Abstract
Objective
Calculate mortality risk that accounts for both severity and recovery of postoperative kidney dysfunction using the pattern of longitudinal change in creatinine.
Summary Background Data
Although the importance of renal recovery after acute kidney injury (AKI) is increasingly recognized, the complex association that accounts for longitudinal creatinine changes and mortality is not fully described.
Methods
We used routinely collected clinical information for 46,299 adult patients undergoing major surgery to develop a multivariable probabilistic model optimized for non-linearity of serum creatinine time series that calculates the risk function for ninety-day mortality. We performed a 70/30 cross validation analysis to assess the accuracy of the model.
Results
All creatinine time series exhibited nonlinear risk function in relation to ninety-day mortality and their addition to other clinical factors improved the model discrimination. For any given severity of AKI, patients with complete renal recovery, as manifested by the return of the discharge creatinine to the baseline value, experienced a significant decrease in the odds of dying within ninety days of admission compared to patients with partial recovery. Yet, for any severity of AKI even complete renal recovery did not entirely mitigate the increased odds of dying as patients with mild AKI and complete renal recovery still had significantly increased odds for dying compared to patients without AKI (odds ratio 1,48 (95% confidence interval 1.30-1.68).
Conclusions
We demonstrate the nonlinear relationship between both severity and recovery of renal dysfunction and ninety-day mortality after major surgery. We have developed an easily applicable computer algorithm that calculates this complex relationship.
Keywords: acute kidney injury, serum creatinine, ninety-day mortality, epidemiology and outcomes, time series, machine learning, renal recovery, postoperative complications
Introduction
Acute kidney injury (AKI) is one of the most common and serious postoperative complications. 1-4 The consensus classification for AKI, RIFLE (Risk, Injury, Failure, Loss, and End-stage Kidney), introduced a simple approach that uses the magnitude of change in routinely measured serum creatinine (sCr) to define three severity stages. The presence of AKI was based on at least a 50% change in sCr relative to the reference value 5 but the recent KDIGO (Kidney Disease: Improving Global Outcomes ) consensus has expanded the criteria to include serum creatinine changes as small as 0.3 mg/dl. 6 Although RIFLE introduced the concept of renal recovery, estimated using the ratio of discharge and reference sCr, most of the initial epidemiological studies using the RIFLE criteria have focused on the effect of AKI severity rather than on renal recovery and rarely the combination of the two.7
The importance of renal recovery after AKI, and recognition that the risk associated with AKI may vary not only with severity but also with subsequent recovery of kidney function, has been shown in several recent studies.8-12 Most of these studies focused on either discharge residual renal function or staging of renal recovery based on the continuing need for renal replacement therapy and a decrease in sCr below an arbitrary cut-off value as defining elements. By considering sCr as a physiological signal, and using it in automated pattern analysis with machine learning, a time series model may better capture the complex association between sCr changes over time and various adverse outcomes. Recently Saria et al. have described a machine learning method that produces a probability score for illness severity using physiological signals including blood pressure, heart rate and oxygen saturation.13 Using a similar computational approach we have developed a probabilistic model that captures the nonlinear relationship between sCr change over time and postoperative mortality. In a large single-center cohort of 46,299 patients undergoing major surgery we have trained and validated this probabilistic model for postoperative ninety-day mortality using the pattern in longitudinal change of sCr in addition to preoperative risk factors.
Material and Methods
Study Subjects
Using the University of Florida (UF) Health Integrated Data Repository we have integrated perioperative clinical, administrative and laboratory databases containing information related to routine clinical care for all patients with age greater or equal to 18 years and admitted to the hospital for longer than 24 hours following any type of inpatient operative procedure between January 1, 2000 and November 30, 2010. We excluded patients with no sCr measurements (n=6636), patients with history of end-stage renal disease prior to admission (n=1904) identified by the previously validated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic and procedure codes,14 and patients who had surgery more than 14 days after the admission date (n=1406). Patients with extreme changes in sCr value (n=94) were excluded based on two criteria: a) patients with three consequent sCr measurements where the third value deviated from the first by no more than by 20% and the second value is either five times greater or smaller than the first one; b) patients with either a ten-fold increase or decrease in two consecutive sCr measurements. For patients with multiple surgeries we chose the first procedure. The final cohort consisted of 46,299 patients. We obtained institutional review board approval through the UF Health Science Center Institutional Review Board and UF Privacy Office (#5-2009).
Covariates and outcomes
Patient survival status was determined using hospital discharges, the Social Security Death Index and Florida Bureau of Statistics. We defined ninety-day mortality as death due to any cause within ninety days of the date of admission. The primary exposure variable was a time series of longitudinal sCr measurements taken during index hospitalization. For each patient we determined additional preoperative risk factors based on clinical availability and univariate analysis: emergent surgery status, type of surgical procedure, age, race, gender, intensive care unit (ICU) admission, Charlson-Deyo comorbidity index (CCI, a measure of the severity of comorbid illness ranging from 0 to 16, with higher scores indicating more comorbidities),15 renal replacement therapy (RRT), time of surgery (days elapsed between admission and the surgical operation), and operating surgeons’ unique identifier.
Serum creatinine Time Series
For all calculations we defined baseline sCr either as the minimum of the sCr values available within six months of admission including the admission day value. The approach using the minimum and mean of the sCr values available within seven days prior to admission including the admission day sCr used for sensitivity analyses did not yield any difference in results.16 Using baseline serum creatinine value and sex, race, and age17 for each patient we calculated baseline estimated glomerular filtration rate (eGFR) by applying the Chronic Kidney Disease Epidemiology Collaboration equation.18 We considered three models based on different representations of sCr time series. The Model1 included the absolute values for the maximum sCr and the last measured sCr during index hospitalization. The last measured creatinine for > 95% of the patients was on the day of discharge while 5% of the patients had values within two days of discharge. The Model2 included differences between maximum sCr, last measured sCr and baseline sCr values during index hospitalization. The Model3 included ratios of maximum sCr and last measured sCr values relative to baseline sCr value during index hospitalization. In addition, we considered a model that included only preoperative data (no sCr time series) referenced as Model0.
