Abstract
Diarrhea in cattle is one of the most economically costly disorders, decreasing milk production and weight gain. In the present study, we established a novel simultaneous detection system using TaqMan real-time PCR designed as a system for detection of microbes from bovine diarrhea using real-time PCR (referred to as Dembo-PCR). Dembo-PCR simultaneously detects a total of 19 diarrhea-causing pathogens, including viruses, bacteria and protozoa. Specific primer–probe sets were newly designed for 7 pathogens and were synthesized on the basis of previous reports for 12 pathogens. Assays were optimized to react under the same reaction conditions. The PCR efficiency and correlation coefficient (R2) of standard curves for each assay were more than 80% and 0.9766, respectively. Furthermore, the sensitivity of Dembo-PCR in fecal sample analysis was measured with feces spiked with target pathogens or synthesized DNA that included specific nucleotide target regions. The resulting limits of detection (LOD) for virus-spiked samples, bacteria and DNA fragments were 0.16–1.6 TCID50 (PFU/reaction), 1.3–13 CFU/reaction and 10–100 copies/reaction, respectively. All reactions showed high sensitivity in pathogen detection. A total of 8 fecal samples, collected from 6 diarrheic cattle, 1 diarrheic calf and 1 healthy cow, were tested using Dembo-PCR to validate the assay’s clinical performance. The results revealed that bovine coronavirus had infected all diarrheic adult cattle and that bovine torovirus had infected the diarrheic calf. These results suggest that Dembo-PCR may be a powerful tool for diagnosing infectious agents in cattle diarrhea.
Keywords: cattle, diagnosis, diarrhea, TaqMan real-time PCR
Diarrhea in cattle is a disorder that causes economic loss by decreasing fertility and productivity, including milk production and weight gain [11, 20]. In particular, young calves are strongly affected by diarrhea and may die from malnutrition and dehydration depending on the situations [25]. The United States Department of Agriculture (USDA) reported that 57% of deaths of weaning calves in the US were because of diarrhea (http:// nahms.aphis.usda.gov/dairy/index.htm). Although the cattle industry has taken measures to prevent diarrhea, such as improvements in hygiene and feeding management, it still occurs throughout the world [5].
One central problem is the fact that the factors that contribute to this disorder are complicated, with diarrhea attributed to both infectious and noninfectious factors. Infectious pathogens are particularly well-known causative agents and include viruses, bacteria and protozoa. Common infectious agents that cause diarrhea in cattle are group A rotavirus (RVA), bovine coronavirus (BCoV), bovine vial diarrhea virus (BVDV), Salmonella sp. and Mycobacterium avium subsp. paratuberculosis (MAP) [1, 4]. Recently, bovine enterovirus (BEV), mammalian orthoreovirus (MRV), group B rotavirus (RVB), group C rotavirus (RVC) and bovine torovirus (BToV) have been reported as potential pathogens causing diarrhea [2, 3, 12, 14, 19, 30]. In addition, Cho et al. reported that more than 50% of the diarrheic calves they tested were concurrently infected with more than one pathogen [5]. Besides infectious diarrhea, noninfectious agents also induce diarrhea due to inadequate uptake of colostrum, unhygienic breeding, inappropriate feeding and numerous other factors [6, 25]. Since several factors concurrently contribute to disease development, it is difficult to identify the specific causative agents for any specific case of diarrhea.
Serodiagnosis, PCR and pathogen isolation are currently used to screen infectious diarrhea. However, these assays are restricted in terms of the detectible number of agents, because they are designed as specific assays that usually detect single target pathogens. For rapid diagnosis, a broad-range detection assay is required. Toward this end, multiplex real-time PCR using a hydrolysis probe has been mainly used to concurrently detect pathogens, because of the method’s high sensitivity, simple procedure and reduced instrumentation requirements compared with other methods of inspection. Several studies have described this method using probes with different fluorescent dyes applied in a single tube [7, 10, 15]. However, these methods can only detect at most 5 target pathogens under the same thermal cycling conditions. In terms of comprehensiveness and rapidity, multiplex real-time PCR is not sufficient for differential diagnosis of diarrhea in cattle, because numerous factors may contribute to this disease, including both noninfectious and infectious agents.
To address this issue, we developed a novel detection system that can simultaneously identify 19 of the known diarrhea-causing pathogens in cattle, including viruses, bacteria and protozoa, in a single reaction. Using this system, clinical samples from cattle with diarrhea were evaluated to identify the specific agents causing an individual animal’s diarrhea.
