Abstract
Interleukin (IL)-17-producing CD4+ T helper (Th17) cells that are known to produce IL-17 have recently been defined as a unique subset of proinflammatory helper cells. Interleukin 17 is an inflammatory cytokine with robust effects on many cells. It can play important roles in the pathogenesis of diverse groups of immune-mediated diseases. In this regard, the present case-control study aimed at determining serum levels of IL-17, IL-6, and transforming growth factor β (TGF-β) in Iranian breast cancer patients. Blood samples were collected from 55 patients with breast cancer and 34 healthy individuals with no history of malignancies or autoimmune disorders, based on simple sampling. The serum levels of IL-17, IL-6 and TGF-β were measured by enzyme-linked immunosorbent assay (ELISA). The serum level of IL-6 was significantly lower in patients with breast cancer compared with healthy individuals (p = 0.0003), and also the IL-17 was lower in the patient group than in controls (p = 0.01). Interestingly, the TGF-β serum level in patients was less than in controls (p < 0.0001). As most of the cases investigated in this study were in their early stages, it can be concluded that reduced IL-17, IL-6, and TGF-β can be used as predictors for clinical stage and prognosis of cancers such as breast carcinoma.
Keywords: breast cancer, interleukin 6, interleukin 17, T-helper 17, transforming growth factor β
Introduction
Two decades ago, Mossman et al. [1] proposed that CD4+ T cells differentiate into two subsets with reciprocal functions and patterns of cytokine secretion, termed T-helper 1 (Th1) and Th2 [2]. Th1 cells are characterised by production of interferon-γ (IFN-γ), and they induce cell-mediated immunity against intracellular pathogens, while Th2 cells produce interleukin (IL)-4 and stimulate humeral immunity against parasitic helminths. This paradigm was maintained until 2005, when a third T-cell subset, known as Th17, was identified [3]. Th17 was reported with its major signature of releasing IL-17 [4]. In recent years, growing reports on the role and function of Th17 indicate that this subset of CD4+ T cells plays a fundamental role in infiltration and recruitment of inflammatory cells against intercellular parasites and fungi [5, 6]. Also, recent data in humans suggest that Th17 cells are important due to their strong association with a diverse group of immune-mediated diseases, including psoriasis, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, cancers, and other autoimmune diseases. Moreover, they seem to be important for controlling these disorders, too [7, 8]. During tumour development, Th17 cells gradually increase in the tumour microenvironment. There are several factors that can play major roles in the induction of Th17 differentiation. These factors include a lot of substances released by tumour cells and tumour stroma, or molecules secreted by tumour-infiltrating immune cells such as transforming growth factor β (TGF-β), IL-6, prostaglandin E2 (PGE2), IL-21, IL-23, osteopontin, IL-1β, and tumour necrosis factor α (TNF-α) [9–11]. Several recent studies have demonstrated that TGF-β and IL-6 are critical factors for murine and human Th17 cell differentiation in vitro. The role of TGF-β in human Th17 cells is still subject to intense debate. In humans, naive T helper cells can be induced to differentiate into Th17 cells in vitro by exposure to IL-6 and TGF-β, also IL-23 promotes proliferation and survival of these cells [12–14]. However, some studies found that the role of TGF-β in differentiation of human Th17 cells to be inhibitory [15, 16].
It is obvious that TGF-β plays an essential role in differentiation of CD4+ T cells toward regulatory T cells (Tregs) or Th17 cells. The combination of TGF-β and IL-6 promotes the differentiation of Th17 cells and inhibits Treg cell differentiation [9, 14, 17], whereas TGF-β plus retinoic acid inhibits Th17 cell differentiation and promotes the Treg cells [18, 19].
There are some studies that show the paradoxes of the protumour and antitumour functions of IL-17. Studies have shown that IL-17 can increase growth and proliferation of cervical cancer cells through IL-6 [20], it can increase blood vessels in ovarian cancer [21], and it has been noticed as a prognostic biomarker in colorectal cancer progression [22]. On the other hand, antitumor functions of IL-17 have been reported. In this connection, Muranski et al. reported that Th17 cells are more effective than Th1 cells in eliminating established tumours [23]. In addition, IL-17 induces the production of IL-6 from a variety of cells. Interleukin 17, via IL-6 and IL-12, is associated with the induction of tumour-specific cytotoxic T lymphocyte (CTL) induction [24] and affects the overexpression of major histocompatibility complex (MHC) classes I and II in dendritic cell maturation [25].