We identified a subcohort of 7,766 patients with either history of chronic kidney disease (CKD) prior to admission (determined using previously validated ICD-9-CM diagnostic codes),14 or with the baseline estimated glomerular filtration rate (eGFR) of less than 60 ml/min/1.73 m2. Since these patients may have different serum creatinine kinetics we performed a separate analysis using models with serum creatinine time series for this cohort.18 The remaining cohort of 38,533 patients included patients whose baseline eGFR was greater or equal to 60 ml/min/1.73 m2.
We applied KDIGO consensus definitions for AKI severity and recovery categories using sCr changes only without urine output criteria. KDIGO defines AKI as either 0.3 mg/dl increase within 48 hours or 50% increase above baseline sCr.6 For all calculations we defined baseline sCr as described for sCr time series. Patients were stratified according to the maximum KIDGO stage reached during the hospital admission by comparing the highest sCr during hospitalization with the baseline sCr. Stage 1 corresponds to a 50% change in sCr, Stage 2 to a doubling in sCr and Stage 3 to a tripling in sCr or increase in sCr to ≥ 4.0 mg/dl or Initiation of RRT. Need for RRT was determined using daily hospital charges in the billing database. Complete renal recovery was considered if the sCr returned to a level less than 50% above baseline sCr, whereas partial renal recovery existed if there was a persistent increase in sCr more than 50% above baseline sCr but no need for RRT. No renal recovery implied there was a need for RRT at the time of hospital discharge.5, 6 For the subgroup of patients without previous history of CKD but with baseline eGFR < 60 ml/min/1.73 m2 that could have represented wither early AKI or undiagnosed CKD, we defined complete recovery as return of discharge creatinine values to the level that corresponds to GFR ≥ 60 ml/min/1.73 m2 .
Statistical analysis
The analytical plan followed the STROBE recommendations for observational cohort studies19 and was performed using SAS software (v.9.3, Cary, N.C.), R software and MATLAB (v 7.12, Natick, Massachusetts, The MathWorks Inc., 2003) by DK, PM, PT, TOB, and AB (Supplemental Digital Content (SDC), Methods). We have developed R code for the final algorithm that is available from authors upon request. The algorithm can utilize clinical data obtained in routine clinical care using variables described in our cohort and can be trained in local environment.
Using a 70/30 cross validation procedure the data was randomly split into 70% used for training and 30% used for validation. Models were learned using the training set only. The reported results were obtained by applying the model to the validation dataset only. Multivariable modeling of the association between the sCr pattern and 90-day mortality was performed using generalized additive models (GAM) with logistic link function and a backward-stepwise selection method based on Akaike information criterion (AIC) (SDC, Methods).20, 21 All variables were tested for univariate association prior to inclusion in the model but the final feature selection was performed using a backward-selection process applied to all variables. The performance of all models was optimized using separate approaches for continuous, nominal and categorical variables. All models were adjusted for non-linearity of all covariates using nonlinear risk functions fi estimated with cubic splines.21 The degrees of freedom for each spline were estimated by maximizing the restricted likelihood function.22 Degrees of freedom characterize a curvature of a spline, with values greater than 1 corresponding to a nonlinear function where higher values indicates functions with more deviation from linearity. Categorical variables were modeled with conditional probabilities for a patient to have a particular variable value conditioning on the outcome. The type of surgical procedure (a nominal variable with more than 2000 discrete values) was modeled based on ICD-9-CM codes for primary procedure with a forest structure, where each node represents a group of procedures, with roots representing most general groups of procedures and leaf nodes representing specific procedures (SDC, Methods). In addition, we performed multivariable analysis using same approach described above for all variables but serum creatinine time series. Instead we used KDIGO AKI stage groups stratified by renal recovery to determine odds ratios (OR) and 95% confidence intervals (CI) for ninety-days mortality while considering patients without AKI as the reference group.
The accuracy of the models was assessed separately for training and validation data sets using area under the receiver operating characteristic curves (AUC ROC) with 95% prediction intervals (PI). For each of the models we performed 100 iterations of 70/30 cross validation procedure. For each iteration the data set was randomly split into 70% training and 30% validation sets. Mean and 95% prediction intervals were calculated using AUCs obtained from 100 iterations. Model fit was tested using Hosmer-Lemeshow goodness-of-fit test. We used Vuong’s closeness test for non-nested models as the statistical measure of the “closeness” betweeen models using the pscl package in R software.23, 24 Large negative or positive test statistics yielding small p-values indicate statistical significance of the difference between models.
Sensitivity analyses
We performed multiple sensitivity analyses. Since patients with baseline eGFR < 60 ml/min/1.73 m2 or CKD prior to admission may have different serum creatinine kinetics we performed a separate analyses using models with serum creatinine time series for this cohort.18 Furthermore as RRT can artificially change serum creatinine all analyses were also performed before and after exclusion of patients requiring RRT. The change in serum creatinine towards the end of index hospitalization was important modifier of the effect of the maximum creatinine in time series analyses. Approximately 99.6% of the cohort had hospital discharge before day 90 when primary outcome was assessed and ~ 50% of all deaths occurred after hospital discharge. Since the last measured creatinine for > 95% of the patients was on the day of discharge, to exclude the fixed effect of discharge creatinine on 90-day mortality for patients who died in hospital we considered two approaches for sensitivity analyses. In order to account for entire cohort we performed a sensitivity analysis using the creatinine value prior to discharge day as the “last measured creatinine” for the model calculations. In the second approach we performed a sensitivity analyses by running models after exclusion of patients who died in hospital prior to day 90 when outcome was assessed.