MATERIALS AND METHODS
Primer and probe design: A total of 19 pathogens were selected as targets based on their virulence and prevalence on cattle. New primer-probe sets were designed for RVA, RVB, RVC, BToV, MRV, Eimeria zuernii and Eimeria bovis using the PrimerQuest software (Integrated DNA Technologies, Inc., Coralville, IA, U.S.A.) on the basis of consensus sequences of each pathogen obtained from the GenBank database. Primer and probe information and their target pathogens are summarized in Table 1. Previously reported qPCR assays were used for 12 pathogen species, including RNA, DNA viruses and bacteria [7, 8, 10, 13, 15,16,17, 22, 23, 28, 29]. Furthermore, as an internal control within the Dembo-PCR reaction, primer-probe sets for β-actin were synthesized as previously reported [28]. All hydrolysis probes were labeled with the reporter dye FAM (6-carboxyfluorecein) at the 5′ end and the fluorescent dye TAMRA (6-carboxytetramethylrhodamine) at the 3′ end. Primers and probes were purchased from Sigma-Aldrich (Sigma Aldrich, St. Louis, MO, U.S.A.), and probes containing a mixed base were produced at Integrated DNA Technologies (Integrated DNA Technologies, Inc.).
Table 1. The nucleotide information of the primer-probe sets used for Dembo-PCR.
| Target name | Target gene name | Probe sequence 5′-3′ (FAM/TAMRA) | Primer sequence 5′-3′ | Reference No. |
|---|---|---|---|---|
| Bovine viral diarrheal virus | 5′UTR | CCAYGTGGACGAGGGCAYGC | GGGNAGTCGTCARTGGTTCG | 17 |
| GTGCCATGTACAGCAGAGWTTTT | ||||
| Bovine enterovirus | 5′UTR | CGCACAATCCAGTGTTGCTACGTCGTAAC | GCCGTGAATGCTGCTAATCC | 13 |
| GTAGTCTGTTCCGCCTCCACCT | ||||
| Bovine coronavirus | Nucleocapsid | CCTTCATATCTATACACATCAAGTTGTT | CTGGAAGTTGGTGGAGTT | 8 |
| ATTATCGGCCTAACATACATC | ||||
| Group A rotavirus | VP6 | ACCAATTCCTCCAGTTTGGAAYTCATTYCC | ACTCCAATGTAAGTGATCTAATTC | This study |
| GAGTTGTTCCAAGTAATCCAAA | ||||
| Group B rotavirus | VP6 | GTCACATGTGTCTCAGGCATGGAAGC | TGGCAGGTGGTCAGGTAATAA | This study |
| ACACCACACGTTCTAGCTTTCAG | ||||
| Group C rotavirus | VP6 | CCAGGATTTCCATGGGAACAGACGT | GCCAATACGAGAAGGGATTC | This study |
| TCTTCACGGATGCAACTAGC | ||||
| Bovine torovirus | Nucleocapsid | CCAGCAGTCACTATCTTTGCCATTTGA | CGTATTCAAAACCAAAGACGTG | This study |
| GTGCAGTCTCATTTGCCATC | ||||
| Mammalian orthoreovirus | λ3 | ATGATCCAGCATCTATCGAAACTRTATAAACG | TCGATATCGGGAATGCAG | This study |
| CTGACGGGAAAGTGGTRGTCA | ||||
| Bovine leukemia virus | pol | GAACGCCTCCAGGCCCTTCA | CCTCAATTCCCTTTAAACTA | 23 |
| GTACCGGGAAGACTGGATTA | ||||
| Bovine herpes virus-1 | gE | TGCGGCCTCCGGGCTTTACGTCT | CAATAACAGCGTAGACCTGGTC | 28 |
| GCTGTAGTCCCAAGCTTCCAC | ||||
| Bovine adenovirus | Hexon | TTCATCWCTGCCACWCAAAGCTTTTTT | CRAGGGAATAYYTGTCTGAAAATC | 29 |
| AAGGATCTCTAAATTTYTCTCCAAGA | ||||
| Salmonella Dublin | VagC | TTTTTCGAGCTGCGCGAACGAGC | GGGTGAGCGAGCTGGAAA | 22 |
| CGCCATAAAGTCCGGGTCA | ||||
| Salmonella Enteritidis | SefA | TGGTGGTGTAGCCACTGTCCCGT | GGTAAAGGGGCTTCGGTATC | 15 |
| TATTGGCTCCCTGAATACGC | ||||
| Salmonella Typhimurium | Fic | ACCTGGGTGCGGTACAGAACCGT | TGCAGAAAATTGATGCTGCT | 15 |
| TTGCCCAGGTTGGTAATAGC | ||||