Breast cancer is the most common cancer among Iranian females, with a mean age onset at least 10 years below than seen in western countries [26]. The role of Th17 cells in the pathogenesis of breast cancer is pivotal.
Due to the importance of IL-6, TGF-β, and IL-17 serum levels in breast cancer disease, we used ELISA to directly measure their serum levels in peripheral blood cells of patients with breast cancer to compare them with healthy blood donors.
Material and methods
Subjects
Seventy-one women who participated in this study were diagnosed with infiltrating ductal carcinoma of the breast, confirmed by histological studies using simple sampling. Fifty-five patients were selected with a mean age of 48 ±10 years, and the rest were excluded from the study because of technical errors in the ELISA method. Patients from the hospitals of Shiraz University of Medical Sciences (Shiraz, Iran) were referred to our laboratory in the Institute for Cancer Research (ICR), which was used as the cancer referral laboratory. Peripheral blood samples (2 ml) were collected before any clinical intervention. Data on age, tumour histology, tumour size, tumour invasion, clinical stage, histological grade, and the presence of other organ metastases were obtained from the hospital pathological records of the patients. The clinical stage was determined with the tumour-node-metastasis (TNM) classification. The TNM classification and tumour diameter were obtained from the pathology reports by one pathologist. In addition, the tumours were graded according to the World Health Organization (WHO) classification criteria as moderately or poorly differentiated. Patients received chemotherapy interventions before this study. Table 1 demonstrates the distribution of patients regarding different clinical criteria. Blood samples from 34 healthy individuals with a mean age of 44 ±7 years without history of malignancies or autoimmune disorders were also obtained as the control group. During sample collection, it was ensured that subjects had neither infection nor any acute or chronic diseases. All the subjects provided informed consent to participate in the study and to allow their biological samples to be analysed. Approval for the study was given by the Ethics Committee of Shiraz University of Medical Sciences (Shiraz, Iran).
Table 1.
Distribution of patients with breast cancer with regard to different clinical criteria
| Pathological characteristic | Status | Frequency n (%) |
| Side | Left | 29 (52) |
| Right | 26 (48) | |
| ER | Positive | 37 (68) |
| Negative | 18 (32) | |
| PR | Positive | 34 (62) |
| Negative | 21 (38) | |
| HER-2 | Positive | 37 (68) |
| Negative | 18 (32) | |
| Necrosis | Positive | 25 (45) |
| Negative | 30 (55) | |
| Calcification | Positive | 37 (67) |
| Negative | 18 (33) | |
| Lymphatic invasion | Positive | 24 (43) |
| Negative | 31 (57) | |
| Vascular invasion | Positive | 11 (20) |
| Negative | 44 (80) | |
| Perineural invasion | Positive | 11 (20) |
| Negative | 44 (80) | |
| Grade | Low | 47 (86) |
| High | 8 (14) | |
| Stage | I | 18 (33) |
| II | 26 (48) | |
| III | 9 (16) | |
| IV | 2 (3) | |
| Metastasis | Positive | 2 (97) |
| Negative | 53 (3) |
Enzyme linked immunosorbent assay (ELISA)
The amounts of cytokines in the patients’ and controls’ sera were measured at the same time by the same technician, using ELISA-kits (eBiosciences, San Diego, CA, USA). Briefly, premixed standards were reconstituted in PBS (pH 7.2), generating stock concentrations of 200, 500, and 1000 pg/ml for IL-6, IL-17, and TGF-β, respectively. Sensitivity for IL-17, IL-6, and TGF-β were 4, 2, and 8 pg/ml, respectively. The standard stocks were serially diluted in Reagent Diluent to generate seven points for the standard curves. Diluted Capture Antibody was added at the final volume of 100 µl. Plates were sealed and incubated overnight at room temperature, then they were washed with Wash Buffer. Premixed standards or samples (100 µl) were added to each well, covered with an adhesive strip, and incubated overnight at 4°C. After incubation and washing, 100 µl of the premixed Detection Antibody was added to each well and incubated for two hours at room temperature. After incubation and washing, Streptavidin-HRP was added to each well (100 µl). The incubation was terminated after 20 minutes at room temperature. Then, 50 µl of Stop Solution was added to each well, and the optical density of each well was immediately determined using a microplate reader set to 450 nm. The results were expressed in pg/ml.
Statistical analysis
The amounts of IL-6, TGF-β, and IL-17 in the peripheral blood were evaluated to the corresponding values from control samples with nonparametric Kruskal-Wallis and Mann-Whitney tests by SPSS software version 11.5 (SPSS, Chicago, IL, 173 USA). Finally, correlations between different statuses were evaluated using Spearman correlation coefficient. The variable levels were evaluated using Prism 4 software (Inc; San Diego CA, USA, 2003). P < 0.05 was regarded as significant in all statistical analysis.
