Abstract
An ELISA kit for detection of antibodies to a nonstructural protein of foot-and-mouth disease (FMDV) was further evaluated using sequentially collected serum samples of experimentally infected animals, because the sensitivity of the kit used in a previous study was significantly low in field animals. The kit fully detected antibodies in infected animals without vaccination; however, the first detections of antibodies by the kit were later than those by the liquid-phase blocking ELISA that is used for serological surveillance in the aftermath of outbreaks in Japan, for detection of antibodies to structural proteins of FMDV. Additionally, although the kit effectively detected antibodies in infected cattle with vaccination, there were several infected pigs with vaccination for which the kit did not detect antibodies during the experimental period. Taken together, the kit may not be suitable for serological surveillance after an FMD outbreak either with or without emergency vaccination in FMD-free countries.
Keywords: antibody, ELISA, foot-and-mouth disease virus, nonstructural protein, vaccine
Foot-and-mouth disease (FMD) is the most important infectious disease in the veterinary field, because it is the most contagious in cloven-hoofed animals, such as cattle, pigs, sheep and goats [4]. Currently, Japan is an FMD-free country where vaccination is not routinely practiced; however, Japan has a significantly high risk to be invaded by FMD, because the disease is endemic in neighboring Asian countries like China and South Korea [13].
In general, when FMD occurs in FMD-free countries where vaccination is not practiced, immediately sacrificing the affected animals and all contact animals, and control of livestock movement inside the affected regions are applied as control measures. In addition, emergency vaccination can also be applied. Vaccines for emergency use, which are stored as concentrated vaccine antigens in national or regional vaccine banks in FMD-free countries where vaccination is not practiced, are generally of high potency and purity [7]. Recent commercial FMD vaccines are purified by industrial ultrafiltration and chromatography in order to remove unnecessary cellular protein contaminants and viral nonstructural proteins (NSPs) [7]. Therefore, differentiating infected animals from vaccinated animals, so-called DIVA, can be performed by detecting antibodies to NSPs, because non-infected animals with vaccination theoretically would not have antibodies to the NSPs [6].
A country can adopt both a “vaccinate-to-die” policy and a “vaccinate-to-live” policy after emergency vaccination is performed in an outbreak [20]. All vaccinated animals must be culled in the “vaccinate-to-die” policy. In the “vaccinate-to-live” policy, instead of destroying vaccinated animals, serological surveillance of vaccinated animals by detecting antibodies to NSPs should be performed in order to confirm that the vaccinated animals are not infected with an FMD virus (FMDV) and to substantiate the absence of occult FMDV infections. This is because, irrespective of vaccination status, FMDV-infected ruminants can be long-term carrier animals of FMDV and discharge viruses intermittently. It is important to detect carrier animals for control of FMD, because carrier animals may be subsequent infectious sources to other susceptible animals although infected animals generally shed fewer viruses during a persistent infection stage than in an acute infection stage [4]. In addition, although a country can adopt both policies, the waiting period required to regain World Organization for Animal Health (OIE) status with regard to FMD differs depending on the selection of the policies. The waiting period for the “vaccinate-to-die” policy is shorter than that for the “vaccinate-to-live” policy; the former is 3 months, while the latter is 6 months [20]. Recently, it has been proposed that the waiting periods for the two policies should be equalized [1, 12, 17]. In addition, destroying a large number of animals in a future outbreak would cause serious problems in terms of environmental contamination, animal welfare, food security and conservation of scarce genetic resources. Therefore, the “vaccinate-to-live” policy has many practical reasons to be favored over the “vaccinate-to-die” policy.
Previously, several commercial ELISA kits that detect antibodies to NSPs (NSP-ELISA) were evaluated for their specificities and sensitivities [10], because the “vaccinate-to-live” policy relies fully upon the capability of the NSP kit that is used. Of the previously evaluated NSP-ELISA kits, the PrioCHECK FMDV NS [19] was thought to be the most convenient, because the PrioCHECK kit is a blocking ELISA and can examine serum samples collected from all animal species. In a previous study [10], the specificity of the PrioCHECK kit was as high as that of a liquid-phase blocking ELISA (LPBE), which is generally used as the antibody test of FMDV in Japan. Although the sensitivity of the PrioCHECK kit to serum samples collected from experimentally infected pigs was high, that to serum samples collected from infected animals in the field was significantly low [10]. Namely, the results for the sensitivity of the PrioCHECK kit were opposite for experimentally infected pigs and infected animals in the field [10]. Therefore, further evaluation of the sensitivity of the PrioCHECK kit needed to be performed using additional samples. The first objective of this study was to evaluate the sensitivity of the PrioCHECK kit using serum samples collected sequentially from several experimental infections of infected animals without vaccination.
In addition to this obscurity, validation tests showed that the sensitivities of the NSP-ELISA kits to infected animals with vaccination were much lower than those to infected animals without vaccination [2, 3, 5, 16]. Because the NSP-ELISA kit was originally used for serological surveillance after emergency vaccination in an outbreak to substantiate absence of infection, evaluation of the sensitivity of the PrioCHECK kit to infected animals with vaccination needs to be performed in the context of the possible adoption of the “vaccinate-to-live” policy in Japan. Therefore, the second objective of this study was an evaluation of the sensitivity of the PrioCHECK kit using serum samples collected sequentially from experimental infections of infected animals with vaccination.
MATERIALS AND METHODS
Ethics: The Animal Care and Use Committee of the National Institute of Animal Health (NIAH) approved all animal procedures prior to initiation of this study (authorization numbers: 12-027, 12-056, 12-081, 13-024, 13-054 and 14-009). All experimental infections were performed in cubicles whose sizes have approximately 14 m2 in a high-containment facility at the NIAH.