| Mycobacterium avium. Ssp. paratuberculosis | IS900 | AAGACCGACGCCAAAGACGCTGCGA | CAGCGGCTGCTTTATATTCC | 16 |
| GCAGAGGCTGCAAGTCGT | ||||
| Clostridium perfringens | Cpb | AACGGATGCCTATTATCACCAACT | ATTTCATTAGTTATAGTTAGTTCAC | 10 |
| TTATAGTAGTAGTTTTGCCTATATC | ||||
| Enterotoxigenic Escherichia coli | K99 | ATTTTAAACTAAAACCAGCGCCCGGCA | GCTATTAGTGGTCATGGCACTGTAG | 7 |
| TTTGTTTTGGCTAGGCAGTCATTA | ||||
| Eimeria zuernii/bovis | ITS1 | TGGCCTGTTGTGGATAGTTACTG (zuernii) | TGTCTAYACACACTMCATCCAAC | This study |
| GCCTTATGGATAGTTAGTGCTCC (bovis) | CT GACCACAGTGTTGGAAATGC | |||
| β-actin | Actin | TCGCTGTCCACCTTCCAGCAGATGT | AGCGCAAGTACTCCGTGTG | 28 |
| CGGACTCATCGTACTCCTGCTT |
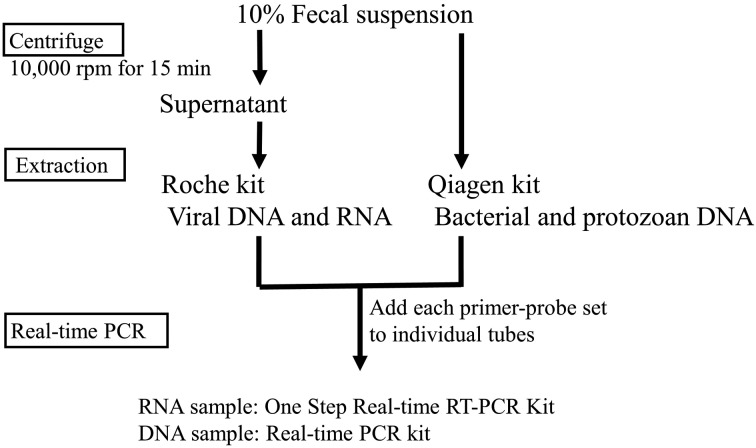
Dembo-PCR workflow: Figure 1 depicts the Dembo-PCR workflow. After pathogen RNA and DNA were extracted, the sample, reagents, and each primer and probe were mixed in individual reaction tubes. Samples were applied at 2 µl per tube. Cycling conditions, reagents and nucleic acid extraction procedures are described below. To verify the absence of nucleic acid loss during the extraction step, the extraction liquids used in the QIAGEN and Roche kits were analyzed as samples using β-actin primer-probe sets. Furthermore, PCR products were used as β-actin positive controls, which were created from Mardin-Darby bovine kidney (MDBK) cells using conventional PCR primers (data not shown). Nuclease-free water was applied as a negative control sample, again using β-actin primer-probe sets. To prepare each sample for assay, 10% fecal suspensions were made in PBS (−). Then, 200 µl of a suspension was used directly for the extraction of bacteria and protozoa nucleic acids with a QIAamp Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany). For virus detection, 200 µl of a suspension was centrifuged for 15 min at 10,000 rpm, and viral DNA and RNA were extracted from the supernatant of the 200 µl of suspension with a High Pure Viral Nucleic Acid Kit (Roche Diagnostics GmbH, Mannheim, Germany). Both nucleic acid extraction procedures were conducted according to the manufacturer’s protocol. A LightCycler Nano (Roche Diagnostics GmbH) was used for all qPCR reactions performed in this study. A one step PrimeScript RT-PCR Kit (Perfect Real time) (TaKaRa Bio, Otsu, Japan) was used for amplification of extracts from RNA viruses, and Premix Ex Taq (Perfect Real time) (TaKaRa Bio) was used for amplification of extracts from DNA viruses, bacteria and protozoa. All reactions were performed in a total volume of 20 µl, which contained the sample nucleic acid, primers, probes (the final concentration of all primers and probes was 0.2 µM) and all other components included in the kits, according to the manufacturers’ protocols. Thermal cycling conditions were as follows: 45°C for 5 min and 95°C for 30 sec, followed by 40 cycles of 95°C for 10 sec, 55°C for 20 sec and 72°C for 20 sec. Fluorescence data were analyzed automatically using LightCycler Nano Software 1.1 (Roche Diagnostics GmbH). The software excluded early cycle data up to 16, and the number of minimum relative amplifications was permuted at zero.
