Abstract
Although the increasing concentration of atmospheric carbon dioxide (CO2) accelerates the accumulation of carbohydrates and increases the biomass and yield of C3 crop plants, it also reduces their nitrogen concentration. The consequent changes in primary and secondary metabolites affect the palatability of host plants and the feeding of herbivorous insects. Aphids are phloem feeders and are considered the only feeding guild that positively responds to elevated CO2. In this review, we consider how elevated CO2 modifies host defenses, nutrients, and water-use efficiency by altering concentrations of the phytohormones jasmonic acid, salicylic acid, ethylene, and abscisic acid. We will describe how these elevated CO2-induced changes in defenses, nutrients, and water statusfacilitate specific stages of aphid feeding, including penetration, phloem-feeding, and xylem absorption. We conclude that a better understanding of the effects of elevated CO2 on aphids and on aphid damage to crop plants will require research on the molecular aspects of the interaction between plant and aphid but also research on aphid interactions with their intra- and inter-specific competitors and with their natural enemies.
Keywords: elevated CO2, aphid, nitrogen metabolism, plant defenses, water potential, legumes
Introduction
Since the industrial revolution, atmospheric CO2 concentrations have increased from 280 ppm to approximately 400 ppm due to anthropogenic effects, i.e., deforestation and fossil fuel combustion. These increases in atmospheric CO2 concentrations have serious implications for global warming and climate change (Stocker et al., 2013). Although changes in climate have been anticipated to greatly affect agricultural ecosystems (Fuhrer, 2003), increases in atmospheric CO2 concentration alone can also be very important because they can directly affect plant physiology and indirectly alter interactions between plants and herbivores and plant pathogens (Robinson et al., 2012). These altered interactions may then lead to more severe and frequent outbreaks of pest insects and plant diseases in agricultural ecosystems (Percy et al., 2002).
To understand how elevated concentrations of atmospheric CO2 could increase pest problems, we must first recognize that increases in CO2 tends to increase the growth of plants by enhancing their photosynthetic rate, resulting in higher yields for most C3 crops (Ainsworth and Rogers, 2007). Under elevated CO2, however, C3 crop plants exhibit decreases in nitrogen (N) and other trace elements, i.e., zinc and iron (Bloom et al., 2010). These decreases reduce the nutritional value for herbivorous insects and may therefore change their feeding behaviors (Myers et al., 2014). For those insects that chew leaves, a reduction in the N concentration in crop tissue and the resulting increase in the carbon/nitrogen ratio (C:N ratio) under elevated CO2 could cause these insect pests to consume more leaves to meet their N needs (Bezemer and Jones, 1998; Sun and Ge, 2011). In addition, leaves grown under elevated CO2 decrease their ability to produce jasmonic acid (JA), a hormone that contributes to plant defenses against chewing insects (Zavala et al., 2008).
Elevated CO2 may also increase the damage to crops caused by phloem-sucking insects including aphids. Aphids feed exclusively on the phloem sap and are very sensitive to changes in plant quality caused by climate change (Pritchard et al., 2007). Recent meta-analysis result shows that aphids tend to perform better under elevated CO2 on average (Robinson et al., 2012). The conclusions from many statsitically signficant researches, however, exhibit idiosyncratic responses of aphids in terms of population abundance, fecundity as well as survival (summarized in Table 1). Although predictions are difficult, it is nevertheless useful to determine why some aphids are more fit while others are less fit under elevated CO2. A mechanistic understanding can help make sense of these contradictory results. Previous study demonstrates that the effect of elevated CO2 on plant, which includes C and N assimilation, secondary metabolism, plant stomatal conductance as well as leaf temperature, could in turn affect aphid population numbers and growth (Ainsworth et al., 2006; May et al., 2013). Futhermore, the feeding behavior of aphids and their interaction with host plant under elevated CO2 are largely ignored but should be crucial to the understanding of idiosyncratic responses. The aim of this review is to highlight overlooked processes and new discoveries that how elevated CO2 affects the components of plant leaves and how these effects alter the different feeding phases of aphids. We also suggest some possible molecular mechanisms underlying the interactions between aphids and their host plants under elevated CO2.
Table 1.
Potential mechanisms regarding aphid performance respond to elevated CO2
| Potential mechanism | Aphid–host plant system | Response | Parameter | Reference |
|---|---|---|---|---|
| Alters absorption of foliar amino acid or | Acyrthosiphon pisum – Medicago sativa | Positive | Population abundance | Ryalls et al., 2015 |
| changes the sap flow of plant | Acyrthosiphon pisum – Medicago truncatula | Positive | Population abundance,feeding efficiency | Guo et al., 2013 |
| Aphis gossypii – Gossypium hirsutum | Unchanged | Growth rate | Sun et al., 2009 | |
| Rhopalosiphum padi – Hordeum vulgare | Positive | Population abundance, intrinsic rate of population | Ryan et al., 2015 | |
| Aphis fabae – Cardamine pratensis | Positive | Population abundance | Salt et al., 1996 | |
| Myzus persicae – Solanum dulcamara | Positive | Population abundance | Salt et al., 1996 | |
| Acyrthosiphon pisum – Medicago sativa | Depend on plant genotypes | Population abundance | Johnson et al., 2014 | |
| Changes of nitrogen concentration or whole plant quality of host plant | Myzus persicae – Bell pepper | Negative | Pre-reproductive period, fecundity | Dáder et al., 2016 |
| Phyllaphis fagi – Fagus sylvatica | Negative | Fecundity, nymph weight, nymph weight | Docherty et al., 1997 | |
| Rhopalosiphum padi – Triticum aestivum | Positive | Weight, relative growth rate, life span | Oehme et al., 2013 | |
| Myzus persicae – Brassica napus | Negative | Weight, relative growth rate, life span | Oehme et al., 2013 | |
| Rhopalosiphum maidis – Hordeum vulgare | Positive | Developmental duration, fecundity | Xie et al., 2014 | |
| Increase of photosynthesis | Myzus persicae – four plant species (Careamine hirsute, Poa annua, Senecio vulgar, Spergula arvensis) | Positive | Population abundance | Bezemer et al., 1998 |
| Plant endophyte induced resistance | Rhopalosiphum padi – Festuca arundinacea | Negative | Population abundance, aphid density | Newman et al., 1999; Ryan et al., 2014a,b |
| Decrease of phytohoemone resistance | Myzus persicae – Arabidopsis | Positive | Population abundance | Sun et al., 2013 |
| Acyrthosiphon pisum – Medicago truncatula | Positive | Mean relative growth rate; feeding efficiency | Guo et al., 2014a,b | |
| R-gene mediated resistance decreased | Amphorophora idaei - Rubus idaeus | Positive | Population abundance, adult mass | Martin and Johnson, 2011 |
| Increase of leaf temperature | Aphis glycines – Glycine max | Positive | Population abundance | O’Neill et al., 2011 |
| Decrease of stomatal aperture | Acyrthosiphon pisum – Medicago truncatula | Positive | Population abundance, feeding efficiency | Sun et al., 2015 |
| Sensitivity to alarm pheromone | Amphorophora idaei – Rubus idaeus | Negative | Escape response to predator | Hentley et al., 2014 |
| Sitobion avenae – Triticum aestivum | Negative | Sensitivity to (E)-β-farnesene | Sun et al., 2010 |
Aphid Feeding Behavior
Recent advances indicate that complex molecular interactions occur when aphids feed on plants. Unlike chewing insects that remove large pieces of plant tissues, aphids use their flexible and long stylets to obtain nutrients from the phloem sap and only inflict slight physical damage (Jaouannet et al., 2014). The specialized feeding behavior of aphids can be detected with electrical penetration graph (EPG) methods, i.e., EPG methods can be used to determine the locations and activities of aphid stylets, including pooled pathway phase activities, probing, salivation into sieve elements, passive uptake of the phloem sap, and xylem absorption (Tjallingii and Esch, 1993). Data on the initiation and duration of these feeding phases provide valuable cues regarding aphid activities and plant responses (Alvarez et al., 2006). Rather than simply withdrawing food from hosts, aphids can change their feeding location to avoid plant defenses or can secrete ‘effector’ proteins to suppress plant defenses (Hogenhout and Bos, 2011). To enhance their feeding, aphids can also alter host physiological traits, e.g., they can induce changes in host primary metabolism and in stomatal movement, and suppress the plant defenses (Giordanengo et al., 2010). Thus, a better understanding of aphid feeding behavior, its effects on hosts, and host responses is critical for understanding how elevated CO2 is likely to affect plant–aphid interactions.
