Abstract
There is considerable interest in the potential of mushrooms in modulating the immune system and/or suppressing tumor growth. Among the studied bioactive compounds in mushrooms, polysaccharides are the most important. Nontoxic fungal polysaccharides have a more important role in immunomodulating and antitumor activities which are related to their effects to act of immune effecter cells such as lymphocytes, macrophages, dendritic cells, and natural killer cells involved in the innate and adaptive immunity. Two mannogalactoglucan-type polysaccharides (WPLE-N-2 and WPLE-A0.5-2), purified from the fruiting bodies of Lentinus edodes, were evaluated for their effects on the cellular immune response of Sarcoma 180 (S-180)-bearing mice. Mice were treated with 100 mg/kg body weight of the polysaccharides for 10 days. Significant tumor regressions of the polysaccharide groups’ mice were observed compared to the control group. These polysaccharides could induce an increase in nitrite oxide (NO) production in peritoneal macrophages, significantly increase macrophage phagocytosis of tumor-bearing mice and augment concanavalin (ConA) and lipopolysaccharide (LPS)-induced splenocytes proliferation. Our results indicated that immunomodulating activity occurred through host mediation in response to lymphocyte proliferation, macrophage phagocytosis and induction of NO production while the antitumor activity occurred through direct cytotoxicity. Our findings suggest that mannogalactoglucan-type polysaccharides from L. edodes can be explored as novel potential immunostimulants. Our research provides essential data to a better understanding of L. edodes bioactive compounds, especially polysaccharides. Our results also confirm the key role of β-linkages in the antitumor and immunomodulating effects of polysaccharides.
Keywords: Lentinus edodes, mannogalactoglucan, immunomodulating activity, polysaccharide
Introduction
Studies on new anticancer treatments and other medicinal substances from mushrooms have been significantly expanded recently. This is mainly because they contain bioactive polymers such as polysaccharides and polysaccharide/protein complexes, secondary metabolites, and enzymes isolated from fruit bodies, mycelia, and culture broth [1]. There are substantial data showing the potential activity of medicinal mushrooms in cancer treatment. Ganoderma lucidum, Agaricus bisporus, Agaricus brasiliensis, Trametes versicolor, Grifola frondosa, Inonotus obliquus, Lentinus edodes, Leucoagaricus americanus, Pleurotus ostreatus, Sparassis crispa etc. have shown the most significant inhibitory effect activity in highly invasive cancer cells [2]. These medicinal mushrooms, along with others that have also been reported to produce bioactive substances, have been tested in vivo and/or in vitro, and have demonstrated cancer inhibitory activity. The antitumor and immunomodulating activities of these compounds related to their effects to act of immune effecter cells such as macrophages, lymphocytes, T cells, hematopoietic stem cells, natural killer cells (NK-cells) and dendritic cells (DCs) involved in the innate and adaptive immunity, resulting in the production of biologic response modifiers [3].
Since 2002 Lentinus edodes, Shiitake, has become the most cultivated edible mushroom in the world with one-quarter of worldwide production [4, 5]. Its importance is attributed to both its nutritional value and medical application [6]. Several bioactive polysaccharides, including (1→3)-β-D-glucans, β-D-glucans, glucans, heteroglycans, heterogalactan, etc. have been isolated and identified from the fruiting bodies, mycelia, and culture medium of L. edodes [5–10]. However, data from studies concerning immunomodulating and antitumor activities often focus on (1→3)-β-D-glucan-type polysaccharides and extracts.
In our previous studies, we successfully purified and identified two mannogalactoglucan-type polysaccharides, named WPLE-N-2 and WPLE-A0.5-2, which exhibited cytotoxicity activity against Sarcoma 180 (S-180) solid tumor and human colorectal cancer cell lines (HT-29 and HCT-116) in vitro [11, 12]. We now report the in vivo antitumor and immunomodulating properties of the above-mentioned polysaccharides. This is the first time to report immunomodulating and in vivo antitumor properties of mannogalactoglucan-type polysaccharides from Lentinus edodes fruiting bodies.
The present research provides essential data for successful interpretation of the bioactivities of L. edodes extracts and thus contributes to a better understanding of its compounds. Our research also confirms the key role of β-linkages in the antitumor and immunomodulating effects of polysaccharides. Furthermore, our finding suggests that mannogalactoglucan-type polysaccharides can be explored as potent immunomodulatory agents for cancer therapy.