Fig. 1.
Dembo-PCR workflow. To prepare each sample for assay, 10% fecal suspensions were made in PBS (−). The suspensions were then used directly for the extraction of bacteria and protozoa nucleic acids with a QIAamp Fast DNA Stool Mini Kit. For virus detection, the suspensions were centrifuged for 15 min at 10,000 rpm, and viral DNA and RNA were extracted from the supernatants with a High Pure Viral Nucleic Acid Kit. After pathogen RNA and DNA were extracted, samples, reagents and each primer and probe were mixed in individual reaction tubes. Samples were applied at 2 µl per tube. A LightCycler Nano was used for all qPCR reactions performed in this study. A one step PrimeScript RT-PCR Kit (Perfect Real time) was used for amplification of extracts from RNA viruses, and Premix Ex Taq (Perfect Real time) was used for amplification of extracts from DNA viruses, bacteria and protozoa.
Evaluation of real-time PCR performance using synthesized DNA: For the purpose of validation, real-time PCR reliability, sensitivity and linearity of standard curves were verified by testing tenfold serial dilutions of synthesized DNA, including each target genome sequence (1 × 10°–1 × 106 or 5 × 10°–5 × 106 copies/reaction). The synthesized DNA was purchased from Integrated DNA Technologies (Integrated DNA Technologies, Inc.). Pathogen dilutions were repeated twice in separate runs, and a standard curve was constructed from the Cq values. The PCR efficiency (E) was calculated using the standard curve slope according to the following formula: E=(10-1/slope(−1)). The correlation co-efficient (R2) was also calculated.
Sensitivity test on fecal samples: The fecal samples included some PCR inhibitors, such as sugars and bile acid [9, 21]. To evaluate the PCR sensitivity of this assay in fecal specimens, each pathogen was spiked into feces collected from healthy cattle and subsequently subjected to real-time PCR after DNA and RNA extraction. Feces collected from healthy cattle were used for a spike test. Bacteria (1.0 × 105 CFU/ml) were prepared by mixing with a fecal suspension, and viruses (1.0 × 105 TCID50 or PFU/ml) were prepared by mixing with the supernatant after centrifugation of a fecal suspension. Tenfold serial dilutions of fecal suspension and supernatant spiked with viruses or bacteria, respectively, were made in PBS (−) within a range of 1.0 × 101 to 105 CFU, TCID50 or PFU/ml. Pathogens were subjected to DNA and RNA extraction using a total elution volume of 25 µl with the High Pure Viral Nucleic Acid Kit and 20 µl with the QIAamp fast DNA Stool Mini Kit. Extracted DNA and RNA were analyzed by Dembo-PCR. The amounts of viruses and bacteria were 1.6 × 10−1 to 103 TCID50 (PFU)/reaction and 1.3 × 10−1 to 103 CFU/reaction, respectively.
MRV, BCoV, RVA, RVB, RVC, Salmonella Typhimurium and 2 species of protozoa could not be analyzed in regard to the sensitivity of fecal samples by Dembo-PCR, because pathogens or infectious materials were not available. In the case of bovine leukemia virus (BLV), provirus DNA extracted from BLV-infected lymphocytes was used in this study. To analyze the sensitivity of each primer and probe for these pathogens in fecal samples, synthesized DNA or BLV proviral DNA was spiked into nucleic acid solutions extracted from healthy feces. The final concentrations of synthesized DNA and BLV proviral DNA were 1.0 × 10° to 106 and 1.08 × 10° to 103 copies/reaction, respectively. The concentration of BLV proviral DNA was equivalent to the Tax gene copy number calculated using a commercial kit for quantification of the BLV Tax gene (TaKaRa Bio).
The limit of detection (LOD) was defined as the lowest concentration at which a fluorescent signal could be detected in all reactions. Reproducibility (inter-assay variance) was assessed using the coefficient value (CV) calculated on the basis of Cq values.
