Abstract
Background
Dimethyl fumarate (DMF) alters the phenotype of circulating immune cells and causes lymphopenia in a subpopulation of treated MS patients.
Objective
To phenotypically characterize circulating leukocytes in DMF-treated MS patients.
Methods
Cross-sectional observational comparisons of peripheral blood from DMF-treated MS patients (n=17 lymphopenic, 24 non-lymphopenic), untreated MS patients (n=17) and healthy controls (n=23) that was immunophenotyped using flow cytometry. Longitudinal samples were analyzed for 13 DMF-treated patients.
Results
Lymphopenic DMF-treated patients had significantly fewer circulating CD8+ and CD4+ T-cells, CD56dim NK cells, CD19+ B-cells and plasmacytoid dendritic cells compared to controls. CXCR3+ and CCR6+ expression was disproportionately reduced among CD4+ T-cells while the proportion of T regulatory cells was unchanged. DMF did not affect circulating CD56hi NK-cells, monocytes or myeloid dendritic cells. Whether lymphopenic or not, DMF-treated patients had a lower proportion of circulating central and effector memory T cells and concomitant expansion of naïve T cells compared to controls.
Conclusions
DMF shifts the immunophenotypes of circulating T cells, causing reduction of memory cells and relative expansion of naïve cells regardless of absolute lymphocyte count. This may represent one mechanism of action of the drug. Lymphopenic patients had disproportionate loss of CD8+ T cells, which may affect their immunocompetence.
Keywords: Dimethyl fumarate, multiple sclerosis, immunology, neuroimmunology
Introduction
Dimethyl fumarate (DMF) was FDA approved in 2013 for the treatment of relapsing multiple sclerosis (MS). In clinical trials, absolute lymphocyte counts (ALC) typically dropped by about 30% and a subset of patients (5-6%) developed severe lymphopenia with an ALC of <500 while on drug (1, 2). DMF-induced lymphopenia is even more common in clinical practice. In a small clinical cohort, half of patients treated for >12 months developed lymphocyte counts below the lower limit of normal; CD8+ T cells were preferentially lost (3). In another study, older patients (over 55 years of age), patients with lower baseline ALC, and patients with recent natalizumab (NTZ) exposure appeared to be at higher risk of lymphopenia (4).
The mechanism by which DMF acts to reduce relapses in MS is multifactorial and may include lymphocyte depletion. Indeed, MS is viewed as a lymphocyte-mediated disease (5). Depleting autoreactive cells and inhibiting their access to the CNS are known mechanisms for other MS disease modifying therapies (DMTs).
Nevertheless, lymphopenia is also a risk factor for infection. While opportunistic infections are uncommon, progressive multifocal leukoencephalopathy (PML) has been identified among patients taking DMF and related fumarate compounds (6-9). Affected patients were lymphopenic and had been treated with fumarates for years. Fatal West Nile encephalitis, disseminated varicella zoster, and Kaposi sarcoma have also been associated with fumarates (10-12).
A better understanding of DMF-induced lymphopenia is necessary to understand the mechanism of this immune-modulating drug and to predict and manage complications that may arise with long term use. To this end, an extensive immunophenotypic analysis of blood leukocytes in lymphopenic and non-lymphopenic DMF-treated patients compared to untreated MS patients and healthy individuals was performed.
Methods
Subject selection
This was a cross-sectional, observational study. We enrolled 41 MS patients who had been stable on DMF (Tecfidera, Biogen, Weston, MA; 240mg p.o. bid) for ≥6 months, including 17 patients with grade 2-3 lymphopenia (ALC of <800 cells/μl) according to the common terminology criteria for adverse events (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). Lymphopenic patients were specifically recruited. We also recruited 17 MS patients who were not taking DMT and 23 healthy volunteers to serve as controls. Patients who had received steroids within the last 3 months were excluded. Blood samples were drawn between 8:30 AM and 1:30 PM. EDSS scores were retrospectively determined for MS patients based on clinical documentation at the time of enrollment. Serial specimens were obtained from 13 subjects. The time between blood draws was 4-6 months (median 6 months). Two patients made their initial donation after taking DMF for only 3-4 months; data from those initial samples were included only in the longitudinal portion of the analysis. This study was approved by the Washington University Human Research Protection Office and all subjects provided written informed consent.