Results
Optimal Model Selection and Model Performance
Among 46,299 patients, 83% (38,533/46,299) had baseline eGFR ≥ 60 ml/min/1.73 m2 while 17% (7,766/46,299) had either history of CKD prior to admission or baseline eGFR < 60 ml/min/1.73 m2. The overall ninety-day mortality was 4% in cohort with eGFR ≥ 60 ml/min/1.73 m2 and 11% in cohort with CKD or eGFR<60 ml/min/1.73 m2. Majority of patients stayed in the hospital less than seven days. About 43% of the all cohort had ICU admission, with 41% and 52% in eGFR≥60 ml/min/1.73 m2 and eGFR<60 ml/min/1.73 m2 cohorts, respectively. Using KDIGO consensus criteria the prevalence of AKI was 33% in patients with eGFR≥60 ml/min/1.73 m2 and 69% among those with CKD or baseline eGFR<60 ml/min/1.73 m2. Non-survivors were more likely to be older, male and to undergo emergent surgery but did not differ in baseline renal function compared to survivors in cohort with eGFR≥60 (Table 1). After excluding 270 and 735 patients requiring RRT in each cohort, the characteristics of the cohort were not significantly changed.
Table 1.
Baseline patients’ characteristics stratified by ninety-day mortality.
| Baseline eGFR<60 ml/min/1.73 m2
(n=7,766) |
Baseline eGFR≥60 ml/min/1.73 m2
(n=38,533) |
|||
|---|---|---|---|---|
|
|
||||
| Non-Survivors (n=848) |
Survivors (n=6,918) |
Non-Survivors (n= 1,597) |
Survivors (n= 36,936) |
|
| Patients’ Characteristics | ||||
| Age in years, median (25th, 75th) | 73 (63, 80) | 67 (55, 75) a | 64 (52, 74) | 54 (41, 65) a |
| Female gender, n (%) | 360 (42) | 3135 (45) | 700 (44) | 18,509 (50) a |
| Race, n (%) | ||||
| Caucasian | 699 (82) | 5617 (81) | 1,319 (83) | 29,860 (81) |
| African-American | 88 (10) | 876 (13) | 168 (11) | 4,339 (12) |
| Hispanic | 14 (2) | 154 (2) | 32 (2) | 1,195 (3) b |
| Other | 14 (2) | 136 (2) | 30 (2) | 810 (2) |
| Missing | 33 (4) | 135 (2) a | 48 (3) | 732 (2) b |
| Charlson comorbidity index, median (25th, 75th) |
3 (1, 4) | 2 (1, 4) a | 2 (1, 4) | 1 (0, 2) a |
| Operative Characteristics | ||||
| Emergent surgery, n (%) | 603 (71) | 3346 (48) a | 1,086 (68) | 15,211 (41) a |
| Operating surgeon (448 physician IDs), n (%) |
603 (71) | 3346 (48) a | 1,086 (68) | 15,211 (41) a |
| First rank | 70 (8) | 403 (6) | 110 (7) | 1,656 (4) |
| Second rank | 49 (6) | 277 (4) | 63 (4) | 1,292 (4) |
| Third rank | 41 (5) | 264 (4) | 57 (4) | 1,267 (3) |
| Surgery type, n (%) | ||||
| Cardiothoracic surgery | 237 (28) | 1518 (22) a | 234 (15) | 4,152 (11) a |
| Non-Cardiac General and Vascular Surgery | 201 (24) | 1537 (22) | 308 (19) | 8,097 (22) c |
| Neurologic Surgery | 107 (13) | 568 (8) a | 534 (33) | 6811 (18) a |
| Specialty Surgeries | 164 (19) | 2,063 (30) a | 259 (16) | 11,725 (32) a |
| Other Surgeries | 139 (16) | 1232 (18) | 262 (16) | 6,151 (17) |
| Time of surgery in days, median (25th, 75th) |
1 (0, 4) | 1 (0, 2) a | 1 (0, 3) | 0 (0, 1) a |
| Number of ICU admissions, median (25th, 75th) |
1 (1, 2) | 0 (0, 1) a | 1 (0, 1) | 0 (0, 1) a |
| ICU Admissions, n (%) | ||||
| 0 | 148 (17) | 3,553 (51) a | 407 (25) | 22,209 (60) a |
| 1 | 459 (54) | 2,614 (38) a | 825 (52) | 12,398 (34) a |
| 2 | 163 (19) | 528 (8) a | 247 (15) | 1,791 (5) a |
| >=3 | 78 (9) | 223 (3) a | 118 (7) | 538 (1) a |
| Hospital length of stay, median (25th, 75th) |
13 (7, 25) | 8 (5, 15) a | 11 (6, 21) | 6 (4, 10) a |
| Renal function | ||||
| Baseline serum creatinine, median (25th, 75th) |
1.5 (1.2, 2.0) | 1.4 (1.2, 1.8)a | 0.8 (0.6, 0.9) | 0.8 (0.6, 0.9) |
| Baseline estimated glomerular filtration rate, ml/min/1.73 m2 , median (25th, 75th) |
43 (29, 53) | 48 (36, 56) a | 91 (78, 105) | 98 (84, 111) a |
| Acute Kidney Injury (KDIGO), n (%) |
||||
| No AKI | 118 (14) | 2,274 (33) a | 497 (31) | 25,420 (69) a |
| Stage 1 | 261 (31) | 2,837 (41) a | 458 (29) | 8,406 (23) a |
| Stage 2 | 119 (14) | 677 (10) a | 275 (17) | 2,259 (6) a |
| Stage 3 | 350 (41) | 1,130 (16) a | 367 (23) | 851 (2) a |
| Renal replacement therapy, n (%) | 224 (26) | 511 (7) a | 153 (10) | 117 (0.3) a |
Abbreviations. ICU, intensive care unit; sCr, serum creatinine, AKI, acute kidney injury; eGFR-Estimated glomerular filtration rate using
All percentage values refer to column percentage.