Clinical samples: In January 2014, an outbreak of diarrhea occurred on a dairy farm in Japan. Six adult cattle were affected by diarrhea and weak. To identify the cause of diarrhea, 6 diarrheal fecal samples and 1 healthy fecal sample, which was collected from a healthy cow at the same farm, were analyzed by Dembo-PCR. In addition, 1 calf was affected by diarrhea at the same farm in March 2014. A fecal sample was collected from this calf and was also analyzed.
RESULTS
LOD and linearity of standard curves using synthesized DNA: Standard curves were constructed using mean Cq values from duplicate 10-fold serial dilutions of synthesized DNA that included the target region of amplification (Fig. S1). Although the PCR efficiency for Salmonella Enteritidis and Eimeria bovis was slightly low (81.6% and 84.0%, respectively), the PCR efficiency in all detection assays was more than 80%, which was sufficient to quantify the target copy number. Furthermore, each assay had a wide dynamic range of at least 5 orders of magnitude.
Sensitivity and accuracy of Dembo-PCR: Table 2 shows the results for LOD numbers and CVs of run-to-run variants. A total of 6 bacteria and 5 viruses were used for optimization and validation of Dembo-PCR. Isolated strains of Salmonella Dublin, Salmonella Enteritidis, MAP, Clostridium perfringens and Enterotoxigenic Escherichia coli (ETEC) were obtained from the National Veterinary Assay Laboratory in Japan. BVDV, bovine herpes virus 1 (BoHV-1) and BToV, which were isolated from field samples, were also used. The test had high sensitivity and steady reproducibility in the spike test, with an LOD of at least 1.6 TCID50 or PFU/reaction for viruses spiked into feces. The LOD of bacteria was at least 13 CFU/reaction. For the BLV provirus, the LOD was equivalent to 10.8 copies/reaction of the Tax gene. The assay using synthesized DNA as a template had an LOD of 10–100 copies/reaction. The coefficient value was 0.1–6.5%.
Table 2. Results of sensitivity testing in feces.
| Type of spiked materials | Pathogens | LOD (/reaction) | Reproducibility CV (%) |
|---|---|---|---|
| Virus | Bovine viral diarrheal virus | 1.6 | 1.6 |
| (TCID50 or PFU) | Bovine enterovirus | 1.6 | 0.5 |
| Bovine torovirus | 1.6 | 0.6 | |
| Bovine adenovirus | 1.6 | 3.0 | |
| Bovine herpes virus-1 | 1.6 | 1.1 | |
| Bacteria | Salmonella Dublin | 13 | 1.7 |
| (CFU) | Salmonella Enteritidis | 1.3 | 2.8 |
| MAP | 1.3 | 1.3 | |
| Clostridium perfringens | 13 | 1.1 | |
| Enterotoxigenic Escherichia coli | 1.3 | 0.1 | |
| DNA | Mammalian orthoreovirus | 100 | 1.5 |
| (copy number) | *Bovine leukemia virus | 10.8 | 0.8 |
| Salmonella Typhimurium | 100 | 3.1 | |
| Eimeria zuernii | 100 | 5.2 | |
| Eimeria bovis | 100 | 0.2 | |
| Bovine coronavirus | 100 | 1.2 | |
| Group A rotavirus | 100 | 0.1 | |
| Group B rotavirus | 10 | 2.2 | |
| Group C rotavirus | 100 | 6.4 |
LOD: Limit of detection. CV: Coefficient value. MAP: Mycobacterium avium Spp. Paratuberculosis. *For bovine leukemia virus, the provirus was spiked into the feces.
Clinical performance: Table 3 shows the information of clinical samples and the results of the Dembo-PCR assay. BCoV was detected in 6 diarrheal fecal samples. In addition, BEV was detected in clinically healthy cattle and in sample No. 5. BToV was detected only in the calf feces. To confirm these results, gel-based PCR assays were conducted on all samples for each pathogen according to previous reports [13, 24, 27]. The gel-based PCR products were subsequently subjected to direct sequencing to compare them with the corresponding NCBI database nucleotide sequences. Gel-based PCR results for BEV, BCoV and BToV were in agreement with the Dembo-PCR results.