Aphid Probing and Penetration Stage and its Relation to Plant Resistance
Influence of Plant Physical Barriers
Once they have arrived on a plant leaf, aphids must conquer host physical defenses including trichomes and waxes before they can insert their stylets into the host (Wang et al., 2004). Surface resistance is the first barrier of plant defense against aphid attack. The time that aphids spend between arriving on a leaf and making their first probe mainly reflects the physical barriers of the leaf surface including trichomes, repellent volatiles, and a thick or tough leaf surface (van Helden and Tjallingii, 1993). Plants can deter aphid attack by releasing secondary metabolites such as glucose esters and sesquiterpenes from glandular trichomes (Avé et al., 1987; Goffreda et al., 1989; Neal et al., 1990). Furthermore, a specifically expressed gene, NtLTP1, in the glandular trichomes of Nicotiana tabacum could enhance the plant’s defense against aphids (Choi et al., 2012). The changes in trichome density in response to CO2 are idiosyncratic. For example, trichome density increased in Brassica rape and Medicago truncatula (Karowe and Grubb, 2011; Guo et al., 2014a) but decreased in Arabidopsis and wheat under elevated CO2 (Masle, 2000; Bidart-Bouzat et al., 2005; Lake and Wade, 2009). In the legume M. truncatula under elevated CO2, the increased density of non-glandular and glandular trichomes caused aphids to spend more time before they made their first probe and to experience a prolonged pathway phase (Guo et al., 2014a). CO2 concentrations may affect trichome development by affecting the levels of gibberellic acid (GA), JA, and the microRNA molecule miR156. Elevated CO2 tends to increase plant GA content and decrease plant JA content (Teng et al., 2006; Zavala et al., 2008) and to decrease expression of miR156 (May et al., 2013). Additional research is needed, however, to clarify whether the effects of elevated CO2 on glandular trichome development and surface resistance to aphids is due to changes in GA, JA, and miR156.
Phytohormone-Mediated Defenses
When the aphid stylet penetrates the plant epidermis and mesophyll, it forms a channel that permits the delivery of saliva into the phloem (Jaouannet et al., 2014). On the one hand, elicitors in aphid saliva could trigger the formation of reactive oxygen species (ROS), which in turn could induce plant defenses (Giordanengo et al., 2010). On the other hand, “effectors” in aphid saliva could suppress plant resistance and manipulate host cell processes to favor aphid feeding and colonization (Bos et al., 2010a,b; McLellan et al., 2013; Gimenez-Ibanez et al., 2014; King et al., 2014). Parameters of aphid feeding behavior revealed by EPG could reflect the intensity of plant resistance; these parameters include the minimum duration of pathway phase activity, the number of test probes, and the total time before phloem ingestion begins (Alvarez et al., 2006). In the M. truncatula–pea aphid system, elevated CO2 increased the number of test probes but decreased the total time before phloem ingestion began (Guo et al., 2014a). The inconsistent effects of elevated CO2 on aphid feeding parameters may result from the contrasting effects of elevated CO2 on the defense signaling pathways involving the phytohormones JA, salicylic acid (SA), and ethylene (ET) (Guo et al., 2014a). Elevated CO2 tends to enhance SA-dependent defense but reduce JA- and ET-dependent defenses in plants (Zavala et al., 2009; Guo et al., 2012; Sun et al., 2013). Furthermore, the enhanced SA signaling pathway under elevated CO2 caused aphids to spend more time before the first probe and reduced aphid fitness (Casteel et al., 2012; Guo et al., 2014a). The suppression of the JA signaling pathway under elevated CO2, however, reduces the time required by aphids to reach the phloem. In addition, elevated CO2 down-regulates the expression of the ET signaling pathway genes ACC, SKL, and ERF in M. truncatula under attack by the pea aphid system; this downregulation, decreases the accumulation of H2O2 and the activities of key enzymes related to ROS (Guo et al., 2014b).
Moreover, elevated CO2 potentially disrupts the homeostatic cross-talk between SA and JA/ET pathways by directly activating the NPR1 (NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1) gene (DeLucia et al., 2012; Zavala et al., 2013). NPR1-mediated suppression of JA signaling is regulated by glutathione biosynthesis (Spoel and Loake, 2011). Elevated CO2 changes the expression of genes that encode thioredoxins and glutathione S-transferase, which may activate the expression of NPR1 (DeLucia et al., 2012). However, Sun et al. (2013) found that when the NPR1 gene was knocked down, the JA-dependent defenses of Arabidopsis were not enhanced by elevated CO2, suggesting that the activation of NPR1 may not explain the response of SA, JA, and ET signaling pathways to elevated CO2. Clearly, the upstream network regulating plant immunity against aphids is complex. The elicitors secreted from aphid salivary glands were recognized by the host co-receptor BRI-ASSOCIATED RECEPTOR KINASE 1 (BAK1) which subsequently phosphorylates BOTRYTIS INDUCED KINASE1 (BIK1). The BAK1 and the BIK1 complexes could jointly modulate the downstream phytohormone-mediated defense signaling pathway (Chaudhary et al., 2014; Lei et al., 2014; Prince et al., 2014). In addition to BAK/BIK, other kinases such as mitogen protein kinases (MAPKs) are also important for regulating plant defense responses against insect herbivores (Hettenhausen et al., 2015). A number of studies reported that MAPKs could regulate the JA, SA, and ET signaling pathways by activating WRKY genes (Zavala et al., 2013). It is still unknown whether elevated CO2 affects the JA- and SA-dependent signaling pathways by regulating upstream BAK/BIK or MAPK signaling. Thus, additional research is needed to determine how elevated CO2 affects these regulatory molecules in phytohormone signaling networks.
Secondary Metabolite-Mediated Resistance
Many plant secondary metabolites may help plants resist aphid attack by negatively affecting the penetration pathway stage of aphid feeding. These secondary metabolites include alkaloids, steroids, foliar phenolic esters (rutin, cholorogenic acid, etc.), terpenoids, cyanogenic glycosides, glucosinolates, saponins, flavonoids, and pyrethrins (Sharma et al., 2000; Urbanska et al., 2002). For example, aphids that fed on high-saponin lines of alfafa required a prolonged time to penetrate the epidermis and mesophyll (pattern C wave) and showed a significant reduction in phloem sap ingestion (Goławska, 2007). Furthermore, different phenolic compounds seem to have different effects on the feeding parameters of aphids. Caffeic and gallic acids in cereals, for example, drastically shortened the probing phase of the grain aphid, whereas catechin prolonged the pathway phase and also decreased the number of probes by the grain aphid (Urbanska et al., 2002). On average, elevated CO2 increases the total phenolicsin plants by an average of 19%, condensed tannins by 22%, and flavonoids by 27% (Robinson et al., 2012). The excess of secondary metabolites in plants may help explain the increased epidermis and mesophyll resistance of plants during pathway and probing feeding stages of aphids under elevated CO2 (Guo et al., 2014a). Despite of increasing tannin content and phenolic compounds in whole host plant leaves, the bird cherry-oat aphid Rhopalosiphum padi performed better under elevated CO2 (Bezemer and Jones, 1998; Zhang et al., 2003). This result suggested that the tricky feeding strategy of the aphid allows it to avoid some potential defensive components. Thus, it is hard to predict the impact on aphid fitness only through surface or pathway effects.