P < 0.001 for comparison between survivors and non-survivors.
P < 0.01 for comparison between survivors and non-survivors.
P < 0.05 for comparison between survivors and non-survivors.
Using backward-stepwise selection for each model we selected sets of significant variables and estimated the degrees of freedom to determine their nonlinear risk function for ninety-day mortality (SDC, Methods). All longitudinal sCr time series and most of the other continuous risk factors exhibited nonlinear risk functions (SDC, Table S1) in relation to mortality. Overall all models had better performance among patients with baseline eGFR ≥ 60 ml/min/1.73 m2 (Table 2). The addition of longitudinal sCr time series provided an improvement to the model discriminatory ability (AUC 0.865 vs. AUC 0.837, p<0.05 for patients with eGFR≥ 60 ml/min/1.73 m2 and 0.809 vs 0.768, p<0.05 for patients with CKD or baseline eGFR<60) and model fit when compared using Vuong’s test for non-nested models (p<0.0001) for ninety-day mortality (Table 2). All three models with sCr time series had very similar discrimination and model fit for ninety-day mortality, likely reflecting the fact that sCr time series in all three models contained similar information. For each patient we calculated mortality probabilities with 95% prediction intervals, using each of the sCr models separately, to determine the number of patients for whom these intervals do not overlap. For the entire cohort this number was less than 0.5%, indicating that all three models produce similar probabilities for ninety-day mortality.
Table 2.
Model accuracy for training and validation cohorts for generalized additive models for ninety-day mortality.
| Area under receiver operating curve (95% Prediction Intervals) | ||||
|---|---|---|---|---|
| Preoperative Model without serum creatinine time series (Model 0a) |
Preoperative Model with serum creatinine time series (Model 3b) |
|||
| Training cohort | Validation cohort | Training cohort | Validation cohort | |
| Baseline eGFR>=60 ml/min/1.73 m2 | ||||
| Excluding RRT patients (N=38,263) | 0.856 (0.850, 0.862) | 0.837 (0.823, 0.852) | 0.881 (0.875, 0.886) | 0.865 (0.852, 0.879) c |
| Using sCr prior to discharge day as the last measured creatinine |
0.856 (0.850, 0.862) | 0.837 (0.823, 0.852) | 0.877 (0.871, 0.883) | 0.861 (0.847, 0.876) c |
| Excluding hospital deaths (N=37,484) | 0.883 (0.875, 0.891) | 0.845 (0.824, 0.867) | 0.885 (0.877, 0.894) | 0.847 (0.824, 0.870) |
| Including RRT patients (N=38,533) | 0.866 (0.860, 0.872) | 0.851 (0.837, 0.866) | 0.888 (0.883, 0.893) | 0.876 (0.864, 0.888)c |
| Excluding hospital deaths (N=37,594) | 0.883 (0.875, 0.892) | 0.845 (0.821, 0.869) | 0.886 (0.877, 0.894) | 0.847 (0.823, 0.871) |
| Baseline eGFR<60 ml/min/1.73 m2 or chronic kidney disease prior to admission |
||||
| Excluding RRT patients (N=7,031) | 0.813 (0.802, 0.824) | 0.768 (0.740, 0.795) | 0.847 (0.836, 0.859) | 0.809 (0.782, 0.836) c |
| Using sCr prior to discharge day as the last measured creatinine |
0.813 (0.802, 0.824) | 0.768 (0.740, 0.795) | 0.840 (0.828, 0.852) | 0.804 (0.778, 0.831) c |
| Excluding hospital deaths (N=6,645) | 0.817 (0.797, 0.838) | 0.744 (0.694, 0.794) | 0.819 (0.802, 0.837) | 0.747 (0.703, 0.790) |
| Including RRT patients (N=7,766) | 0.828 (0.818, 0.839) | 0.795 (0.773, 0.818) | 0.854 (0.842, 0.866) | 0.823 (0.796, 0.850) c |
| Excluding hospital deaths (N=7,168) | 0.805 (0.791, 0.820) | 0.737 (0.698, 0.776) | 0.810 (0.790, 0.829) | 0.737 (0.688, 0.785) |
Abbreviations. eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy; sCR, serum creatinine. All models showed sufficient fit using Hosmer-Lemeshow goodness of fit test.
Model 0 included only preoperative data and no serum creatinine time series data.
Model 3 included ratios of maximum serum creatinine and last measured serum creatinine values relative to baseline serum creatinine during index hospitalization.
p<0.05 compared to area under receiver operating curve of Model 0 fit on validation cohort.
The results of sensitivity analyses are summarized in Table 2. For all analyses the results were not significantly changed when performed in cohorts that included patients requiring renal replacement therapy, regardless of baseline eGFR. No significant difference in model performance was observed after using the creatinine value prior to discharge day as the “last measured creatinine” for the model calculations or after exclusion of the patients who died in hospital.
