Abstract
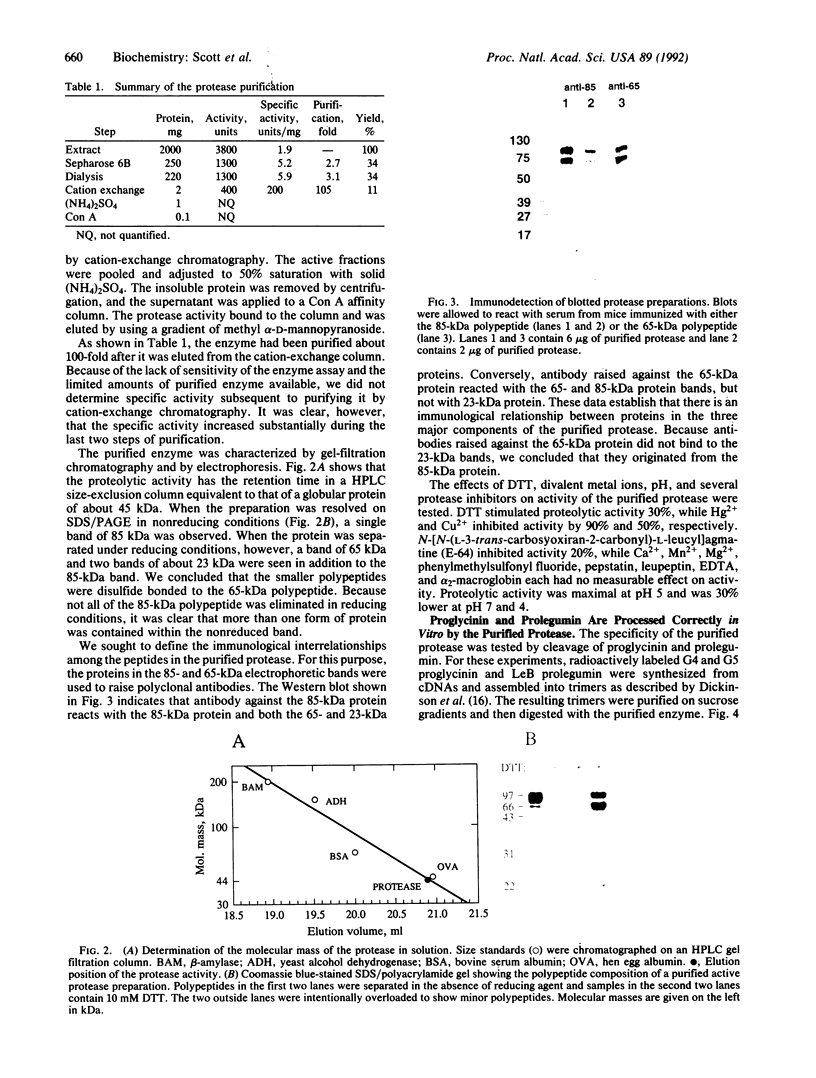
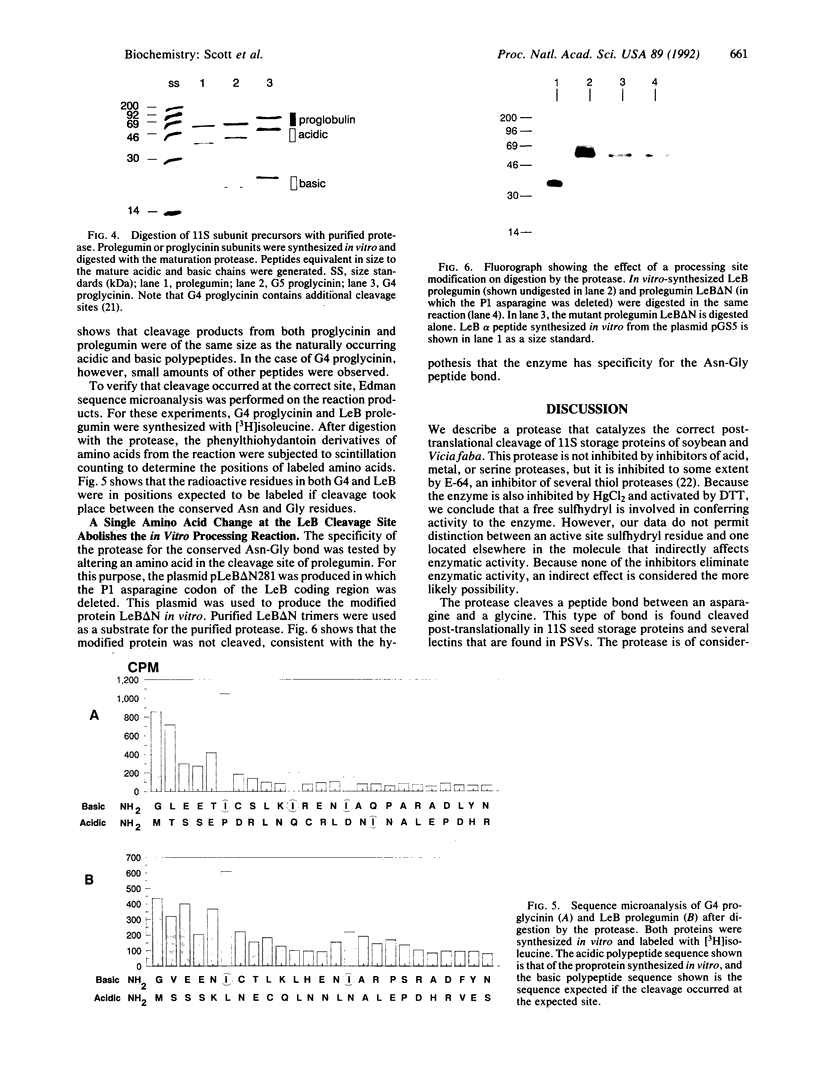
The assembly of 11S globulin seed storage proteins in plants is regulated in part by the activity of a protease that cleaves between asparagine and glycine residues. Post-translational cleavage of subunit precursors into acidic and basic polypeptides is associated with the ability of subunits in trimers to aggregate into hexamers in vitro. An activity is present in extracts from immature soybean seeds that specifically cleaves immature 11S seed storage proteins of soybean and Vicia faba into the polypeptides of the mature proteins. Sequence microanalysis has been used to demonstrate that proglycinin and prolegumin are cut at the legitimate site when proteins synthesized in vitro are used as substrates. A single amino acid change in the cleavage site renders the substrate uncleavable. The protease responsible for this activity also hydrolyzes a synthetic octapeptide whose sequence reproduces four amino acids on either side of the glycinin subunit G4 cleavage site. This assay permitted the purification and characterization of the protease. It is a glycosylated enzyme with an acidic pH optimum and a molecular mass of about 45 kDa in solution.