Material and methods
Chemicals and reagents
All the plates used in this study were purchased from Nunc (Rochester, NY, USA). Penicillin/streptomycin was from the Tina Jin Hao Yang Biological Manufacture Co. Griess reagent was purchased from Jiancheng Biological Engineering Co. Ltd. (Nanjing, China). Trypsin and PMSF were from Amersco. D-Hanks solution, Roswell Park Memorial Institute 1640 medium (RPMI 1640 medium), Dulbecco's modified Eagle medium: Nutrient Mixture F-12(DEME/F12) medium, calf serum, sodium dodecyl sulfate (SDS) and dimethyl sulfoxide (DMSO) were purchased from Gibco (Grand Island, NY, USA). 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), concanavalin A (ConA), lipopolysaccharide (LPS), HEPES were purchased from Sigma (St. Louis, MO, USA). All other chemicals reagents and solvents used were of analytical grade made in China.
Cell line
S-180 Sarcoma cells obtained from American Type Culture Collection (stocks cultures) were maintained in RPMI-1640 medium supplemented with 10% calf serum and 100 IU/ml penicillin and streptomycin, under a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Animals
Male Kunming mice (6-8 weeks old, 19.4 ±0.8 g), purchased from the Pharmacology Experimental Center of Jilin University (Changchun, China) were housed under normal laboratory conditions, i.e., 12/12-hour light-dark cycle, room temperature and with free access to standard rodent chow and water ad libitum during the experiments. Animal handling procedures were conducted under the animal care and use guidelines of the National Institutes of Health (China legislation). All efforts were made to minimize the animals’ suffering and to reduce the number of animals used. All experiments were performed in strict compliance with the institutional regulations and national criteria for use of laboratory animals. All procedures in our study were approved by the Institution Animal Ethics Committee.
Treatment
S-180 cells (0.2 ml, 5 × 106 cells) were passed into mice ascites, then ascites were inoculated subcutaneously into the right axilla of each mouse. The mice inoculated with S-180 were divided into three groups of 6 mice each. The mice were treated as follows: control group (Group I) received pure water and tested groups (Group II and Group III) received polysaccharides WPLE-N-2 and WPLE-A0.5-2 (100 mg/kg body weight), respectively. The polysaccharides were dissolved in pure water and administered intragastrically (i.g.) for 10 days at 24 hours after tumor inoculation. The dose volume was 0.2 ml. After 10 days, mice were dispatched by cervical dislocation and tumor weights were measured. The antitumor activity of the tested samples was expressed as an inhibition ratio (%) calculated as
[(A – B)/A] × 100, where A and B are the average tumor weight of the control and treated group, respectively.
Macrophage phagocytosis assay
Macrophages were prepared from Kunming mice according to the reported procedure [13]. Macrophages phagocytosis was measured by a neutral red uptake method as described in the reported data [14, 15]. Peritoneal exudate cells were collected from the S-180-bearing mice by lavage of the peritoneal cavity with sterile physiological saline. After centrifugation (370 g, 10 min), the erythrocytes were lysed with Tris-NH4Cl, and the cells were then washed three times and re-suspended in complete medium at 2 × 106 cells/ml. Cell suspension (100 µl) was added in each well of 96-well microwell plates. After a 3-hour incubation to allow the cells to attach to the plate bottom, the supernatant was discarded, and 0.075% of neutral red dye was added to each well (100 µl per well). The plates were incubated for another 1 to 2 hours. The plates were then washed two times with phosphate buffered saline (PBS) and were patted gently on tissues to let them drain. Finally, 100 µl of lysis solution (0.1 mol/l acetic acid and ethanol in the ratio of 1: 1) was pipetted into each well. The mixtures were blended completely and evaluated at a wavelength of 540 nm on a Bio-Rad microplate reader. Each experiment was performed in triplicate.
Nitrite oxide (NO) assay
Peritoneal exudate cells were prepared as described above. Exudate cells were seeded at 2 × 106 cells/ml in 48-well plates. Macrophages were adhered to the plate at 37°C for 3 hours. Non-adherent cells were removed by washing with warm D-Hanks solution. Adherent macrophages were cultured for 48 hours at 37°C. At the end of the incubation, culture supernatant was collected. The isolated supernatants were mixed with an equal volume of Griess reagent (1% sulfanilamide in ultrapure water, 0.1% naphthyl ethylene diamine dihydrochloride in 5% phosphoric acid) and incubated at room temperature for 10 minutes. Absorbance was measured at 540 nm with a microplate reader. The nitrite concentration was determined by extrapolation based on a standard sodium nitrite curve [16–18]. Each experiment was performed in triplicate.