Experimental infections in non-vaccinated animals: Full details of experimental infections (i), (ii) and (iii) have already been published [11, 14]. Brief details of the experimental infections that provided serum samples for this study are as follows: (i) Two 6-month-old Holstein cattle housed in separate cubicles were inoculated with 1 ml of 106.2 50% tissue culture infectious dose (TCID50) of the FMDV O/JPN/2010-1/14C [9] by an intradermal route. At 1 day post-infection (dpi), two additional 6-month-old Holstein cattle were housed with the infected cattle. They were housed in the same cubicle for approximately 1 month [14]. (ii) Two 4-month-old Japanese Saanen goats were inoculated with 1 ml of 106.2 TCID50 of the O/JPN/2010-1/14C by an intradermal route. At 1 dpi, 2 additional 4-month-old Japanese Saanen goats were housed with the infected goats. They were housed in the same cubicle for approximately 1 month [14]. (iii) Thirteen 2-month-old pigs were inoculated with 1 ml of 103 or 106 TCID50 of the FMDV O/JPN/2010-1/14C by intranasal or intraoral route. They were housed in separate cubicles for approximately 2 weeks [11].
Experimental infections in vaccinated animals: (iv) Seven 3-month-old Holstein cattle were administered intramuscularly an inactivated FMDV vaccine (six 50% protection dose (PD50), serotype O, O Manisa strain, Aftpor, Merial, Lyon, France). At 3 or 30 days post-vaccination (dpv), the vaccinated cattle were inoculated with 1 ml of 106 TCID50 of the FMDV O/JPN/2010-1/14C by an intradermal route. They were observed for approximately 2 weeks to 1 month after the infection. (v) Six 2-month-old pigs were administered intramuscularly the FMDV vaccine (6 PD50). At 3 or 30 dpv, the vaccinated pigs were inoculated with 1 ml of 106 TCID50 of the FMDV O/JPN/2010-1/14C by an intraoral route. They were observed for approximately 1 month after the infection.
Collection of clinical samples from the animals: The clinical samples were collected routinely as shown in Tables 1 , 2 , 3, 4, 5. Sera were collected from cervical veins using a vacuum blood collection tube (Venoject II, Terumo Corporation, Tokyo, Japan). Saliva was collected from oral cavities using a roll-shaped synthetic saliva collector (Salivette, Sarstedt KK, Tokyo, Japan) and forceps. Nasal swabs were collected from nasal cavities using a cotton swab (Men-tip, JCB Industry Limited, Tokyo, Japan).
Table 1. Detection of viral genes from clinical samples by RT-PCR assay and of antibodies by LPBE and the PrioCHECK kit in infected cattle without vaccination.
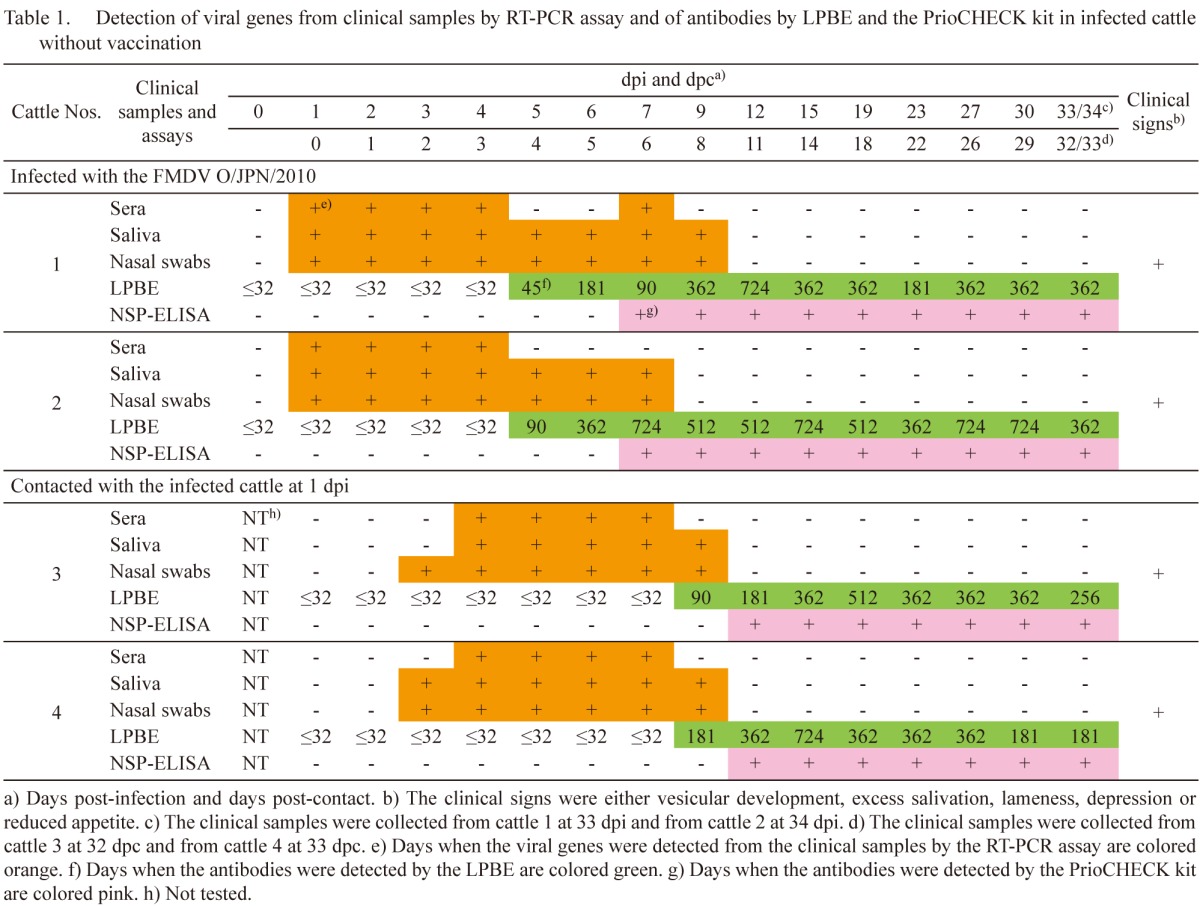
Table 2. Detection of viral genes from clinical samples by RT-PCR assay and of antibodies by LPBE and the PrioCHECK kit in infected goats without vaccination.
