Abstract
Background
Excessive antibiotic use in cold and flu season is costly and contributes to antibiotic resistance. The study objective was to develop an index of excessive antibiotic use in cold and flu season and determine its correlation with other indicators of prescribing quality.
Methods and Findings
We included Medicare beneficiaries in the 40% random sample denominator continuously enrolled in fee for service benefits for 2010 and/or 2011 (7,961,201 person-years (PY)) and extracted data on prescription fills for oral antibiotics that treat respiratory pathogens. We collapsed the data to the state-level so that it could be merged with monthly flu activity data from the Centers for Disease Control and Prevention (CDC). Linear regression, adjusted for state-specific mean antibiotic use and demographic characteristics, was used to estimate how antibiotic prescribing responded to state-specific flu activity. There was considerable geographic variation in flu-associated antibiotic use across states—lowest in Vermont and Connecticut and highest in Mississippi and Florida. There was a robust positive correlation between medications that often cause adverse events in the elderly and flu-associated prescribing (0.755; p<0.001), while there was a strong negative correlation with beta-blocker use after a myocardial infarction (MI) (−0.413; p=0.003).
Conclusion
Adjusted flu-associated antibiotic use was positively correlated with high-risk medications to the elderly and negatively correlated with beta-blocker use post MI. These findings suggest excessive antibiotic use reflects low quality prescribing, and imply that practice and policy solutions should go beyond narrow, antibiotic-specific, approaches to encourage evidence-based prescribing for the elderly Medicare population.
Keywords: Antibiotic Use, Antibiotic Resistance, Influenza, Evidence-Based Prescribing
Introduction
An estimated 2 million people are infected with drug-resistant organisms annually in the United States, resulting in 23,000 deaths and over 20 billion dollars in excess costs.1,2 The use of antibiotics for viral-associated upper respiratory infections contributes to the spread of antibiotic resistance.3 Although seasonal and influenza-related trends in antibiotic prescribing have been described,4–8 to date, there are few studies that look at excessive antibiotic use at the population-level. 5,9–11 Those that do use methods which are vulnerable to the critique that risk-adjusted geographic variation does not necessarily indicate inappropriate use.12 Furthermore, excessive antibiotic use could reflect a tendency by providers to prescribe all types of medications—including those that are clinically indicated— and therefore would not be synonymous with poor prescribing quality.
To better understand patterns of antibiotic overuse and whether such patterns reflect the quality of prescribing, we developed a novel index that isolates antibiotic prescribing in response to local influenza activity. We constructed our index using data from Medicare Part D—including beneficiaries in the 40% random sample denominator continuously enrolled in fee for service benefits for 2010 and/or 2011. We focus on the elderly since nationally representative data on antibiotic use are readily available and because polypharmacy and drug interactions are particularly important for this population. We extracted data on prescription fills for oral antibiotics that treat respiratory pathogens and collapsed the data to the state-level so that it could be merged with monthly flu activity data from the Centers for Disease Control and Prevention (CDC). We estimated a linear regression of antibiotic prescribing on the calendar month (thus capturing seasonal patterns of antibiotic use), the state (reflecting permanent differences in state-level antibiotic prescriptions), and monthly state-level CDC influenza activity as well as state-level demographics. The coefficient on the CDC influenza activity, which is allowed to vary by state, is our index of state-level excessive antibiotic prescribing.
To determine whether excessive antibiotic use reflects low prescribing quality to the elderly more broadly, we correlate our index with the use of medications that can often cause adverse events in the elderly and clinically indicated prescriptions, such as beta-blocker use after a myocardial infarction. If overuse of antibiotics is positively correlated with both of these indicators, this would imply physicians in some places are simply prescribing more frequently. If, on the other hand, inappropriate use of one medication is correlated with failure to use a different medication when indicated, this would suggest that antibiotic overuse is symptomatic of low prescribing quality.
Methods
Study Cohorts and Data Sources
We used a 40% random-sample of 2010–2011 fee-for-service Medicare Parts A (inpatient), B (outpatient) and D (prescription) beneficiaries, and identified 9,335,698 person-years representing individuals over age 65 who were enrolled for at least a full calendar year. To exclude those who might justifiably have unique antibiotic use patterns or medication fills not apparent in Part D claims, we removed 1,282,862 with a cancer diagnosis (other than nonmelanoma skin cancer patients); 79,294 enrolled in hospice and 12,341 with end-stage renal disease as the original eligibility for Medicare enrollment. Our final cohort therefore included 7,961,201 person-years.