Pattern of longitudinal creatinine change and ninety-day mortality
The risk factors describing sCr time series represent four separate properties of renal function: baseline renal function (as measured by baseline sCr and baseline eGFR), severity of AKI (defined either using ratio or absolute difference between highest and baseline sCr), degree of renal recovery (defined either using ratio or absolute difference between last measured and baseline sCr) and discharge renal function (as measured by last measured sCr). In order to illustrate the relationship and degree to which severity and renal recovery for any degree in longitudinal change in creatinine are associated with mortality, we used Model3 to compute adjusted odds ratios as functions of the ratio of highest and baseline serum creatinine (maxCr/baseCr) and the ratio of the last measured and baseline serum creatinine (lastCr/baseCr) while adjusting for other preoperative risk factors. Model3 was selected as it was the simplest, while providing accuracy and model fit equal to the other two models (baseline sCr was eliminated during feature selection as a separate variable in this model). An odds ratio of 1 corresponds to a patient with no longitudinal change in serum creatinine during hospitalization.
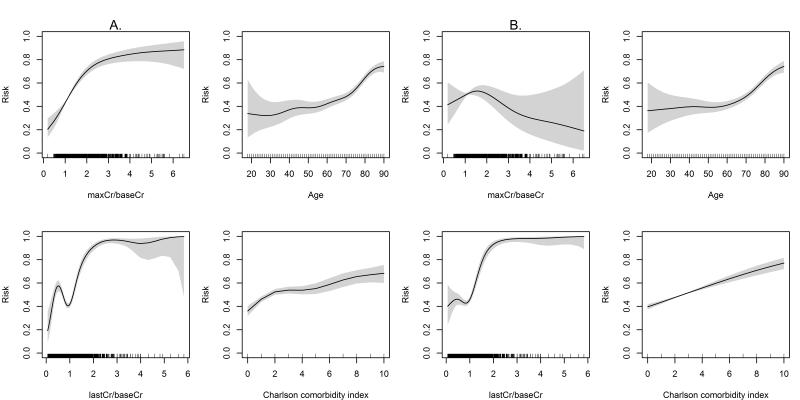
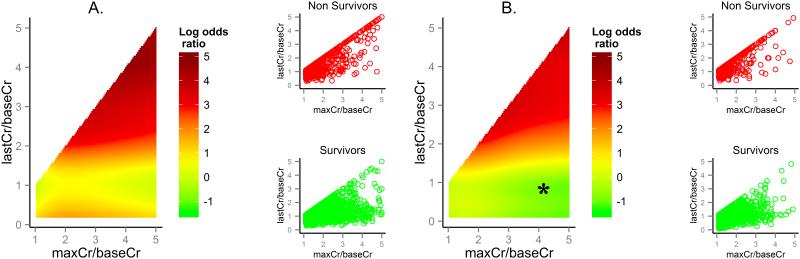
Figures 1 and 2 illustrate the unadjusted and adjusted nonlinear functions fi for the association between ninety-day mortality and serum creatinine changes, age, and Charlson comorbidity index after exclusion of RRT patients, calculated separately for the cohort with baseline eGFR ≥ 60 ml/min/1.73 and the cohort including CKD patients and those with eGFR < 60 ml/min/1.73 m2, respectively. In adjusted analyses, for any given value of serum creatinine change both severity and recovery of renal dysfunction contributed to the associated increased risk of ninety-day mortality in non-linear and non-additive fashion. Furthermore, the increase in last measured serum creatinine compared to baseline creatinine reflecting lack of renal recovery was important modifier of the magnitutide of the adverse effect of the maximum change in creatinine that was not evident in unadjusted analysis. Any degree of incomplete renal recovery as manifested by the persistent increase in the last measured creatinine above the baseline value contributed to increased risk for associated mortality over the whole spectrum of severity of AKI (Figures 1-3).
Figure 1.
Nonlinear functions for the association between maximum creatinine and baseline creatinine ratio (maxCr/baseCr), last measured creatinine and baseline creatinine ratio (lastCr/baseCr), age, and Charlson comorbidity index and ninety-day mortality for the patients with baseline estimated glomerular filtration rate ≥ 60 ml/min/1.73 m2 after excluding patients on renal replacement therapy. Panels (A) and (B) show unadjusted and adjusted nonlinear functions, respectively. The Y axis represents risk probability for ninety-days mortality ranging from 0 to 1. The shaded areas represent 95% prediction intervals for the function values.
Figure 2.
Nonlinear functions for the association between maximum creatinine and baseline creatinine ratio (maxCr/baseCr), last measured creatinine and baseline creatinine ratio (lastCr/baseCr), age, and Charlson comorbidity index and ninety-day mortality for the patients with chronic kidney disease or baseline estimated glomerular filtration rate < 60 ml/min/1.73 m2 after excluding patients on renal replacement therapy. Panels (A) and (B) show unadjusted and adjusted nonlinear functions, respectively. The Y axis represents risk probability for ninety-days mortality ranging from 0 to 1. The shaded areas represent 95% prediction intervals for the function values.
Figure 3.
Association between pattern in serum creatinine change and ninety-day mortality adjusted for preoperative clinical factors among (A) patients with baseline estimated glomerular filtration rate ≥ 60 ml/min/1.73 m2 and (B) patients with chronic kidney disease or baseline estimated glomerular filtration rate < 60 ml/min/1.73 m2. Left panel: The log odds ratios for ninety-day mortality based on adjusted nonlinear functions for maximum creatinine and baseline creatinine ratio (maxCr/baseCr) and last measured creatinine and baseline creatinine ratio (lastCr/baseCr) with respect to a pattern with no change in serum creatinine. Right panel: Distribution of maxCr/baseCr and lastCr/baseCr values for non-survivors and survivors. *There were no patients in the dataset with the combination of the maxCr/baseCr and lastCr/baseCr in the marked range.