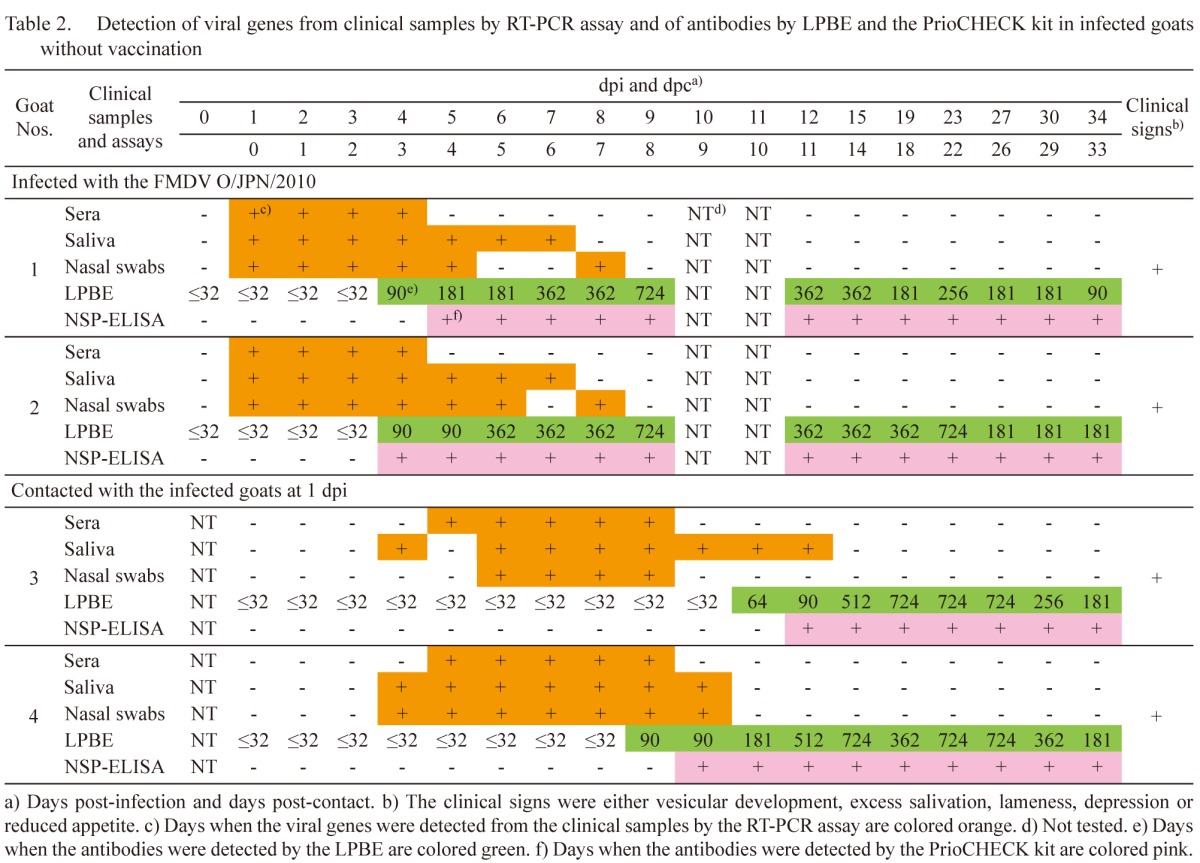
Table 3. Detection of antibodies by LPBE and the PrioCHECK kit in infected pigs by the intranasal and intraoral routes without vaccination.
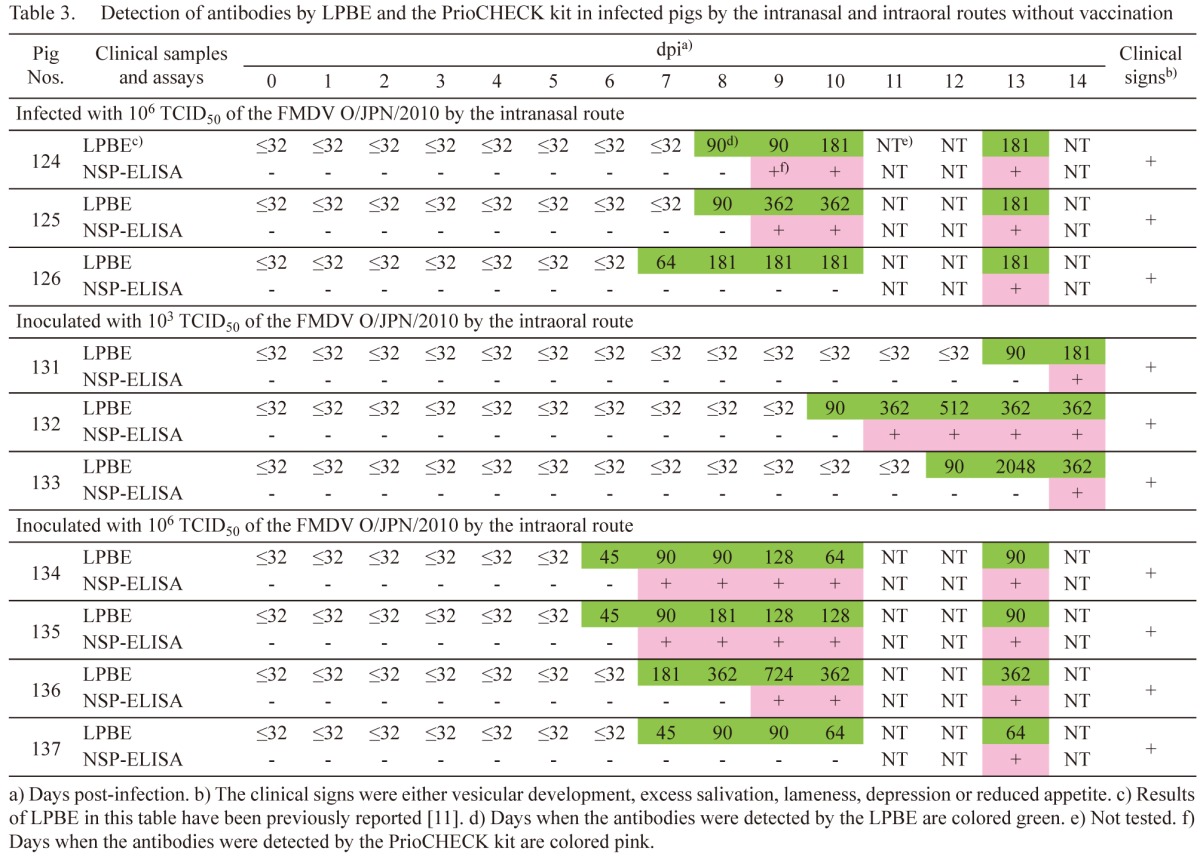
Table 4. Detection of viral genes from clinical samples by RT-PCR assay and of antibodies by LPBE and the PrioCHECK kit in infected cattle with vaccination.