Respiratory Antibiotic Data
The Medicare Prescription Drug Event (PDE) data files include national drug codes (NDC), prescription fill date, and type of antibiotic filled. We defined antibiotics used for respiratory infections as Cephalosporins, Macrolides, Tetracyclines, Penicillins and Quinolones. We limited our analysis to oral antibiotics. We excluded Sulfonamides, Nitrofurantoin, Fosfomycin, Methenamine and Trimethoprim which are often used to treat urinary tract infections. We used ZIP codes to assign each beneficiary to a given state. To calculate the number of respiratory antibiotics prescriptions filled by state and month over the two-year period of the analysis, we summed the total number of respiratory antibiotic fills by month for each state. We used this measure to construct the number of respiratory antibiotics per 100 Medicare Part D Beneficiaries for the 2010–2011 period.
Influenza Activity Data
The US Outpatient Influenza-like Illness Program Surveillance Network (ILINet) coordinated by the CDC, and consisting of more than 2,900 outpatient providers, measures influenza activity across US states. Each week, these providers report to the CDC the total number of patients and the number of those patients with ILI,13 defined as a fever (temperature of 100°F or greater), cough, and/or sore throat without a known cause other than influenza. The CDC determines influenza activity by comparing the ILI prevalence in a given week with a region-specific baseline. This procedure yields 10 activity levels ranging from minimal (level 1–3), low (levels 4–5), moderate (levels 6–7) to high (levels 8–10) activity. An activity level of 1 corresponds to values that are below the mean and values of 2–10 correspond to activity levels above the mean. Further details of the CDC methodology can be found on their website.13 We used the ILINet data to construct state-level monthly measures of influenza, which was the finest temporal level we could use without encountering problems of censorship for low frequency antibiotic cell values, and accounted for the nonlinearity of the activity levels scale by using its natural logarithm in the analysis.
High-Risk and Beta-Blocker Prescribing Data
The Dartmouth Atlas of Health Care provides a measure of the proportion of the Medicare population filling at least one prescription for a high-risk medication by state.14 This measure is constructed using drug utilization rates from a 40% Medicare random sample for the year 2010. The numerator is the number of beneficiaries filling one or more prescriptions that have been classified as high-risk for individuals over 65 years of age by the Healthcare Effectiveness Data and Information Set (HEDIS),15,16 while the denominator is the number of eligible people in the sample. The HEDIS list includes prescription drugs that often result in adverse drug events that contribute to hospitalization, increased duration of illness, nursing home placement, falls and fractures. A similar measure is constructed for the proportion of the Medicare population receiving a beta-blocker within six months of a myocardial infarction (MI).
Demographic data from Medicare
We used Medicare data to calculate the percent of beneficiaries who are minority (Black and Hispanic), male, percent old (65–69), medium old (70–79) and very old (80+), percent on a low income subsidy, and percent with a diagnosis of chronic pulmonary disease or chronic tobacco use. The pulmonary/tobacco category included individuals with ICD-9 diagnostic codes 415 (acute cor pulmonale), 416.8 (chronic pulmonary heart disease), 416.9 (chronic pulmonary heart disease, unspecified), 491 (chronic bronchitis), 492 (emphysema), 494 (bronchiectasis), 496 (chronic airway obstruction), 305.1 (tobacco use disorder), V15.82 (history of tobacco use), and 989.84 (tobacco use). These data enter as time-varying, state-level controls in the analysis.
Analysis
We constructed a longitudinal state by month dataset of respiratory antibiotic prescriptions and flu activity (approximately 24 observations per state for a total of 1188 observations; 12 observations were dropped due to missing flu observations). A linear regression on these data estimated how the number of respiratory antibiotic prescription fills per 100 Medicare Part D beneficiaries responded to changes in state-specific flu activity. In the regression, the sensitivity of antibiotic prescribing to changes in flu activity was allowed to vary across each state by interacting the flu variable with a state-specific indicator variable.17 By also including indicator variables for each state and month, the regression explicitly controls for state-level average effects (that is, antibiotic use in Mississippi may be different from its use in Vermont because of higher underlying disease burdens) and for monthly average effects (all states may experience higher rates of bronchitis in January). In addition, we adjust for demographic factors also believed to influence prescribing behavior, such as age, chronic pulmonary disease, tobacco use as well as minority and socioeconomic status, which is proxied for by the percent of the beneficiary population on a low-income subsidy.