Leukocyte phenotyping
Fresh whole blood was labeled with CD25, CD45RA, CD45RO, CD86 (clone FUN-1), CCR7 (clone 150503; all from BD Biosciences), Foxp3 (clone 259D), CXCR3 (clone G025H7), BDCA-2 (clone 201), BDCA-4 (clone 12C2), Lin-1, CD1c (clone L161; all from Biolegend), CCR6 (clone R6H1), CD56 (clone CMSSB), C14 (clone 61D3), CD3 (clone SK7), CD62L (clone DREG-56; all from eBioscience), HLA-DR, CD4 and CD8 (Beckman Coulter). Fluorescent eBeads (eBioscience) were added to allow calculation of absolute cell numbers and flow cytometry was performed using a Beckman Coulter Gallios instrument with 10 fluorescent channels.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (La Jolla, CA) and SPSS version 23.0 (IBM Corp, Armonk, NY). Because most of the data were not normally distributed, comparisons between groups were performed using Kruskal-Wallis ANOVA with Dunn's multiple comparison test for differences between groups. We also performed multiple linear regression analyses to control for age between the groups. Results were considered significant at p<0.05.
Results
Flow cytometric analysis of peripheral immune cells was performed in 41 DMF treated MS patients, 17 untreated MS controls and 23 healthy controls (HC) (Supplementary Table 1). DMF treated MS patients included 17 patients with grade 2-3 lymphopenia based on routine laboratory testing (DMF-L; mean ALC 594 cells/μl, range 300-800) and 24 patients with normal lymphocyte counts (DMF-N; mean ALC 1493 cells/μl, range 900-3300). Groups had similar baseline demographics including age (mean 46, range 25-69), sex, and EDSS (median 2.75, range 0-6.5) (Supplementary Table 1). There were no significant differences between DMF groups in terms of prior treatments (most patients had transitioned to DMF from interferons or glatiramer) or number of prior disease modifying therapies. Lymphopenic patients had a slightly longer exposure to DMF compared to non-lymphopenic patients and had carried a diagnosis of MS for longer than untreated MS controls (Supplementary Table 1). The duration of MS did not differ between lymphopenic and non-lymphopenic DMF treated patients.
Absolute cell counts for all T-lymphocyte populations, including CD4+, CD8+ and T regulatory cells (T-regs; CD4+ CD25+ Foxp3+), were significantly reduced in lymphopenic DMF-treated patients compared to HC and MS controls (Figure 1A-C, Table 1). Non-significant trends for lower T-lymphocyte counts in non-lymphopenic DMF-treated patients compared to the control groups were observed. Despite reduced absolute numbers of circulating T-regs among lymphopenic patients, the proportion of T-regs did not change between treatment groups (Table 2, Figure 1B, H). Relative reduction in CD8+ T-cells was particularly marked in lymphopenic patients, evidenced by an increased CD4/CD8 ratio (Figure 1I, Table 1).
Figure 1. Frequencies of circulating leukocytes in DMF treated MS patients compared to controls.
Absolute numbers of immune subsets were compared among healthy controls (n=23), untreated MS controls (n=17), DMF-treated patients without lymphopenia (DMF-N; n=24), and lymphopenic DMF-treated patients (DMF-L; n=17) using flow cytometry (A-G). CD4/CD8 ratio (I) was calculated based on % of total CD3+ gated cells. Error bars represent mean and standard deviation. Kruskal Wallis ANOVA with Dunn's multiple comparison test was used to compare between groups. T-reg: T regulatory cell; NK: natural killer cell; DC: dendritic cell * p<0.05, ** p<0.01, *** p<0.001; **** p<0.0001
Table 1.