Phenylalanine ammonialyase (PAL) and polyphenol oxidase (PPO) are two key enzymes involved in the synthesis of phenolic compounds that may be absorbed by the salivary sheath of the aphid stylet. The further polymerization of phenolic compounds causes browning of cells in contact with the saliva; such browning was associated with aphid probing activity during penetration of the epidermal and mesophyll tissues (Jiang and Miles, 1993; Urbanska et al., 1998; Han et al., 2009). PAL and PPO activities are changed by elevated CO2. For example, elevated CO2 tends to increase PAL activity but decrease PPO activity in M. truncatula. However, it is still unknown how changes in PPO and PAL activities under elevated CO2 affect the penetration phase of aphid feeding (Guo et al., 2014b).
Resistance Expressed in the Phloem
After overcoming defenses associated with the plant epidermis and mesophyll, the aphid stylet may finally reach the phloem, but plants have ways to prevent or reduce the ingestion of phloem sap. Phloem sap contains carbohydrates, proteins, and amino acids that are essential for plant development (Gündüz and Douglas, 2009). If the phloem is impaired, plants could suffer loss of nutrients, disturbance of translocation, and increased vulnerability to infection by microbial pathogens (Dinant et al., 2010). Therefore, plants have evolved a range of defenses to inhibit phloem feeding by aphids (Will et al., 2013). The most common defense involves the occlusion of sieve tubes by the plugging of sieve pores (Knoblauch and van Bel, 1998). Two groups of sieve-tube occlusion mechanisms can be found in plants: callose deposition and protein plugging (e.g., Will and van Bel, 2006; Furch et al., 2007). The Ca2+ signaling pathway in plants plays a key role in sieve-tube occlusion during aphid penetration. When the stylet penetrates the sieve membrane, the high concentration gradient of Ca2+ between the apoplast and the sieve element lumen leads to an influx of Ca2+into the sieve element lumen, which induces occlusion (Knoblauch and van Bel, 1998). When this occurs, aphids must secret watery saliva into the phloem; the saliva contains proteins that bind Ca2+ and counteract sieve element occlusion. Thus, the time spent during salivary secretion into sieve elements reflects the defenses located in the phloem (Will et al., 2013).
Phloem resistance against aphids may be affected by elevated CO2. The key gene involved in callose biosynthesis is up-regulated in Arabidopsis under elevated CO2 (Li et al., 2008). Furthermore, cytosolic free Ca2+ is increased by elevated CO2 in Commelina communis (Webb et al., 1996). The increased production of callose and free Ca2+ in cells may cause aphids to spend more time in overcoming phloem resistance. EPG data consistently showed that elevated CO2 increased the time of salivary secretion into sieve elements when pea aphids fed on M. truncatula (Guo et al., 2013). Still, there is no direct evidence confirming that elevated CO2 increases the phloem resistance against pea aphids because of increases in callose deposition and in the Ca2+ signaling pathway.
Aphid Phloem Ingestion and its Relation to Plant Nutrition
The efficiency with which aphids feed on phloem sap is determined by the nutritional composition of the sap (Douglas, 2003). Sucrose is the dominant organic compound in the phloem sap and is a crucial C source for aphids (Fisher and Cash-Clark, 2000; Douglas, 2003). Sucrose is the principal energy source for aphids and also provides the C skeleton for lipid, amino acid, and protein synthesis (Rhodes et al., 1996; Febvay et al., 1999). In potato, a mutation of the sucrose transporter StSUT1 gene reduced the phloem sucrose content and simultaneously reduced the performance of the potato aphid (Pescod et al., 2007). Nevertheless, high concentrations of soluble carbohydrates in plant tissues often reduce aphid performance because they dilute other nutrients such as amino acids and proteins; as a consequence, the aphids must increase their consumption of phloem sap and excrete the excess sucrose as honeydew (Wilkinson et al., 1997). In contrast to carbohydrate-based nutrients, N nutrition is a limiting factor for aphid growth. The phloem sap ingested by aphids has a protein/carbohydrate ratio (w/w) as low as 0.1 while the leaf tissue ingested by chewing insects has a protein/carbohydrate ratio ranging from 0.8 to 1.5 (Behmer, 2008).
Increases in atmospheric CO2 accelerate photosynthesis and synthesize and transport of sucrose into the phloem, which dilutes the N concentration and increases the C:N ratio in the phloem of non-legumes (Barbehenn et al., 2004). The decreased nitrogen concentration of plants could prolonged the pre-reproductive period and decrease the fecundity of some aphids under elevated CO2 (Dáder et al., 2016). However, Sun et al. (2009) found that although amino acid relative concentration in the phloem of cotton plants was lower under elevated CO2 than under ambient CO2, higher amounts of free amino acids were found in cotton aphids fed on cotton grown in elevated CO2. These results suggested that cotton aphids under elevated CO2 will ingest increased quantities of phloem sap to satisfy their nutritional requirements. Moreover, the relative concentrations of predominantly essential amino acids in the phloem of barley are increased under elevated CO2 (Ryan et al., 2015). The latter result is consistent with the large increases in the levels of minor amino acids (most of which are considered essential) in tobacco seedlings under elevated CO2 (Geiger et al., 1998). These results suggest that although the total N concentration of plants is decreased, amino acids biosynthesis and translation in some non-legumes may increase under elevated CO2. Moreover, the mathematic model constructed by Newman et al. (2003) predicted that aphid populations tend to be larger under elevated CO2 if host plants have higher N supplementation, that the nitrogen requirement of aphids is low and that the density-dependent response is weak. Thus, a general explanation for the species-specific responses of aphids to elevated CO2 remains to be elucidated.
In legumes, elevated CO2 leads to a 38% increase in the quantity of N fixed from the atmosphere, which can compensate for decreases in plant N under elevated CO2 and cause the legumes to maintain a C:N ratio similar to that under ambient CO2 (Lam et al., 2012). When M. truncatula was infested by pea aphids, elevated CO2 significantly increased the concentration of total amino acids in leaves and of most individual amino acids in the phloem by enhancing the enzyme activities of N transamination (Guo et al., 2013). The increased amino acids, however, are mostly nonessential, and require the aphid endosymbiont Buchnera to convert them into essential amino acids (Nikoh et al., 2010). When the N-fixation ability was reduced by artificially induced mutation, the individual amino acid relative concentration in the phloem of M. truncatula was decreased such that Buchnera could no longer convert the nonessential amino acids into essential amino acids (Guo et al., 2013). These results with legumes suggest that elevated CO2 may increase the phloem feeding time of the pea aphid by altering amino acid metabolism, and that this response depends on a functional N fixation system. Responses of different cultivars, varieties, or genotypes of the same species to elevated CO2, however, can also vary. For example, Johnson et al. (2014) found elevated CO2 increased 86% and 56% essential amino acid concentrations and pea aphid colonization success on the high resistant cultivar ‘Sequel’ of M. sativa. However, elevated CO2 decreased 53% and 33% essential amino acid concentrations and aphid colonization on the moderate resistant cultivar ‘Genesis’. This result suggested some cultivars may become more or less susceptible to aphid attack under climate change conditions, an important consideration for determining future outcomes (McKenzie et al., 2013).
The ability to fix N is regulated by several hormone signaling pathways including the ET signaling pathway (Ma et al., 2002; Penmetsa et al., 2008). When the key gene Mtskl in the ET-perception pathway was mutated in M. truncatula, the nitrogenase activity was increased about two times (Penmetsa and Cook, 1997). Previous study has shown that elevated CO2 decreases the ET signaling pathway in Arabidopsis (Sun et al., 2013). The suppression of the ET signaling pathway in M. truncatula increased nodulation and N fixation ability, which thereby satisfied the increased demand for N by plants growing under elevated CO2. The down-regulation of the ET signaling pathway, however, is accompanied by decreased ET-mediated host resistance against the pea aphid (Guo et al., 2014b). This result suggested that in the M. truncatula–pea aphid system under elevated CO2, both nutritional and resistance effects would increase the fitness of the pea aphid by suppressing the ET signaling pathway (Figure 1).
