Abstract
Echinacea purpurea-containing remedies are herbal medicines used in respiratory tract infections and several inflammatory conditions as enhancers of non-specific and modulators of specific cellular immunity. They also exert anti-inflammatory, anti-viral, and anti-microbial activity. The aim of the present study was to compare the in vivo influence of orally administered three Echinacea purpurea-based remedies (IMMUNAL drops, ECHINACEA FORTE drops, IMMUNAL FORTE tablets) on some parameters of cellular and humoral immunity in mice.
Results
Feeding mice for seven days with IMMUNAL drops resulted in enhanced anti-SRBC antibody production and modulatory effect on proliferative response to PHA of their splenic lymphocytes. No stimulatory effect was observed on splenocytes chemokinesis.
Mice fed with ECHINACEA FORTE drops presented enhanced response to PHA of their splenocytes. However, contrary to the previous group, no enhancement of antibody production was observed. In this group, lymphocyte-induced immunological angiogenesis (LIA) and chemokinesis (spontaneous migration – SM) of spleen lymphocytes was diminished after feeding mice with both doses (LIA) or with a higher dose (SM) of remedy. Lymphocyte-induced immunological angiogenesis activity of splenocytes collected from animals fed with prophylactic and therapeutic IMMUNAL FORTE tablet doses did not differ from the controls.
Keywords: mice, Echinacea purpurea, cellular and humoral immunity
Introduction
Herbs, which possess immunostimulatory, antimicrobial, antiviral, anti-parasitic, anti-inflammatory, and pro-regenerative properties might be beneficial in the treatment of infections and in wound healing, and they might also be valuable as biological enhancers after surgical procedures. Extracts obtained from Echinacea species fulfil these criteria. The results of experiments performed by Samochowiec et al. [1] have proven that the herb Echinacea angustifolia contains active substances exerting an in vitro inhibitory effect on Trichomonas vaginalis. Bany et al. reported the effect of an Echinacea purpurea-containing remedy, Alchinal, on the development of Trichinella spiralis infection in mice. After administering this remedy for 10 days, the number of adult forms and muscular larvae significantly decreased [2].
Extracts of Echinacea purpurea were also reviewed for their antiviral and anti-bacterial properties. After treatment of mouse L 929 cells with juice or extracts of E. purpurea herbs and roots, the cells became resistant to influenza, herpes (HSV), and vesicular stomatitis viruses [3]. None of the fractions, however, exhibited any anti-rhinovirus activity [4]. Extracts of Echinacea were found to show antiviral activity against HSV type 1 in vitro when exposed to visible and UV-A light [5]. In a chronic inflammatory disorder of skin follicles (acne) caused by Propionibacterium acnes, which induces the secretion of proinflammatory cytokines, Echinacea purpurea inhibited the proliferation of bacteria and suppressed cytokine levels [6]. Recent studies have revealed that standardised E. purpurea preparations show strong antimicrobial activity [7]. It was reported by Bany et al. that in mice E. purpurea and Echinacea-containing remedy Alchinal stimulated some parameters of cellular immunity mediated by lymphocytes and granulocytes, and increased animals’ defence against Pseudomonas aeruginosa infection [8, 9].
In vitro and animal studies have shown that Echinacea increases levels of interferon [10] and may increase phagocytosis, cellular respiratory activity, and lymphocyte activation through release of tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), interferon β (IFN-β) [11]. It was discovered in HIV-infected patients that co-administration of E. purpurea with darunavir-ritonavir was safe and well tolerated [12]. Echinacea had also an anti-fungal effect, which disrupted fungal cell walls [13]. In in vitro studies Echinacea reduced human cancer cell viability [14]. Some studies in cancer patients undergoing chemotherapy showed that polysaccharide fraction isolated from Echinacea purpurea may counteract chemotherapy-induced leukopaenia [15]. The anti-inflammatory effect of essential oils from extracts of E. purpurea was evaluated in vivo in mice and rats by using various experimental models. It was shown that the pro-inflammatory cytokines in the blood were reduced in the treated groups [16].
The aim of the present study was to evaluate the in vivo influence of three orally administered Echinacea-based remedies (IMMUNAL drops, IMMUNAL FORTE tablets, and ECHINACEA FORTE drops) on some parameters of cellular and humoral immunity in mice.
Material and methods
Drugs
1. IMMUNAL drops (succus of Echinacea purpurea herbae with sorbitol and 20% ethyl alcohol); 2. IMMUNAL FORTE tablets (1 tablet contains 80 mg of Echinaceae purpurea herbae succus siccum, producer LEK Slovenia); 3. ECHINACEA FORTE drops (juice squeezed from fresh flowers – blooming species Echinacea purpurea with 22% of ethyl alcohol, Dr Theiss Germany). Mice were fed IMMUNAL drops for seven days in daily doses of 2 µl, 6 µl, or 12 µl diluted with distilled water times two (controls received 10% alcohol), or with IMMUNAL FORTE tablets in daily doses of 160, 320, 640, or 1280 µg dissolved in 40 µl of distilled water, and water as a control, or with ECHINACEA FORTE drops in daily doses 6 and 18 µl diluted with distilled water times two (controls received 10% alcohol). These doses correspond to 1, 3, or 6 (recommended therapeutic dose) ml of IMMUNAL drops; or 80, 160 (recommended therapeutic dose), 320, and 640 mg of IMMUNAL tablets; or 3 (recommended therapeutic dose) and 9 ml of ECHINACEA FORTE drops, given to a person that weighs 70 kg (the factor of seven was applied for the differences between mouse and human in relation to the surface to body mass).