DMF effects on absolute numbers of circulating leukocytes.
| Cells/μl | ||||
|---|---|---|---|---|
| Markers Examined by Flow Cytometry (No. of subjects) | Healthy Controls (n=23) | MS Controls (n=17) | DMF-N (n=24) | DMF-L (n=17) |
| T-lymphocytes | ||||
| CD3+CD4+ | 474 (216-822) | 482 (105-959) | 346 (150-709) | 140 (35-302)4, d |
| CD4+ CD25+ Foxp3+ (T-reg) | 34 (5-77) | 37 (14-85) | 23 (7-73) | 12 (4-42)3, d |
| CD3+CD8+ | 145 (60-320) | 140 (66-295) | 105 (27-301) | 20 (5-129)4, d |
| CD4/CD8 (mean, SD) | 3.5 (2.3) | 3.9 (2.6) | 4.6 (2.8) | 6.6 (3.2) 2, a |
| B-lymphocytes | ||||
| CD19+ | 122 (46-247) | 131 (31-257) | 129 (54-311) | 79 (31-193)1, a |
| Monocytes | ||||
| CD14+ | 176 (65-330) | 167 (59-313) | 122 (29-352) | 145 (56-242) |
| NK cells | ||||
| CD3− CD56lo | 111 (34-272) | 88 (28-396) | 51 (19-136)2 | 46 (14-98)4, a |
| CD3− CD56hi | 8 (2-18) | 6 (1-13) | 9 (3-20) | 8 (2-19) |
| Dendritic cells | ||||
| Lin1− HLADR+ CD1c− BDCA4+ BDCA2+ (Plasmacytoid) | 2 (1-4) | 2 (0-4) | 2 (0-5) | 1 (0-2)2, a |
| Lin1− HLADR+ CD1c+ BDCA4− BDCA2− (Myeloid) | 4 (0-8) | 4 (1-14) | 3 (0-9) | 3 (0-8) |
Table 2.
DMF effects on circulating T-cell subpopulations.
| Percent | ||||
|---|---|---|---|---|
| Markers Examined by Flow Cytometry (No. of subjects) | Healthy Controls (n=23) | MS Controls (n=17) | DMF-N (n=24) | DMF-L (n=17) |
| CD4+ Subpopulations | ||||
| CD4+ CD25+ Foxp3+ (T-reg) | 7 (4-11) | 8 (3-12) | 7 (4-12) | 8 (5-17) |
| CD4+ CD45RA+ CCR7+ (naïve) | 42 (27-65) | 35 (9-70) | 57 (27-87)a | 70 (30-82)2, d |
| CD4+ CD45RA− CCR7+ (TCM) | 40 (21-58) | 38 (25-63) | 24 (11-62)1, a | 16 (2-48)4, d |
| CD4+ CD45RA− CCR7− (TEM) | 7 (3-13) | 7 (3-17) | 4 (1-18)a | 3 (1-10)3, c |
| CD3+CD4+CXCR3+ | 40 (21-50) | 39 (20-75) | 25 (14-58)2, a | 23 (13-49)3, b |
| CD3+CD4+CCR6+ | 13 (2-20) | 11 (1-32) | 7 (2-46) | 5 (3-22)a |
| CD4+ CD62L+ | 86 (80-94) | 88 (79-94) | 95 (76-99)3, b | 97 (85-98)4, c |
| CD8+ Subpopulations | ||||
| CD8+ CD45RA+CCR7+ (naïve) | 39 (18-70) | 36 (5-63) | 58 (17-88)1, b | 46 (20-88) |
| CD8+ CD45RA− CCR7+ (TCM) | 10 (2-23) | 12 (2-41) | 4 (1-27)2, b | 2 (1-17)4, d |
| CD8+ CD45RA− CCR7− (TEM) | 26 (8-49) | 22 (9-35) | 10 (4-47)4, b | 12 (1-46)1 |
| CD3+CD8+CXCR3+ | 87 (73-95) | 90 (63-96) | 86 (55-98) | 88 (84-96) |
| CD3+CD8+CCR6+ | 11 (4-32) | 8 (2-23) | 10 (1-48) | 10 (3-27) |
| CD8+ CD62L+ | 61 (37-86) | 68 (31-81) | 80 (39-97) | 76 (41-97) |
Among other immune cell subsets, lymphopenic DMF-treated patients had significantly fewer CD56dim natural killer cells (NK cells; CD3− CD56dim) compared to HC and MS controls. Numbers of CD56dim NK cells were also reduced in non-lymphopenic DMF treated patients compared to HC (Figure 1E). CD19+ B cells and plasmacytoid dendritic cells (pDCs; Lin1− HLADR+ CD1c− BDCA4+ BDCA2+) were reduced compared to controls in lymphopenic DMF-treated patients (Figure 1D, G). There were no differences in the absolute numbers of CD14+ monocytes, CD56hi NK cells, or myeloid DCs (Lin1− HLADR+ CD1c+ BDCA4− BDCA2−) compared to controls (Figure 1F, H; Table 1).