Splenocytes preparation
Spleens collected under aseptic conditions, from S-180-bearing mice, were minced in lymphocytes separation medium using a pair of scissors and passed through gauze to obtain homogeneous cell suspensions. After centrifugation (370 g, 10 min), the supernatant was collected and an equal volume of D-Hanks was added and the mixture centrifuged (280 g, 10 min). The cell pellet was then suspended in 5 ml Tris-NH4Cl to lyse red cells and the cells were then washed three times and re-suspended in complete medium (RPMI 1640 supplemented with 25 mM HEPES, 10% heat-inactivated calf serum, 1 × 105 IU/l penicillin G, and 100 mM streptomycin).
Spleen lymphocyte proliferation assay
To investigate the effects of mannogalactoglucan on the cellular immune response, we evaluated the proliferation of spleen lymphocytes in S-180-bearing mice, including the control group (that received water) and the treated groups (that received the polysaccharides). The spleen lymphocytes proliferation was determined using the MTT cellular viability assay method [19, 20]. Splenocytes were seeded into 96-well flat-bottom microtiter plates at 5 × 106 cells/ml and cultured with RPMI 1640 medium, ConA (5.0 µg/ml), or LPS (10.0 µg/ml). The plates were incubated at 37°C in a humidified atmosphere with 5% CO2. After 48 hours, 20 µl of MTT solution (5 mg/ml) was added to each well of the culture plate and then further incubated for 4 hours at 37°C. After aspirating the supernatant from the wells,100 µl 20% SDS containing 0.04 M solution was added and shaken for 20 min to dissolve the colored material (formazan crystals), and the optical density of each well was then measured in a Bio-Rad (Hercules, CA, USA) using a measurement wavelength of 570 nm [21]. Each experiment was performed in triplicate.
Statistical analysis
All experiments were conducted in triplicate. Data are presented as the mean ± standard deviation (SD). Statistical analysis was performed with SPSS version 17.0 software and GraphPad Prism version 5.0 software. One-way analysis of variance (ANOVA) test was used to make a statistical comparison between the treatment and the control groups. The differences were considered significant when *p < 0.05; **p < 0.01.
Results and discussion
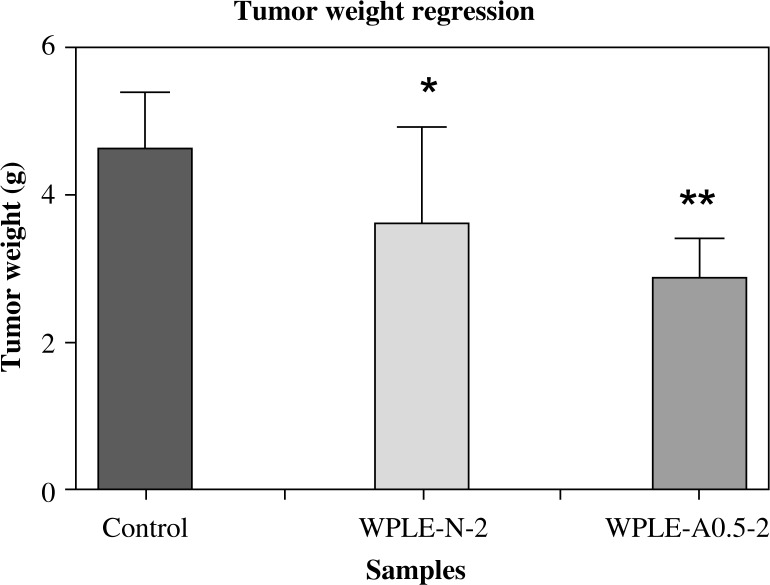
Tumor weight regression
The results of the in vivo antitumor activity assay of WPLE-N-2 and WPLE-A0.5-2 (The Mannogalactoglucan-type polysaccharides consisted of Glc-Gal-Man in a molar ratio of 63: 27: 10 and 72: 18: 10, respectively; with a Mw of 21 × 103 Da and 18 × 103 Da, respectively; as described in our previous papers [11, 12]) against Sarcoma 180 solid tumor grown in Kunming mice at the dosage of 100 mg/kg for 10 days showed that all the polysaccharides exhibited an inhibition against the tumor cell.