Animals
The study was performed on 219 female inbred Balb/c mice 6-8 weeks old, approximately 20-22 g of body mass, delivered from the Polish Academy of Sciences breeding colony, and on 36 F1 hybrids Balb/c × C3H, females, four weeks old, weighing about 18-20 g. For all performed experiments animals were handled according to the Polish law on the protection of animals and NIH (National Institutes of Health) standards. All experiments were accepted and conducted according to the ethical guidance of the Local Bioethical Committee. Mice were housed 4-5 per cage and maintained under conventional conditions (room temperature 22.5-23.0°C, relative humidity 50-70%, 12-hour day/night cycle) with free access to standard rodent diet and water.
Cell culture experiments
Balb/c mice were fed for seven days with the tested remedies. On day eight the mice were bled in anaesthesia (ketamine 100 mg/kg and xylazine 10 mg/kg, BIOWET, Puławy, Poland) and sacrificed by cervical dislocation. Splenocytes were isolated from their spleens under sterile conditions by straining through a stainless steel sieve and cotton gauze followed by centrifugation on a Histopaque 1077 (Sigma-Aldrich, USA) for eight minutes at 400 g in order to remove erythrocytes. Isolated splenocytes were resuspended in Parker culture medium (TC 199, BIOMED, Lublin, Poland).
Mitogen-induced (PHA) splenocyte proliferation assay was performed as previously described [17]. Before establishing cultures, splenocytes from 2-3 Balb/C mice were pooled. Spleen cells cultures (in multiple repetitions) were incubated in Costar 96-well microplates (105 cells in 0.2 ml RPMI-1640 medium, Biomed Lublin, with 2 mM L-glutamine, 10% FCS and antibiotics) with mitogen PHA (Murex, G. B.) at a concentration of 0.5, 1, and 2 µg/ml, in a humidified atmosphere, at 37°C, with 5% CO2. After 48 hours of incubation 10 µl of tritiated thymidine (3HTdR, 0.2 mCi/ml, specific act. 2 Ci/mM) was added. After a further 24 hours the cells were harvested (Skatron) and incorporation of tritiated thymidine was measured using a β-scintillation counter (Rack Beta 1218, LKB Wallac). The arithmetical mean of the quadruplicate count was calculated and expressed as counts per minute (CPM).
Spleen cells chemokinesis (spontaneous migration) assay was performed in vitro according to the Sandberg method [18] with some modifications [19]. Splenocytes were resuspended in Parker culture medium with 5% inactivated FCS, at a final concentration of 30 × 106 cells/ml. Afterwards, siliconised capillary tubes were filled with cell suspension, sealed with plasticine, centrifuged (5 minutes, 450 g), and fixed on glass plates. Cell levels were marked. After 24 hours of incubation (37°C, 5% CO2, humidified atmosphere) the distances of migration were measured in millimetres (mm) at a magnification of 6.5× and presented as migration units (1 MU = 0.18 mm).
Antibody production
Mice were immunised with 10% SRBC (0.2 ml intraperitoneally), fed tested remedies for seven days beginning on the day of immunisation, bled in anaesthesia (3.6% chloral hydrate) from retro-orbital plexus on the day eight after immunisation, and sacrificed with Morbital (Biowet Pulawy, Poland). The antibody level was evaluated with haemagglutination assay in inactivated (56°C, 30 minutes) sera. After performing a series of sera dilutions, 1% SRBC in PBS was added and the mixture was centrifuged (5 minutes, 150 g) incubated for 120 minutes at room temperature, and shaken. The haemagglutination titre was evaluated under a light microscope – as the last dilution in which at least three cell conglomerates were present in at least three consecutive fields at objective magnification 20×. For statistical purposes the results were transformed into logarithm inversions of the antibody titre.
