensitive and useful screening device for ASD in toddlers, both when compared to typically-developing children and those with other psychiatric conditions (Muratori et al., 2011; Narzisi et al., 2013; Sikora et al., 2008). Moreover, this subscale has been found to be strongly correlated with other autism screening measures such as the Gilliam Autism Rating Scale (GARS; Sikora et al., 2008). Similarly, the CBCL affective problems scale demonstrates criterion validity with referral for mental health services (DSM IV checklists completed by professionals), with referred children scoring significantly higher on the affective problems scale than nonreferred children (Dekker et al., 2007; Achenbach & Rescorla, 2000). Parent report contains some bias and shows modest correlation with other raters (Najman et al., 2001). On the other hand, there are few alternatives to parental assessment of behavior in early childhood, it is a practical solution for large samples, and parents are aware of young children’s behavior across development and across a range of situations.
Our study demonstrates that during the early preschool years, autistic-like traits tend to occur alongside affective problems in some children. The causes of this co-occurrence appear to be due to partly the same genes and environments that make children vulnerable to affective problems also influencing autistic-like traits. Research about causal pathways in early childhood can feed into the knowledge base from which intervention and early prevention strategies are developed.
Acknowledgements
This research is supported by grants R01 HD068435 and R01 MH062375 from the National Institutes of Health to Dr. Saudino.
Footnotes
There are no conflicts of interest to report.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2000. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, Charman T. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: The Special Needs and Autism Project (SNAP) Lancet. 2006;368:210–215. doi: 10.1016/S0140-6736(06)69041-7. doi:10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Pickles A, Murphy M, Rutter M. Autism, affective and other psychiatric disorders: Patterns of familial aggregation. Psychological Medicine. 1998;28:385–395. doi: 10.1017/s0033291797006004. doi:10.1017/S0033291797006004. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Van Beijsterveldt CEM, Hudziak JJ. Genetic and environmental influences on anxious/depression during childhood: A study from the Netherlands Twin Register. Brain and Behavior. 2005;4:466–481. doi: 10.1111/j.1601-183X.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, Krueger RF, Iacono WG. How are parent-child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Development and Psychopathology. 2005;17:145–165. doi: 10.1017/S095457940505008X. doi:10.1017/S095457940505008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, Bolton P. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.3028. doi 10.1001/jamapsychiatry.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran S, Dworzynski K, Happe F, Ronald A, Allison C, Baron-Cohen S, Bolton PF. No major effect of twinning on autistic traits. Autism Research. 2011;4:377–382. doi: 10.1002/aur.207. doi: 10.1002/aur.207. [DOI] [PubMed] [Google Scholar]
- Dekker MC, Ferdinand RF, Van Lang ND, Bongers IL, Van Der Ende J, Verhulst FC. Developmental trajectories of depressive symptoms from early childhood to late adolescence: Gender differences and adult outcome. Journal of Child Psychology and Psychiatry. 2007;48:657–666. doi: 10.1111/j.1469-7610.2007.01742.x. doi:10.1111/j.1469-7610.2007.01742.x. [DOI] [PubMed] [Google Scholar]
- Evans DM, Martin NG. The validity of twin studies. GeneScreen. 2000;1:77–79. [Google Scholar]
- Gau JS, Silberg JL, Erickson MT, Hewitt JK. Childhood behavior problems: A comparison of twin and non-twin samples. Acta Geneticae Medicae Et Gemellologiae: Twin Research. 1992;41:53–63. doi: 10.1017/s0001566000002518. [DOI] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Rijsdijk F, Happé F. Association of autistic-like and internalizing traits during childhood: A longitudinal twin study. The American Journal of Psychiatry. 2010;167:809–817. doi: 10.1176/appi.ajp.2009.09070990. doi:10.1176/appi.ajp.2009.09070990. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. The four-factor index of social status. Unpublished manuscript, Yale University; New Haven, CT: 1975. [Google Scholar]