WPLE-A0.5-2, containing β-linkages, exhibited a higher inhibition ratio (29%) than WPLE-N-2 (22%), containing α-linkages. Significant (p < 0.05 and p < 0.01) tumor regression was observed at the dose of 100 mg/kg of the polysaccharides WPLE-N-2 and WPLE-A0.5-2, respectively, as shown in Fig. 1. This result implied that WPLE-A0.5-2 had stronger direct cytotoxicity to cancerous cells than WPLE-N-2. The result implied also that mannogalactoglucan could have direct cytotoxicity to cancerous cells.
Fig. 1.
Effect of the mannogalactoglucan-type polysaccharides WPLE-N-2 and WPLE-A0.5-2 on tumor regression of tumor-bearing mice. The polysaccharides were dissolved in pure water and were administered i.g.; while control group received pure water. The dose volume was 0.2 ml. Values are means ± SD of 6 mice; *p < 0.05 and **p < 0.01 vs. control
Our results were in accordance with literature reporting that some components and extracts of many mushrooms, including L. edodes, A. blazei, Ganoderma lucidum, and Grifola frondosa inhibit or suppress tumor growth [22–24].
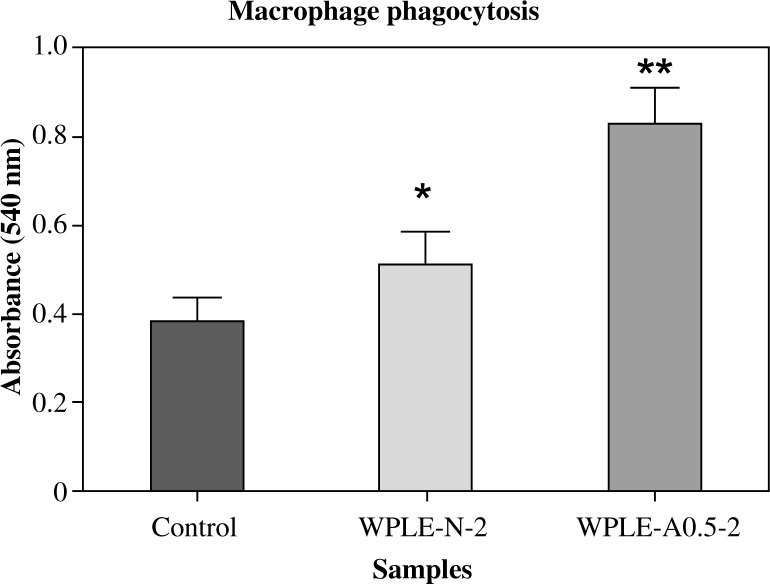
Phagocytosis of macrophage
As the first step towards understanding the immunomodulatory activity of the mannogalactoglucan-type polysaccharides (WPLE-N-2 and WPLE-A0.5-2) we investigated the effect on phagocytosis of macrophage. Phagocytosis ability of macrophage of the treated group mice increased as compared with the control group. A significant increase was observed with the polysaccharides compared with the control group (Fig. 2). WPLE-A0.5-2 exhibited higher phagocytosis ability than WPLE-N-2.
Fig. 2.
Effect of mannogalactoglucan-type polysaccharides WPLE-N-2 and WPLE-A0.5-2 on phagocytosis of macrophage. Phagocytosis activity was expressed as the absorption at 540 nm. Values are means ± SD of six mice; *p < 0.05 and **p < 0.01 vs. control
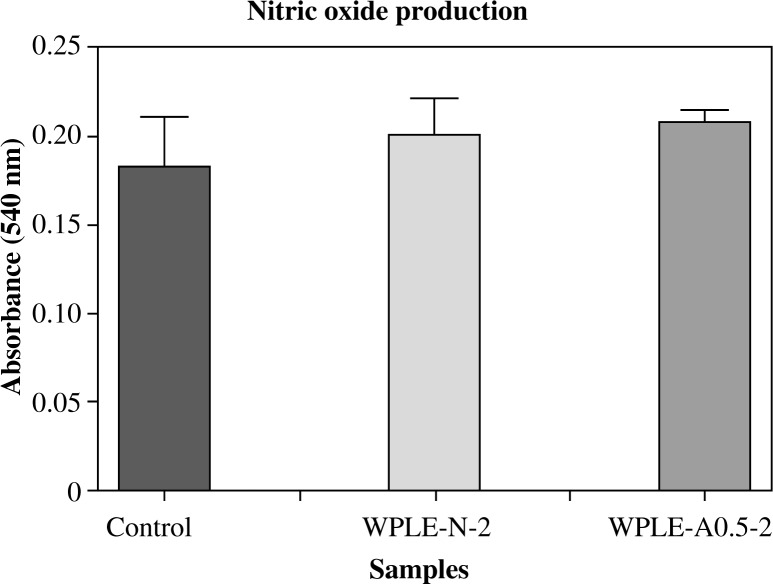
Nitrite oxide production
Nitrite oxide is one of the major reactive oxygen species (ROS) which is produced by macrophage for destroying the foreign body. Since the final product of NO is either nitrite or nitrate, the sum of nitrite and nitrate level provides an indirect measurement of NO level. Macrophages were incubated with mannogalactoglucan-type polysaccharides for 24 hours and NO concentrations in the culture supernatants were assessed by the Griess reagent. The polysaccharides presented a higher effect on NO production at the dose of 100 mg/kg body weight when compared with the control. The effect of WPLE-A0.5-2 was slightly higher than that of WPLE-N-2, but the difference was not significant as shown in Fig. 3.