Lymphocyte-induced angiogenesis test (LIA) [20, 21]
Balb/c mice were supplemented for seven days with tested remedies as described previously. After this, mice were bled in anaesthesia (ketamine 100 mg/kg prep. Ketamina 10%, Biowet Pulawy, Poland, and xylazine 10 mg/kg, prep Sedazin, BIOWET, Pulawy, Poland) and sacrificed by cervical dislocation. Splenocytes were isolated from their spleens under sterile conditions by straining through a stainless steel sieve and cotton gauze and centrifugation on a Histopaque 1077 (Sigma-Aldrich, USA) for eight minutes at 400 g in order to remove erythrocytes. Isolated splenocytes were resuspended in Parker culture medium (TC 199, BIOMED, Lublin,Poland) and pooled within the groups of four (IMMUNAL FORTE tablets) or of three (ECHINACEA FORTE drops) mice. A local GVH reaction (lymphocyte-induced angiogenesis, LIA test) was performed according to [21]. Shortly afterwards, spleen cell suspensions were grafted intradermally (1 million cells in 0.05 ml of Parker medium per graft) into F1 (Balb/c × C3H) recipients. Before performing injections mice were anaesthetised intraperitoneally with 3.6% chloral hydrate (Sigma Aldrich, USA; 0.1 ml per 10 g of body mass). Both