- Holmboe K, Rijsdijk FV, Hallett V, Happe F, Plomin R, Ronald A. Strong genetic influences on the stability of autistic traits in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:221–230. doi: 10.1016/j.jaac.2013.11.001. doi: 10.1016/j.jaac.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Szatmari P, Bryson SE, Streiner DL, Wilson FJ. The prevalence of anxiety and mood problems among children with autism and asperger syndrome. Autism. 2000;4:117–132. doi:10.1177/1362361300004002002. [Google Scholar]
- Lainhart JE, Folstein SE. Affective disorders in people with autism: A review of published cases. Journal of Autism and Developmental Disorders. 1994;24:587–601. doi: 10.1007/BF02172140. doi:10.1007/BF02172140. [DOI] [PubMed] [Google Scholar]
- Lake JK, Perry A, Lunsky Y. Mental health services for individuals with high functioning autism spectrum disorder. Autism Research and Treatment. 20142014:502420. doi: 10.1155/2014/502420. doi: 10.1155/2014/502420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Viding E, Rijsdijk FV, Plomin R. Relationships between parental negativity and child antisocial behavior over time: A bidirectional effects model in a longitudinal genetically informative design. Journal of Abnormal Child Psychology. 2008;36:633–645. doi: 10.1007/s10802-007-9151-2. doi: 10.1007/s10802-007-9151-2. [DOI] [PubMed] [Google Scholar]
- Lau JF, Eley TC. Changes in genetic and environmental influences on depressive symptoms across adolescence and young adulthood. The British Journal of Psychiatry. 2006;189:422–427. doi: 10.1192/bjp.bp.105.018721. doi:10.1192/bjp.bp.105.018721. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Wray NR. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature Genetics. 2013;45:984–994. doi: 10.1038/ng.2711. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom S, Chang Z, Kerekes N, Gumpert CH, Rastam M, Gillberg C, Anckarsater H. Autistic-like traits and their association with mental health problems in two nationwide twin cohorts of children and adults. Psychological Medicine. 2011;41:2423–2433. doi: 10.1017/S0033291711000377. doi: 10.1017/S0033291711000377. [DOI] [PubMed] [Google Scholar]
- Lundstrom S, Chang Z, Rastam M, Gillberg C, Larsson H, Anckarsater H, Lichtenstein P. Autism spectrum disorders and autistic like traits: Similar etiology in the extreme end and the normal variation. Archives of Genetic Psychiatry. 2012;69:46–52. doi: 10.1001/archgenpsychiatry.2011.144. doi: 10.1001/archgenpsychiatry.2011.144. [DOI] [PubMed] [Google Scholar]
- Man KC, Tong HY, Wong LL, Chan EW, Simonoff E, Wong IK. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: A systematic review and meta-analysis of observational studies. Neuroscience and Biobehavioral Reviews. 2015:4982–4989. doi: 10.1016/j.neubiorev.2014.11.020. doi:10.1016/j.neubiorev.2014.11.020. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Muratori F, Narzisi A, Tancredi R, Cosenza A, Calugi S, Saviozzi I, Calderoni S. The CBCL 1.5–5 and the identification of preschoolers with autism in Italy. Epidemiology and Psychiatric Sciences. 2011;20:329–338. doi: 10.1017/s204579601100045x. doi:10.1017/S204579601100045X. [DOI] [PubMed] [Google Scholar]
- Najman JM, Williams GM, Nikles J, Spence S, Bor W, O'Callaghan M, Shuttlewood GJ. Bias influencing maternal reports of child behaviour and emotional state. Social Psychiatry and Psychiatric Epidemiology. 2001;36:186–194. doi: 10.1007/s001270170062. [DOI] [PubMed] [Google Scholar]
- Narzisi A, Calderoni S, Maestro S, Calugi S, Mottes E, Muratori F. Child Behavior Checklist 1½–5 as a tool to identify toddlers with autism spectrum disorders: A case-control study. Research in Developmental Disabilities. 2013;34:1179–1189. doi: 10.1016/j.ridd.2012.12.020. doi:10.1016/j.ridd.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 7th. Department of Psychiatry, Virginia Commonwealth University; Richmond, VA: 2006. [Computer software] [Google Scholar]
- Nivard MG, Dolan CV, Kendler KS, Kan KJ, Willemsen G, van Beijsterveldt CE, Boomsma DI. Stability in symptoms of anxiety and depression as a function of genotype and environment: A longitudinal twin study from ages 3 to 63 years. Psychological Medicine. 2014:1–11. doi: 10.1017/S003329171400213X. doi: 10.1017/S003329171400213X. [DOI] [PubMed] [Google Scholar]
