Abstract
Neutrophil extracellular traps (NETs) are threads of nuclear DNA complexed with antimicrobial proteins released by neutrophils to extracellular matrix to bind, immobilise, and kill different pathogens. NET formation is triggered by different physiological and non-physiological stimulants. It is also suggested that antibiotics could be non-physiological compounds that influence NET release.
The aim of the study was to investigate the effect of clindamycin and amoxicillin on NET release and the phagocyte function of neutrophils.
Neutrophils isolated from healthy donors by density centrifugation method were incubated with amoxicillin or clindamycin for two hours, and then NET release was stimulated with phorbol 12-myristate 13-acetate (PMA). After three hours of incubation with PMA NETs were quantified as amount of extracellular DNA by fluorometry and visualised by immunofluorescent microscopy. The percent of phagocyting cells was measured by flow cytometry.
We showed that amoxicillin induces NET formation (increase of extracellular DNA fluorescence, p = 0.03), while clindamycin had no influence on NET release (p > 0.05), as confirmed by quantitative measurement and fluorescent microscopy. Regarding phagocyte function, both antibiotics increased bacterial uptake (43.3% and 61.6% median increase for amoxicillin and clindamycin, respectively).
We concluded that the ability of antibiotics to modulate NET release depends on the antibiotic used and is not associated with their ability to influence phagocytosis.
Keywords: amoxicillin, antibiotics, clindamycin, neutrophil extracellular traps, phagocytosis
Introduction
Neutrophil extracellular traps (NETs), described for the first time in 2004, are threads of extracellular DNA complexed with antimicrobial proteins released from neutrophils to fight and kill pathogens, such as bacteria and fungi [1]. Pathogens are trapped and immobilised within a viscous web-like structure, and influenced by high concentrations of antimicrobial compounds, such as neutrophil elastase, myeloperoxidase, and histones [1–3]. Many physiological and non-physiological agents induce NET release, including S. aureus, C. glabrata, HIV, interleukin-8, tumour necrosis factor α (TNF-α), calcium ions, phorbol myristate acetate (PMA), or calcium ionophore [4]. In line with phagocytosis and oxidative burst, release of NETs is an efficient mechanism of innate immunity [2, 3, 5]. On the other hand, NETs were found to be a source of antigens for production of anti-nuclei auto-antibodies. Significant formation of NETs accompanied by insufficient clearance of DNA from extracellular matrix are two important mechanisms involved in many autoimmune diseases [4].
Antibiotics are widely used for treatment of bacterial infections. Depending on their biochemical structure and mechanisms of action, they can act not only as bactericidal compounds but may also influence neutrophil or lymphocyte functions [6, 7]. Moreover, they can influence release of cytokines or delayed-type hypersensitivity reactions [6, 8, 9]. However, the influence of antibiotics on NET release is largely unknown. Jerjomiceva et al. found that enrofloxacin increases NET release from bovine granulocytes [10]. Clindamycin was found to inhibit nuclease activity of Staphylococcus aureus, which resulted in increased human NET efficacy in bacteria elimination [11]. Recently, Konstantinidis et al. showed that clarithromycin may induce NET formation in human both in vivo and in vitro [12].
Clindamycin belongs to the group of antibiotics called lincosamides. It has a bacteriostatic effect, and after binding to bacterial ribosomes it acts as an inhibitor of protein synthesis. It was found that clindamycin influences chemotaxis of neutrophils in a dose-dependent manner; the concentration that is obtained physiologically during infection treatment with the antibiotic enhances chemotaxis. Moreover, clindamycin increases oxidative burst of neutrophils [13]. In several studies clindamycin showed a positive effect on phagocytosis [7, 14, 15]. Amoxicillin belongs to the β-lactam antibiotics, and its action is based on inhibition of the synthesis of bacterial cell wall. No influence of amoxicillin on phagocytosis was previously reported in in vitro studies. Interestingly, amoxicillin showed a positive effect on neutrophil chemotaxis in vitro [7]. No direct influence of both antibiotics on neutrophil extracellular traps formation was studied so far.
The aim of this study was to investigate whether amoxicillin and clindamycin influence NET release and phagocytosis of human neutrophils.
Material and methods
Neutrophil isolation
Whole blood from six healthy volunteers was collected into tubes containing 3.2% sodium citrate. Then, neutrophils were isolated using the density gradient centrifugation method. After rich-platelet plasma was discarded after centrifugation for 10 minutes at 200 g, a double plasma volume of 0.9% solution of NaCl was added and diluted blood was layered onto a Histopaque 1077 (Sigma Aldrich) and centrifuged for 30 minutes at 400 g. The supernatant (mononuclear cells and isolation media layers) was carefully removed and the layer containing polymorphonuclear and red blood cells was mixed with 5 ml of 1% polyvinyl alcohol and left for 20 minutes for erythrocyte sedimentation. The top layer consisting of alcohol and neutrophils was collected and centrifuged. The remaining red blood cells were lysed with distilled water for 40 seconds and the lysis was stopped by adding two-times-concentrated phosphate buffered saline (2 × PBS). Granulocytes were then washed twice and the cell pellet was resuspended in a cell culture medium (RPMI 1640 without phenol red, supplemented with 10 mM HEPES; Gibco, Waltham, USA).