Given the variable treatment intervals, we subdivided DMF treated patients into those treated for 6-12 months (n=11 for DMF-N and n=5 for DMF-L) and those treated for >12 months (n=16 for DMF-N and n=12 for DMF-L; three patients contributed samples to both the 6-12 month subgroup and the >12 month subgroup). No significant differences between these groups were observed for any lymphocyte subset (data not shown).
Within CD4+ and CD8+ lymphocyte subsets, we assessed the proportion of naïve, central memory (TCM) and effector memory (TEM) populations. DMF treatment was associated with relative expansion of the CD4+ and CD8+ naïve (CD45RA+ CCR7+) T cell subset and loss of TCM(CD45RA− CCR7+) and TEM(CD45RA− CCR7−) subsets for both lymphopenic and non-lymphopenic patients (Table 2, Figure 2A, B). We confirmed these findings using an alternate method of identifying TCM(CD45RO+ CD62L+) and TEM(CD45RO+ CD62L−). Using this definition, we again observed that the fractions of circulating TCM and TEM were significantly decreased for both groups of DMF-treated patients when compared to healthy and MS controls with concurrent relative expansion of naïve T cell populations (Supplementary Figure 1). Analyses of serial blood samples from a subset of our cohort demonstrated that these lymphocyte populations were generally stable over time (Figure 2 C-D). A trend for recovery of the effector memory CD4+ T-cell population with time was observed in some subjects; all other subsets were unchanged during ongoing DMF exposure.
Figure 2. DMF effects on naïve and memory T cell distribution.
Proportions of CD4+ and CD8+ naïve (CD45RA+ CCR7+), central memory (CD45RA− CCR7+) and effector memory (CD45RA− CCR7−) T cells were identified using flow cytometry. Distributions for each subset are shown in A-B. A subset of DMF-treated patients (n=13) provided longitudinal samples, and the naïve and memory T-cell distributions are shown over time (C-D). Solid lines represent the group mean for MS control group and dotted lines represent the group mean for the healthy control group. Stars represent samples obtained from lymphopenic patients (C-D). Error bars represent mean and standard deviation. Kruskal Wallis ANOVA with Dunn's multiple comparison test was used to compare between groups. * p<0.05, ** p<0.01, *** p<0.001; **** p<0.0001.
L-selectin (CD62L) is downregulated on T cells as they differentiate and become activated. Moreover, low numbers of circulating CD62L+ CD4+ T cells may be a biomarker for PML risk among NTZ treated patients (13). Thus, circulating CD62L+ CD4+ lymphocytes were compared between groups. Both lymphopenic and non-lymphopenic DMF-treated patients had expansion of the CD62L+ CD4+ lymphocyte population compared to untreated MS controls and HC (Table 2).
Our cohort included a wide age range, and age has previously been linked to DMF-induced lymphopenia (4). Therefore, we performed multiple linear regression models to account for age. After controlling for age, DMF treatment remained a significant predictor for numbers of CD4+ and CD8+ T-cells, T-reg, CD56dim NK (p≤0.001 for all), and pDC (p=0.004) as well as for percentages of CD4+ and CD8+ naïve, TCM and TEM (p<0.001 for all). However, DMF was not a significant predictor for CD19+ cell number after controlling for age (p=0.123). Interestingly, age was an independent predictor of CD8+ (including naïve and TEM subsets) and plasmacytoid DC cells but did not contribute to other circulating lymphocyte subpopulations.