flanks of each mouse were finely shaved with a razor blade and injected with cells 1-3 times. Cell suspensions were supplemented with 0.05 ml/1 ml of 0.01% trypan blue in order to facilitate recognition of injection sites later on. After 72 hours the mice were treated with a lethal dose of pentobarbital (Morbital, Biowet Pulawy, Poland). All newly-formed blood vessels were identified and counted under a dissection microscope on the inner skin surface, at a magnification of 6×, in 1/3 central area of the microscopic field. Identification was based on the fact that new blood vessels are thin, directed to the point of cells injection, with ramifications, and some of them are tortuous.
Statistical analysis
Statistical evaluation of the results was performed by two-way or one-way ANOVA, and the significance of differences between the groups was verified with a Bonferroni or Tukey Multiple Comparison Post Test and unpaired t test (Graph Pad Prism).
Results
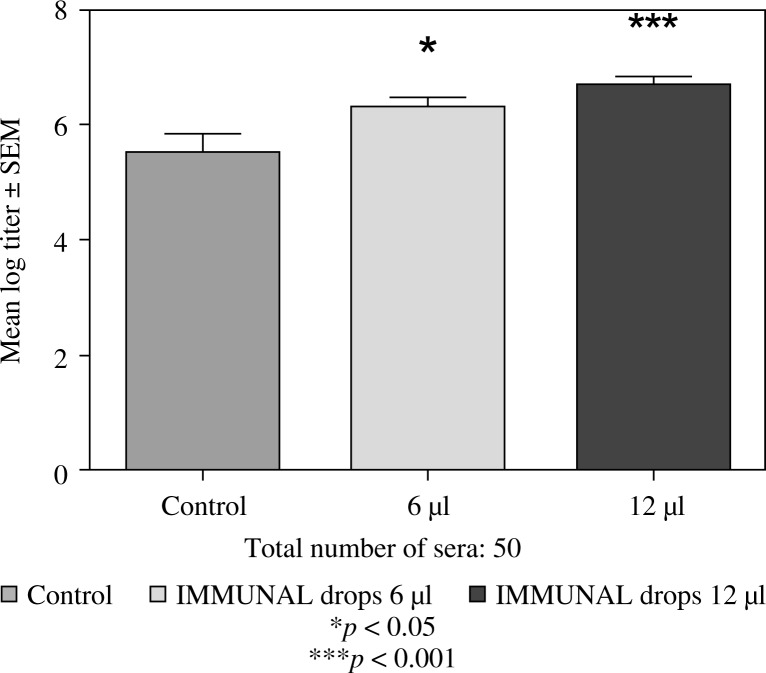
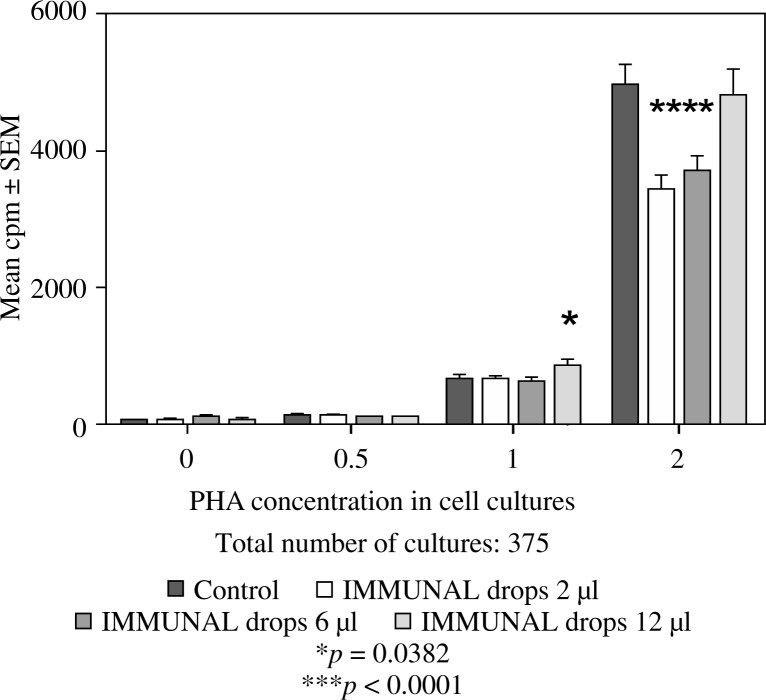
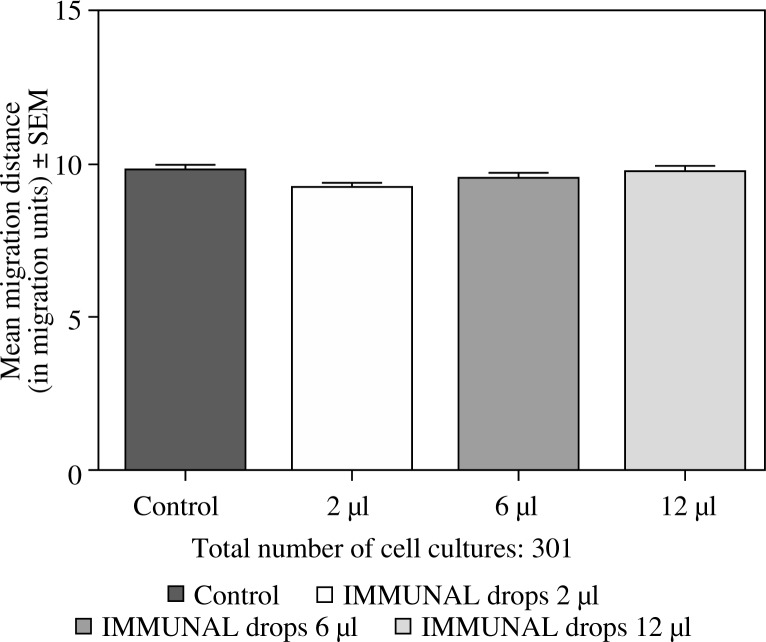
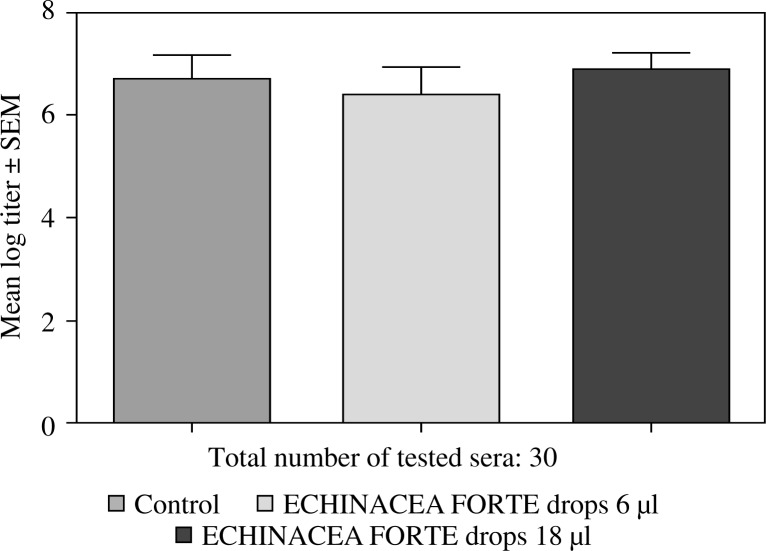
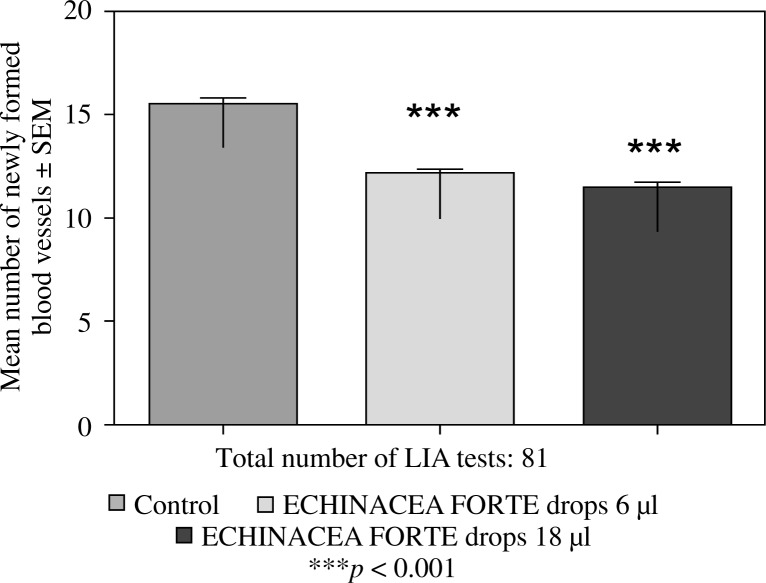
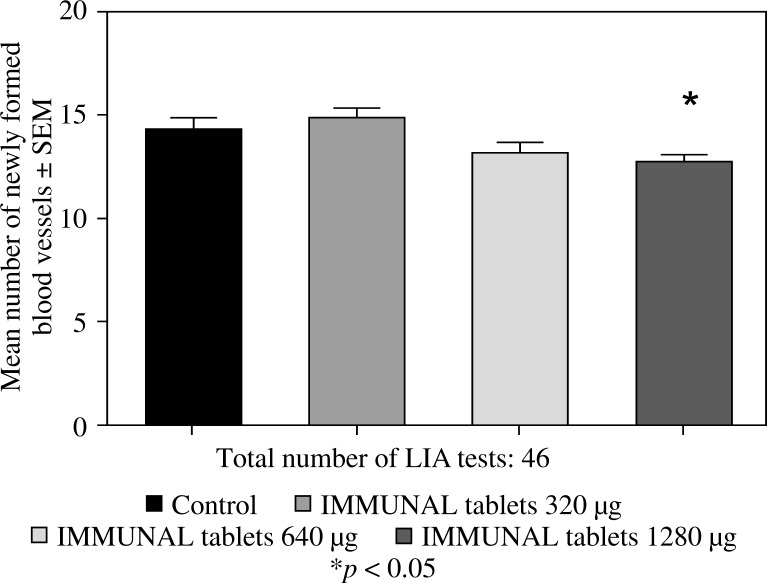