Phagocytosis
Neutrophils (5 × 105/ml) were incubated for 2 hours at 37°C, 5% CO2 with clindamycin (1 mg/l, Sigma Aldrich) or amoxicillin (50 µg/ml, Sigma Aldrich), with or without addition of cytochalasin D (10 µg/ml, Sigma Aldrich), an inhibitor of phagocytosis, and negative control. Subsequently, cells were incubated with Escherichia coli (5 µl, BioParticles, fluorescein conjugate) for 30 minutes at 37°C, 5% CO2. Then the cells were washed with PBS, treated with 0.4% Trypan Blue (Sigma Aldrich) to quench fluorescence of non-phagocyted, adherent bacteria and centrifuged for five minutes at 200 g. Fluorescence of neutrophils was analysed with a Cytomics FC500 flow cytometer (Beckman Coulter, USA) equipped with an argon laser at the first channel of fluorescence.
Neutrophil extracellular traps quantification
Isolated neutrophils were seeded into 96-well black plates at density 1 × 105 cells/well, treated with clindamycin, amoxicillin, or RPMI 1640 alone and incubated for two hours at 37°C, 5% CO2. After incubation, NET formation was stimulated with 100 nM PMA (Sigma Aldrich) for three hours at 37°C, 5% CO2. Unstimulated neutrophils were used as a control. Following incubation, 500 mIU of micrococcal nuclease (ThermoFisher Scientific, Waltham, USA) was added to detach the DNA from the cell surface and the plate was incubated for 20 minutes at 37°C, 5% CO2. After 20 minutes of incubation, the reaction was stopped with 5 mM EDTA and the plates were centrifuged (10 minutes at 415 g). Subsequently, supernatant was collected and 100 nM Sytox green fluorescent dye (Life Technologies, Waltham, USA) was added. The amount of extracellular DNA release was measured in a FluroStar OMEGA plate reader (BMG Labtech, Ortenberg, Germany) and the results were expressed in relative fluorescence units (rfu).
Neutrophil extracellular traps visualization
Neutrophils were seeded in eight-well Lab-Tek chamber slides (2.5 × 104 cells/well; Nunc, Waltham, USA) and treated with clindamycin, amoxicillin, or RPMI 1640 for two hours at 37°C, 5% CO2. After the incubation period the cells were stimulated with 100 nM PMA for three hours at 37°C, 5% CO2. Then the samples were fixed with 4% paraformaldehyde, blocked with 0.1% Triton X (Sigma Aldrich), and incubated overnight with FITC-conjugated MPO antibody (0.1 mg/ml, Abcam ab11729). Post incubation, DNA was stained with 100 µM Sytox Orange fluorescent dye (Life Technologies, Waltham, USA). NETs were visualised using a Leica DMi8 fluorescent microscope.
The study was approved by the Local Ethical Committee at Medical University of Warsaw. Informed, written consent was obtained from all subjects enrolled in the study.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v. 5.0 (GraphPad Software, La Jolla California USA). The results have been expressed as mean ± SD. Nonparametric analysis was performed using Mann-Whitney test for unpaired data and Wilcoxon matched paired test for paired data. Results were considered statistically significant at p < 0.05
Results
Effect of clindamycin and amoxicillin on phagocytosis
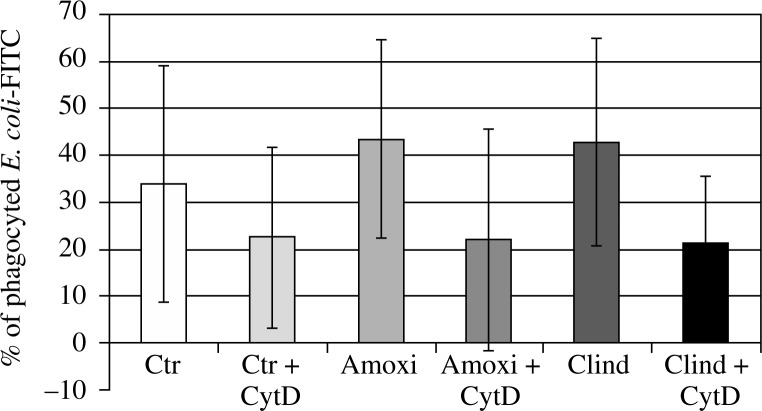
Cytochalasin D, as expected, inhibited the uptake of bacteria in each case, causing a decrease of cells fluorescence of 30 ±25%, 53 ±8%, and 55 ±24% in samples with culture media, clindamycin, and amoxicillin, respectively. Both of the antibiotics influenced the phagocytic function of neutrophils. Incubation with amoxicillin resulted in 74.65 ±122.9% higher phagocytic activity of neutrophils, and with clindamycin in 48.09 ±40.69% higher phagocytic activity of isolated cells (Fig. 1) than in samples pre-incubated only with culture medium. No significant difference between both antibiotics with respect to the control was observed.