Table 3. Information about the clinical samples analyzed in this study.
| Sample No. | Breed | Age (years) | Sex | Symptom | Sampling date | Detected pathogens* |
|---|---|---|---|---|---|---|
| No.1 | Holstein | 4.3 | Female | Severe diarrhea, anorexia | January, 2014 | BCV |
| No.2 | Holstein | 2.3 | Female | Severe diarrhea, anorexia | January, 2014 | BCV |
| No.3 | Holstein | 7.0 | Female | Severe diarrhea, anorexia | January, 2014 | BCV |
| No.4 | Holstein | 3.5 | Female | Mild diarrhea | January, 2014 | BCV |
| No.5 | Holstein | 4.9 | Female | Severe diarrhea, anorexia | January, 2014 | BCV, BEV |
| No.6 | Holstein | 3.7 | Female | Severe diarrhea, anorexia | January, 2014 | BCV |
| No.7 | Holstein | 6.0 | Female | Healthy | January, 2014 | BEV |
| No.8 | Holstein | 0.1 | Female | Severe diarrhea, weakeness | March, 2014 | BToV |
BCV: bovine coronavirus. BEV: bovine enterovirus; BToV: bovine torovirus. *The reults were analyzed by Dembo-PCR and gel-based PCR for each pathogen.
DISCUSSION
In this study, a new system for simultaneous detection of cattle diarrhea-associated pathogens was developed. This novel system was designated as a detection system for microbes from bovine diarrhea by real-time PCR (referred to as Dembo-PCR). Dembo-PCR can detect a total of 19 pathogens in a single run, including 9 RNA viruses (BLV is targeted as a provirus), 2 DNA viruses, 6 bacteria and 2 protozoa, within 3 hr.
In 2014, an outbreak of severe diarrhea occurred on a farm in Japan in the winter, and decrease in milk production was observed in the affected cattle. BCoV is a pathogen that causes “winter dysentery” and is one of the major infectious agents that causes epidemic outbreaks in adult cattle [26]. Cattle infected with this virus occasionally present with severe diarrhea and reduced milk production with weakening during the winter. In a previous report, BCoV was detected in more than 57.8% of adult cattle suffering from diarrhea [18]. Our results showed that BCoV was detected in all diarrheal samples collected in January. Judging from the results, gel-based PCR and the clinical findings, the epidemic outbreak of diarrhea in January 2014 was caused by BCoV. In addition, diarrhea occurred after introducing one cow (No. 6). Therefore, the outbreak is thought to have been caused by cow No. 6.
On the other hand, both BCoV and BEV were detected in samples from cow No. 5 and healthy cow (No. 7). Some studies have claimed that BEV infection may cause diarrhea in cattle [2, 30]. Conversely, other studies have shown that BEV infection is noncritical in cattle because of its high prevalence in healthy cattle [1, 5]. The latter opinion agrees with our results showing that the healthy cow sample was positive for BEV. Therefore, there is a low possibility that BEV caused diarrhea in the cow. However, the pathogenesis of this virus remains to be clarified. Additional evaluation of BEV pathogenicity should be conducted.
One calf (No. 8) was affected by diarrhea at 2 months after the first incidence of diarrhea, although the outbreak of diarrhea in adult cattle had been stamped out. The results showed that only BToV was detected in this calf. Previous studies showed that BToV produces mild-to-moderate diarrhea in calves under both experimental and field conditions [12, 24]. The results obtained from Dembo-PCR suggested that diarrhea in the calf in this study was caused by BToV. However, diarrhea can also be caused by noninfectious agents, such as environmental factors and the condition of cattle immunity [6]. Moreover, BToV can be detected occasionally in healthy cattle. The association of BToV with diarrhea in this case was not obvious. A further study of how BToV causes diarrhea in cattle should be conducted.
In this study, we describe the development and validation of a novel tool for differential diagnosis of infectious diarrhea in cattle. Dembo-PCR has the advantage of being able to detect known and unknown diarrheal pathogens. This system will be a powerful tool for rapidly diagnosing the causes of this nuisance disease.
Supplementary Material
Acknowledgments
We would like to acknowledge Prof. Kenji Murakami of the Microbiology Laboratory of Iwate University for the provision of virus strains and technical assistance. We also thank Dr. Harutaka Katano (National Institute of Infectious Diseases, Tokyo, Japan) for his technical assistance. This work was supported in part by a grant-in-Aid for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labour and Welfare, Japan (grant H24-shinkou-ippan-005).