Table 5. Detection of viral genes from clinical samples by RT-PCR assay and of antibodies by LPBE and the PrioCHECK kit in infected pigs with vaccination.
RNA extraction and RT-PCR: Viral RNAs were extracted from the clinical samples using the High Pure Viral RNA kit (Roche Diagnostics, Basel, Switzerland). The FMDV-specific genes were detected by an RT-PCR assay using primers FM8 and FM9, which can amplify a portion of a 3D region of an FMDV genome [18].
LPBE and NSP-ELISA: An LPBE (Institute for Animal Health, Surrey, U.K.) was performed for detection of antibodies to structural proteins (SPs) of FMDV according to manufacturer’s instructions. The FMDV O Manisa strain was used as the antigen of the LPBE. The PrioCHECK FMDV NS (Thermo Fisher Scientific, Waltham, MA, U.S.A.) [19] was performed for detection of antibodies to the non-structural protein 3ABC of FMDV according to the manufacturer’s instructions.
RESULTS
Infected cattle without vaccination: In cattle 1 and 2, which were inoculated with the FMDV, clinical signs, such as vesicular development on the tongues, inside lips, snouts and feet, excess salivation, and lameness, were initially found between 1 and 6 dpi. Viral genes were detected from the clinical samples between 1 and 9 dpi (Table 1). Antibodies were detected from 5 dpi in the LPBE. Antibody titers were between 45 and 724. In the PrioCHECK kit, antibodies were detected from 7 dpi. The days when the antibodies were first detected in the PrioCHECK kit were delayed for 2 days from those in the LPBE.
In cattle 3 and 4, which were housed with the infected cattle, clinical signs, such as vesicular development on the tongues, inside lips, snouts and feet, and excess salivation, were initially found between 4 and 6 days post-contact (dpc). Viral genes were detected from the clinical samples between 2 and 8 dpc (Table 1). Antibodies were detected from 8 dpc in the LPBE. Antibody titers were between 90 and 724. In the PrioCHECK kit, the antibodies were detected from 11 dpc. The days when the antibodies were first detected in the PrioCHECK kit were delayed for 3 days from those in the LPBE.
Infected goats without vaccination: In goats 1 and 2, which were inoculated with the FMDV, clinical signs, such as vesicular development on the feet, were initially found between 3 and 4 dpi. Viral genes were detected from the clinical samples between 1 and 8 dpi (Table 2). Antibodies were detected from 4 dpi in the LPBE. Antibody titers were between 90 and 724. In the PrioCHECK kit, the antibodies were detected from 5 and 4 dpi, respectively. The day when the antibody was first detected in the PrioCHECK kit was 1 day after that by the LPBE in goat 1; in goat 2, antibodies were detected by both tests on the same day.
In goats 3 and 4, which were housed with the infected goats, clinical signs, such as vesicular development on the tongue, inside lip, snout and feet, and excess salivation, were initially found between 6 and 11 dpc. Viral genes were detected from the clinical samples between 3 and 11 dpc (Table 2). Antibodies were detected from 10 and 8 dpc in the LPBE, respectively. Antibody titers were between 64 and 724. In the PrioCHECK kit, the antibodies were detected from 11 and 9 dpc, respectively. The antibodies were first detected 1 day later by the PrioCHECK kit than by the LPBE.
Infected pigs by the intranasal route without vaccination: In pigs 124 to 126, which were inoculated with 106 TCID50 of the FMDV, clinical signs, such as vesicular development on the inside lips, snouts, teat and feet, excess salivation, lameness, depression and reduced appetite, were initially found between 3 and 9 dpi [11]. The days when viral genes were detected in pigs 124 to 126 were as described previously [11]. As previously reported [11], antibodies were detected from 8, 8 and 7 dpi in the LPBE, respectively (Table 3). Antibody titers were between 64 and 362. In the PrioCHECK kit, the antibodies were detected from 9, 9 and 13 dpi, respectively. The days when the antibodies were first detected in the PrioCHECK kit were delayed for 1, 1 and 6 days from those in the LPBE, respectively.
In pigs 121 to 123, viral genes and LPBE antibodies were not detected during the experimental period as described previously [11]. In the PrioCHECK kit, antibodies were not also detected in this study.
Infected pigs by the intraoral route without vaccination: In pigs 131 to 133, which were inoculated with 103 TCID50 of the FMDV, clinical signs, such as vesicular development on the tongue, inside lips, snouts and feet, excess salivation, lameness and depression, were initially found between 7 and 10 dpi [11]. The days when viral genes were detected in pigs 131 to 133 were as described previously [11]. As previously reported [11], antibodies were detected from 13, 10 and 12 dpi in the LPBE, respectively (Table 3). Antibody titers were between 90 and 2,048. In the PrioCHECK kit, the antibodies were detected from 14, 11 and 14 dpi, respectively. The days when the antibodies were first detected in the PrioCHECK kit were delayed for 1, 1 and 2 days from those in the LPBE, respectively.