To illustrate common clinical scenarios we imposed cut-offs proposed for KDIGO severity stages and renal recovery categories (Table 3). For any given severity of AKI, those patients who had more complete renal recovery, as manifested by the return of the last measured creatinine to the baseline value, experienced a significant decrease in the odds of dying up to ninety days after admission. The most dramatic difference was for patients with the most severe stage 3 AKI. Patients who remained dialysis dependent throughout the hospitalization as well as those with persistent increase in serum creatinine but no initiation of RRT had comparable increase in the odds of dying compared to patients with no AKI (OR 14.73, 95% CI 12.10 - 17.94 for patients on RRT and OR 16.36, 95% CI 13.58 - 19.71 for group with persistent creatinine increase and no RRT initiation). However, for patients with Stage 3 AKI who had complete renal recovery the odds of dying, although still high, were improved (OR 5.73, 95% CI 4.74 – 6.93). However, for any stage of AKI even complete renal recovery did not entirely mitigate the increased odds of dying. Patients with mild AKI (KDIGO stage 1) with complete renal recovery had significantly increased odds for dying compared to patients with no AKI (OR 1.48, 95% CI 1.30-1.68). Age, Charlson comorbidity index, operating surgeon, emergent surgery status, and type of surgical procedure were all associated with increased risk for mortality, in addition to serum creatinine factors, although the magnitude of the association was the highest for sCr time series.
Table 3.
Patterns of renal recovery and 90-day mortality.
| Number of patients, N | Crude Mortality, n (%) | Adjusted Odds Ratios (95% CI) | |
|---|---|---|---|
| No AKI | 28,309 | 615 (2) | 1 (Reference) |
| Complete Recovery | |||
| Stage 1 AKI | 9,441 | 481 (5) | 1.48 (1.30, 1.68) a |
| Stage 2 AKI | 2,613 | 214 (8) | 2.16 (1.81, 2.56) a |
| Stage 3 AKI | 1,193 | 199 (17) | 5.73 (4.74, 6.93) a |
| Partial Recovery | |||
| Stage 1 AKI | 2,521 | 238 (9) | 2.73 (2.31, 3.23) a |
| Stage 2 AKI | 717 | 180 (25) | 8.81 (7.17, 10.82) a |
| Stage 3 AKI | 792 | 283 (36) | 16.36 (13.58, 19.71) a |
| No recovery | 713 | 235 (33) | 14.73 (12.10, 17.94) a |
Adjusted odds ratios for ninety-day mortality were obtained in all cohort of 46,299 patients, including the patients with renal replacement therapy and using general additive models while adjusting for age, gender, race, Charlson comorbidity index, emergent surgery status, type of surgical procedure, operating surgeon, and intensive care unit admission.
P < 0.001 for comparison with respect to no AKI group.
Discussion
The pattern of longitudinal change in serum creatinine during hospitalization in a large single-center cohort of postoperative patients is significantly associated with ninety-day mortality, independently of other clinical factors. We have developed an algorithm that calculates a complex non-linear function based on both the magnitude of longitudinal change in serum creatinine during hospitalization and its return to a baseline value for any given set of serum creatinine time series data adjusted for other clinical factors. The two-dimensional graphical representation of this relationship demonstrates that the increased risk for mortality is a function not only of how severe AKI is when it occurs but also how complete is renal recovery after AKI. This relationship is continuous and non-linear and can be implemented in real time on serial creatinine values through a computer algorithm. Our study expands previous findings of the association between the severity of AKI in surgical patients with postoperative complications, hospital mortality and cost by adding the dimension of renal recovery into consideration.
This study demonstrates for the first time that the risk-adjusted association between longitudinal change in postoperative serum creatinine and ninety-day mortality is continuous over the whole spectrum of measured creatinine values and duration of time series, and is independent of the need for renal replacement therapy after AKI.6 Using empirical cut-offs for renal recovery proposed in the consensus criteria we have demonstrated that complete renal recovery at the time of discharge significantly lessens the risk for mortality even among patients with the most severe AKI while lack of complete recovery maintains the increased risk for patients with the least severe AKI. Recent study in cohort of patients with pneumonia has reported similar findings.
Most of the studies of renal recovery after AKI have focused on the liberation from renal replacement therapy during or after hospitalization. 25 It has long been recognized that the severity of AKI, reflecting the magnitude of decline in glomerular filtration rate, is determinant of adverse outcomes associated with AKI. We confirm the importance of renal recovery, estimated either by the degree of successful renal recovery 8-12 or by the estimated residual renal function at discharge 9-11, 26-28 in minimizing adverse outcomes associated with AKI. Recently Engoren et al. combined RIFLE AKI stage with discharge creatinine to demonstrate a strong association with adverse outcomes for the interaction of these two factors. 29 This study integrates all three dimensions - severity of functional decline, renal recovery and discharge residual renal function while accounting for co-linearity and non-linearity between these measurements, and confirms that patients without complete renal recovery have worse survival for any level of renal functional decline. More importantly, even patients with complete renal recovery at the time of discharge have increased mortality over both the short and long-term. 9, 11, 26, 27, 30, 31 In the cohort of 32,045 critically ill patients, Kellum et al demonstrated that short- and long-term risk of death or RRT is greatest when patients meet both the serum creatinine level and urine output criteria for AKI and when these abnormalities persist.32 Consensus clinical guidelines use a categorical cut-off to define renal recovery, but they also utilize the unfortunate label of “partial renal recovery” for patients with a persistent increase in serum creatinine but no need for renal replacement therapy, when in fact their serum creatinine likely does not reflect true recovery of renal function.5, 6 For elderly patients with longer hospitalizations even normal creatinine values may overestimate actual glomerular filtration rate due to the multifactorial decrease in creatinine generation in acute illness.33
In contemporary clinical practice the awareness for AKI is low and only 20% of patients with AKI carry this diagnosis in their discharge summary.26 Not surprisingly any follow-up of creatinine within the first three months of hospitalization occurs in less than 50% of patients with the most severe AKI, and one can assume even less frequently after less severe AKI.34 Among AKI survivors with persistent renal dysfunction at discharge the referral rates for outpatient nephrology consultation are as low as 11%.34, 35 We provide a simple graphic representation of the association between multiple creatinine measurements and ninety-day mortality that may help identify patients with the highest risk of adverse outcome who may benefit from timely nephrology follow-up and repeat creatinine measurements after discharge. Importantly most of the patients who would have an increased risk by this algorithm would not be picked up by their age, comorbidities or surgery type alone or by isolated measurement of creatinine.