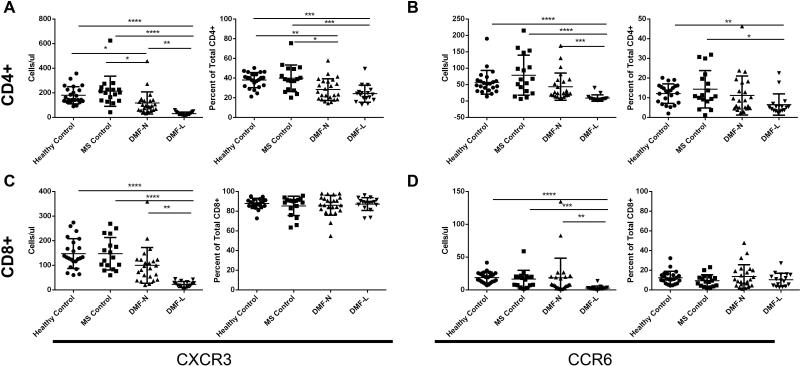
CXCR3 has been considered a surrogate marker for CD4+ T-helper (Th) 1-type pro-inflammatory lymphocytes, while CCR6 has been considered a surrogate marker for IL-17 producing Th17-type lymphocytes. Both subsets are thought to be pathogenic in MS (14-16). We examined expression of these chemokine receptors on CD4+ and CD8+ T cells. Among lymphopenic DMF-treated patients, a smaller fraction of circulating CD4+ T cells expressed CXCR3 and CCR6 when compared to controls (Table 2; Figure 3A-B). Despite reductions in CD8+ numbers with DMF treatment, there were no changes in the proportions of CXCR3 or CCR6-expressing CD8+ T cells (Figure 3C-D).
Figure 3. DMF effects on circulating CXCR3+ and CCR6+ on T cells.
Expression of the Th1 chemokine receptor CXCR3 and the Th17 chemokine receptor CCR6 was determined on CD4+ (A, B) and CD8+ (C, D) lymphocytes. Error bars represent mean and standard deviation. Kruskal Wallis ANOVA with Dunn's multiple comparison test was used to compare between groups. * p<0.05, ** p<0.01, *** p<0.001; **** p<0.0001.
Discussion
We performed extensive immune phenotyping of peripheral immune cells in DMF-treated patients with and without lymphopenia as well as in HC and untreated MS controls and demonstrated reductions of multiple circulating immune cell subsets, particularly CD4+ and CD8+ T lymphocytes, among lymphopenic DMF-treated patients. Moreover, we demonstrated that DMF shifts the immunophenotype of circulating T cells, causing selective reductions of circulating central and effector memory CD4+ and CD8+ lymphocytes regardless of absolute lymphocyte counts. These data may help elucidate the mechanism of action for DMF as well as provide additional information about the immunocompetence of patients taking DMF.
Memory T lymphocytes are responsible for mounting a rapid, robust immune response to a previously encountered antigen. In the healthy immune system, antigen-specific TEM rapidly migrate to areas of infection or inflammation and have immediate effector function while TCM home to secondary lymphoid tissue, proliferate in response to antigenic stimulation and differentiate into TEM. Presumably, the majority of autoreactive T cells in MS are memory cells (17). Indeed, this is the premise behind recent trials of stem cell transplant in MS; by eradicating autoreactive memory populations and “rebooting” the immune system using autologous stem cells, patients have achieved long lasting remission and in some cases, reversal of disability (18, 19). Depleting memory T cells may therefore be a key mechanism for DMF efficacy analogous to an established mechanism of action for other MS therapies. Fingolimod depletes circulating naïve T cells and TCM (20) and the number of circulating TCM is a biomarker of response to fingolimod (21). Similarly, we have now shown that DMF reduces circulating TCM in MS patients. Unlike fingolimod, DMF expands the proportion of naïve T cells in the circulation and reduces TEM.
Reduction in circulating CD62L+ CD4+ lymphocytes has been proposed as a possible biomarker of PML risk for NTZ treated patients. Schwab and colleagues showed that this T cell subset was markedly diminished in NTZ-treated patients who subsequently developed PML when compared to controls and to NTZ-treated patients who did not develop PML (13). Given recent concerns about possible PML risk with DMF therapy, we assessed this T cell population among our cohort and found that CD62L+ CD4+ lymphocytes were relatively expanded among patients taking DMF. The role of L-selectin (CD62L) in MS is controversial (22); it is an adhesion molecule that is downregulated as T cells mature and become activated (23). The expansion of CD62L+ populations in DMF-treated individuals thus parallels the expansion of naïve T cells. Whether loss of circulating CD62L+ CD4+ T cells is a biomarker for PML risk in DMF-treated patients deserves investigation. Indeed, the mechanism of DMF-induced T cell redistribution requires further study. In vitro data suggest that fumarates induce T cell apoptosis as well as impair lymphocyte proliferation and DC differentiation (24, 25). Future studies should investigate lymphocyte differentiation and maturation in patients taking DMF to further explore the phenomenon of drug-induced T lymphocyte redistribution.