We calculate flu-associated respiratory antibiotic prescriptions as the product of the state-specific interaction coefficient and moderate flu activity (levels 6–7). The state-specific response for New Hampshire could not be calculated since the flu activity data did not vary over the analysis period within the state. A negative coefficient suggests that certain states are responding to the heightened flu activity by prescribing a substitute (such as an antiviral) or symptomatic relief, while a positive sign reflects increased antibiotic prescribing within a state in response to upward deviations in flu activity. The exact equations used to estimate the relationship between flu and antibiotic use as well as to calculate the predicted values are available in the technical appendix. Analyses were conducted using STATA version 13.0 (StataCorp, College Station, TX). Robust standard errors were clustered at the state level.
Ethical Approval
The analyses, which required the creation of de-identified aggregate Medicare Data, was approved by the Dartmouth Institutional Review Board (IRB). The Stanford University and University of British Colombia researchers, who only had access to the de-identified aggregate data, were exempt from IRB approval.
Results
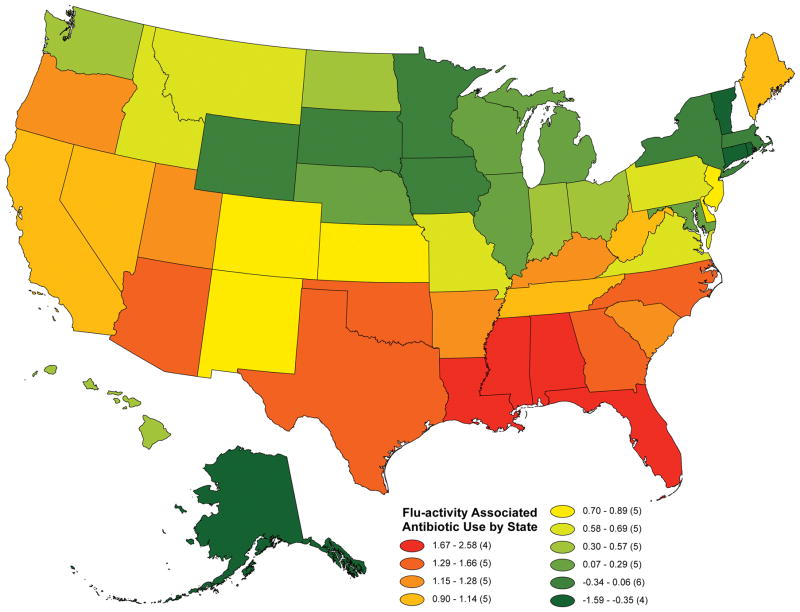
There is considerable heterogeneity in our measure of excessive antibiotic use across the United States. Mississippi and Florida had the greatest prescribing response to flu activity, with increases of antibiotic prescriptions per 100 Medicare Part D beneficiaries of 2.58 and 1.91, respectively (see Appendix Table 1 for all values). In contrast, states particularly in the Northeast responded to heightened flu activity by decreasing their use of antibiotic prescriptions, perhaps reflecting greater patient and/or provider awareness of the likelihood of a viral versus bacterial etiology for respiratory tract symptoms. These results are shown in Figure 1 which is a map demonstrating the predicted, adjusted flu-associated antibiotic use per 100 Medicare Part D beneficiaries. Red states correspond to heightened antibiotic prescribing responsiveness to flu activity and blue states correspond to less responsiveness. The different colors represent deciles of the adjusted flu-associated antibiotic use index.
Figure 1. Geographic Variation in Adjusted Flu-Predicted Antibiotic Use per 100 Medicare Part D Beneficiaries.
Notes: Deciles of predicted, adjusted flu-associated antibiotic use per 100 Medicare beneficiaries are represented in varying colors. Predicted values are obtained following linear regression that controls for mean antibiotic use by state (using state fixed effects) and time-varying demographic characteristics including the percent of beneficiaries that are in certain age categories, percent on a low-income subsidy, percent male, percent minority and percent with chronic pulmonary disease/tobacco use. The red states are those with greater antibiotic prescribing responsiveness to flu activity and the blue states are those with less responsiveness. The value for each state is provided in Appendix Table 1.