REFERENCES
- 1.Bartels C. J. M., Holzhauer M., Jorritsma R., Swart W. A. J. M., Lam T. J. G. M.2010. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves. Prev. Vet. Med. 93: 162–169. doi: 10.1016/j.prevetmed.2009.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blas-Machado U., Saliki J. T., Boileau M. J., Goens S. D., Caseltine S. L., Duffy J. C., Welsh R. D.2007. Fatal ulcerative and hemorrhagic typhlocolitis in a pregnant heifer associated with natural bovine enterovirus type-1 infection. Vet. Pathol. 44: 110–115. doi: 10.1354/vp.44-1-110 [DOI] [PubMed] [Google Scholar]
- 3.Chang K. O., Parawani A. V., Smith D., Saif L. J.1997. Detection of group B rotaviruses in fecal samples from diarrheic calves and adult cows and characterization of their VP7 genes. J. Clin. Microbiol. 35: 2107–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi J., VanLeeuwen J. A., Weersink A., Keefe G. P.2002. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev. Vet. Med. 55: 137–153. doi: 10.1016/S0167-5877(02)00094-6 [DOI] [PubMed] [Google Scholar]
- 5.Cho Y. I., Han J. I., Wang C., Cooper V., Schwartz K., Engelken T., Yoon K. J.2013. Case-control study of microbiological etiology associated with calf diarrhea. Vet. Microbiol. 166: 375–385. doi: 10.1016/j.vetmic.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho Y. I., Yoon K. J.2014. An overview of calf diarrhea − infectious etiology, diagnosis, and intervention. J. Vet. Sci. 15: 1–17. doi: 10.4142/jvs.2014.15.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho Y. I., Kim W. I., Liu S., Kinyon J. M., Yoon K. J.2010. Development of a panel of multiplex real-time polymerase chain reaction assays for simultaneous detection of major agents causing calf diarrhea in feces. J. Vet. Diagn. Invest. 22: 509–517. doi: 10.1177/104063871002200403 [DOI] [PubMed] [Google Scholar]
- 8.Decaro N., Elia G., Campolo M., Desario C., Mari V., Radogna A., Colaianni M. L., Cirone F., Tempesta M., Buonavoglia C.2008. Detection of bovine coronavirus using a TaqMan-based real-time RT-PCR assay. J. Virol. Methods 151: 167–171. doi: 10.1016/j.jviromet.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flekna G., Schneeweiss W., Smulders F. J., Wagner M., Hein I.2007. Real-time PCR method with statistical analysis to compare the potential of DNA isolation methods to remove PCR inhibitors from samples for diagnostic PCR. Mol. Cell. Probes 21: 282–287. doi: 10.1016/j.mcp.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 10.Gurjar A. A., Hegde N. V., Love B. C., Jayarao B. M.2008. Real-time multiplex PCR assay for rapid detection and toxintyping of Clostridium perfringens toxin producing strains in feces of dairy cattle. Mol. Cell. Probes 22: 90–95. doi: 10.1016/j.mcp.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 11.Heuer C., Healy A., Zerbini C.2007. Economic effects of exposure to bovine viral diarrhea virus on dairy herds in New Zealand. J. Dairy Sci. 90: 5428–5438. doi: 10.3168/jds.2007-0258 [DOI] [PubMed] [Google Scholar]
- 12.Hoet A. E., Nielsen P. R., Hasoksuz M., Thomas C., Wittum T. E., Saif L. J.2003. Detection of bovine torovirus and other enteric pathogens in feces from diarrhea cases in cattle. J. Vet. Diagn. Invest. 15: 205–212. doi: 10.1177/104063870301500301 [DOI] [PubMed] [Google Scholar]
- 13.Jiménez-Clavero M. A., Escribano-Romero E., Mansilla C., Gómez N., Córdoba L., Roblas N., Ponz F., Ley V., Sáiz J. C.2005. Survey of bovine enterovirus in biological and environmental samples by a highly sensitive real-time reverse transcription-PCR. Appl. Environ. Microbiol. 71: 3536–3543. doi: 10.1128/AEM.71.7.3536-3543.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leary T. P., Erker J. C., Chalmers M. L., Cruz A. T., Wetzel J. D., Desai S. M., Mushawar I. K., Dermody T. S.2002. Detection of mammalian reovirus RNA by using reverse transcription-PCR: sequence diversity within the lambda3-encoding L1 gene. J. Clin. Microbiol. 40: 1368–1375. doi: 10.1128/JCM.40.4.1368-1375.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S. H., Jung B. Y., Rayamahji N., Lee H. S., Jeon W. J., Choi K. S., Kweon C. H., Yoo H. S.2009. A multiplex real-time PCR for differential detection and quantification of Salmonella spp., Salmonella enterica serovar Typhimurium and Enteritidis in meats. J. Vet. Sci. 10: 43. doi: 10.4142/jvs.2009.10.