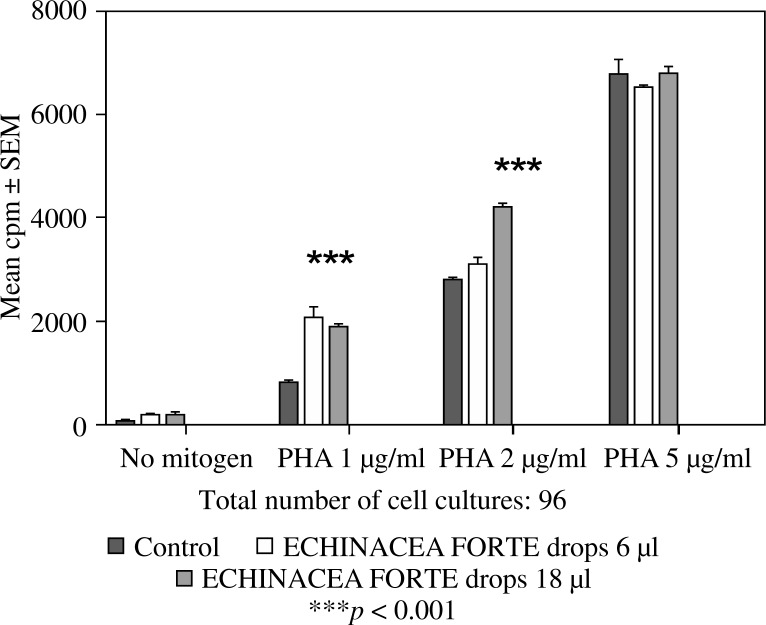
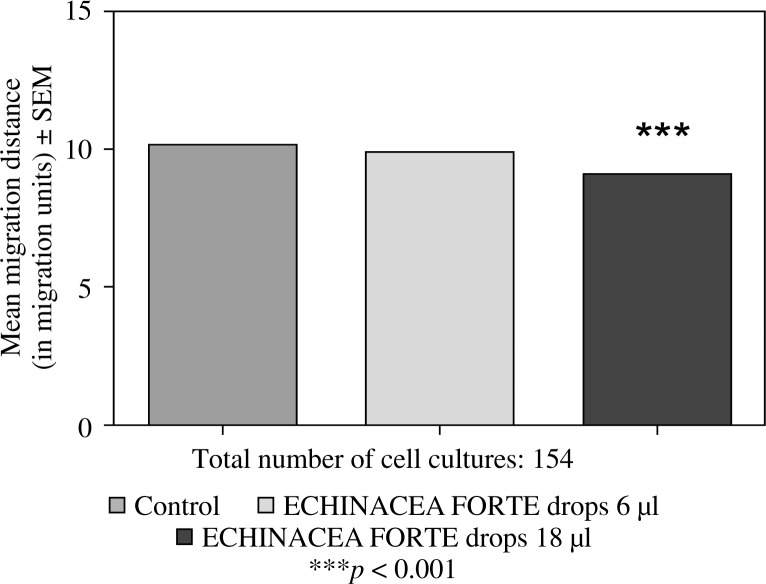
Feeding mice for seven days with 6 or 12 µl of Echinacea purpurea succus stabilised with alcohol (IMMUNAL drops) resulted in enhanced anti-SRBC antibody production (Fig. 1) and modulated (enhanced in lower concentration of mitogen, suppressed in higher) proliferative response to PHA of their splenic lymphocytes (Fig. 2). No stimulatory effect was observed on splenocytes chemokinesis (Fig. 3). Mice fed another formulation of Echinacea purpurea succus stabilised with alcohol (ECHINACEA FORTE drops) presented enhanced response to PHA of their splenocytes. However, contrary to the previous group, no enhancement of antibody production was observed. In this group, lymphocyte-induced immunological angiogenesis (LIA) and chemokinesis (spontaneous migration – SM) of spleen lymphocytes was diminished after feeding mice with both doses (LIA) or with higher dose (SM) of remedy (Figs. 4–7). LIA activity of splenocytes collected from animals fed with prophylactic and therapeutic doses of IMMUNAL FORTE tablets did not differ from the controls (Fig. 8).
Fig. 1.
Stimulatory effect of IMMUNAL drops on the anti-SRBC antibody production
Fig. 2.