FIGURE 1.
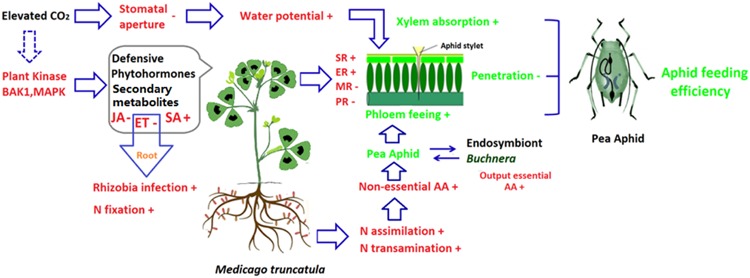
Potential effects of elevated CO2 on host plant, and the cascading effects on aphid feeding using Medicago truncatula-pea aphid as examples. Elevated CO2 affects aphid feeding efficiency in three ways. First, elevated CO2 modifies the phytohormone-dependent induced defenses and plant secondary metabolites derived defense. Enhancement of the salicylic acid-dependent defense pathway increased surface and epidermis resistance while the impairment of the jasmonic acid/ethylene-dependent defense pathway decreased mesophyll and phloem resistance. The changes of resistance facilitate the penetration feeding phase (the feeding phase that occurs before the stylet reaches the phloem). Second, the impairment of ethylene signaling pathway enhanced N fixation inroot, elevated CO2 tends to increase N assimilation and non-essential amino acid supple in the phloem. Furthermore, the aphid endosymbiont Buchnera could transform non-essential amino acid into essential amino acids, which increases the output of essential amino acid for aphids. Therefore, the increased amino acid supply benefits the phloem feeding of aphids. Third, elevated CO2 decreases stomatal conductance and transpiration, which increases the water potential in M. truncatula. As a result, aphid xylem feeding and osmotic pressure regulation are enhanced under elevated CO2. These three effects of elevated CO2 (alteration of host plant defenses, of amino acid supply in the phloem, and of host and aphid water status) greatly affect aphid feeding efficiency. AA, amino acid; BAK1, BRI-ASSOCIATED RECEPTOR KINASE 1; ER, epidermis resistance; ET, Ethylene; MAPK, mitogen protein kinases; JA, jasmonic acid; MR, mesophyll resistance; PR, phloem resistance; SA, Salicylic acid; SR, surface resistance; +, positively affected by elevated CO2; -, negatively affected by elevated CO2.
Aphid Xylem Absorption and its Relation to Plant Water Status
Aphids occasionally ingest xylem to increase their phloem feeding efficiency (Tjallingii and Esch, 1993; Douglas, 2006). Because the sugar-enriched phloem sap has an osmotic pressure that is as much as 4 to 5 times greater than that of the aphid’s haemolymph, continuously passive uptake of the phloem sap could result in aphid dehydration. To avoid self-dehydration and osmotic stress in the haemolymph during the phloem-feeding phase (i.e., to balance haemolymph osmolarity), aphids must consume a certain amount of xylem sap, which has a lower osmolarity than phloem sap (Pompon et al., 2011). This xylem-feeding behavior requires that the host plant has a relatively high plant water potential because the feeding is passive, i.e., fluid moves from plant to aphid because of a water potential gradient (Huberty and Denno, 2004; Daniels et al., 2009; Nalam et al., 2012). Aphids, like pathogens, can trigger stomatal closure, decrease leaf transpiration, and maintain the water content of the host plant by up-regulating the ABA signaling pathway. This manipulation of host stomata helps aphids absorb water from the xylem to neutralize phloem osmotic pressure (Sun et al., 2015).
Under elevated CO2, plants also exhibit reduced stomatal apertures and stomatal conductance. In FACE experiments, elevated CO2 has decreased stomatal conductance by an average of 22% for five functional plant groups that include 285 plant species (Ainsworth and Rogers, 2007). As noted, the decreased stomatal conductance reduces water loss from plants and increases plant water potential and water content (Wullschleger et al., 2002; Pritchard et al., 2007). Sun et al. (2015) found that the decreases in stomatal aperture and increases in plant water potential induced by elevated CO2 facilitated xylem feeding by aphids and thereby decreased aphid haemolymph osmolarity, which indicated a decreased cost of osmoregulation in aphids under elevated CO2.
Conclusion and Perspectives
Recent studies have provided evidence that elevated CO2 alters plant resistance, nutritional value, and water status and that these changes affect certain feeding stages of aphids (Figure 1). The evidence also indicates that such changes and effects could be mediated by the phytohormones JA, SA, ET, and ABA (Guo et al., 2013, 2014a,b; Sun et al., 2015). In these and related studies, elevated CO2 stimulated the SA signaling pathway and thereby increased the epidermis and mesophyll resistance of plants. However, elevated CO2 decreased JA and ET signaling pathways, which reduced the total time required by aphids to reach the phloem. The decreased ET signaling pathway also increased the N fixation ability of legumes and thereby increased their synthesis of amino acids, which in turn increased amino acid acquisition by aphids (Guo et al., 2013). Moreover, elevated CO2 decreased stomatal aperture and increased plant water potential, which thereby increased aphid xylem absorption and enhanced aphid osmoregulation (Sun et al., 2015). Nevertheless, transcriptomic evidence shows that elevated CO2 has a wide range of effects on plant metabolism (including C and N assimilation, secondary metabolism, and transportation), all of which may affect aphid performance (Ainsworth et al., 2006; May et al., 2013). Thus, the effects of elevated CO2 on the interaction between plants and aphids cannot be understood by simply relating one aspect of plant quality to a specific feeding phase of the aphid. Because the responses to elevated CO2 differ among plant species, it is currently difficult to generalize about how further increases in concentrations in atmospheric CO2 affects aphid feeding and damage. An increased understanding of the molecular mechanisms underlying the recognition and interactions between plants and aphids should increase our ability to predict aphid damage under elevated CO2.
In addition to changes in aphid feeding behavior, changes in aphid physiology must be considered to understand how aphid performance is affected by elevated CO2. Some studies have reported increases in aphid growth rate and fecundity under elevated CO2, which suggests that elevated CO2 increases aphid fitness and increases the probability of aphid outbreaks. At present, we have some understanding of what happens but we do not know how it happens. Like chewing insects, aphids could sense and respond to nutritional changes in host plants by regulating a complex regulatory network involving the insulin-related peptides, the target of rapamycin (TOR), ecdysteroids, and juvenile hormone (Badisco et al., 2013). For example, TOR acts as a central regulator of protein synthesis by sensing and integrating signals from amino acid nutrition, while the insulin signaling pathway is responsible for sensing carbohydrate-derived nutrients (Grewal, 2009). Thus, research is needed on how these two nutrient-sensing and regulatory pathways in aphids affect vitellogenins and juvenile hormone/ecdysone when aphids feed on plants with increased C:N ratios under elevated CO2.
Herbivorous insects can be affected by environmental change via changes in host physiology and chemical composition or via changes in competitors or natural enemies (Awmack and Leather, 2002). Elevated CO2 affects aphid performance from the level of individual physiology or even molecular function to the level of the ecosystem (Sun and Ge, 2011). The effects of elevated CO2 on individual plants and aphids may differ from the effects involving the entire ecosystem and multiple trophic levels because responses to elevated CO2 may differ among trophic levels. It is well known that elevated CO2 has bottom-up effects on the feeding behavior and population size of aphids, but the situation becomes more complicated when aphid–aphid interactions or top–down effects involving natural enemies are considered. For example, aphid species, or even different genotypes within the same species, differ in their responses to elevated CO2 (Mondor et al., 2005), and these differences might affect the outcome of intra- or inter-specific competition between aphid species or genotypes (Stacey and Fellowes, 2002; Sun et al., 2009). Furthermore, some reports indicate that parasitoids and predators are more abundant or effective under elevated CO2 (Percy et al., 2002; Chen et al., 2005) and that aphids are less sensitive to alarm pheromones under elevated CO2 (Awmack et al., 1997; Mondor et al., 2004). It seems that enhanced top-down effects on aphids under elevated CO2 may strongly alter the effects of aphids on host plants (Hentley et al., 2014).