- Pickles A, Rowe R, Simonoff E, Foley D, Rutter M, Silberg J. Child psychiatric symptoms and psychosocial impairment: Relationship and prognostic significance. The British Journal of Psychiatry. 2001;179:230–235. doi: 10.1192/bjp.179.3.230. doi:10.1192/bjp.179.3.230. [DOI] [PubMed] [Google Scholar]
- Piven J, Chase GA, Landa R, Wzorek M, Gayle J, Cloud D, Folstein S. Psychiatric disorders in the parents of autistic individuals. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:471–478. doi: 10.1097/00004583-199105000-00019. doi:10.1097/00004583-199105000-00019. [DOI] [PubMed] [Google Scholar]
- Piven J, Gayle J, Chase GA, Fink B, Landa R, Wzorek MM, Folstein SE. A family history study of neuropsychiatric disorders in the adult siblings of autistic individuals. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29:177–183. doi: 10.1097/00004583-199003000-00004. doi:10.1097/00004583-199003000-00004. [DOI] [PubMed] [Google Scholar]
- Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nature Reviews Genetics. 2009;10:872–878. doi: 10.1038/nrg2670. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Polderman TJ. What have we learned from recent twin studies about the etiology of neurodevelopmental disorders? Current Opinion in Neurology. 2013;26:111–121. doi: 10.1097/WCO.0b013e32835f19c3. doi: 10.1097/WCO.0b013e32835f19c3. [DOI] [PubMed] [Google Scholar]
- Price TS, Freeman B, Craig L, Ebersole L, Plomin R. Infant zygosity can be assigned by parent questionnaire data. Twin Research. 2000;3:129–133. doi: 10.1375/136905200320565391. doi: http://dx.doi.org/10.1375/twin.3.3.129. [DOI] [PubMed] [Google Scholar]
- Rijsdijk F, Sham P. Analytic approaches to twin data using structural equation models. Bioinformatics. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happé F, Ronald A. Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%) Archives of Genetic Psychiatry. 2011;68:1113–1121. doi: 10.1001/archgenpsychiatry.2011.119. doi: 10.1001/archgenpsychiatry.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Edelson LR, Asherson P, Saudino KJ. Exploring the relationship between autistic-like traits and ADHD behaviors in early childhood: Findings from a community twin study of 2-year-olds. Journal of Abnormal Child Psychology. 2010;38:185–196. doi: 10.1007/s10802-009-9366-5. doi: 10.1007/s10802-009-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Happé F, Bolton P, Butcher LM, Price TS, Wheelwright S, Plomin R. Genetic heterogeneity between the three components of the autism spectrum: A twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:691–699. doi: 10.1097/01.chi.0000215325.13058.9d. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: A decade of new twin studies. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156B:255–274. doi: 10.1002/ajmg.b.31159. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of Child Psychology and Psychiatry. 2008;49:535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- Sikora DM, Hall TA, Hartley SL, Gerrard-Morris AE, Cagle S. Does parent report of behavior differ across ADOS-G classifications: Analysis of scores from the CBCL and GARS. Journal of Autism and Developmental Disorders. 2008;38:440–448. doi: 10.1007/s10803-007-0407-z. doi:10.1007/s10803-007-0407-z. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, Sullivan PF. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pourcain B, Skuse DH, Mandy WP, Wang K, Hakonarson H, Timpson NJ, Smith GD. Variability in the common genetic architecture of social-communication spectrum phenotypes during childhood and adolescence. Molecular Autism. 2014;5:18. doi: 10.1186/2040-2392-5-18. doi: 10.1186/2040-2392-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilp RH, Gernsbacher MA, Schweigert EK, Arneson CL, Goldsmith HH. Genetic variance for autism screening items in an unselected sample of toddler-age twins. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:267–276. doi: 10.1016/j.jaac.2009.11.012. doi:10.1097/00004583-201003000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczuk MA, Zavos HM, Gregory AM, Eley TC. The phenotypic and genetic structure of depression and anxiety disorder symptoms in childhood, adolescence, and young adulthood. JAMA Psychiatry. 2014;71:905–916. doi: 10.1001/jamapsychiatry.2014.655. doi: 10.1001/jamapsychiatry.2014.655. [DOI] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: A tool for genome-wide complex trait analysis. American Journal of Human Genetics. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]