Fig. 3.
Effect of the mannogalactoglucan-type polysaccharides WPLE-N-2 and WPLE-A0.5-2 on NO production in macrophages. Values are means ± SD of 6 mice; *p < 0.05 and **p < 0.01 vs. control
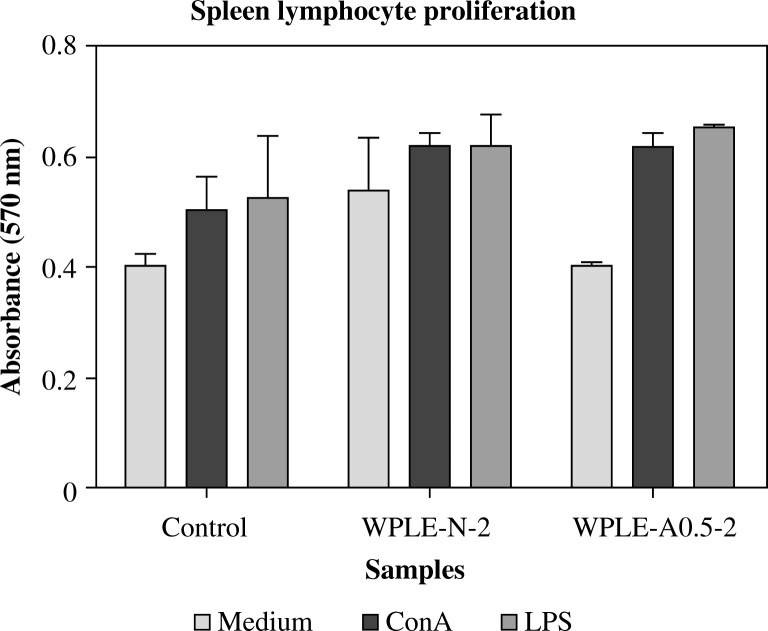
Lymphocyte proliferation
Lymphocytes are the key effector cells of the mammalian immune system. To confirm the effect these polysaccharides have on the cellular immune response we evaluated the proliferation of splenocytes from mice in response to ConA and LPS. The effect of mannogalactoglucan-type polysaccharides was higher than that of the control group, with WPLE-A0.5-2 having a slightly higher effect than that of WPLE-N-2, but the difference was not significant (p < 0.05 and p < 0.01) as shown in Fig. 4. The results, given in Fig. 4, indicate that the mannogalactoglucan-type polysaccharides are able to stimulate proliferation of splenocytes in S-180-bearing mice.
Fig. 4.
Effect of the mannogalactoglucan-type polysaccharides WPLE-N-2 and WPLE-A0.5-2 on splenocytes proliferation. Proliferation activity was expressed as the absorption at 570 nm. Values are means ± SD of 6 mice; *p < 0.05 and **p < 0.01 vs. control
Since the isolation and identification of Lentinan, a (1→3)-β-D-glucan with immunomodulating and antitumor activities from L. edodes, researches on polysaccharides have attracted much attention as functional and natural sources of antitumor drugs [1–3, 25]. Indeed, several research studies have reported a variety of bioactive compounds isolated from L. edodes. Extracts of many mushrooms, including Grifola frondosa, Agaricus blazei, Ganoderma lucidum, and Lentinus edodes modulate the immune system of the host and suppress tumor growth [24]. In published papers, most of L. edodes polysaccharides showed their immunomodulatory and anticancer activities based on (1→3)-β-D-glycosidic linkage structures. We have made a tumor-bearing animal model to research the in vivo antitumor and immunostimulating activities of mannogalactoglucan-type polysaccharides according to pharmacology.