The effect of feeding mice IMMUNAL drops for 7 days in daily doses 2, 6, or 12 µl on mitogen-induced proliferation of their splenic lymphocytes in 72-hour cell cultures, measured by 3H thymidine incorporation
Fig. 3.
No effect of feeding mice with IMMUNAL drops for 7 days in daily doses 2, 6, or 12 µl on chemokinetic activity of their splenocytes in 24-hour cell culture
Fig. 4.
No effect of ECHINACEA FORTE drops on anti-SRBC antibody production
Fig. 7.
Inhibitory effect of ECHINACEA FORTE drops on the local graft-versus-host reaction in mice. Balb/c mice were fed ECHINACEA FORTE drops for 7 days in 6 or 18 µl daily doses, then their splenocytes were grafted intradermally to F1 (Balb/c × C3H) recipients (lymphocyte induced angiogenesis, LIA test)
Fig. 8.
Inhibitory (in the highest dose) effect of IMMUNAL FORTE tablets on immunological angiogenesis in the course of the local graft-versus-host reaction in mice. Balb/c mice were fed drug for 7 days in 320, 640, or 1280 µg daily doses, then their splenocytes were grafted intradermally to F1 (Balb/c × C3H) recipients (lymphocyte induced angiogenesis, LIA test)
Fig. 5.
The effect of feeding mice ECHINACEA FORTE drops for 7 days in daily doses 6 or 18 µl on mitogen-induced proliferation of their splenic lymphocytes in 72-hour cell cultures, measured by 3H thymidine incorporation
Fig. 6.
No effect of feeding mice with ECHINACEA FORTE drops for 7 days in daily doses 6 µl on chemokinetic activity of their splenocytes in 24-hour cell culture, and diminished chemokinetic activity in mice fed 18 µl daily
Discussion
The results of the study revealed some differences between in vivo immunotropic effects exerted by two liquid Echinacea remedies: IMMUNAL drops and ECHINACEA FORTE drops. Both remedies have modulated splenocytes mitogen-induced proliferation. In the case of ECHINACEA FORTE drops the obtained effect was stimulatory in both dosage groups but disappeared in the highest concentration of PHA. In the case of IMMUNAL drops the situation was more complicated: stimulation was observed in the lower PHA concentration – the one that contained cultures of splenocytes collected from mice fed the highest dose of remedy only. In the higher PHA concentration we observed inhibition (when splenocytes derive from 2 or 6 µl dosage groups of animals) or no effect when splenocytes were derived from the highest dosage group. In the previous study, we observed stimulation of PHA-induced splenocyte proliferation after feeding mice IMMUNAL FORTE tablets with a 0.32 mg daily dose for seven days. In that study, similarly to the present findings obtained with anti-SRBC antibody response and IMMUNAL drops and contrary to the results of experiments with ECHINACEA FORTE drops, anti-SRBC antibody production was stimulated [22]. Lack of stimulation of antibody response to the antigen in the case of ECHINACEA FORTE drops is difficult to understand because other formulations of Echinacea evaluated by us earlier constantly have stimulated this parameter [22, 23]. Maybe the reason of this exceptional situation (perhaps stimulation of suppressor mechanisms – regulatory lymphocytes and their products) will be resolved during further studies. In the present and earlier ex vivo and in vitro studies of cell-mediated parameters (response to mitogens, spontaneous cell migration) we observed various effects, stimulation, inhibition, or no effect. In the majority of situations, stimulation was connected with lower, and inhibition with higher, doses of Echinacea (or other herbs), but not always. In our previous study of IMMUNAL FORTE tablets, chemokinesis of spleen lymphocytes was enhanced in mice fed with a 0.32 mg daily dose, and it was suppressed by higher doses [22]. In the present experiments, no effect (IMMUNAL drops) or slight inhibition in higher dose (ECHINACEA FORTE) was observed.
Previously, immunological angiogenesis experiments performed with IMMUNAL drops revealed significant stimulation of angiogenic activity of splenic lymphocytes in both dosage groups of mice [24]. In the present work we compared groups of mice fed IMMUNAL FORTE tablets and ECHINACEA FORTE drops. We obtained highly significant inhibition with the lower dose (6 µl) of ECHINACEA FORTE drops (calculated from the human dose prescribed for adults), as well as by the higher dose. In the case of IMMUNAL FORTE tablets no effects in its prophylactic and therapeutic doses were observed. A slightly suppressive effect was seen in the very high dosage group (1280 µg) only.
Looking at these and previous results, one may hypothesise that the ability of Echinacea succus to stimulate or inhibit immunological angiogenesis disappears after the drying process. On the other hand, it also might be possible that drying has the potential to increase in lower concentration, and decrease in higher concentration, the splenic lymphocytes motility, manifested as increased or decreased chemokinesis in ex vivo tissue culture.
One important group of active Echinacea compounds are alkamides. Unsaturated N-alkylamide lipids capable of activating cannabinoid receptor type-2 are thought to possess anti-inflammatory and immunomodulatory activities [25]. They exert pleiotropic effects, synergistic or antagonistic with the action of other active Echinacea compounds [26]. Processing Echinacea herb slightly alters the levels of some alkamides, whereas drying has no effect [27, 28]. There is no significant difference in the bioavailability of alkamides from the liquid and siccum Echinacea forms.