We attempted to control for selection bias with multivariable statistical methods and risk adjustment but the retrospective design precludes any conclusion on causal inference. The major strength of our study was the ability to use mathematically optimized serum creatinine time series rather than relying on previously established cut-offs for severity and recovery of renal function or use of statistical methods that do not account for non-linearity of creatinine measurements. Furthermore our analyses demonstrate that all continuous variables had non-linear associations with an increased risk of ninety-day mortality. This analysis required the use of generalized additive models, as commonly used logistic regression models would be inadequate to demonstrate such associations. The performance of all models was optimized using separate approaches for continuous, nominal and categorical variables. We assessed comorbidities using previously validated criteria. 15 This approach relies on accurate coding leading to potential risk underassessment. Although this is a single institution report, it comprises a large cohort of patients with morbidity and mortality that is comparable to other reports in the literature for the same procedures. The administration of intravenous fluids during surgery may have influenced serum creatinine through dilution, but we do not have data on overall fluid balance to further examine this potential effect. The performance of the model depends on the initial training of the model using the institutional distribution of surgical procedures and operating surgeons. The internal validity of analyses is assured by our use of the methodology recommended by the STROBE guidelines including the use of a validation cohort and sensitivity analyses addressing missing data, selection bias and effect of baseline creatinine .19
The recovery of renal function after an episode of AKI is dependent upon both the structural changes in the kidney that occur after AKI and the repair potential of the kidney. Both adaptive and maladaptive cellular changes persist for weeks after the induction of kidney injury. 36 A beneficial adaptation is acquired cytoresistance that protects the kidney against repeated injury, while a maladaptive adaptation includes tubular up-regulation of toll-like receptor responses with resulting exaggerated cytokine production and increased susceptibility to recurrent infections.37 Persistent up-regulation of pro-inflammatory, pro-fibrotic and vasoconstrictive genes leads to progressive renal injury. Thus the important period between injury induction and the onset of renal repair can ultimately impact renal functional recovery, extra-renal injury and the possible transition of AKI into progressive renal disease. 36, 38
With the recent emergence of validated urinary biomarkers to detect early AKI clinicians now have the prospect of interventions to reduce the effects of AKI. 39, 40 Prevention of new AKI and mitigation of injury from newly detected AKI are logical goals for primary and secondary prevention in surgical patients, and will likely depend on the development and adoption of clinical guidelines and “kidney protective strategies” by intensivists, surgeons and anesthesiologists as the primary caregivers for these patients. 6, 40 Successful renal recovery emerges as a key goal for the management of patients with established AKI that will fundamentally affect their chances for survival and long-term recovery, and will require synergistic action between primary surgical caregivers and nephrologists and must extend beyond the hospital discharge. 41, 42 Available evidence from randomized controlled trials, large observational studies and comparative analysis of their results suggest that the avoidance of intermittent hemodialysis and of a cumulative positive fluid balance are likely to increase the speed of renal recovery and may prevent end-stage renal failure in selected high-risk patients with acute kidney injury. 43-46
Conclusions
Our results expand upon previous findings to demonstrate the non-linear relationship between the severity and recovery of renal dysfunction and ninety-day mortality after major surgery. We have developed an algorithm that calculates this complex relationship. Even complete recovery to baseline renal function in patients with the smallest change is serum creatinine does not mitigate the risk for adverse outcomes. The development of clinical guidelines for standardized primary and secondary prevention of postoperative AKI using clinical risk stratification and urinary biomarkers are urgently needed.
Supplementary Material
Acknowledgments
We want to thank Gigi Lipori, Christine Bono and Yue Du for assistance with data retrieval.
Source of Funding: AB was supported by a Vision Grant from the Society of Critical Care Medicine (SCCM) and Center for Sepsis and Critical Illness Award P50 GM-111152 from the National Institute of General Medical Sciences and has received research grants from Astute Medical, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or SCCM.
Footnotes
Supplemental Digital Content 1. Methods
Supplemental Digital Content 2. Table S1. Degree of freedom for the variables included in all general additive models for ninety-day mortality.