A significant tumor weight regression of our polysaccharide (100 mg/kg) group mice was observed compared with the control group (Fig. 1). Direct cytotoxicity to cancerous cells of many mushroom polysaccharides has been reported [26]. These studies indicated that incubation of polysaccharides together with tumor cells could change the expression of signal within tumor cells. This can arrest the cell cycle and generate apoptosis, which can explain the inhibition of tumor growth. Aqueous extracts of L. edodes highly inhibited growth of tumors in Swiss albino implanted Sarcoma 180 ascites cells [27]. The strongest tumor regression activity of the mannogalactoglucan-type polysaccharides compared to that of the control might be attributed to the presence of Glc and Man. Indeed, a polysaccharide receptor has been found on human macrophages, which has demonstrated high specificity for Glc and Man [28]. Thus, mushroom polysaccharides consisting of Glc and Man may have some antitumor action. In addition, a multiple linear regression analysis study reported that Man and Gal were identified as the monosaccharides that could be related to the macrophage stimulatory activities [29].
The mannogalactoglucan-type polysaccharides WPLE-N-2 and WPLE-A0.5-2 can induce the increase of NO production in peritoneal macrophage (Fig. 3). Our outcome is in agreement with reported studies stipulating that in the evaluation of the immunomodulating activity of macrofungi extracts containing polysaccharides, it is demonstrated that L. edodes increased the NO production [1]. This implied that the immunoregulation mechanism of mannogalactoglucan might be related to the induction of NO production.
The phagocytes (neutrophils, monocytes and macrophages), one of the earliest cell types to respond to invasion by pathogenic organisms, are key participants in the innate immune response [30, 31]. Neutrophils and macrophages represent the first line of host defense after the epithelial barrier. They are involved in tissue remodeling during embryogenesis, wound repair, clearance of apoptotic cells and hematopoiesis [32, 33]. Macrophages can kill the tumor cells either by macrophage-mediated tumor cytotoxicity or antibody-dependent cellular cytotoxicity (ADCC). Both processes will end up releasing cytotoxic mediators including TNF-α, IL-1, NO and reactive oxygen intermediates or phagocytosis [32]. It is reported that the feeding of fruit bodies administered orally to mice could augment both ability of macrophage to phagocytose latex particles and the spreading activity of the macrophages [34]. We have demonstrated that mannogalactoglucan-type polysaccharides WPLE-N-2 and WPLE-A0.5-2 can increase macrophage phagocytosis (Fig. 2). This ability might be due to their structural features including monosaccharide component, anomeric configuration and glycosidic linkages [28, 29]. However, some researchers reported that aqueous extracts from Lentinus edodes could not increase phagocytosis of macrophage. Our purified molecule can have a more favorable exposure of the anticancer effect sites (several immune receptors including Dectin-1, complement receptor (CR3) and TLR-2/6) and easily trigger a group of immune cells including macrophages, neutrophils, monocytes, natural killer cells and dendritic cells [35]. CPFN-G-I (a β-(1→6)-branched-β-(1→4)-glucan), CPBN-G and CPBA-G (both heteromannans), purified from the fruiting body and culture cell-free broth of L. edodes, were found to stimulate the functional activation of macrophages including NO production, cytokines expression and increase the phagocytic uptake [36, 37].
MPSSS, a polysaccharide fraction (extracted with hot water and sequentially ethanol precipitation) mainly consisting of (1→6)-β-linked-glucan branched at C-4 with side chains of (1→6)-β-linked-glucans, was found to inhibit tumor growth of McgR32 tumor cells, which was accompanied by a strong stimulating effect on splenocytes in mice (C57BL/6 and BALB/c) [38].
In our research, we found that the mannogalactoglucan-type polysaccharides could augment ConA and LPS-induced splenocyte proliferation, suggesting that these polysaccharides were able to stimulate both T-Cells and B-Cells proliferations [15]. It is reported that mushroom polysaccharides with biological activities differ greatly in their structural features including chemical composition and configuration, as well as physical properties [2]. The differences in bioactivity of WPLE-N-2 and WPLE-A0.5-2, as observed during our study, could be correlated with their molecular sizes, branching rate, and anomeric configuration. The strongest activity of WPLE-A0.5-2 compared to that of WPLE-N-2 might be due to the presence of structural features such as (1→3), (1→4) and (1→6)-linked β-D-Glcp residues [1–3]. Thus, our results confirmed that the presence of such structural features are essential factors for antitumor and immunomodulating actions.