Fig. 1.
The effect of cytochalasin and antibiotics on phagocytosis. Cells treated with cytochalasin D showed decreased ability to phagocyte FITC-conjugated E. coli, as they served as negative control. Neutrophils incubated for 2 hours with amoxicillin and clindamycin showed increased phagocytosis of FITC-conjugated E. coli
Neutrophil extracellular traps and visualisation quantification assay
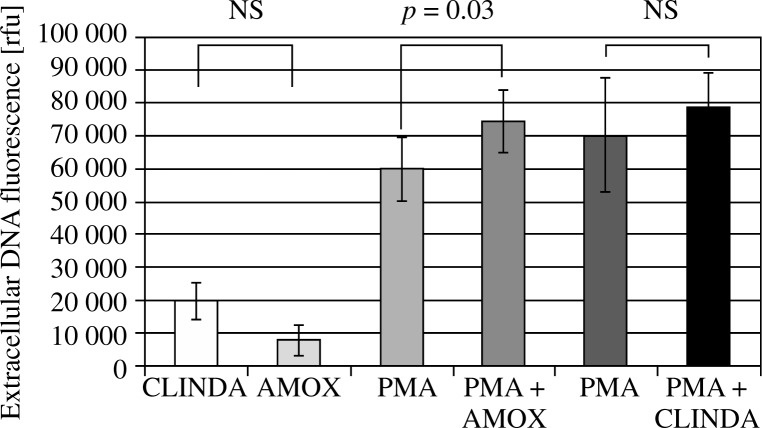
The quantity of released DNA was analysed using a fluorescence reader. The results show that clindamycin does not affect PMA-induced NET formation (78,628 ±17,290 rfu for PMA-stimulated sample vs. 78,628 ±10,249 rfu for amoxicillin-pretreated, PMA-stimulated sample p = 0.2), while amoxicillin increases NETS release (59,972 ±9755 rfu for PMA-stimulated sample and 74,306 ±9519 rfu for amoxicillin-pretreated, PMA-stimulated sample, p = 0.03) (Fig. 2). Neutrophil extracellular traps visualisation using immunofluorescent microscopy confirmed these observations (Fig. 3).
Fig. 2.
The amount of extracellular DNA released from antibiotic- pretreated neutrophils without or after stimulation with 100 nM PMA. Amoxicillin pretreated cells showed increased release of NETs, while clindamycin did not affect NET release. Samples treated only with antibiotics served as negative control for NET formation
Fig. 3.
Immunostaining of NETs. (A) Control unstimulated neutrophils, (B) neutrophils stimulated with 100 nM PMA, (C) Amoxicillin-pretreated, PMA-stimulated (100 nM) neutrophils, (D) Clindamycin-pretreated, PMA-stimulated (100 nM) neutrophils. Red colour – Sytox Orange-stained extracellular DNA, green colour – myeloperoxidase stained with FITC-conjugated anti-myeloperoxidase antibodies
Discussion
In the present study we showed that antibiotics can affect NET release and phagocyte functions of human neutrophils. Previously, an effect of different antibiotics on physiological functions of neutrophils was described [7]. Only a few papers describe the influence of different antibiotics on neutrophil extracellular trap release, none regarding β-lactams [10–12]. The results presented in our paper may shed new light on the immunomodulatory effect of antibiotics. We showed that clindamycin does not affect NET release, despite influencing phagocytosis. On the contrary, we found that amoxicillin may enhance NET formation, which could be an additional mechanism of bactericidal action of a studied β-lactam.
In our study, neutrophils that were preincubated with clindamycin showed higher intake of fluorescein-conjugated Escherichia coli. This is in line with previous reports describing the influence of lincosamides on granulocyte functions [14, 15]. However, we did not find any influence on NET formation in vitro after stimulation with PMA. This is in agreement with the findings of Schilcher et al. They found that clindamycin does not directly change neutrophil ability to release NETs, but it inhibits bacterial nucleases activity, thus decreasing degradation of webs by trapped bacteria [11].