References
- 1.Bihorac A, Brennan M, Ozrazgat-Baslanti T, et al. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med. 2013;41:2570–83. doi: 10.1097/CCM.0b013e31829860fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakar CV. Perioperative Acute Kidney Injury. Advances in Chronic Kidney Disease. 2013;20:67–75. doi: 10.1053/j.ackd.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Bihorac A, Delano MJ, Schold JD, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg. 2010;252:158–65. doi: 10.1097/SLA.0b013e3181deb6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group Clinical Practice Guideline for Acute Kidney Injury. Kidney inter., Suppl. 2012;2:1–138. [Google Scholar]
- 7.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538–46. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 8.Brown JR, Cochran RP, MacKenzie TA, et al. Long-Term Survival After Cardiac Surgery is Predicted by Estimated Glomerular Filtration Rate. Ann Thorac Surg. 2008;86:4–11. doi: 10.1016/j.athoracsur.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Brown JR, Kramer RS, Coca SG, et al. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–8. doi: 10.1016/j.athoracsur.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell NJ, Freemantle N, Bonser RS, et al. Subtle changes in renal function are associated with differences in late survival following adult cardiac surgery. Eur J Cardiothorac Surg. 2012;41:e38–42. doi: 10.1093/ejcts/ezr329. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RH, Honeycutt E, Patel UD, et al. Impact of Recovery of Renal Function on Long-Term Mortality After Coronary Artery Bypass Grafting. The American Journal of Cardiology. 2010;106:1728–1734. doi: 10.1016/j.amjcard.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Piccinni P, Cruz DN, Gramaticopolo S, et al. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT) Minerva Anestesiologica. 2011;77:1072–83. [PubMed] [Google Scholar]
- 13.Saria S, Rajani AK, Gould J, et al. Integration of early physiological responses predicts later illness severity in preterm infants. Sci Transl Med. 2010;2:48ra65. doi: 10.1126/scitranslmed.3001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wald R, Waikar SS, Liangos O, et al. Acute renal failure after endovascular vs open repair of abdominal aortic aneurysm. Journal of Vascular Surgery. 2006;43:460–466. doi: 10.1016/j.jvs.2005.11.053. discussion 466. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Siew ED, Ikizler T, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. J Am Soc Nephrol. 2012;7:8. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–9. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Annals of Internal Medicine. 2007;147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 20.Hastie T, Tibshirani R, Friedman JH. 2nd Springer; New York: 2009. The elements of statistical learning : data mining, inference, and prediction. [Google Scholar]
- 21.Hastie T, Tibshirani R. 1st Chapman and Hall; London; New York: 1990. Generalized additive models. [Google Scholar]
- 22.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society Series B-Statistical Methodology. 2011;73:3–36. [Google Scholar]
- 23.Vuong QH. Likelihood Ratio Tests for Model Selection and Non-Nested Hypotheses. Econometrica. 1989;57:307–333. [Google Scholar]
- 24.Jackman S. Journal. Stanford University, Stanford; California: pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory. [serial online]. 2012. Available from: Department of Political Science. [Google Scholar]
- 25.Schneider AG, Bellomo R, Bagshaw SM, et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2013;39:987–97. doi: 10.1007/s00134-013-2864-5. [DOI] [PubMed] [Google Scholar]
- 26.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Annals of Surgery. 2009;249:851–8. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 27.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 28.Tian J, Barrantes F, Amoateng-Adjepong Y, et al. Rapid reversal of acute kidney injury and hospital outcomes: a retrospective cohort study. Am J Kidney Dis. 2009;53:974–81. doi: 10.1053/j.ajkd.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Engoren M, Habib RH, Arslanian-Engoren C, et al. The effect of acute kidney injury and discharge creatinine level on mortality following cardiac surgery*. Crit Care Med. 2014;42:2069–74. doi: 10.1097/CCM.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 30.Loef BG, Epema AH, Smilde TD, et al. Immediate Postoperative Renal Function Deterioration in Cardiac Surgical Patients Predicts In-Hospital Mortality and Long-Term Survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 31.Liano F, Felipe C, Tenorio MT, et al. Long-term outcome of acute tubular necrosis: a contribution to its natural history. Kidney Int. 2007;71:679–86. doi: 10.1038/sj.ki.5002086. [DOI] [PubMed] [Google Scholar]
- 32.Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by Urine Output versus Serum Creatinine Level. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2014070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heimburger O, Stenvinkel P, Barany P. The enigma of decreased creatinine generation in acute kidney injury. Nephrol Dial Transplant. 2012;27:3973–4. doi: 10.1093/ndt/gfs459. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Renal Data System . USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. [Google Scholar]
- 35.Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23:305–12. doi: 10.1681/ASN.2011030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zager RA. 'Biologic memory' in response to acute kidney injury: cytoresistance, toll-like receptor hyper-responsiveness and the onset of progressive renal disease. Nephrology, Dialysis, Transplantation. 2013;28:1985–93. doi: 10.1093/ndt/gft101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bihorac A, Baslanti TO, Cuenca AG, et al. Acute kidney injury is associated with early cytokine changes after trauma. J Trauma Acute Care Surg. 2013;74:1005–13. doi: 10.1097/TA.0b013e31828586ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bihorac A, Kellum JA. Acute kidney injury in 2014: A step towards understanding mechanisms of renal repair. Nat Rev Nephrol. 2015;11:74–5. doi: 10.1038/nrneph.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bihorac A, Chawla LS, Shaw AD, et al. Validation of Cell-Cycle Arrest Biomarkers for Acute Kidney Injury Using Clinical Adjudication. Am J Respir Crit Care Med. 2014 doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 41.Lai CF, Wu VC, Huang TM, et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care. 2012;16:R123. doi: 10.1186/cc11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glassford NJ, Bellomo R. Acute kidney injury: how can we facilitate recovery? Curr Opin Crit Care. 2011;17:562–8. doi: 10.1097/MCC.0b013e32834cd334. [DOI] [PubMed] [Google Scholar]
- 44.Renal Replacement Therapy Study Investigators. Bellomo R, Cass A, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–38. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 45.VA NIH Acute Renal Failure Trial Network. Palevsky PM, Zhang JH, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wald R, Shariff SZ, Adhikari NK, et al. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study*. Crit Care Med. 2014;42:868–77. doi: 10.1097/CCM.0000000000000042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.