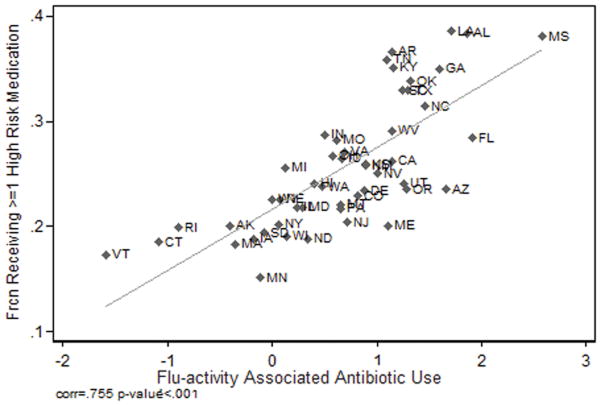
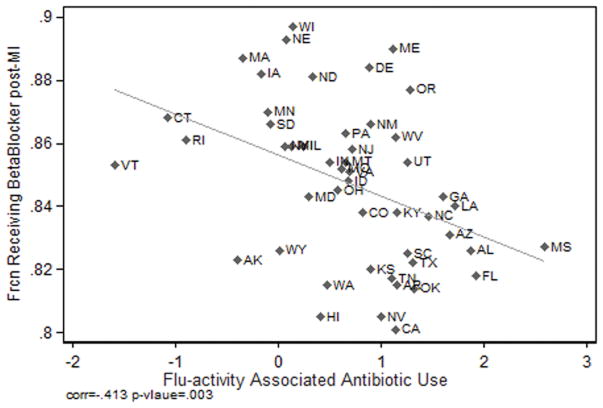
Excessive antibiotic use appears to reflect poor prescribing quality. Evidence to support this hypothesis is presented in Figures 2 and 3. Figure 2 presents the relationship between the adjusted flu-associated respiratory antibiotic fills per 100 Medicare Part D beneficiaries and an independent measure of poor quality prescribing: the fraction of the eligible Medicare population receiving one or more high-risk non-antibiotic drugs. The correlation coefficient is positive and significant, (0.755; p < .001). Figure 3 demonstrates that the adjusted flu-associated antibiotic use per 100 Medicare Part D beneficiaries correlates negatively with the fraction of the elderly Medicare population receiving beta blockers within 6 months of a myocardial infarction (correlation coefficient of, −0.413; p =.003).
Figure 2. High Risk Medications and Flu-adjusted Antibiotic Use.
Notes: The adjusted flu-associated index of respiratory antibiotic is positively correlated to an independent measure of inappropriate prescribing: the fraction of the eligible Medicare population receiving one or more high-risk drugs as defined by the HEDIS high-risk drug list (Dartmouth Atlas, 2013).
Figure 3. Beta-Blocker and Flu-adjusted Responsive Antibiotic Use.
Notes: The adjusted flu-associated index of respiratory antibiotic is negatively correlated to an independent measure of appropriate prescribing: the fraction of the of the eligible Medicare population receiving a beta-blocker within six months of a myocardial infarction (Dartmouth Atlas, 2013).
Discussion
Our study makes two contributions. First, we develop a novel method of measuring excessive antibiotic use. We found wide geographic variation in this index across the United States. Our findings concur with those of Zhang et al., who also demonstrated considerable variation in antibiotic use across regions of the United States which was highest in the South. Our study differs from Zhang et al. by focusing on state rather than regional measures, and by the use of an independent CDC measure of monthly flu activity to capture variation in the responsiveness of antibiotic prescribing to viral etiologies.
Our second contribution is to correlate our index of excessive antibiotic use with prescribing for other medications. We find these adjusted flu-associated antibiotic prescriptions are tightly positively associated with distinct geographical practices of high-risk prescribing in the elderly and negatively associated with the use of beta-blockers after a myocardial infraction. These findings provide support for the hypothesis that excessive antibiotic use is symptomatic of low quality prescribing more broadly.