Results
Serum cytokine level in patient and healthy groups
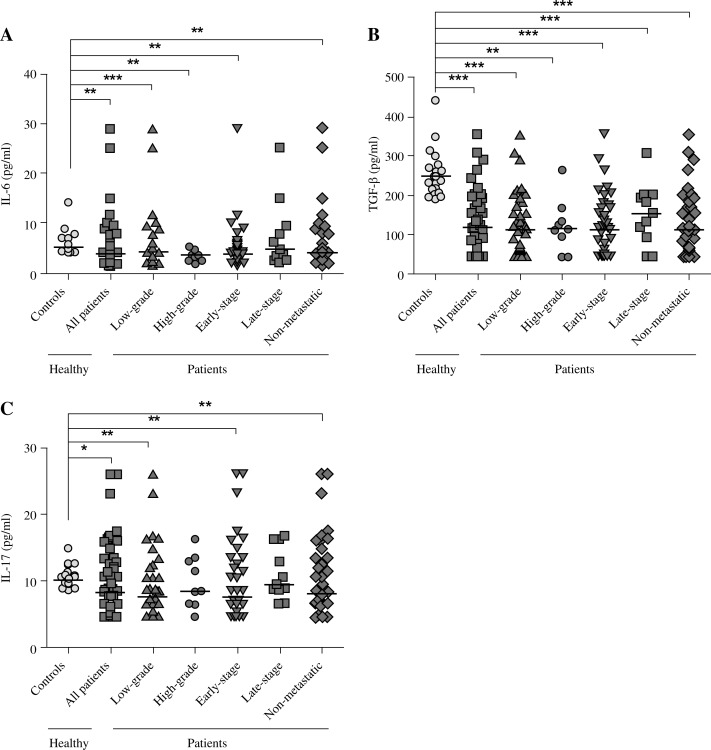
It was shown that the serum level of IL-6 among patients was significantly low compared to the control group (Fig. 1A). We noted significant differences in the low-grade, high-grade, early disease stages (stages I and II), and non-metastatic status compared to controls. These results are summarised in Table 2. In addition, no correlation was found between IL-6 serum level and clinicopathological data of the cases (data not shown). Interleukin 6 was not significantly related to depth of tumour, and there were no significant differences between negative and positive invasion status (Table 3).
Fig. 1.
Serum level of IL-6, TGF-β, and IL-17 in the peripheral blood of breast cancer patients and normal controls. Significant differences were found in the serum level of IL-6 (A), TGF-β (B), and IL-17 (C) in peripheral blood of patients with breast cancer patients compared to healthy controls. Presented data were analysed with the nonparametric two-tailed Mann-Whitney test; the horizontal lines show the median of the groups
Table 2.
The serum levels of IL-6, TGF-β, and IL-17 in peripheral blood of patients with breast cancer and normal individualsa,b
| IL-6 | TGF-β | IL-17 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ±SEM | Median | p | Mean ±SEM | Median | p | Mean ±SEM | Median | p | |
| Controls (n = 34) | 5.70 ±0.31 | 5.13 | 249.4 ±8.05 | 247.7 | 10.17 ±0.21 | 10.0 | |||
| All patients (n = 55) | 5.30 ±0.67 | 3.74 | 0.0003 | 130.1 ±10.11 | 118.1 | < 0.0001 | 9.67 ±0.71 | 8.05 | 0.01 |
| Low-grade (n = 38) | 5.80 ±0.91 | 4.14 | 0.007 | 126.3 ±12.78 | 111.8 | < 0.0001 | 8.94 ±0.82 | 7.40 | 0.001 |
| High-grade (n = 17) | 4.18 ±0.76 | 3.47 | 0.0002 | 138.8 ±16.12 | 133.0 | 0.0002 | 11.35 ±1.35 | 9.27 | > 0.05 |
| Early-stage (n = 42) | 4.84 ±0.67 | 3.71 | < 0.0001 | 120.2 ±11.24 | 111.8 | < 0.0001 | 9.26 ±0.86 | 7.40 | 0.003 |
| Late-stage (n = 13) | 7.45 ±2.11 | 4.59 | > 0.05 | 152.4 ±23.26 | 154.2 | < 0.0001 | 10.92 ±1.16 | 9.27 | > 0.05 |
| Non-metastatic (n = 53) | 5.46 ±0.72 | 3.90 | 0.0008 | 125.2 ±10.48 | 113.9 | < 0.0001 | 9.52 ±0.75 | 7.83 | 0.003 |
pg/ml
The presented data was analysed with the nonparametric two-tailed Mann-Whitney test. Early-stage and late-stage patients were in stages I, II and III, IV with a localised tumour in breast and low-grade and high-grade patients were in grades 1, 2 and 3, 4 with good or moderate differentiation.