In pigs 134 to 137, which were inoculated with 106 TCID50 of the FMDV, clinical signs, such as vesicular development on the snout and feet, excess salivation, lameness, depression and reduced appetite, were initially found between 1 and 6 dpi [11]. The days when viral genes were detected in pigs 134 to 137 were as described previously [11]. As previously reported [11], antibodies were detected from 6, 6, 7 and 7 dpi in the LPBE, respectively (Table 3). Antibody titers were between 45 and 724. In the PrioCHECK kit, the antibodies were detected from 7, 7, 9 and 13 dpi, respectively. The days when the antibodies were first detected in the PrioCHECK kit were delayed for 1, 1, 2 and 6 days from those in the LPBE, respectively.
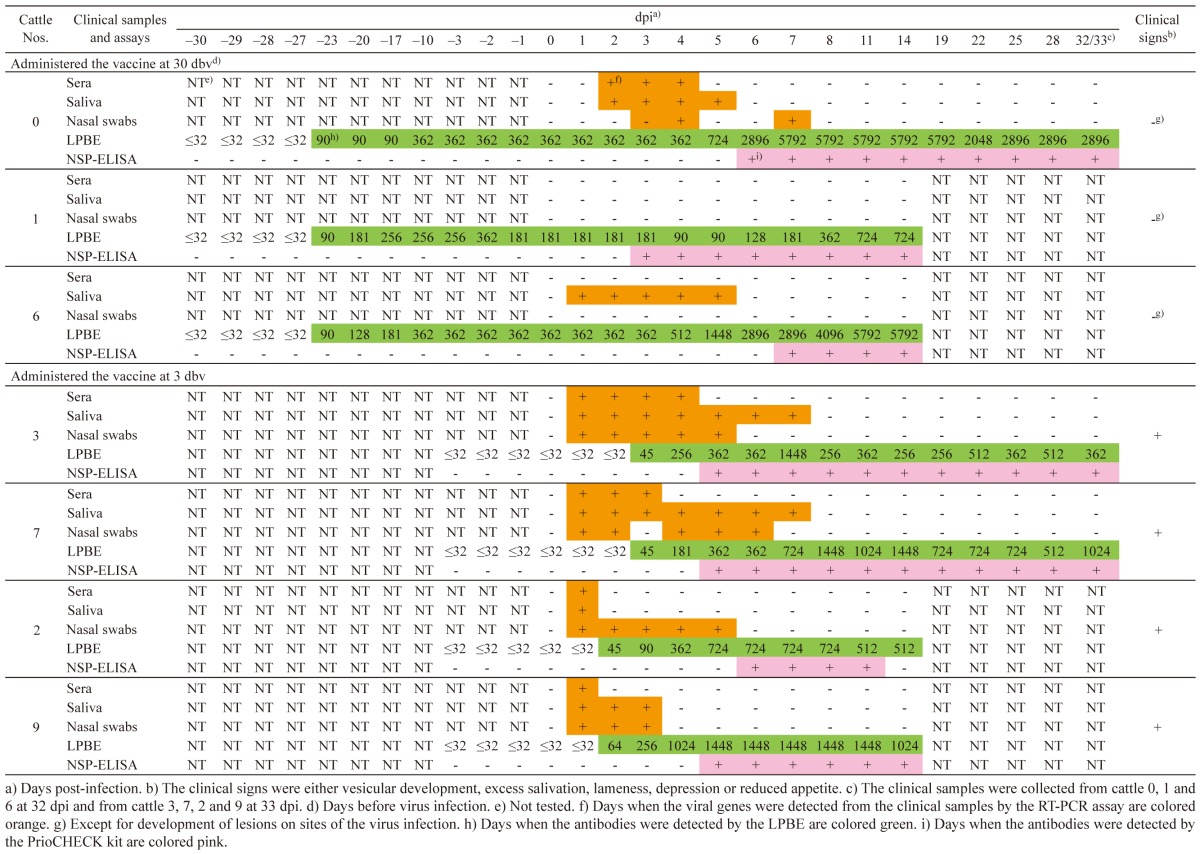
Infected cattle with vaccination: In cattle 0, 1 and 6, which were vaccinated at 30 days before virus infection (dbv), any clinical signs, such as vesicular development, excess salivation, lameness, depression and reduced appetite, were not found, except for development of lesions on sites of the virus infection. Viral genes were detected from the clinical samples between 1 and 7 dpi; however, they were not detected in cattle 1 during the experimental period (). Antibodies were detected from 23 dbv in the LPBE. Antibody titers were between 90 and 5792. Using the PrioCHECK kit, the antibodies were detected from 6, 3 and 7 dpi, respectively.
In cattle 3, 7, 2 and 9, which were vaccinated at 3 dbv, clinical signs, such as vesicular development on the tongues, inside lips, snouts and feet, were initially found between 1 and 6 dpi. Viral genes were detected from the clinical samples between 1 and 7 dpi (Table 4). Antibodies were detected from 3, 3, 2 and 2 dpi in the LPBE, respectively. Antibody titers were between 45 and 1448. In the PrioCHECK kit, the antibodies were detected from 5, 5, 6 and 5 dpi, respectively. However, in cattle 2, no antibody was detected at 14 dpi in the PrioCHECK kit.