We observed loss of several other immune subsets in DMF-treated patients when compared to controls. NK cells are subdivided into the CD56dim population, which has enhanced killing activity, and the CD56hi population, which secretes large amounts of cytokines but is not cytotoxic (26). Recent work has illustrated that CD56hi NK-cells are immunoregulatory; these are expanded by daclizumab, a monoclonal antibody currently undergoing trials for MS. CD56hi NK cell expansion is thought to contribute to the efficacy of daclizumab in MS (27). Patients taking DMF maintained this immunomodulatory CD56hi population. In contrast, CD56dim NK-cells were decreased in both lymphopenic and non-lymphopenic patients. Little is known about the role of CD56dim NK cells in MS, although one study suggested that this population expands in progressive MS (28).
We also observed a modest decrease in pDCs among lymphopenic DMF-treated patients compared to HC. This versatile cell type performs many immune functions including production of type 1 interferons; pDCs may be either immunogenic or tolerogenic, depending on the specific microenvironment (29). pDCs have been identified in MS lesions, but their role in the disease remains controversial (30). Further study is needed to confirm these findings and to explore the possible functional consequences.
In addition to altering T-lymphocyte subpopulations and depleting multiple types of circulating immune cells, DMF selectively reduced the proportion of circulating CXCR3+ CD4+ and CCR6+ CD4+ cells, but not T-regs, among lymphopenic patients. The CXCR3+ CD4+ population was also reduced in non-lymphopenic DMF-treated patients. These chemokine receptors are sometimes considered surrogate markers for Th1 and Th17 type pro-inflammatory lymphocytes, respectively; both T cell subsets have been implicated in MS pathogenesis (14-16). Selective depletion of Th1 and Th17 cells would be expected to be protective in MS and could contribute to the therapeutic effect of DMF. Nevertheless, despite the loss of CXCR3+ and CCR6+ cells among lymphopenic patients taking DMF, these patients have not shown a superior clinical response to the drug to date (4, 31)
Spencer and colleagues recently addressed DMF-induced immunophenotypic changes in a small cohort of patients that was followed longitudinally (3). Like us, they found a disproportionate reduction in CD8+ T cells relative to CD4+ T cells. They also reported time-dependent reductions in CD8+, CD4+ and CD19+ lymphocytes, with no alteration in the levels of CD56+ NK cells, monocytes and other WBC types. They did not examine T cell subsets (i.e. regulatory or memory T cell populations). Our cross-sectional observational study covered a larger group of subjects and was designed to include DMF-treated patients with and without clinical lymphopenia as well as untreated MS controls and HC. We confirmed reductions in CD8+ T cells and CD4+ T cells as well as a slight reduction in CD19+ B cell numbers when compared to controls; after controlling for age, however, DMF treatment did not affect CD19+ numbers. Although Spencer et al. demonstrated a reduction in CD19+ cells over time, the absolute cell numbers typically remained within normal limits. Thus, the apparent discrepancy between our results is likely attributable to the longitudinal versus cross-sectional study design. Our phenotyping of T-regs, naïve, central memory and effector memory T cell subsets, CD56hi vs CD56dim NK cells and dendritic cells greatly extends the findings previously reported.
Few opportunistic infections have been reported with DMF. Despite this, the observed reductions of several WBC types suggest that clinicians should maintain vigilance. Concerns remain about the long term safety of reducing T lymphocytes in general and memory T cells in particular. CD4+ T cell depletion is associated with a variety of opportunistic infections (32, 33). CD8+ T cells are major effectors of viral immunity and contribute to immune surveillance of the CNS (34). It is possible that viral infections may become more prevalent in the setting of DMF-associated CD8+ T cell reduction; recent reports of West Nile encephalitis and disseminated varicella zoster in DMF treated patients substantiate this (10, 11). Redistributing naïve and memory T lymphocyte populations relative to one another is also likely to have functional consequences (35) .