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logar K., Kopinč R., Bandelj P., Starič J., Lapanje A., Ocepek M.2012. Evaluation of combined high-efficiency DNA extraction and real-time PCR for detection of Mycobacterium avium subsp. paratuberculosis in subclinically infected dairy cattle: comparison with faecal culture, milk real-time PCR and milk ELISA. BMC Vet. Res. 8: 49. doi: 10.1186/1746-6148-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahlum C. E., Haugerud S., Shivers J. L., Rossow K. D., Goyal S. M., Collins J. E., Faaberg K. S.2002. Detection of bovine viral diarrhea virus by TaqMan reverse transcription polymerase chain reaction. J. Vet. Diagn. Invest. 14: 120–125. doi: 10.1177/104063870201400205 [DOI] [PubMed] [Google Scholar]
- 18.Mawatari T., Hirano K., Ikeda H., Tsunemitsu H., Suzuki T.2014. Surveillance of diarrhea-causing pathogens in dairy and beef cows in Yamagata Prefecture, Japan from 2002 to 2011. Microbiol. Immunol. 58: 530–535. doi: 10.1111/1348-0421.12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mawatari T., Taneichi A., Kawagoe T., Hosokawa M., Togashi K., Tsunemitsu H.2004. Detection of a bovine group C rotavirus from adult cows with diarrhea and reduced milk production. J. Vet. Med. Sci. 66: 887–890. doi: 10.1292/jvms.66.887 [DOI] [PubMed] [Google Scholar]
- 20.McKenna S. L. B., Keefe G. P., Tiwari A., VanLeeuwen J., Barkema H. W.2006. Johne’s disease in Canada part II: disease impacts, risk factors, and control programs for dairy producers. Can. Vet. J. 47: 1089–1099. [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro L., Bonnemaison D., Vekris A., Petry K. G., Bonnet J., Vidal R., Cabrita J., Mégraud F.1997. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J. Clin. Microbiol. 35: 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson S., Jacobsen T., Olsen J. E., Olsen K. E., Hansen F.2012. A new real-time PCR method for the identification of Salmonella Dublin. J. Appl. Microbiol. 113: 615–621. doi: 10.1111/j.1365-2672.2012.05378.x [DOI] [PubMed] [Google Scholar]
- 23.Rola-Łuszczak M., Finnegan C., Olech M., Choudhury B., Kuźmak J.2013. Development of an improved real time PCR for the detection of bovine leukaemia provirus nucleic acid and its use in the clarification of inconclusive serological test results. J. Virol. Methods 189: 258–264. doi: 10.1016/j.jviromet.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 24.Smits S. L., Lavazza A., Matiz K., Horzinek M. C., Koopmans M. P., de Groot R. J.2003. Phylogenetic and evolutionary relationships among torovirus field variants: evidence for multiple intertypic recombination events. J. Virol. 77: 9567–9577. doi: 10.1128/JVI.77.17.9567-9577.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson C., Lundborg K., Emanuelson U., Olsson S. O.2003. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Prev. Vet. Med. 58: 179–197. doi: 10.1016/S0167-5877(03)00046-1 [DOI] [PubMed] [Google Scholar]
- 26.Tråvén M., Näslund K., Linde N., Linde B., Silván A., Fossum C., Hedlund K. O., Larsson B.2001. Experimental reproduction of winter dysentery in lactating cows using BCV–comparison with BCV infection in milk-fed calves. Vet. Microbiol. 81: 127–151. doi: 10.1016/S0378-1135(01)00337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsunemitsu H., Smith D. R., Saif L. J.1999. Experimental inoculation of adult dairy cows with bovine coronavirus and detection of coronavirus in feces by RT-PCR. Arch. Virol. 144: 167–175. doi: 10.1007/s007050050493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wernike K., Hoffmann B., Kalthoff D., König P., Beer M.2011. Development and validation of a triplex real-time PCR assay for the rapid detection and differentiation of wild-type and glycoprotein E-deleted vaccine strains of Bovine herpesvirus type 1. J. Virol. Methods 174: 77–84. doi: 10.1016/j.jviromet.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 29.Wong K., Xagoraraki I.2010. Quantitative PCR assays to survey the bovine adenovirus levels in environmental samples. J. Appl. Microbiol. 109: 605–612. [DOI] [PubMed] [Google Scholar]
- 30.Zhu L., Xing Z., Gai X., Li S., San Z., Wang X.2014. Identification of a novel enterovirus E isolates HY12 from cattle with severe respiratory and enteric diseases. PLoS ONE 9: e97730. doi: 10.1371/journal.pone.0097730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.