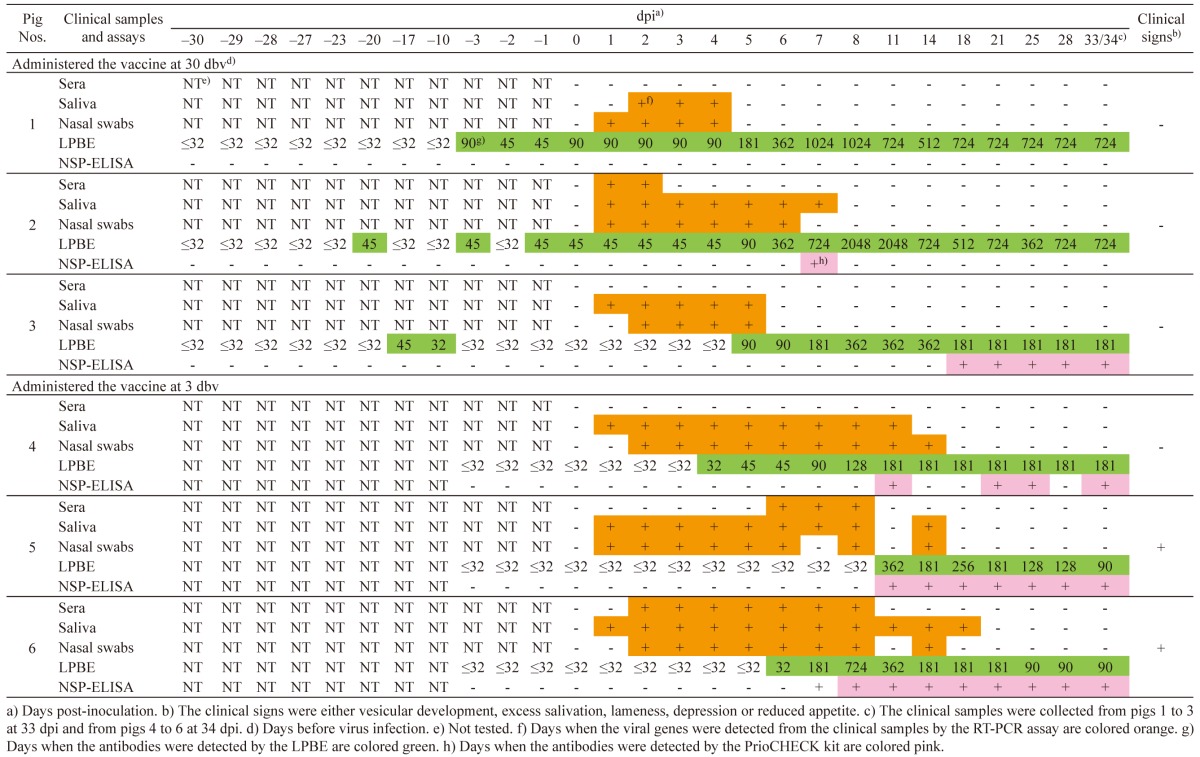
Infected pigs with vaccination: In pigs 1 to 3, which were vaccinated at 30 dbv, any clinical signs, such as vesicular development, excess salivation, lameness, depression and reduced appetite, were not found. Viral genes were detected from the clinical samples between 1 and 7 dpi (Table 5). Antibody responses of the pigs by LPBE varied. Pig 1 was the latest of the pigs to show antibody response. An antibody was first detected at 3 dbv. In pig 2, although an antibody was first detected at 20 dbv, there were several days when antibodies were not detected until 1 dbv. In pig 3, antibodies were detected at 10 and 17 dbv; however, antibodies were not detected from 3 dbv to 4 dpi. In the PrioCHECK kit, antibody responses of the pigs also varied. In pigs 1 and 2, antibodies were not detected during the experimental period in this study, except for 7 dpi in pig 2. An antibody was first detected at 18 dpi in pig 3.
In pigs 5 and 6, which were vaccinated at 3 dbv, clinical signs, such as vesicular development on the tongues, snout and feet, were initially found between 4 and 11 dpi. However, any clinical signs, such as vesicular development, excess salivation, lameness, depression and reduced appetite, were not found in pig 4. Viral genes were detected from the clinical samples between 1 and 18 dpi (Table 5). Antibodies were detected from 4, 11 and 6 dpi in the LPBE, respectively. Antibody titers were between 32 and 724. In the PrioCHECK, antibodies were detected from 11 and 7 dpi in pigs 5 and 6, respectively; however, the antibodies were detected intermittently between 11 and 34 dpi in pig 4.
DISCUSSION
Recommended serological tests for FMD surveillance are described in the OIE manual [21]. The tests are in principle divided into 2 kinds of tests; those detecting antibodies to SPs and to NSPs of FMDV, respectively. There are 7 serotypes in FMDV: A, O, C, SAT1, SAT2, SAT3 and Asia1 [4]. Tests for SPs therefore are dependent on serotypes. An appropriate antigen needs to be used for measuring antibodies to a seeking serotype. In contrast to tests for SPs, tests for NSPs are independent of serotypes. Tests for NSPs therefore may be more convenient in countries where several serotypes of FMDVs are prevalent. According to the Terrestrial Animal Health Code [20], tests for both SPs and NSPs can be used for serological surveillance in areas where animals are not vaccinated. In practice, tests for NSPs have been frequently used for serological surveillance of cattle in South American countries [1, 2]. However, the results in this study showed that the first detection of antibodies by the PrioCHECK kit was delayed compared to that by the LPBE. Therefore, tests for SPs should be used for surveillance, especially in an outbreak in FMD-free countries, because tests for SPs may be able to detect FMDV infections earlier than tests for NSPs.
In our previous study [10], the sensitivities of three NSP-ELISA kits were significantly low in infected animals without vaccination tested in the 2010 epidemic in Japan. In contrast to our results, the sensitivities of several NSP-ELISA kits were reported to be high in infected animals without vaccination [2, 3, 5, 16]. Both of the results were completely inconsistent with each other. In our previous study, the antibody titers of serum samples in the LPBE were the focus of inquiry regarding the inconsistent results, because precise dates when the animals had been infected with the FMDV in the field were unknown. Serum samples that showed relatively high antibody titers were speculated to have been collected from animals a longer time after virus infection. However, many serum samples that showed relatively high antibody titers in the LPBE showed negative results in the NSP-ELISA kits. To explore the reasons for this inconsistency, we focused on the relationships between the results of the RT-PCR assay, LPBE and NSP-ELISA kit in this study.
As mentioned in Tables 1 and 2, and the chapter of the results of infected pigs by the intranasal and intraoral routes without vaccination, basically, the viral genes by the RT-PCR assay, the antibodies by the LPBE and the antibodies by the PrioCHECK kit were sequentially detected in infected animals without vaccination. Detection of the viral genes by the RT-PCR assay therefore meant that the samples were collected early after the FMDV infection. In contrast, relatively high antibody titers did not usually mean that the samples were collected late after the animals were infected with FMDV, because high antibody titers were observed even at early dpi when the viral genes were detected simultaneously by the RT-PCR assay. From these results, early collection of samples after the animals were infected with an FMDV therefore may be the reason for the significantly low sensitivities of the NSP-ELISA kits in the serum samples collected from the animals in the field in the 2010 epidemic in Japan.