Conclusions
In summary, according to our experiments it was demonstrated that the immunoregulation/immunostimulating activity of the mannogalactoglucan-type polysaccharides from L. edodes occurred through induction of NO production, macrophage phagocytosis and lymphocyte proliferation which participates in the antitumor activity.
Thus, mannogalactoglucan-type polysaccharides can be explored as potential immunomodulatory agents for cancer therapy. There is no doubt that to exploit the application of these polysaccharides in medicinal and food industries, more detailed studies are still needed.
The authors declare no conflict of interest.
The authors gratefully acknowledge the financial support from the Chinese New Drug Creation and Manufacturing Program (2012ZX09502001-001), the National Natural Science Foundation of China (No. 31170770), the Key Scientific Program of Jilin Province (Nos. 20110242, 20140101122JC), and the Doctoral Fund of Ministry of Education of China (20120043130001).
References
- 1.Mohammad-Fata M, Hossein M, Shirin G, Ghorban-Ali H. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi) Int Immunopharmacol. 2007;7:701–724. doi: 10.1016/j.intimp.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;10:13–32. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Cui SW, Cheung PCK, Wang Q. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trend Food Sci Tech. 2007;18:4–19. [Google Scholar]
- 4.Peter CKC. Mushrooms as functional foods. John Wiley & Sons, Inc; 2008. p. 14. ISBN 978-0-470-05406. [Google Scholar]
- 5.Huoliang C, Ying J, Junjie L, Min Y. Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. Int J Biol Macromol. 2012;50:214–218. doi: 10.1016/j.ijbiomac.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Carbonero ER, Gracher AHP, Komura DL, et al. Lentinus edodes heterogalactan: Antinociceptive and anti-inflammatory effects. Food Chemistry. 2008;111:531–537. [Google Scholar]
- 7.Ruan Z, Su J, Dai H, Wu M. Characterization and immunomodulating activities of polysaccharide from Lentinus edodes. Int Immunopharmacol. 2005;5:811–820. doi: 10.1016/j.intimp.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Xiaojuan X, Pan C, Lina Z, Hitoshi A. Chain structures of glucans from Lentinus edodes and their effects on NO production from RAW 264.7 macrophages. Carbohydrate Polymers. 2012;87:1855–1862. [Google Scholar]
- 9.Unursaikhan S, Lina Z, Xiaojuan X, et al. Effects of molecular structure on antitumor activities of (1→3)-β-D-glucans from different Lentinus edodes. Carbohydrate Polymers. 2006;63:97–104. [Google Scholar]
- 10.Zhang Y, Gu M, Wang K, et al. Structure, chain conformation and antitumor activity of a novel polysaccharide from Lentinus edodes. Fitoterapia. 2010;81:1163–1170. doi: 10.1016/j.fitote.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Iteku BJ, Shanshan L, Xiaoxia P, et al. Purification, structural elucidation and antitumor activity of a novel mannogalactoglucan from the fruiting bodies of Lentinus edodes. Fitoterapia. 2013;84:338–346. doi: 10.1016/j.fitote.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Iteku BJ, Xiaowen Y, Lin S, et al. Purification and in vitro anti-proliferative effect of novel polysaccharides from Lentinus edodes. Int J Biol Macromol. 2013;52:99–106. doi: 10.1016/j.ijbiomac.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Kim GY, Choi GS, Lee SH, Park YM. Acidic polysaccharide isolated from Phellinus linteus enhances through the up-regulation of nitric oxide and tumor necrosis factor-alpha from peritoneal macrophages. J Ethnopharmacol. 2004;95:69–76. doi: 10.1016/j.jep.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Weeks BA, Keisler AS, Myrvik QN, Warinner JE. Differential uptake of neutral red by macrophages from three species of estuarine fish. Dev Comp Immunol. 1987;11:117–124. doi: 10.1016/0145-305x(87)90013-9. [DOI] [PubMed] [Google Scholar]
- 15.Ni W, Zhang X, Bi H, et al. Preparation of a glucan from the roots of Rubus crataegifolius Bge. and its immunological activity. Carbohydrate Research. 2009;344:2512–2518. doi: 10.1016/j.carres.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Flick DA, Gifford GE. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984;68:167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- 17.Keller R, Geiges M, Keist R. L-arginine-dependent reactive nitrogen intermediates as mediators of tumor cell killing by activated macrophages. Cancer Res. 1990;50:1421–1425. [PubMed] [Google Scholar]