The different feeding strategies evident in aphid responses to environmental changes are possibly driven by synchronous adaptation to host and environment. Because it directly affects herbivorous only weakly, elevated CO2 mainly influences herbivorous insect by altering the host plant (Yin et al., 2010). Thus, understanding plant–aphid interactions is likely to be central to understanding how aphids respond to elevated CO2. We suggest that molecular tools be used to better understand how the host plant ‘recognizes’ the aphid and vice versa; this research might focus on salivary secretions (the most obvious ‘signal’ available), which could trigger various molecular responses in the host that then affect the aphid in various ways. Although the knowledge from literatures shows that aphids may have species-specific molecular interaction with the hosts, it is believed that the genetics and physiology governing plant–aphid interactions have many commonalities rooted in their phylogenies so that understanding the complexity of interaction will provide meaningful insights into aphids acting on different kinds of plants and aid us in using them to our best advantage. Given increasing concentrations of atmospheric CO2 and climate change, new crop varieties will be needed that can produce sustainable yields in spite of the changing environment and the potential for increased pressure from aphids and other pests. The development of such crop varieties will be facilitated by a better understanding of the interactions between plants and aphids at molecular, community, and ecosystem levels.
Author Contributions
YS and HG wrote this article, FG revised it.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB11050400) and the National Nature Science Fund of China (nos. 31500332 and 31221091).
References
- Ainsworth E. A., Rogers A. (2007). The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 30 258–270. 10.1111/j.1365-3040.2007.01641.x [DOI] [PubMed] [Google Scholar]
- Ainsworth E. A., Rogers A., Vodkin L. O., Walter A., Schurr U. (2006). The effects of elevated CO2 concentration on soybean gene expression. An analysis of growing and mature leaves. Plant Physiol. 142 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez A. E., Tjallingii W. F., Garzo E., Vleeshouwers V., Dicke M., Vosman B. (2006). Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomol. Exp. Appl. 121 145–157. 10.1111/j.1570-8703.2006.00464.x [DOI] [Google Scholar]
- Avé D. A., Gregory P., Tingey W. M. (1987). Aphid repellent sesquiterpenes in glandular trichomes of Solanum berthaultii and S. tuberosum. Entomol. Exp. Appl. 44 131–138. 10.1111/j.1570-7458.1987.tb01057.x [DOI] [Google Scholar]
- Awmack C., Harrington R., Leather S. (1997). Host plant effects on the performance of the aphid Aulacorthum solani (Kalt.) (Homoptera: Aphididae) at ambient and elevated CO2. Glob. Chang. Biol. 3 545–549. 10.1046/j.1365-2486.1997.t01-1-00087.x [DOI] [Google Scholar]
- Awmack C. S., Leather S. R. (2002). Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47 817–844. 10.1146/annurev.ento.47.091201.145300 [DOI] [PubMed] [Google Scholar]
- Badisco L., Van Wielendaele P., Broeck J. V. (2013). Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front. Physiol. 4:202 10.3389/fphys.2013.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbehenn R. V., Chen Z., Karowe D. N., Spickard A. (2004). C3 grasses have higher nutritional quality than C4 grasses under ambient and elevated atmospheric CO2. Glob. Chang. Biol. 10 1565–1575. 10.1111/j.1365-2486.2004.00833.x [DOI] [Google Scholar]
- Behmer S. T. (2008). Insect herbivore nutrient regulation. Ann. Rev. Entomol. 54 165–187. 10.1146/annurev.ento.54.110807.090537 [DOI] [PubMed] [Google Scholar]
- Bezemer T. M., Jones T. H. (1998). Plant-insect herbivore interactions in elevated atmospheric CO2: quantitative analyses and guild effects. Oikos 82 212–222. 10.2307/3546961 [DOI] [Google Scholar]
- Bezemer T. M., Jones T. H., Knight K. J. (1998). Long-term effects of elevated CO2 and temperature on populations of the peach potato aphid Myzus persicae and its parasitoid Aphidius matricariae. Oecologia 116 128–135. 10.1007/s004420050571 [DOI] [PubMed] [Google Scholar]
- Bidart-Bouzat M. G., Mithen R., Berenbaum M. R. (2005). Elevated CO2 influences herbivory-induced defense responses of Arabidopsis thaliana. Oecologia 145 415–424. 10.1007/s00442-005-0158-5 [DOI] [PubMed] [Google Scholar]
- Bloom A. J., Burger M., Asensio J. S. R., Cousins A. B. (2010). Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328 899–903. 10.1126/science.1186440 [DOI] [PubMed] [Google Scholar]
- Bos J. I. B., Armstrong M. R., Gilroy E. M., Boevink P. C., Hein I., Taylor R. M., et al. (2010a). Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc. Natl. Acad. Sci. U.S.A. 107 9909–9914. 10.1073/pnas.0914408107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. I. B., Prince D. C., Pitino M., Maffei M. E., Win J., Hogenhout S. A. (2010b). A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (Green Peach Aphid). PLoS Genet. 6:e1001216 10.1371/journal.pgen.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteel C. L., Segal L. M., Niziolek O. K., Berenbaum M. R., DeLucia E. H. (2012). Elevated carbon dioxide increases salicylic acid in Glycine max. Environ. Entomol. 41 1435–1442. 10.1603/EN12196 [DOI] [PubMed] [Google Scholar]
- Chaudhary R., Atamian H. S., Shen Z., Briggs S. P., Kaloshian I. (2014). GroEL from the endosymbiont Buchnera aphidicola betrays the aphid by triggering plant defense. Proc. Natl. Acad. Sci. U.S.A. 111 8919–8924. 10.1073/pnas.1407687111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. J., Ge F., Parajulee M. N. (2005). Impact of elevated CO2 on tri-trophic interaction of Gossypium hirsutum, Aphis gossypii, and Leis axyridis. Environ. Entomol. 34 37–46. 10.1603/0046-225X-34.1.37 [DOI] [PubMed] [Google Scholar]
- Choi Y. E., Lim S., Kim H. J., Han J. Y., Lee M. H., Yang Y., et al. (2012). Tobacco NtLTP1, a glandular-specific lipid transfer protein, is required for lipid secretion from glandular trichomes. Plant J. 70 480–491. 10.1111/j.1365-313X.2011.04886.x [DOI] [PubMed] [Google Scholar]
- Dáder B., Fereres A., Moreno A., Trębicki P. (2016). Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 6:19120 10.1038/srep19120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M., Bale J. S., Newbury H. J., Lind R. J., Pritchard J. (2009). A sublethal dose of thiamethoxam causes a reduction in xylem feeding by the bird cherry-oat aphid (Rhopalosiphum padi), which is associated with dehydration and reduced performance. J. Insect Physiol. 55 758–765. 10.1016/j.jinsphys.2009.03.002 [DOI] [PubMed] [Google Scholar]