Antibody responses were influenced by viral doses for infection in the pigs (Table 3). The antibodies in the LPBE were detected earlier in pigs 134 to 137 inoculated with 106 TCID50 of the isolate than in pigs 131 to 133 inoculated with 103 TCID50 of the isolate. In the antibody titers, the serum sample collected from pig 133 at 13 dpi showed the highest antibody titer, as 2048. The pig and pig 131 were assumed to be infected by horizontal transmission from pig 132 [14]. Therefore, high viral dose infection from pig 132 may stimulate the highest antibody titer in pig 133.
In previous studies [2, 3, 5, 16], sensitivities of several evaluated NSP-ELISA kits were relatively high in infected animals without vaccination. On the other hand, sensitivities of the NSP-ELISA kits were considerably low in infected animals with vaccination in the studies. As a reason for these differential sensitivities, it was speculated that viral replication is limited in infected animals with vaccination and that serological conversion to NSPs occurs more slowly and in a lower proportion than in infected animals without vaccination. However, the precise reason why there are cases in which antibodies to NSPs are not detected in infected animals with vaccination has not yet been confirmed. A report proposed that studies should be encouraged to define pathogenicity including antibody responses to NSPs in infected animals with vaccination [2]. In this study, antibodies were detected fully from serum samples collected from infected cattle with vaccination by the PrioCHECK kit, although the first detection dates were later than those in the LPBE (Table 4). The PrioCHECK kit therefore may be applicable for serological surveillance in cattle after emergency vaccination would be practiced in an FMD outbreak. However, it should be noted that tests for NSPs are generally considered reliable at a herd level [1, 16]. In addition, antibody responses to NSPs in infected cattle with vaccination need to be further evaluated in different experimental conditions, such as viral infected doses and routes.
On the other hand, the study of the infected pigs with vaccination showed the different results. The PrioCHECK kit did not detect antibodies in pig 1 during the experimental period, and in pig 2, it detected antibodies at only 7 dpi, although both the pigs were confirmed to be infected with the FMDV by the results of the detection of the viral genes from the clinical samples (Table 5). Well-vaccinated animals may become subclinically infected, even if they are exposed to a sufficient virus challenge [8, 15]. Similarly, in this study, pigs 1 to 4 did not show any clinical signs, even though viral infection occurred (Table 5). The PrioCHECK kit therefore may not be suitable to apply for serological surveillance in pigs after emergency vaccination would be practiced in an FMD outbreak, because infected pigs with vaccination may not be detected by either serological or clinical surveillance. If the PrioCHECK kit would be applied as an assay for serological surveillance in pigs after emergency vaccination would be practiced in an FMD outbreak, statistical sufficient numbers of pigs should be examined by the kit. In addition, a high sensitive assay, such as an RT-PCR assay, should be applied simultaneously, because other pigs, which are in an acute stage of infection, may exist in the same premise. However, rapid sampling from the large numbers of pigs would be hard to perform during an outbreak. To reveal availability for serological surveillance in pigs, the PrioCHECK kit needs to be further evaluated using infected pigs with vaccination before it is used for surveillance purposes. In addition, evaluation of these two ELISA kits for antibody detection in infected goats with vaccination might be needed, although goats along with sheep are not major domestic animals in Japan.
In conclusion, the following was confirmed in this study: (i) the LPBE can detect antibodies in infected animals without vaccination earlier than the PrioCHECK kit; (ii) the significantly low sensitivities of the NSP-ELISA kits in the previous study may have been due to the early collection dates of samples after animals were infected with FMDV; and (iii) the PrioCHECK kit may not be able to detect antibodies in infected pigs with vaccination. These results will be valuable for authorities in choosing adequate control measures for possible FMD outbreaks in the future.
Acknowledgments
We are grateful to Mr. Yoshihiro Ohtake (Tochigi Prefectural Central District Animal Hygiene Service Center, Tochigi, Japan) and Mr. Masaki Kato (Nagano Prefectural Matsumoto Animal Hygiene Service Center, Nagano, Japan) for technical assistance. The authors would also like to thank Mr. Hiroki Kimura, Mr. Masayuki Kanda, Mr. Shinya Sato, Mr. Kenichi Ishii, Mr. Tatsuo Nakamura and Mr. Shigeo Mizumura for their care of the animals. This study was supported by a research project on improving food and animal health of the Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- 1.Barnett P. V., Geale D. W., Clarke G. W., Davis J., Kasari T. R.2015. A review of OIE country status recovery using vaccinate-to-live versus vaccinate-to-die foot-and-mouth disease response policies I: benefits of higher potency vaccines and associated NSP DIVA test systems in post-outbreak surveillance. Transbound. Emerg. Dis. 62: 367–387. doi: 10.1111/tbed.12166 [DOI] [PubMed] [Google Scholar]