Table 3.
The serum level of IL-6, TGF-β, and IL-17 in different clinic pathological parameters of patients with breast cancer
| IL-6 [pg/ml] | TGF-β [pg/ml] | IL-17 [pg/ml] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ±SEM | Median | p | Mean ±SEM | Median | p | Mean ±SEM | Median | p | |
| Lymphatic invasion | |||||||||
| Negative | 4.89 ±0.88 | 3.37 | NS | 120.9 ±13.31 | 111.8 | NS | 9.38 ±0.97 | 7.69 | NS |
| Positive | 6.24 ±1.19 | 4.81 | 135.8 ±16.76 | 124.5 | 10.13 ±1.15 | 8.63 | |||
| Vascular invasion | |||||||||
| Negative | 5.53 ±0.87 | 3.93 | NS | 125.3 ±12.48 | 111.8 | NS | 9.69 ±0.92 | 7.69 | NS |
| Positive | 4.99 ±1.09 | 3.47 | 130.9 ±17.64 | 128.7 | 9.56 ±0.97 | 8.99 | |||
| Perineural invasion | |||||||||
| Negative | 5.04 ±0.86 | 3.47 | NS | 125.1 ±12.98 | 116.0 | NS | 10.1 ±0.77 | 7.69 | NS |
| Positive | 6.65 ±1.45 | 4.81 | 138.9 ±19.92 | 126.6 | 9.01 ±0.87 | 8.41 | |||
| ER | |||||||||
| Negative | 3.67 ±0.26 | 3.47 | NS | 95.37 ±13.35 | 90.62 | NS | 9.87 ±1.33 | 7.40 | NS |
| Positive | 5.83 ±0.89 | 4.43 | 147.8 ±14.64 | 143.6 | 10.07 ±0.97 | 8.34 | |||
| PR | |||||||||
| Negative | 5.10 ±1.14 | 3.74 | NS | 108.5 ±15.25 | 103.3 | NS | 10.40 ±1.23 | 8.4 | NS |
| Positive | 4.85 ±0.54 | 3.53 | 135.3 ±14.36 | 116.0 | 9.53 ±0.97 | 7.69 | |||
| HER-2 | |||||||||
| Negative | 5.76 ±0.94 | 4.43 | NS | 109.4 ±18.05 | 99.08 | NS | 9.72 ±1.17 | 9.85 | NS |
| Positive | 5.06 ±0.99 | 3.32 | 141.4 ±16.56 | 149.9 | 10.10 ±1.16 | 8.12 | |||
| Necrosis | |||||||||
| Negative | 6.66 ±1.45 | 3.71 | NS | 124.6 ±15.62 | 116.0 | NS | 9.43 ±0.98 | 8.12 | NS |
| Positive | 4.30 ±0.36 | 3.98 | 135.7 ±14.88 | 122.4 | 10.15 ±1.23 | 8.63 | |||
| Calcification | |||||||||
| Negative | 6.33 ±1.16 | 4.11 | NS | 135.4 ±12.05 | 128.7 | NS | 10.19 ±1.05 | 8.41 | NS |
| Positive | 4.16 ±0.44 | 3.53 | 121.7 ±22.08 | 94.85 | 9.195 ±1.19 | 7.40 | |||
| Involvement | |||||||||
| Right | 5.11 ±0.64 | 3.95 | NS | 137.7 ±17.83 | 116.0 | NS | 9.41 ±1.05 | 8.12 | NS |
| Left | 5.68 ±1.24 | 3.53 | 118.8 ±11.98 | 122.4 | 9.92 ±1.08 | 7.83 | |||
| Grade | |||||||||
| Low | 5.80 ±0.91 | 4.14 | NS | 126.3 ±12.78 | 111.8 | NS | 8.94 ±0.82 | 7.40 | NS |
| High | 4.18 ±0.76 | 3.47 | 138.8 ±16.12 | 133.0 | 11.35 ±1.35 | 9.27 | |||
| Stage | |||||||||
| Early | 4.84 ±0.67 | 3.71 | NS | 120.2 ±11.24 | 111.8 | NS | 9.26 ±0.86 | 7.40 | NS |
| Late | 7.45 ±2.11 | 4.59 | 152.4 ±23.26 | 154.2 | 10.92 ±1.16 | 9.27 | |||
| Metastasis | |||||||||
| Negative | 5.46 ±0.72 | 3.90 | NS | 125.2 ±10.48 | 113.9 | NS | 9.52 ±0.75 | 7.83 | NS |
| Positive | 3.20 ±0.58 | 3.05 | 193.5 ±22.77 | 198.8 | 11.62 ±1.72 | 11.51 | |||
NS – not significant
As shown in Fig. 1B, specimens in the control group showed substantially higher levels of TGF-β than all of those from the patients (Table 2). In addition, among the patients in low-grade, high-grade, early disease stages (stages I and II), late disease stages, and non-metastasis status, the serum level of IL-6 was lower than in healthy volunteers.