- DeLucia E. H., Nabity P. D., Zavala J. A., Berenbaum M. R. (2012). Climate change: resetting plant-insect interactions. Plant Physiol. 160 1677–1685. 10.1104/pp.112.204750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinant S., Bonnemain J. L., Girousse C., Kehr J. (2010). Phloem sap intricacy and interplay with aphid feeding. C. R. Biol. 333 504–515. 10.1016/j.crvi.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Docherty M., Wade F., Hurst D., Whittaker J., Lea P. (1997). Responses of tree sap-feeding herbivores to elevated CO2. Glob. Chang. Biol. 3 51–59. 10.1046/j.1365-2486.1997.00096.x [DOI] [Google Scholar]
- Douglas A. E. (2003). The nutritional physiology of aphids. Adv. Insect Physiol. 31 73–140. 10.1016/S0065-2806(03)31002-1 [DOI] [Google Scholar]
- Douglas A. E. (2006). Phloem-sap feeding by animals: problems and solutions. J. Exp. Bot. 57 747–754. 10.1093/jxb/erj067 [DOI] [PubMed] [Google Scholar]
- Febvay G., Rahbé Y., Rynkiewicz M., Guillaud J., Bonnot G. (1999). Fate of dietary sucrose and neosynthesis of amino acids in the pea aphid, Acyrthosiphon pisum, reared on different diets. J. Exp. Biol. 202 2639–2652. [DOI] [PubMed] [Google Scholar]
- Fisher D. B., Cash-Clark C. E. (2000). Sieve tube unloading and post-phloem transport of fluorescent tracers and proteins injected into sieve tubes via severed aphid stylets. Plant Physiol. 123 125–138. 10.1104/pp.123.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer J. (2003). Agroecosystem responses to combinations of elevated CO2, ozone, and global climate change. Agric. Ecosyst. Environ. 97 1–20. 10.1016/S0167-8809(03)00125-7 [DOI] [Google Scholar]
- Furch A. C. U., Hafke J. B., Schulz A., van Bel A. J. E. (2007). Ca2+ mediated remote control of reversible sieve tube occlusion in Vicia faba. J. Exp. Bot. 58 2827–2838. 10.1093/jxb/erm143 [DOI] [PubMed] [Google Scholar]
- Geiger M., Walch-Liu P., Engels C., Harnecker J., Schulze E.-D., Ludewig F., et al. (1998). Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ. 21 253–268. 10.1046/j.1365-3040.1998.00277.x [DOI] [Google Scholar]
- Gimenez-Ibanez S., Boter M., Fernández-Barbero G., Chini A., Rathjen J. P., Solano R. (2014). The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol. 12:e1001792 10.1371/journal.pbio.1001792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordanengo P., Brunissen L., Rusterucci C., Vincent C., Van Bel A., Dinant S., et al. (2010). Compatible plant-aphid interactions: how aphids manipulate plant responses. C. R. Biol. 333 516–523. 10.1016/j.crvi.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Goffreda J. C., Mutschler M. A., Avé D. A., Tingey W. M., Steffens J. C. (1989). Aphid deterrence by glucose esters in glandular trichome exudate of the wild tomato, Lycopersicon pennellii. J. Chem. Ecol. 15 2135–2147. 10.1007/BF01207444 [DOI] [PubMed] [Google Scholar]
- Goławska S. (2007). Deterrence and toxicity of plant saponins for the pea aphid Acyrthosiphon pisum Harris. J. Chem. Ecol. 33 1598–1606. 10.1007/s10886-007-9333-y [DOI] [PubMed] [Google Scholar]
- Grewal S. S. (2009). Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int. J. Biochem. Cell Biol. 41 1006–1010. 10.1016/j.biocel.2008.10.010 [DOI] [PubMed] [Google Scholar]
- Gündüz E. A., Douglas A. E. (2009). Symbiotic bacteria enable insect touse a nutritionally inadequate diet. Proc. R. Soc. Lond. B Biol. Sci. 276 987–991. 10.1098/rspb.2008.1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Sun Y., Li Y., Liu X., Wang P., Zhu-Salzman K., et al. (2014a). Elevated CO2 alters the feeding behaviour of the pea aphid by modifying the physical and chemical resistance of Medicago truncatula. Plant Cell Environ. 37 2158–2168. 10.1111/pce.12306 [DOI] [PubMed] [Google Scholar]
- Guo H., Sun Y., Li Y., Liu X., Zhang W., Ge F. (2014b). Elevated CO2 decreases the response of the ethylene signaling pathway in Medicago truncatula and increases the abundance of the pea aphid. New Phytol. 201 279–291. 10.1111/nph.12484 [DOI] [PubMed] [Google Scholar]
- Guo H., Sun Y., Li Y., Tong B., Harris M., Zhu-Salzman K., et al. (2013). Pea aphid promotes amino acid metabolism both in Medicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2. Glob. Chang. Biol. 19 3210–3223. 10.1111/gcb.12260 [DOI] [PubMed] [Google Scholar]
- Guo H., Sun Y., Ren Q., Zhu-Salzman K., Kang L., Wang C., et al. (2012). Elevated CO2 reduces the resistance and tolerance of tomato plants to Helicoverpa armigera by suppressing the JA signaling pathway. PLoS ONE 7:e41426 10.1371/journal.pone.0041426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Wang Y., Bi J. L., Yang X. Q., Huang Y., Zhao X., et al. (2009). Constitutive and induced activities of defense-related enzymes in aphid-resistant and aphid-susceptible cultivars of wheat. J. Chem. Ecol. 35 176–182. 10.1007/s10886-009-9589-5 [DOI] [PubMed] [Google Scholar]
- Hentley W. T., Vanbergen A. J., Hails R. S., Jones T. H., Johnson S. N. (2014). Elevated atmospheric CO2 impairs aphid escape responses to predators and conspecific alarm signals. J. Chem. Ecol. 40 1110–1114. 10.1007/s10886-014-0506-1 [DOI] [PubMed] [Google Scholar]
- Hettenhausen C., Schuman M. C., Wu J. (2015). MAPK signaling: a key element in plant defense response to insects. Insect Sci. 22 157–164. 10.1111/1744-7917.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenhout S. A., Bos J. I. (2011). Effector proteins that modulate plant–insect interactions. Cur. Opin. Plant Biol. 14 422–428. 10.1016/j.pbi.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Huberty A. F., Denno R. F. (2004). Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology 85 1383–1398. 10.1890/03-0352 [DOI] [Google Scholar]
- Jaouannet M., Rodriguez P. A., Thorpe P., Lenoir C. J., MacLeod R., Escudero-Martinez C., et al. (2014). Plant immunity in plant–aphid interactions. Front. Plant Sci. 5:663 10.3389/fpls.2014.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Miles P. W. (1993). Responses of a compatible lucerne variety to attack by spotted alfalfa aphid: changes in the redox balance in affected tissues. Entomol. Exp. Appl. 67 263–274. 10.1111/j.1570-7458.1993.tb01677.x [DOI] [Google Scholar]
- Johnson S. N., Ryalls J. M. W., Karley A. J. (2014). Global climate change and crop resistance to aphids: contrasting responses of lucerne genotypes to elevated atmospheric carbon dioxide. Ann. Appl. Biol. 165 62–72. 10.1111/aab.12115 [DOI] [Google Scholar]
- Karowe D. N., Grubb C. (2011). Elevated CO2 increases constitutive phenolics and trichomes, but decreases inducibility of phenolics in Brassica rapa (Brassicaceae). J. Chem. Ecol. 37 1332–1340. 10.1007/s10886-011-0044-z [DOI] [PubMed] [Google Scholar]
- King S. R. F., Mclellan H., Boevink P. C., Armstrong M. R., Bukharova T., Sukarta O., et al. (2014). Phytophthora infestans RXLR effector PexRD2 interacts with host MAPKKKε to suppress plant immune signaling. Plant Cell 26 1345–1359. 10.1105/tpc.113.120055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch M., van Bel A. J. E. (1998). Sieve tubes in action. Plant Cell 10 35–50. 10.1105/tpc.10.1.35 [DOI] [Google Scholar]