Our data are limited by the cross sectional, observational design of this study. Although serial data from a subset of our study group on DMF for 3 months or more demonstrated stability of lymphocyte subsets during DMF therapy, pre-treatment immunophenotyping was not available. Examination of the recovery of immune cell phenotypes after discontinuation of DMF over time would have been desirable as well, but these data were not interpretable as patients who stopped DMF due to lymphopenia typically began other DMTs immediately thereafter. Moreover, we assessed circulating lymphocytes. It is possible that DMF alters immune cell compartmentalization and that lymphocytes are merely being redirected to another location rather than being permanently lost. Additional work will need to identify the mechanisms of DMF-induced lymphopenia and selective memory T cell loss as well as any functional consequences.
In summary, we have shown that DMF induces redistribution of CD4+ and CD8+ naïve and memory T cells with relative loss of circulating TCM and TEM cells in patients exposed to the drug, regardless of their absolute lymphocyte count. Moreover, it causes loss of CD4+ and CD8+ T-lymphocytes from the bloodstream, with particular reductions of Th1 and Th17-like cells, in a subset of MS patients.
Supplementary Material
Acknowledgements
The authors would like to thank Drs Gregory Wu, David Clifford, Marina Cella, Becky Jo Parks, Robert Naismith, Robyn Klein, & Haina Shin for assistance recruiting patients, discussion of the data, and critique of the manuscript. Dr. Longbrake is funded by a Sylvia Lawry Fellowship from the National MS Society and NIH training grant UL1 TR000448. Dr. Cross was supported by The Manny & Rosalyn Rosenthal – Dr. John L. Trotter MS Center Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation. Dr. Piccio was supported by the Harry Weaver Neuroscience Scholar award of the National MS Society (JF 2144A2/1).
This study received no corporate funding. It was supported in part by NIH training grant UL1 TR000448.
References
- 1.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 2.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 3.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BA, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e76. doi: 10.1212/NXI.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longbrake EE, Naismith RT, Parks BJ, Wu GF, Cross AH. Dimethyl fumarate-associated lymphopenia: Risk factors and clinical significance. Multiple Sclerosis Journal – Experimental, Translational and Clinical. 2015;1 doi: 10.1177/2055217315596994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122(4):1180–8. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenkranz T, Novas M, Terborg C. PML in a Patient with Lymphocytopenia Treated with Dimethyl Fumarate. N Engl J Med. 2015;372(15):1476–8. doi: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- 7.van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013;368(17):1658–9. doi: 10.1056/NEJMc1215357. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwkamp DJ, Murk JL, Cremers CH, Killestein J, Viveen MC, Van Hecke W, et al. PML in a Patient without Severe Lymphocytopenia Receiving Dimethyl Fumarate. N Engl J Med. 2015;372(15):1474–6. doi: 10.1056/NEJMc1413724. [DOI] [PubMed] [Google Scholar]
- 9.Ermis U, Weis J, Schulz JB. Case reports of PML in patients treated for psoriasis. N Engl J Med. 2013;369(11):1081. doi: 10.1056/NEJMc1307680. [DOI] [PubMed] [Google Scholar]
- 10.van Kester MS, Bouwes Bavinck JN, Quint KD. PML in Patients Treated with Dimethyl Fumarate. N Engl J Med. 2015;373(6):583–4. doi: 10.1056/NEJMc1506151. [DOI] [PubMed] [Google Scholar]
- 11.Berkovich R, Weiner LP. Effects of dimethyl fumarate on lymphocyte subsets. Mult Scler Relat Disord. 2015;4(4):339–41. doi: 10.1016/j.msard.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Philipp S, Kokolakis G, Hund M, Witte E, Witte K, Kunz S, et al. Immunological changes in psoriasis patients under long-term treatment with fumaric acid esters: risk of Kaposi sarcoma occurrence? Eur J Dermatol. 2013;23(3):339–43. doi: 10.1684/ejd.2013.2014. [DOI] [PubMed] [Google Scholar]
- 13.Schwab N, Schneider-Hohendorf T, Posevitz V, Breuer J, Göbel K, Windhagen S, et al. L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81(10):865–71. doi: 10.1212/WNL.0b013e3182a351fb. [DOI] [PubMed] [Google Scholar]
