Abstract
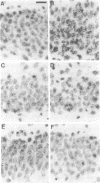
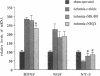
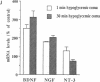
In situ hybridization was used to study expression of mRNAs for members of the nerve growth factor (NGF) family in the rat brain after 2 and 10 min of forebrain ischemia and 1 and 30 min of insulin-induced hypoglycemic coma. Two hours after the ischemic insults, the level of brain-derived neurotrophic factor (BDNF) mRNA was markedly increased in the granule cells of the dentate gyrus, and at 24 h it was still significantly elevated. NGF mRNA showed a pronounced increase 4 h after 2 min of ischemia but had returned to a control level at 24 h. Both 2 and 10 min of ischemia caused a clear reduction of the level of mRNA for neurotrophin 3 (NT-3) in the dentate granule cells and in regions CA2 and medial CA1 of the hippocampus 2 and 4 h after the insults. The increase of BDNF mRNA could be partially blocked by the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist NBQX but was not influenced by the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801. Both NBQX and MK-801 attenuated the decrease of NT-3 mRNA after ischemia. One and 30 min of hypoglycemic coma also induced marked increases in BDNF and NGF mRNA in dentate granule cells with maximal levels at 2 h. If the changes of mRNA expression lead to alterations in the relative availability of neurotrophic factors, this could influence functional outcome and neuronal necrosis following ischemic and hypoglycemic insults.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson R. F., Alterman A. L., Barde Y. A., Lindsay R. M. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990 Sep;5(3):297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Auer R. N., Olsson Y., Siesjö B. K. Hypoglycemic brain injury in the rat. Correlation of density of brain damage with the EEG isoelectric time: a quantitative study. Diabetes. 1984 Nov;33(11):1090–1098. doi: 10.2337/diab.33.11.1090. [DOI] [PubMed] [Google Scholar]
- Auer R. N., Siesjö B. K. Biological differences between ischemia, hypoglycemia, and epilepsy. Ann Neurol. 1988 Dec;24(6):699–707. doi: 10.1002/ana.410240602. [DOI] [PubMed] [Google Scholar]
- Ayer-LeLievre C., Olson L., Ebendal T., Seiger A., Persson H. Expression of the beta-nerve growth factor gene in hippocampal neurons. Science. 1988 Jun 3;240(4857):1339–1341. doi: 10.1126/science.2897715. [DOI] [PubMed] [Google Scholar]
- Ballarín M., Ernfors P., Lindefors N., Persson H. Hippocampal damage and kainic acid injection induce a rapid increase in mRNA for BDNF and NGF in the rat brain. Exp Neurol. 1991 Oct;114(1):35–43. doi: 10.1016/0014-4886(91)90082-n. [DOI] [PubMed] [Google Scholar]
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Buzsàki G., Freund T. F., Bayardo F., Somogyi P. Ischemia-induced changes in the electrical activity of the hippocampus. Exp Brain Res. 1989;78(2):268–278. doi: 10.1007/BF00228898. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988 Oct;11(10):465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Curran T., Gordon M. B., Rubino K. L., Sambucetti L. C. Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2(1):79–84. [PubMed] [Google Scholar]
- Ebendal T. NGF in CNS: experimental data and clinical implications. Prog Growth Factor Res. 1989;1(3):143–159. doi: 10.1016/0955-2235(89)90008-2. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Bengzon J., Kokaia Z., Persson H., Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991 Jul;7(1):165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Ibáez C. F., Ebendal T., Olson L., Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Wetmore C., Olson L., Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990 Oct;5(4):511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Ernfors Patrik, Persson Håkan. Developmentally Regulated Expression of HDNF/NT-3 mRNA in Rat Spinal Cord Motoneurons and Expression of BDNF mRNA in Embryonic Dorsal Root Ganglion. Eur J Neurosci. 1991;3(10):953–961. doi: 10.1111/j.1460-9568.1991.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Friedman W. J., Ernfors P., Persson H. Transient and persistent expression of NT-3/HDNF mRNA in the rat brain during postnatal development. J Neurosci. 1991 Jun;11(6):1577–1584. doi: 10.1523/JNEUROSCI.11-06-01577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman Wilma J., Olson Lars, Persson Håkan. Cells that Express Brain-Derived Neurotrophic Factor mRNA in the Developing Postnatal Rat Brain. Eur J Neurosci. 1991 Jul;3(7):688–697. doi: 10.1111/j.1460-9568.1991.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Gall C. M., Isackson P. J. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989 Aug 18;245(4919):758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- Gall C., Murray K., Isackson P. J. Kainic acid-induced seizures stimulate increased expression of nerve growth factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 1991 Jan;9(1-2):113–123. doi: 10.1016/0169-328x(91)90136-l. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Morrell F., deToledo-Morrell L. Remodeling of synaptic architecture during hippocampal "kindling". Proc Natl Acad Sci U S A. 1988 May;85(9):3260–3264. doi: 10.1073/pnas.85.9.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T., Vahlsing H. L., Manthorpe M., Varon S. Nerve growth factor infusion into the denervated adult rat hippocampal formation promotes its cholinergic reinnervation. J Neurosci. 1990 Sep;10(9):3087–3092. doi: 10.1523/JNEUROSCI.10-09-03087.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbök F., Ibáez C. F., Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991 May;6(5):845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986 Aug;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M. M., Barde Y. A. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988 Jan 21;331(6153):261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S. R., Hohn A., Leibrock J., Barde Y. A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990 Aug;9(8):2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn A., Leibrock J., Bailey K., Barde Y. A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990 Mar 22;344(6264):339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Hyman C., Hofer M., Barde Y. A., Juhasz M., Yancopoulos G. D., Squinto S. P., Lindsay R. M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991 Mar 21;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Barde Y. A., Schwab M., Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986 Oct;6(10):3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. R., Reichardt L. F. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho Y., Yoshimura K., Nakahama K. Cloning and expression of a cDNA encoding a novel human neurotrophic factor. FEBS Lett. 1990 Jun 18;266(1-2):187–191. doi: 10.1016/0014-5793(90)81536-w. [DOI] [PubMed] [Google Scholar]