- Lake J. A., Wade R. N. (2009). Plant–pathogen interactions and elevated CO2: morphological changes in favour of pathogens. J. Exp. Bot. 60 3123–3131. 10.1093/jxb/erp147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S. K., Chen D., Norton R., Armstrong R. (2012). Does phosphorus stimulate the effect of elevated [CO2] on growth and symbiotic nitrogen fixation of grain and pasture legumes? Crop Pasture Sci. 63 53–62. 10.1071/CP11296 [DOI] [Google Scholar]
- Lei J., Finlayson S. A., Salzman R. A., Shan L., Zhu-Salzman K. (2014). BOTRYTIS-INDUCED KINASE1 modulates Arabidopsis resistance to green peach aphids via PHYTOALEXIN DEFICIENT4. Plant Physiol. 165 1657–1670. 10.1104/pp.114.242206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Ainsworth E. A., Leakey A. D., Ulanov A., Lozovaya V., Ort D. R., et al. (2008). Arabidopsis transcript and metabolite profiles: ecotype-specific responses to open air elevated [CO2]. Plant Cell Environ. 31 1673–1687. 10.1111/j.1365-3040.2008.01874.x [DOI] [PubMed] [Google Scholar]
- Ma W., Penrose D. M., Glick B. R. (2002). Strategies used by rhizobia to lower plant ethylene levels and increase nodulation. Can. J. Microbiol. 48 947–954. 10.1139/w02-100 [DOI] [PubMed] [Google Scholar]
- Martin P., Johnson S. N. (2011). Evidence that elevated CO2 reduces resistance to the European large raspberry aphid in some raspberry cultivars. J. Appl. Entomol. 135 237–240. 10.1111/j.1439-0418.2010.01544.x [DOI] [Google Scholar]
- Masle J. (2000). The effects of elevated CO2 concentrations on cell division rates, growth patterns, and blade anatomy in young wheat plants are modulated by factors related to leaf position, vernalization, and genotype. Plant Physiol. 122 1399 10.1104/pp.122.4.1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P., Liao W., Wu Y., Shuai B., McCombie W. R., Zhang M. Q., et al. (2013). The effects of carbon dioxide and temperature on microRNA expression in Arabidopsis development. Nat. Commun. 4:2145 10.1038/ncomms3145 [DOI] [PubMed] [Google Scholar]
- McKenzie S. W., Hentley W. T., Hails R. S., Jones T. H., Vanbergen A. J., Johnson S. N. (2013). Global climate change and above-belowground insect herbivore interactions. Front. Plant Sci. 4:412 10.3389/fpls.2013.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan H., Boevink P. C., Armstrong M. R., Pritchard L., Gomez S., Morales J., et al. (2013). An RxLR effector from Phytophthora infestans prevents localisation of two plant NAC transcription factors from the endoplasmic reticulum to the nucleus. PLoS Pathog. 9:e1003670 10.1371/journal.ppat.1003670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondor E. B., Tremblay M., Awmack C. S., Lindroth R. L. (2004). Divergent pheromone-mediated insect behaviour under global atmospheric change. Glob. Chang. Biol. 10 1820–1824. 10.1111/j.1365-2486.2004.00838.x [DOI] [Google Scholar]
- Mondor E. B., Tremblay M. N., Awmack C. S., Lindroth R. L. (2005). Altered genotypic and phenotypic frequencies of aphid populations under enriched CO2 and O3 atmospheres. Glob. Chang. Biol. 11 1990–1996. [Google Scholar]
- Myers S. S., Zanobetti A., Kloog I., Huybers P., Leakey A. D. B., Bloom A. J., et al. (2014). Increasing CO2 threatens human nutrition. Nature 510 139–143. 10.1038/nature13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalam V. J., Keeretaweep J., Sarowar S., Shah J. (2012). Root-derived oxylipins promote green peach aphid performance on Arabidopsis foliage. Plant Cell 24 1643–1653. 10.1105/tpc.111.094110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal J. J., Tingey W. M., Steffens J. C. (1990). Sucrose esters of carboxylic acids in glandular trichomes of Solanum berthaultii deter settling and probing by green peach aphid. J. Chem. Ecol. 16 487–497. 10.1007/BF01021780 [DOI] [PubMed] [Google Scholar]
- Newman J. A., Gibson D. J., Hickam E., Lorenz M., Adams E., Bybee L., et al. (1999). Elevated carbon dioxide results in smaller populations of the bird cherry- oat aphid Rhopalosiphum padi. Ecol. Entomol. 24 486–489. 10.1046/j.1365-2311.1999.00210.x [DOI] [Google Scholar]
- Newman J. A., Gibson D. J., Parsons A. J., Thornley J. H. M. (2003). How predictable are aphid population responses to elevated CO2. J. Anim. Ecol. 72 556–566. 10.1046/j.1365-2656.2003.00725.x [DOI] [PubMed] [Google Scholar]
- Nikoh N., McCutcheon J. P., Kudo T., Miyagishima S. Y., Moran N. A., Nakabachi A. (2010). Bacterial genes in the aphid genome: absence of functional gene transfer from Buchnera to its host. PLoS Genet. 6:e1000827 10.1371/journal.pgen.1000827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehme V., Högy P., Zebitz C. P., Fangmeier A. (2013). Effects of elevated atmospheric CO2 concentrations on phloem sap composition of spring crops and aphid performance. J. Plant Interact. 8 74–84. 10.1080/17429145.2012.736200 [DOI] [Google Scholar]
- O’Neill B. F., Zangerl A. R., DeLucia E. H., Casteel C., Zavala J. A., Berenbaum M. R. (2011). Leaf temperature of soybean grown under elevated CO2 increases Aphis glycines (Hemiptera: Aphididae) population growth. Insect Sci. 18 419–425. 10.1111/j.1744-7917.2011.01420.x [DOI] [Google Scholar]
- Penmetsa R. V., Cook D. R. (1997). A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275 527–530. 10.1126/science.275.5299.527 [DOI] [PubMed] [Google Scholar]
- Penmetsa R. V., Uribe P., Anderson J., Lichtenzveig J., Gish J. C., Nam Y. W., et al. (2008). The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 55 580–595. 10.1111/j.1365-313X.2008.03531.x [DOI] [PubMed] [Google Scholar]
- Percy K. E., Awmack C. S., Lindroth R. L., Kubiske M. E., Kopper B. J., Isebrands J. G., et al. (2002). Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature 420 403–407. 10.1038/nature01028 [DOI] [PubMed] [Google Scholar]
- Pescod K. V., Quick W. P., Douglas A. E. (2007). Aphid responses to plants with genetically manipulated phloem nutrient levels. Physiol. Entomol. 32 253–258. 10.1111/j.1365-3032.2007.00577.x [DOI] [Google Scholar]
- Pompon J., Quiring D., Goyer C., Giordanengo P., Pelletier Y. (2011). A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. J. Insect Physiol. 57 1317–1322. 10.1016/j.jinsphys.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Prince D. C., Drurey C., Zipfel C., Hogenhout S. A. (2014). The leucine-rich repeat receptor-like kinase BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1 and the cytochrome P450 PHYTOALEXIN DEFICIENT3 contribute to innate immunity to aphids in Arabidopsis. Plant Physiol. 164 2207–2219. 10.1104/pp.114.235598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J., Griffiths B., Hunt E. J. (2007). Can the plant-mediated impacts on aphids of elevated CO2 and drought be predicted? Glob. Chang. Biol. 13 1616–1629. 10.1111/j.1365-2486.2007.01401.x [DOI] [Google Scholar]
- Rhodes J., Croghan P., Dixon A. (1996). Uptake, excretion and respiration of sucrose and amino acids in the pea aphid Acyrthosiphon pisum. J. Exp. Biol. 199 1269–1276. [DOI] [PubMed] [Google Scholar]
- Robinson E. A., Ryan G. D., Newman J. A. (2012). A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 194 321–336. 10.1111/j.1469-8137.2012.04074.x [DOI] [PubMed] [Google Scholar]