Our findings are subject to several limitations. First, the index we develop is at the state level. The same index could be developed at a more disaggregated level of analysis if finer data on influenza were publicly available. Second, some geographic differences in antibiotic prescribing are warranted and may reflect changes in the incidence of bacterial pneumonia. However, our empirical strategy is designed specifically to measure only respiratory antibiotic fills associated with differences in flu activity within states controlling for demographic characteristics. An alternative approach to capturing pneumonia-based differences in prescribing would be to include physician billing codes for bacterial infections in office visits as an additional control in the analysis. We did not follow this approach because it raises a concern about potential reverse causation as physicians prescribing antibiotics may be more likely to code for bacterial infection. Using hospitalizations for pneumonia instead of outpatient visits is also problematic given secular changes in diagnostic coding.18 Third, part of the state-level variation in antibiotic overuse may be the consequence of patient demand.19–21 Disentangling the extent to which geographic variation reflects patient demand versus provider supply side factors is an active area of research.22–24 While patient demand for inappropriate antibiotic prescriptions may require public education campaigns, ultimately the physician, as society’s medication steward, must authorize the prescription.
Jumps in antibiotic use associated with influenza-like illness provide a valuable index of excessive antibiotic use. There have been calls for new policies designed to address potential public health threats (and excess costs) arising from the overuse of antibiotics.2,25,26 However, this study suggests that monitoring antibiotic overuse should not be done in isolation but rather part of a broader agenda of improving safe and evidence-based prescribing to the elderly.27–31
Acknowledgments
Funding/Support: Financial support for this research was provided by the National Institutes of Aging (P01-AG019783).
Footnotes
Author Contributions: All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study: Marcella Alsan, Jon Skinner and Nancy Morden.
Collection, management, analysis, and interpretation of the data: Marcella Alsan, Jon Skinner, and Nancy Morden.
Preparation, review, or approval of the manuscript: All authors.
Conflict of Interest Disclosures: Skinner is an investor in Dorsata, Inc., a startup clinical pathway software company. No other authors have conflicts of interest with regard to this study.
References
- 1.Centers for Disease Control and Prevention. Adult Appropriate Antibiotic Use Summary: Physician Information Sheet (Adults) 2012 Retrieved from http://www.cdc.gov/getsmart/campaign-materials/info-sheets/adult-approp-summary.html.
- 2.Centers for Disease Control and Prevention. World Health Day Media Fact Sheet: Antimicrobial Resistance No Action Today No Cure Tomorrow. 2011 Retrieved from http://www.cdc.gov/media/releases/2011/f0407_antimicrobialresistance.html.
- 3.Centers for Disease Control and Prevention. Cold and Flu Season: No Reason for Antibiotics. 2009 Retrieved from http://www.cdc.gov/getsmart/campaign-materials/press_kit/Cold-Flu_Season-508.pdf.
- 4.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Taylor TH. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrobial Agents and Chemotherapy. 2014;58(5):2763–2766. doi: 10.1128/AAC.02239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Steinman MA, Kaplan CM. Geographic variation in outpatient antibiotic prescribing among older adults. Archives of Internal Medicine. 2012;172(19):1465–1471. doi: 10.1001/archinternmed.2012.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun L, Klein EY, Laxminarayan R. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clinical Infectious Diseases. 2012;55(5):687–694. doi: 10.1093/cid/cis509. [DOI] [PubMed] [Google Scholar]
- 7.Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278(11):901–904. [PubMed] [Google Scholar]
- 8.Ciesla G, Leader S, Stoddard J. Antibiotic prescribing rates in the US ambulatory care setting for patients diagnosed with influenza, 1997–2001. Respiratory Medicine. 2004;98(11):1093–1101. doi: 10.1016/j.rmed.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996–2010. JAMA. 2014;311(19):2020–2022. doi: 10.1001/jama.2013.286141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA. 2005;294(18):2315–2322. doi: 10.1001/jama.294.18.2315. [DOI] [PubMed] [Google Scholar]
- 11.Linder JA, Stafford RS. Antibiotic Treatment of Adults With Sore Throat by Community Primary Care Physicians. JAMA. 2001;286(10):1181–1186. doi: 10.1001/jama.286.10.1181. [DOI] [PubMed] [Google Scholar]
- 12.McWilliams JM, Dalton JB, Landrum MB, Frakt AB, Pizer SD, Keating NL. Geographic Variation in Cancer-Related Imaging: Veterans Affairs Health Care System Versus Medicare. Annals of Internal Medicine. 2014;161(11):794–802. doi: 10.7326/M14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. [Accessed October 14, 2014];Overview of Influenza Surveillance in the United States. 2014 Retrieved from http://www.cdc.gov/flu/weekly/overview.htm.