- 18.Xia L, Xiaoyan X, Mengyao Y, et al. Characterization and immunostimulatory activity of an α-(1→6)-D-glucan from the cultured Armillariella tabescens mycelia. Food Chemistry. 2008;111:357–363. doi: 10.1016/j.foodchem.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;63:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Xu Z, Shanshan L, Lin S, et al. Further analysis of the structure and immunological activity of an RG-I type pectin from Panax ginseng. Carbohydrate Polymers. 2012;89:519–525. doi: 10.1016/j.carbpol.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Fei L, Qipeng Y, Farzana R. Isolation, purification and immunobiological activity of a new water-soluble bee pollen polysaccharide from Crataegus pinnatifida Bge. Carbohydrate Polymers. 2009;78:80–88. [Google Scholar]
- 22.Smith JE, Rowan NJ, Sullivan R. Biotechnol Lett. 2002;24:184–198. [Google Scholar]
- 23.Yu R, Yin Y, Yang W, et al. Structural elucidation and biological activity of a novel polysaccharide by alkaline extraction from cultured Cordyceps military. Carbohydrate Polymer. 2008;75:166–171. [Google Scholar]
- 24.Igor AS, Mark TQ. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Yangyang Z, Sheng L, Xiaohua W, et al. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocolloids. 2011;25:196–206. [Google Scholar]
- 26.Wang YY, Khoo KH, Chen ST, et al. Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides; functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorganic Medical Chemistry. 2002;10:1057–1062. doi: 10.1016/s0968-0896(01)00377-7. [DOI] [PubMed] [Google Scholar]
- 27.Breene WM. Nutritional and medicinal value of specialty mushrooms. J Food Prot. 1990;53:883–890. doi: 10.4315/0362-028X-53.10.883. [DOI] [PubMed] [Google Scholar]
- 28.Lombard YJ. A new method for studying the binding and ingestion of zymosan particles by macrophages. J Immunol Methods. 1994;174:155–163. doi: 10.1016/0022-1759(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 29.Tiffany CTL, Yi HJ, Anne LJC, Cheng AC. Use of statistical methods to find the polysaccharide structural characteristics and the relationships between monosaccharide composition ratio and macrophage stimulatory activity of regionally different strains of Lentinus edodes. Analytica Chimica Acta. 2007;684:50–56. doi: 10.1016/j.aca.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 30.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 31.Uthaisangsook S, Day NK, Bahna SL, et al. Innate immunity and its role against infections. Ann Allergy Asthma Immunol. 2002;88:253–264. doi: 10.1016/S1081-1206(10)62005-4. [DOI] [PubMed] [Google Scholar]
- 32.Klimp AH, de Vries EGE, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. 2002;44:143–161. doi: 10.1016/s1040-8428(01)00203-7. [DOI] [PubMed] [Google Scholar]
- 33.Lingen MW. Role of leucocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch Pathol Lab Med. 2001;125:67–71. doi: 10.5858/2001-125-0067-ROLAEC. [DOI] [PubMed] [Google Scholar]
- 34.Nanba H, Mori K, Toyomasu T, Kuroda H. Antitumor action of Shiitake (Lentinus edodes) fruit bodies orally administered to mice. Chem Pharm Bull. 1987;35:2453–2458. doi: 10.1248/cpb.35.2453. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey C-FC, Wing KC, Daniel M-YS. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HH, Lee JS, Cho JY, et al. Structural characteristics of immunostimulating polysaccharides from Lentinus edodes. J Microbiol Biotechnol. 2009;19:455–461. doi: 10.4014/jmb.0809.542. [DOI] [PubMed] [Google Scholar]
- 37.Lee HH, Lee JS, Cho JY, et al. Study on immunostimulating activity of macrophage treated with purified polysaccharides from liquid culture and fruiting body of Lentinus edodes. J Microbiol Biotechnol. 2009;19:566–572. doi: 10.4014/jmb.0809.541. [DOI] [PubMed] [Google Scholar]
- 38.Hao W, Ning T, Xiaoman L, et al. Polysaccharide from Lentinus edodes inhibits the immunosuppressive function of myeloid-derived suppressor cells. PLOS One. 2012;7:1–9. doi: 10.1371/journal.pone.0051751. [DOI] [PMC free article] [PubMed] [Google Scholar]