- Knüsel B., Winslow J. W., Rosenthal A., Burton L. E., Seid D. P., Nikolics K., Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer L. F. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987 Jan 9;235(4785):214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Lorez H., Keller F., Ruess G., Otten U. Nerve growth factor increases in adult rat brain after hypoxic injury. Neurosci Lett. 1989 Apr 10;98(3):339–344. doi: 10.1016/0304-3940(89)90425-4. [DOI] [PubMed] [Google Scholar]
- Lu B., Buck C. R., Dreyfus C. F., Black I. B. Expression of NGF and NGF receptor mRNAs in the developing brain: evidence for local delivery and action of NGF. Exp Neurol. 1989 Jun;104(3):191–199. doi: 10.1016/0014-4886(89)90029-0. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Friedman B., Alderson R. F., Wiegand S. J., Furth M. E., Lindsay R. M., Yancopoulos G. D. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990 Oct;5(4):501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Messenheimer J. A., Harris E. W., Steward O. Sprouting fibers gain access to circuitry transsynaptically altered by kindling. Exp Neurol. 1979 Jun;64(3):469–481. doi: 10.1016/0014-4886(79)90225-5. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Woo J. E., Edwards R. H., Riopelle R. J., Longo F. M., Weskamp G., Otten U., Valletta J. S., Johnston M. V. Developmental regulation of nerve growth factor and its receptor in the rat caudate-putamen. Neuron. 1989 Nov;3(5):655–664. doi: 10.1016/0896-6273(89)90276-6. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986 Aug 7;322(6079):552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Phillips H. S., Hains J. M., Laramee G. R., Rosenthal A., Winslow J. W. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990 Oct 12;250(4978):290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- Popovici T., Represa A., Crépel V., Barbin G., Beaudoin M., Ben-Ari Y. Effects of kainic acid-induced seizures and ischemia on c-fos-like proteins in rat brain. Brain Res. 1990 Dec 17;536(1-2):183–194. doi: 10.1016/0006-8993(90)90024-6. [DOI] [PubMed] [Google Scholar]
- Pulsinelli W. A., Brierley J. B., Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982 May;11(5):491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Represa A., Le Gall La Salle G., Ben-Ari Y. Hippocampal plasticity in the kindling model of epilepsy in rats. Neurosci Lett. 1989 May 8;99(3):345–350. doi: 10.1016/0304-3940(89)90471-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Goeddel D. V., Nguyen T., Lewis M., Shih A., Laramee G. R., Nikolics K., Winslow J. W. Primary structure and biological activity of a novel human neurotrophic factor. Neuron. 1990 May;4(5):767–773. doi: 10.1016/0896-6273(90)90203-r. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R., Freund T. F. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40(3):599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Shigeno T., Mima T., Takakura K., Graham D. I., Kato G., Hashimoto Y., Furukawa S. Amelioration of delayed neuronal death in the hippocampus by nerve growth factor. J Neurosci. 1991 Sep;11(9):2914–2919. doi: 10.1523/JNEUROSCI.11-09-02914.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö B. K., Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab. 1989 Apr;9(2):127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Mechanisms of ischemic brain damage. Crit Care Med. 1988 Oct;16(10):954–963. doi: 10.1097/00003246-198810000-00006. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Auer R. N., Siesjö B. K. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol. 1984;64(4):319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Bendek G., Dahlgren N., Rosén I., Wieloch T., Siesjö B. K. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand. 1984 Jun;69(6):385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- Sutula T., He X. X., Cavazos J., Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988 Mar 4;239(4844):1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Bandtlow C., Heumann R. The physiological function of nerve growth factor in the central nervous system: comparison with the periphery. Rev Physiol Biochem Pharmacol. 1987;109:145–178. doi: 10.1007/BFb0031026. [DOI] [PubMed] [Google Scholar]
- Wetmore C., Ernfors P., Persson H., Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990 Aug;109(2):141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- Whittemore S. R., Friedman P. L., Larhammar D., Persson H., Gonzalez-Carvajal M., Holets V. R. Rat beta-nerve growth factor sequence and site of synthesis in the adult hippocampus. J Neurosci Res. 1988 Aug;20(4):403–410. doi: 10.1002/jnr.490200402. [DOI] [PubMed] [Google Scholar]
- Whittemore S. R., Seiger A. The expression, localization and functional significance of beta-nerve growth factor in the central nervous system. Brain Res. 1987 Nov;434(4):439–464. doi: 10.1016/0165-0173(87)90008-7. [DOI] [PubMed] [Google Scholar]
- Williams L. R., Varon S., Peterson G. M., Wictorin K., Fischer W., Bjorklund A., Gage F. H. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F., Hengerer B., Leibrock J., Thoenen H., Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990 Nov;9(11):3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]