- 14.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Callahan MK, Huang D, Ransohoff RM. Chemokine receptor CXCR3: an unexpected enigma. Curr Top Dev Biol. 2005;68:149–81. doi: 10.1016/S0070-2153(05)68006-4. [DOI] [PubMed] [Google Scholar]
- 16.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9(6):393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giunti D, Borsellino G, Benelli R, Marchese M, Capello E, Valle MT, et al. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J Leukoc Biol. 2003;73(5):584–90. doi: 10.1189/jlb.1202598. [DOI] [PubMed] [Google Scholar]
- 18.Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2015;313(3):275–84. doi: 10.1001/jama.2014.17986. [DOI] [PubMed] [Google Scholar]
- 19.Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Griffith LM, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsingremitting multiple sclerosis (HALT-MS): a 3-year interim report. JAMA Neurol. 2015;72(2):159–69. doi: 10.1001/jamaneurol.2014.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71(16):1261–7. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- 21.Song ZY, Yamasaki R, Kawano Y, Sato S, Masaki K, Yoshimura S, et al. Peripheral blood T cell dynamics predict relapse in multiple sclerosis patients on fingolimod. PLoS One. 2014;10(4):e0124923. doi: 10.1371/journal.pone.0124923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li O, Liu JQ, Zhang H, Zheng P, Liu Y, Bai XF. CD62L is required for the priming of encephalitogenic T cells but does not play a major role in the effector phase of experimental autoimmune encephalomyelitis. Scand J Immunol. 2006;64(2):117–24. doi: 10.1111/j.1365-3083.2006.01783.x. [DOI] [PubMed] [Google Scholar]
- 23.Lefrançois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 24.Treumer F, Zhu K, Gläser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol. 2003;121(6):1383–8. doi: 10.1111/j.1523-1747.2003.12605.x. [DOI] [PubMed] [Google Scholar]
- 25.Peng H, Guerau-de-Arellano M, Mehta VB, Yang Y, Huss DJ, Papenfuss TL, et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J Biol Chem. 2012;287(33):28017–26. doi: 10.1074/jbc.M112.383380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 27.Pfender N, Martin R. Daclizumab (anti-CD25) in multiple sclerosis. Exp Neurol. 2014;262(Pt A):44–51. doi: 10.1016/j.expneurol.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Plantone D, Marti A, Frisullo G, Iorio R, Damato V, Nociti V, et al. Circulating CD56dim NK cells expressing perforin are increased in progressive multiple sclerosis. J Neuroimmunol. 2013;265(1-2):124–7. doi: 10.1016/j.jneuroim.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Karrich JJ, Jachimowski LC, Uittenbogaart CH, Blom B. The plasmacytoid dendritic cell as the Swiss army knife of the immune system: molecular regulation of its multifaceted functions. J Immunol. 2014;193(12):5772–8. doi: 10.4049/jimmunol.1401541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lande R, Gafa V, Serafini B, Giacomini E, Visconti A, Remoli ME, et al. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol. 2008;67(5):388–401. doi: 10.1097/NEN.0b013e31816fc975. [DOI] [PubMed] [Google Scholar]
- 31.Fox R, Chan A, Gold R, Phillips J, Selmaj K, Zhang R, et al. Characterization of absolute lymphocyte count profiles in MS patients treated with delayed-release dimethyl fumarate: Considerations for patient management. 2015 [Google Scholar]
- 32.McBath A, Stafford R, Antony SJ. Idiopathic CD4 lymphopenia associated with neuroinvasive West Nile disease: case report and review of the literature. J Infect Public Health. 2014;7(2):170–3. doi: 10.1016/j.jiph.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Delgado-Alvarado M, Sedano MJ, González-Quintanilla V, de Lucas EM, Polo JM, Berciano J. Progressive multifocal leukoencephalopathy and idiopathic CD4 lymphocytopenia. J Neurol Sci. 2013;327(1-2):75–9. doi: 10.1016/j.jns.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Young KG, Maclean S, Dudani R, Krishnan L, Sad S. CD8+ T cells primed in the periphery provide time-bound immune-surveillance to the central nervous system. J Immunol. 2011;187(3):1192–200. doi: 10.4049/jimmunol.1100695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254(1):54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.