Another important group of active compounds are polyphenols, among them cichoric acid. This phenolic acid is very sensitive to drying temperature, with a greater loss from aerial plant parts than from the root [29–31]. We previously reported that various phenolic acids may exert stimulatory or inhibitory effects on angiogenesis [32, 33]. It would be interesting to see whether there is a difference between the level of cichoric and other phenolic acids in IMMUNAL drops and in ECHINACEA FORTE drops, and we plan to perform some experiments on this topic in the future.
In conclusion, Echinacea purpurea succus from aerial plant parts is a wide-spectrum immunomodulator, with various forms that differ greatly between each other. They can contain a number of active compounds that in specific proportions can either work synergistically or oppose one another. Hence every medication that is based on those substances should be thoroughly tested prior to being approved for use in various immune system malfunctions.
The authors declare no conflict of interest.
References
- 1.Samochowiec E, Urbańska L, Mańka W, Stolarska E. Assessment of the action of Calendula officinalis and Echinacea angustifolia extracts on Trichomonas vaginalis in vitro. Wiadomości Parazytologiczne. 1979;25:77–81. [PubMed] [Google Scholar]
- 2.Bany J, Zdanowska D, Zdanowski R, Skopińska-Różewska E. The effect of herbal remedy on the development of Trichinella spiralis infection in mice. Pol J Vet Sci. 2003;6(3 Suppl):6–8. [PubMed] [Google Scholar]
- 3.Egert D, Beuscher N. Virus-inhibition by Echinacea purpurea. Planta Med. 1992;58:163–165. doi: 10.1055/s-2006-961420. [DOI] [PubMed] [Google Scholar]
- 4.Binns SE, Hudson J, Merali S, Arnason JT. Antiviral activity of characterized extracts from Echinacea spp. (Heliantheae:Asteraceae) against herpes simplex virus (HSV-I) Planta Med. 2002;68:780–783. doi: 10.1055/s-2002-34397. [DOI] [PubMed] [Google Scholar]
- 5.Vimalanathan s, Kang L, Amiguet V, et al. Echinacea purpurea aerial parts contain multiple antiviral compounds. Pharmac Biol. 2005;43:740–745. [Google Scholar]
- 6.Sharma M, Schoop R, Suter A, Hudson JB. The potential use of Echinacea in acne: control of Propionibacterium acnes growth and inflammation. Phytother Res. 2011;25:517–521. doi: 10.1002/ptr.3288. [DOI] [PubMed] [Google Scholar]
- 7.Hudson JB. Applications of the phytomedicine Echinacea purpurea (purple coneflower) in infectious diseases. J Biomed Biotechnol. 2012;2012:769896. doi: 10.1155/2012/769896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bany J, Siwicki AK, Zdanowska D, et al. Echinacea purpurea stimulates cellular immunity and anti-bacterial defense independently of the strain of mice. Pol J Vet Sci. 2003;6(suppl 3):3–5. [PubMed] [Google Scholar]
- 9.Bany J, Skopinska-Rozewska E, Joanna Chorostowska-Wynimko, et al. The effect of complex herbal remedy on the angiogenic activity of L-1 sarcoma cells, L-1 sarcoma tumor growth and on the bacterial infection in mice. Centr Eur J Immunol. 2004;29:29–34. [Google Scholar]
- 10.Hayashi I, Ohotsuki M, Suzuki I, et al. Effects of oral administration of Echinacea purpurea (American herb) on incidence of spontaneous leukemia caused by recombinant leukemia viruses in AKR/J mice. Nihon Rinsho Meneki Gakkai Kaishi. 2001;20:10–20. doi: 10.2177/jsci.24.10. [DOI] [PubMed] [Google Scholar]
- 11.Robbers JE, Tyler VE. Herbs of choise: the therapeutic use of phytomedicinals. New York: Haworth Herbal Press; 1999. [Google Scholar]
- 12.Molto J, Valle M, Miranda C, et al. Herb-drug interaction between Echinacea purpurea and darunavir-ritonavir in HIV- infected patients. Antimicrob Agents Chemother. 2011;55:326–330. doi: 10.1128/AAC.01082-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mir-Rashed N, Cruz I, Jessulat M, et al. Disruption of fungal cell wall by antifungal Echinacea extracts. Med Mycol. 2010;48:949–958. doi: 10.3109/13693781003767584. [DOI] [PubMed] [Google Scholar]
- 14.Chicca A, Adinolfi B, Martinotti E, et al. Cytotoxic effects of Echinacea root hexanic extracts on human cancer cell lines. J Ethnopharmacol. 2007;110:148–153. doi: 10.1016/j.jep.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Melchart D, Clemm C, Weber B, et al. Polysaccharides isolated from Echinacea purpurea herba cell cultures to counteract undesired effects of chemotherapy – a pilot study. Phytother Res. 2002;16:138–142. doi: 10.1002/ptr.888. [DOI] [PubMed] [Google Scholar]