Keywords: protein maturation, endopeptidase, purification, Glycine max, 11S globulins
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton K. A., Thompson J. F., Madison J. T., Rosenthal R., Jarvis N. P., Beachy R. N. The biosynthesis and processing of high molecular weight precursors of soybean glycinin subunits. J Biol Chem. 1982 Jun 10;257(11):6089–6095. [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Spencer D. Assembly of storage protein oligomers in the endoplasmic reticulum and processing of the polypeptides in the protein bodies of developing pea cotyledons. J Cell Biol. 1982 May;93(2):306–313. doi: 10.1083/jcb.93.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. M., Suzuki E., Van Donkelaar A. The structure of cucurbitin: subunit symmetry and organization in situ. Eur J Biochem. 1980 Feb;103(3):585–588. doi: 10.1111/j.1432-1033.1980.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Dickinson C. D., Floener L. A., Lilley G. G., Nielsen N. C. Self-assembly of proglycinin and hybrid proglycinin synthesized in vitro from cDNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5525–5529. doi: 10.1073/pnas.84.16.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson C. D., Hussein E. H., Nielsen N. C. Role of posttranslational cleavage in glycinin assembly. Plant Cell. 1989 Apr;1(4):459–469. doi: 10.1105/tpc.1.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorel C., Voelker T. A., Herman E. M., Chrispeels M. J. Transport of proteins to the plant vacuole is not by bulk flow through the secretory system, and requires positive sorting information. J Cell Biol. 1989 Feb;108(2):327–337. doi: 10.1083/jcb.108.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I., Nishimura M. Proglobulin processing enzyme in vacuoles isolated from developing pumpkin cotyledons. Plant Physiol. 1987 Oct;85(2):440–445. doi: 10.1104/pp.85.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. R., Snyder S. H. Neuropeptides: multiple molecular forms, metabolic pathways, and receptors. Annu Rev Biochem. 1986;55:773–799. doi: 10.1146/annurev.bi.55.070186.004013. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Nielsen N. C., Dickinson C. D., Cho T. J., Thanh V. H., Scallon B. J., Fischer R. L., Sims T. L., Drews G. N., Goldberg R. B. Characterization of the glycinin gene family in soybean. Plant Cell. 1989 Mar;1(3):313–328. doi: 10.1105/tpc.1.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach G., Jung R., Kunze G., Saalbach I., Adler K., Müntz K. Different legumin protein domains act as vacuolar targeting signals. Plant Cell. 1991 Jul;3(7):695–708. doi: 10.1105/tpc.3.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E., Hermodson M. A., Nielsen N. C. Identification of the acidic and basic subunit complexes of glycinin. J Biol Chem. 1981 Aug 25;256(16):8752–8755. [PubMed] [Google Scholar]
- Staswick P. E., Hermodson M. A., Nielsen N. C. Identification of the cystines which link the acidic and basic components of the glycinin subunits. J Biol Chem. 1984 Nov 10;259(21):13431–13435. [PubMed] [Google Scholar]
- Strauss M., Streuli C. H., Griffin B. E. Efficient oligodeoxyribonucleotide-directed deletion mutagenesis using pEMBL vectors: removal of early region introns from polyoma virus mutants. Gene. 1986;49(3):331–340. doi: 10.1016/0378-1119(86)90369-0. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Kossiakoff A. A., Chambers J. L. Mechanisms of zymogen activation. Annu Rev Biophys Bioeng. 1977;6:177–193. doi: 10.1146/annurev.bb.06.060177.001141. [DOI] [PubMed] [Google Scholar]
- Vonder Haar R. A., Allen R. D., Cohen E. A., Nessler C. L., Thomas T. L. Organization of the sunflower 11S storage protein gene family. Gene. 1988 Dec 30;74(2):433–443. doi: 10.1016/0378-1119(88)90176-x. [DOI] [PubMed] [Google Scholar]