- 2.Bergmann I. E., Malirat V., Neitzert E.2005. Non-capsid proteins to identify foot-and-mouth disease viral circulation in cattle irrespective of vaccination. Biologicals 33: 235–239. doi: 10.1016/j.biologicals.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 3.Brocchi E., Bergmann I. E., Dekker A., Paton D. J., Sammin D. J., Greiner M., Grazioli S., De Simone F., Yadin H., Haas B., Bulut N., Malirat V., Neitzert E., Goris N., Parida S., Sørensen K., De Clercq K.2006. Comparative evaluation of six ELISAs for the detection of antibodies to the non-structural proteins of foot-and-mouth disease virus. Vaccine 24: 6966–6979. doi: 10.1016/j.vaccine.2006.04.050 [DOI] [PubMed] [Google Scholar]
- 4.Brown F.2003. The history of research in foot-and-mouth disease. Virus Res. 91: 3–7. doi: 10.1016/S0168-1702(02)00268-X [DOI] [PubMed] [Google Scholar]
- 5.Chen S. P., Ellis T. M., Lee M. C., Cheng I. C., Yang P. C., Lin Y. L., Jong M. H., Robertson I. D., Edwards J. R.2007. Comparison of sensitivity and specificity in three commercial foot-and-mouth disease virus non-structural protein ELISA kits with swine sera in Taiwan. Vet. Microbiol. 119: 164–172. doi: 10.1016/j.vetmic.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 6.Clavijo A., Wright P., Kitching P.2004. Development in diagnostic techniques for differentiating infection from vaccination in foot-and-mouth disease. Vet. J. 167: 9–22. doi: 10.1016/S1090-0233(03)00087-X [DOI] [PubMed] [Google Scholar]
- 7.Doel T. R.2003. FMD vaccines. Virus Res. 91: 81–99. doi: 10.1016/S0168-1702(02)00261-7 [DOI] [PubMed] [Google Scholar]
- 8.Eblé P. L., Weerdmeester K., van Hemert-Kluitenberg F., Dekker A.2009. Intradermal vaccination of pigs against FMD with 1/10 dose results in comparable vaccine efficacy as intramuscular vaccination with a full dose. Vaccine 27: 1272–1278. doi: 10.1016/j.vaccine.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 9.Fukai K., Morioka K., Yoshida K.2011. An experimental infection in pigs using a foot-and-mouth disease virus isolated from the 2010 epidemic in Japan. J. Vet. Med. Sci. 73: 1207–1210. doi: 10.1292/jvms.11-0063 [DOI] [PubMed] [Google Scholar]
- 10.Fukai K., Morioka K., Onozato H., Yoshida K., Sakamoto K.2013. Comparative evaluation of three commercial ELISA kits for detection of antibodies to a nonstructural protein of foot-and-mouth disease virus. J. Vet. Med. Sci. 75: 693–699. doi: 10.1292/jvms.12-0430 [DOI] [PubMed] [Google Scholar]
- 11.Fukai K., Yamada M., Morioka K., Ohashi S., Yoshida K., Kitano R., Yamazoe R., Kanno T.2015. Dose-dependent responses of pigs infected with foot-and-mouth disease virus O/JPN/2010 by the intranasal and intraoral routes. Arch. Virol. 160: 129–139. doi: 10.1007/s00705-014-2239-4 [DOI] [PubMed] [Google Scholar]
- 12.Geale D. W., Barnett P. V., Clarke G. W., Davis J., Kasari T. R.2015. A review of OIE country status recovery using vaccinate-to-live versus vaccinate-to-die foot-and-mouth disease response policies II: waiting periods after emergency vaccination in FMD free countries. Transbound. Emerg. Dis. 62: 388–406. doi: 10.1111/tbed.12165 [DOI] [PubMed] [Google Scholar]
- 13.OIE/FAO Foot-and-Mouth Disease Reference Laboratory Network. 2013. Annual Report 2013 [cited 2015 March 9]. Available from http://www.wrlfmd.org/ref_labs/ref_lab_reports/OIE-FAO%20FMD%20Ref%20Lab%20Network%20Report%202013.pdf.
- 14.Onozato H., Fukai K., Kitano R., Yamazoe R., Morioka K., Yamada M., Ohashi S., Yoshida K., Kanno T.2014. Experimental infection of cattle and goats with a foot-and-mouth disease virus isolate from the 2010 epidemic in Japan. Arch. Virol. 159: 2901–2908. doi: 10.1007/s00705-014-2135-y [DOI] [PubMed] [Google Scholar]
- 15.Parida S., Fleming L., Oh Y., Mahapatra M., Hamblin P., Gloster J., Doel C., Gubbins S., Paton D. J.2007. Reduction of foot-and-mouth disease (FMD) virus load in nasal excretions, saliva and exhaled air of vaccinated pigs following direct contact challenge. Vaccine 25: 7806–7817. doi: 10.1016/j.vaccine.2007.08.058 [DOI] [PubMed] [Google Scholar]
- 16.Paton D., Sammin D., Dyrting K., Chow M., Fleming L., Verin B., Ferris N., McDonagh G., O’Connor M., Hamblin P., Gibson D., Parida S.2006. Comparative evaluation of serological tests for the detection of foot-and-mouth disease virus infection in vaccinated pigs. 2006 Session of the Research Group of the Standing Technical Committee of EuFMD [cited 2015 March 18]. Available from http://www.fao.org/ag/againfo/commissions/docs/research_group/paphos/App51.pdf.
- 17.Paton D. J., Füssel A. E., Vosloo W., Dekker A., De Clercq K.2014. The use of serosurveys following emergency vaccination, to recover the status of “foot-and-mouth disease free where vaccination is not practiced”. Vaccine 32: 7050–7056. doi: 10.1016/j.vaccine.2014.10.064 [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto K., Kanno T., Yamakawa M., Yoshida K., Yamazoe R., Murakami Y.2002. Isolation of foot-and-mouth disease virus from Japanese black cattle in Miyazaki Prefecture, Japan, 2000. J. Vet. Med. Sci. 64: 91–94. doi: 10.1292/jvms.64.91 [DOI] [PubMed] [Google Scholar]
- 19.Sørensen K. J., de Stricker K., Dyrting K. C., Grazioli S., Haas B.2005. Differentiation of foot-and-mouth disease virus infected animals from vaccinated animals using a blocking ELISA based on baculovirus expressed FMDV 3ABC antigen and a 3ABC monoclonal antibody. Arch. Virol. 150: 805–814. doi: 10.1007/s00705-004-0455-z [DOI] [PubMed] [Google Scholar]
- 20.World Organization for Animal Health. 2014. Chapter 8.7. Foot and mouth disease. Terrestrial Animal Health Code [cited 2015 February 17]. Available from http://www.oie.int/international-standard-setting/terrestrial-code/access-online/.
- 21.World Organization for Animal Health. 2014. Chapter 2.1.5. Foot and mouth disease. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2014 [cited 2015 March 20]. Available from http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.05_FMD.pdf.