- 16.Yu D, Yuan Y, Jiang L, et al. Anti-inflammatory effects of essential oil in Echinacea purpurea L. Pak J Pharm Sci. 2013;26:403–408. [PubMed] [Google Scholar]
- 17.Skopińska-Różewska E, Sokolnicka I, Siwicki AK, et al. Dose-dependent in vivo effect of Rhodiola and Echinacea on the mitogen-induced lymphocyte proliferation in mice. Pol J Vet Sci. 2011;14:265–272. doi: 10.2478/v10181-011-0040-9. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg G. The sealed capillary migration technique and thymocyte migration in vitro. J Immunol Meth. 1976;12:365–368. doi: 10.1016/0022-1759(76)90058-2. [DOI] [PubMed] [Google Scholar]
- 19.Skopińska-Różewska E, Bychawska M, Białas-Chromiec B, Sommer E. The in vivo effect of Rhodiola rosea and Rhodiola quadrifida hydro-alcoholic extracts on chemokinetic activity of spleen lymphocytes in mice. Cent Eur J Immunol. 2009;34:42–45. [Google Scholar]
- 20.Skopińska-Różewska E, Wasiutyński A, Sommer E, et al. Modulatory effect of Echinacea pallida on cellular immunity and angiogenesis in mice. Cent Eur J Immunol. 2011;36:18–23. [Google Scholar]
- 21.Skopiński P, Lewicki S, Bałan BJ, et al. In vivo inhibitory effect of Aloe vera gel on the ability of mouse parental splenic lymphocytes to induce cutaneous angiogenesis in recipient F1 mice. Pol J Vet Sci. 2014;17:131–136. doi: 10.2478/pjvs-2014-0017. [DOI] [PubMed] [Google Scholar]
- 22.Bałan BJ, Nartowska J, Skopińska-Różewska E, et al. Wpływ wyciągów Echinacea purpurea na reakcje odpornościowe oraz procesy angiogenezy. In: Skopińska-Różewska E, Siwicki AK, editors. Endogenne i egzogenne modulatory odporności i angiogenezy. Olsztyn: Wyd. EDYCJA; 2007. pp. 27–60. [Google Scholar]
- 23.Sokolnicka I, Skopińska-Różewska E, Strzelecka H, et al. Adaptacja testów biologicznych do oceny aktywności preparatów jeżówki purpurowej (Echinacea purpurea). 1. Badania in vivo. Terapia. 2001;3:38–42. [Google Scholar]
- 24.Skopińska-Różewska E, Furmanowa M, Guzewska J, et al. The effect of Centella asiatica,Echinacea purpurea and Melaleuca alternifolia on cellular immunity in mice. Cent Eur J Immunol. 2002;27:142–148. [Google Scholar]
- 25.Woelkart K, Bauer R. The role of alkamides as an active principle of echinacea. Planta Medica. 2007;73:615–623. doi: 10.1055/s-2007-981531. [DOI] [PubMed] [Google Scholar]
- 26.Chicca A, Raduner S, Pellati F, et al. Synergistic immunopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int Immunopharmacol. 2009;9(7-8):850–858. doi: 10.1016/j.intimp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Perry NB, van Klink JW, Burgess EJ, Parmenter GA. Alkamide levels in Echinacea purpurea: effects of processing, drying and storage. Planta Med. 2000;66:54–56. doi: 10.1055/s-2000-11111. [DOI] [PubMed] [Google Scholar]
- 28.Kim HO, Durance TD, Scaman CH, Kitts DD. Retention of alkamides in dried Echinacea purpurea. J Agric Food Chem. 2000;48:4187–4192. doi: 10.1021/jf000246n. [DOI] [PubMed] [Google Scholar]
- 29.Livesey J, Awang DV, Amason JT, et al. Effect of temperature on stability of marker constituents in Echinacea purpurea root formulations. Phytomedicine. 1999;6:347–349. doi: 10.1016/S0944-7113(99)80057-9. [DOI] [PubMed] [Google Scholar]
- 30.Stuart DL, Wills RB. Effect of drying temperature on alkylamide and cichoric acid concentrations of Echinacea purpurea. J Agric Food Chem. 2003;51:1608–1610. doi: 10.1021/jf026213k. [DOI] [PubMed] [Google Scholar]
- 31.Matthias A, Addison RS, Agnew LL, et al. Comparison of Echinacea alkylamide pharmacokinetics between liquid and tablet preparations. Phytomedicine. 2007;14:587–590. doi: 10.1016/j.phymed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Glinkowska G, Bałan B, Sommer E, et al. The effect of phenolic compounds of poplar leaves extract on cutaneous angiogenesis reaction induced in mice by human mononuclear leukocytes. Acta Pol Pharm. 1997;54:151–154. [PubMed] [Google Scholar]
- 33.Bałan BJ, Skopińska-Różewska E, Barcz E, et al. Wpływ wybranych kwasów fenolowych na aktywność angiogenną komórek raka jajnika – doniesienie wstępne. Onkol Pol. 1999;2:203–208. [Google Scholar]