- Ryalls J. M., Moore B. D., Riegler M., Gherlenda A. N., Johnson S. N. (2015). Amino acid-mediated impacts of elevated carbon dioxide and simulated root herbivory on aphids are neutralized by increased air temperatures. J. Exp. Bot. 66 613–623. 10.1093/jxb/eru439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. D., Emiljanowicz L., Haerri S. A., Newman J. A. (2014a). Aphid and host-plant genotype × genotype interactions under elevated CO2. Ecol. Entomol. 39 309–315. 10.1111/een.12101 [DOI] [Google Scholar]
- Ryan G. D., Shukla K., Rasmussen S., Shelp B. J., Newman J. A. (2014b). Phloem phytochemistry and aphid responses to elevated CO2, nitrogen fertilization and endophyte infection. Agri. For. Entomol. 16 273–283. 10.1111/afe.12055 [DOI] [Google Scholar]
- Ryan G. D., Sylvester E. V. A., Shelp B. J., Newman J. A. (2015). Towards an understanding of how phloem amino acid composition shapes elevated CO2-induced changes in aphid population dynamics. Ecol. Entomol. 40 247–257. 10.1111/een.12181 [DOI] [Google Scholar]
- Salt D. T., Fenwick P., Whittaker J. B. (1996). Interspecific herbivore interactions in a high CO2 environment: root and shoot aphids feeding on Cardamine. Oikos 77 182–237. 10.2307/3546072 [DOI] [Google Scholar]
- Sharma H. C., Sharma K. K., Seetharama N., Ortiz R. (2000). Prospects for using transgenic resistance to insects in crop improvement. Electron. J. Biotechnol. 3 21–22. 10.2225/vol3-issue2-fulltext-3 [DOI] [Google Scholar]
- Spoel S. H., Loake G. J. (2011). Redox-based protein modifications: the missing link in plant immune signalling. Cur. Opin. Plant Biol. 14 358–364. 10.1016/j.pbi.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Stacey D. A., Fellowes M. E. (2002). Influence of elevated CO2 on interspecfic interactions at higher trophic levels. Glob. Chang. Biol. 8 668–678. 10.1046/j.1365-2486.2002.00506.x [DOI] [Google Scholar]
- Stocker T. F., Qin D., Plattner G. K., Tignor M., Allen S. K., Boschung J., et al. (2013). IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, MA: Cambridge University Press. [Google Scholar]
- Sun Y., Ge F. (2011). How do aphids respond to elevated CO2? J. Asia-Pac. Entomol. 14 217–220. 10.1016/j.aspen.2010.08.001 [DOI] [Google Scholar]
- Sun Y., Guo H., Yuan L., Wei J., Zhang W., Ge F. (2015). Plant stomatal closure improves aphid feeding under elevated CO2. Glob. Chang. Biol. 21 2739–2748. 10.1111/gcb.12858 [DOI] [PubMed] [Google Scholar]
- Sun Y., Guo H., Zhu-Salzman K., Ge F. (2013). Elevated CO2 increases the abundance of the peach aphid on Arabidopsis by reducing jasmonic acid defenses. Plant Sci. 210 128–140. 10.1016/j.plantsci.2013.05.014 [DOI] [PubMed] [Google Scholar]
- Sun Y., Su J. W., Ge F. (2010). Elevated CO2 reduces the response of Sitobion avenae (Homoptera: Aphididae) to alarm pheromone. Agric. Ecosyst. Environ. 135 140–147. 10.1016/j.agee.2009.09.011 [DOI] [Google Scholar]
- Sun Y. C., Jing B. B., Ge F. (2009). Response of amino acid changes in Aphis gossypii (Glover) to elevated CO2 levels. J. Appl. Entomol. 133 189–197. 10.1111/j.1439-0418.2008.01341.x [DOI] [Google Scholar]
- Teng N., Wang J., Chen T., Wu X., Wang Y., Lin J. (2006). Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 172 92–103. 10.1111/j.1469-8137.2006.01818.x [DOI] [PubMed] [Google Scholar]
- Tjallingii W. F., Esch T. H. (1993). Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 18 317–328. 10.1111/j.1365-3032.1993.tb00604.x [DOI] [Google Scholar]
- Urbanska A., Leszczynski B., Tjallingii W. F., Matok H. (2002). Probing behaviour and enzymatic defence of the grain aphid against cereal phenolics. Electron. J. Polish Agric. Univ. Biol. 5 [Google Scholar]
- Urbanska A., Tjallingii W. F., Dixon A. F. G., Leszczynski B. (1998). Phenol oxidising enzymes in the grain aphid’s saliva. Entomol. Exp. Appl. 86 197–203. 10.1046/j.1570-7458.1998.00281.x [DOI] [Google Scholar]
- van Helden M., Tjallingii W. F. (1993). Tissue localisation of lettuce resistance to the aphid Nasonovia ribisnigri using electrical penetration graphs. Entomol. Exp. Appl. 68 269–278. 10.1111/j.1570-7458.1993.tb01713.x [DOI] [Google Scholar]
- Wang E., Hall J. T., Wagner G. J. (2004). Transgenic Nicotiana tabacum L. with enhanced trichome exudate cembratrieneols has reduced aphid infestation in the field. Mol. Breed. 13 49–57. 10.1023/B:MOLB.0000012328.04974.fb [DOI] [Google Scholar]
- Webb A. A. R., McAinsh M. R., Mansfield T. A., Hetherington A. M. (1996). Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J. 9 297–304. 10.1046/j.1365-313X.1996.09030297.x [DOI] [Google Scholar]
- Wilkinson T., Ashford D., Pritchard J., Douglas A. (1997). Honeydew sugars and osmoregulation in the pea aphid Acyrthosiphon pisum. J. Exp. Biol. 200 2137–2143. [DOI] [PubMed] [Google Scholar]
- Will T., Furch A. C., Zimmermann M. R. (2013). How phloem-feeding insects face the challenge of phloem-located defenses. Front. Plant Sci. 4:336 10.3389/fpls.2013.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will T., van Bel A. J. E. (2006). Physical and chemical interactions between aphids and plants. J. Exp. Bot. 57 729–737. 10.1093/jxb/erj089 [DOI] [PubMed] [Google Scholar]
- Wullschleger S. D., Tschaplinski T. J., Norby R. J. (2002). Plant water relations at elevated CO2–implications for water-limited environments. Plant Cell Environ. 25 319–331. 10.1046/j.1365-3040.2002.00796.x [DOI] [PubMed] [Google Scholar]
- Xie H., Zhao L., Wang W., Wang Z., Ni X., Cai W., et al. (2014). Changes in life history parameters of Rhopalosiphum maidis (Homoptera: Aphididae) under four different elevated temperature and CO2 combinations. J. Econ. Entomol. 107 1411–1418. 10.1603/EC13302 [DOI] [PubMed] [Google Scholar]
- Yin J., Sun Y., Wu G., Ge F. (2010). Effects of elevated CO2 associated with maize on multiple generations of the cotton bollworm, Helicoverpa armigera. Entomol. Exp. Appl. 136 12–20. 10.1111/j.1570-7458.2010.00998.x [DOI] [Google Scholar]
- Zavala J. A., Casteel C. L., DeLucia E. H., Berenbaum M. R. (2008). Anthropogenic increase in carbon dioxide compromises plant defense against invasive insects. Proc. Natl. Acad. Sci. U.S.A. 105 5129–5133. 10.1073/pnas.0800568105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala J. A., Casteel C. L., Nabity P. D., Berenbaum M. R., DeLucia E. H. (2009). Role of cysteine proteinase inhibitors in preference of Japanese beetles (Popillia japonica) for soybean (Glycine max) leaves of different ages and grown under elevated CO2. Oecologia 161 35–41. 10.1007/s00442-009-1360-7 [DOI] [PubMed] [Google Scholar]
- Zavala J. A., Nabity P. D., DeLucia E. H. (2013). An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 58 79–97. 10.1146/annurev-ento-120811-153544 [DOI] [PubMed] [Google Scholar]
- Zhang J., Xing G. M., Liao J. X., Hou Z. D., Wang G. X., Wang Y. F. (2003). Effects of different atmospheric CO2 concentrations and soil moistures on the populations of bird cherry-oat aphid (Rhopalosiphum padi) feeding on spring wheat. Eur. J. Entomol. 100 521–530. 10.14411/eje.2003.080 [DOI] [Google Scholar]