- 14.The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice. 2014 Available from http://www.dartmouthatlas.org/
- 15.National Committee for Quality Assurance. HEDIS 2014 Final NDC Lists. 2014 Retrieved from http://www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures/HEDIS2014/HEDIS2014FinalNDCLists.aspx.
- 16.Munson JC, Morden NE. The Dartmouth Atlas of Medicare Prescription Use. 2013 Retrieved from http://www.dartmouthatlas.org/downloads/reports/Prescription_Drug_Atlas_101513.pdf. [PubMed]
- 17.United States Census Bureau. Census, Regions and Divisions of the United States. Retrieved from https://www.census.gov/geo/maps-data/maps/pdfs/reference/us_regdiv.pdf.
- 18.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of Diagnostic Coding With Trends in Hospitalizations and Mortality of Patients With Pneumonia, 2003–2009. JAMA. 2012;307(13):1405–1413. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane J, Holmes W, Macfarlane R, Britten N. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ. 1997;315(7117):1211–1214. doi: 10.1136/bmj.315.7117.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cals JW, Boumans D, Lardinois RJ, Gonzales R, Hopstaken RM, Buttler CC, Dinant GJ. Public beliefs on antibiotics and respiratory tract infections: an internet-based questionnaire study. British Journal of General Practice. 2007;57(545):942–947. doi: 10.3399/096016407782605027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coenen S, Michiels B, Renard D, Denekens J, Van Royen P. Antibiotic prescribing for acute cough: the effect of perceived patient demand. British Journal of General Practce. 2006;56(524):183–190. [PMC free article] [PubMed] [Google Scholar]
- 22.Cutler D, Stern AD, Skinner J, Wennberg D. Physician Beliefs and Patient Preferences: A New Look at Regional Variation in Health Care Spending. National Bureau of Economic Research; 2013. Working Paper No.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelstein A, Gentzkow M, Williams H. Sources of Geographic Variation in Health Care : Evidence from Patient Migration. National Bureau of Economic Research; 2014. Working Paper No. w20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemens J, Gottlieb JD. Physicians’ Financial Incentives Affect Medical Treatment and Patient Health? The American Economic Review. 2014;104(4):1320–1349. doi: 10.1257/aer.104.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, … Cars O. Antibiotic resistance — the need for global solutions. Lancet Infectious Disease. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 26.The White House. Executive Order--Combatting Antibiotic-Resistant Bacteria. Office of the Press Secretary; 2014. Retrieved from http://www.whitehouse.gov/the-press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. [Google Scholar]
- 27.Liu GG, Christensen DB. The continuing challenge of inappropriate prescribing in the elderly: an update of the evidence. Journal of the American Pharmaceutical Association (Washington, DC:1996) 2001;42(6):847–857. doi: 10.1331/108658002762063682. [DOI] [PubMed] [Google Scholar]
- 28.Barry PJ, O’Keefe N, O’Connor KA, O’Mahony D. Inappropriate prescribing in the elderly: a comparison of the Beers criteria and the improved prescribing in the elderly tool (IPET) in acutely ill elderly hospitalized patients. Journal of Clinical Pharmacy and Therapeutics. 2006;31(6):617–626. doi: 10.1111/j.1365-2710.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- 29.Curtis LH, Østbye T, Sendersky V, Hutchinson S, Dans PE, Writgh A, … Schulman KA. Inappropriate prescribing for elderly Americans in a large outpatient population. Archives of Internal Medicine. 2004;164(15):1621–1625. doi: 10.1001/archinte.164.15.1621. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher P, Barry P, O’Mahony D. Inappropriate prescribing in the elderly. Journal of Clinical Pharmacy and Therapeutics. 2007;32(2):113–121. doi: 10.1111/j.1365-2710.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 31.Lai HY, Hwang SJ, Chen YC, Chen TJ, Lin MH, Chen LK. Prevalence of the prescribing of potentially inappropriate medications at ambulatory care visits by elderly patients covered by the Taiwanese National Health Insurance program. Clinical Therapeutics. 2209;31(8):1859–1870. doi: 10.1016/j.clinthera.2009.08.023. [DOI] [PubMed] [Google Scholar]