So far there have been no papers regarding the influence of amoxicillin on NET formation. Of all β-lactams cefotaxime was shown to influence oxidative burst and decrease release of cytokines, especially interleukin-8 and TNF-α, which are important proinflammatory cytokines triggering NET formations [13]. In the present paper we have shown that amoxicillin increases NET release after stimulation with PMA. The study was performed in vitro, so no effect on proinflammatory cytokines, the release of which might be affected by an antibiotic, could be associated with the observed NET release. We have also indicated that phagocyte activity after incubation with amoxicillin was enhanced. Taken together, our observations point to a strong immunomodulatory effect of β-lactams. In our previous study (in press) we did not find any effect of cefotaxime on NET release. Thus, it is probable that amoxicillin acts differentially from cephalosporins, despite their similar antimicrobial effect.
Studies regarding the influence of antibiotics on neutrophil extracellular traps may profit from new findings, which may widen our knowledge regarding immumodulatory effects of antimicrobial drugs. Further analysis will be performed with wider analysis of mechanisms of action and other groups of antibiotics.
This project was financed by the Grant for Young Scientists No. 1WW/PM11/15 funded by the Dean of the First Faculty of Medicine, Medical University of Warsaw.
The authors declare no conflict of interest.
References
- 1.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Arazna M, Pruchniak MP, Zycinska K, Demkow U. Neutrophil extracellular trap in human diseases. Adv Exp Med Biol. 2013;756:1–8. doi: 10.1007/978-94-007-4549-0_1. [DOI] [PubMed] [Google Scholar]
- 3.Manda A, Pruchniak MP, Araźna M, Demkow UA. Neutrophil extracellular traps in physiology and pathology. Cent Eur J Immunol. 2014;39:116–121. doi: 10.5114/ceji.2014.42136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pruchniak MP, Kotuła I, Manda-Handzlik A. Neutrophil extracellular traps (Nets) impact upon autoimmune disorders. Cent Eur J Immunol. 2015;40:217–224. doi: 10.5114/ceji.2015.52836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araźna M, Pruchniak MP, Demkow U. Reactive Oxygen Species, Granulocytes, and NETosis. Adv Exp Med Biol. 2015;836:1–7. doi: 10.1007/5584_2014_12. [DOI] [PubMed] [Google Scholar]
- 6.Minić S, Bojić M, Vukadinov J, et al. Immunomodulatory actions of antibiotics. Med Pregl. 2009;62:327–330. doi: 10.2298/mpns0908327m. [DOI] [PubMed] [Google Scholar]
- 7.Van Vlem B, Vanholder R, De Paepe P, et al. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–291. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]
- 8.Roszkowski W, Ko HL, Roszkowski K, et al. Antibiotics and immunomodulation: effects of cefotaxime, amikacin, mezlocillin, piperacillin and clindamycin. Med Microbiol Immunol. 1985;173:279–289. doi: 10.1007/BF02124944. [DOI] [PubMed] [Google Scholar]
- 9.Ziegeler S, Raddatz A, Hoff G, et al. Antibiotics modulate the stimulated cytokine response to endotoxin in a human ex vivo, in vitro model. Acta Anaesthesiol Scand. 2006;50:1103–1110. doi: 10.1111/j.1399-6576.2006.01112.x. [DOI] [PubMed] [Google Scholar]
- 10.Jerjomiceva N, Seri H, Völlger L, et al. Enrofloxacin enhances the formation of neutrophil extracellular traps in bovine granulocytes. J Innate Immun. 2014;6:706–712. doi: 10.1159/000358881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schilcher K, Andreoni F, Uchiyama S, et al. Increased neutrophil extracellular trap-mediated Staphylococcus aureus clearance through inhibition of nuclease activity by clindamycin and immunoglobulin. J Infect Dis. 2014;210:473–482. doi: 10.1093/infdis/jiu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konstantinidis T, Kambas K, Mitsios A, et al. Immunomodulatory role of Clarithromycin in Acinetobacter baumannii infection via Neutrophil Extracellular Traps formation. Antimicrob Agents Chemother. 2015;60:1040–1048. doi: 10.1128/AAC.02063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guz K, Bugla-Płoskońska G. The immunomodulatory and anti-inflammatory properties of different antimicrobial agents. Postepy Hig Med Dosw (Online) 2007;61:828–837. [PubMed] [Google Scholar]
- 14.Eicka S, Pfistera W, Fiedlerb D, Straubea E. Clindamycin promotes phagocytosis and intracellular killing of periodontopathogenic bacteria by crevicular granulocytes: an in vitro study. J. Antimicrob. Chemother. 2000;46:583–588. doi: 10.1093/jac/46.4.583. [DOI] [PubMed] [Google Scholar]
- 15.Wittmann S, Arlt M, Rothe G, Fröhlich D. Differential effects of clindamycin on neutrophils of healthy donors and septic patients. International Immunopharmacology. 2004;4:929–937. doi: 10.1016/j.intimp.2004.04.003. [DOI] [PubMed] [Google Scholar]