The IL-17 serum level showed a significantly lower level among patients compared to the control group. Remarkably, patients in low-grade, early-stage, and non-metastatic status of the disease showed significant differences in the mean serum level of IL-17 (Fig. 1C). Nevertheless, no correlation was found between IL-17 serum level and any clinicopathological parameters in the patient group (data not shown). In addition, patients in none of clinicopathological parameters showed significant differences in cytokine level. These results are summarised in Table 3.
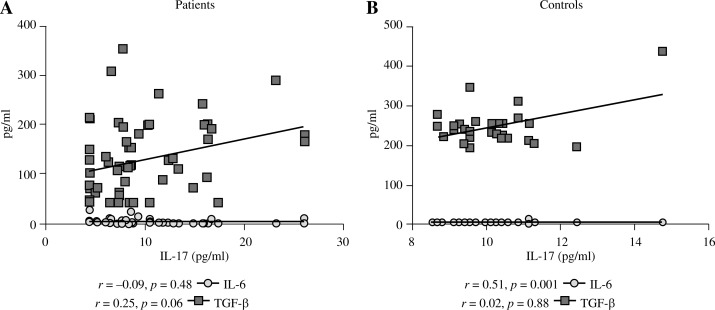
Our study was extended by performing the correlation analysis between IL-6 and TGF-β serum level with IL-17 concentration in patient and control groups (Fig. 2). The IL-17 serum level positively correlated with the IL-6 level in the control group (r = 0.51, p = 0.001).
Fig. 2.
The correlation between serum level of IL-6 and TGF-β with IL-17 in the peripheral blood of patients with breast cancer and healthy individuals. The IL-17 serum level positively correlates with IL-6 level in control group (r = 0.51, p = 0.001). Results were analysed with the Spearman test
Discussion
Current understanding about IL-17 reveals that it is produced from Th17 cells and plays a crucial role in inflammation. It has also been shown that this cytokine plays an important role in allergies and autoimmune diseases [27, 28]. One of the most important cytokines in the development of Th17 is TGF-β, which stimulates the formation of Treg cells. However, a combination of IL-6 and TGF-β plays a leading role in the differentiation and development of Th17 cells [29]. In addition to its important physiological roles, the roles of Th17 are considerable in both pathological conditions including cancer and autoimmune disorders [8, 27]. In the present study the levels of IL-17, IL-6, and TGF-β as the stimulators of Th-17 differentiation in peripheral blood of patients with breast cancer were measured. Our findings revealed that the serum level of these cytokines were significantly decreased in patients in comparison with the control group. In addition, a positive correlation between IL-6 and IL-17 was demonstrated. A number of studies have been done regarding IL-17 in different cancers. For instance, Baharlou's findings showed that IL-17 and TGF-β were reduced in patients with bladder cancer, and that they can be important indicators for following the course and clinical stages of bladder carcinoma progression [30]. A study on IL-17 expression by Zhang et al. showed high expression levels of IL-17 and IL-23 mRNA in tumour tissues from patients with gastric cancer, suggesting that Th17 cell differentiation may increase in gastric cancer [31]. A different study also confirmed that IL-17 contributes to the growth of tumour via upregulation of proangiogenic and proinflammatory cytokines [32]. A study on ovarian cancer emphasised high expression of IL-17 with a significant role in tumour growth via angiogenesis [21]. Moreover, Wang et al. showed a significant increase of IL-17 and IL-6 mRNA expression in the tumour, which promoted tumour growth. It also seems that VEGF and TGF-β contribute to tumour progression by IL-17 [33]. However, a study by Radosavljevic et al. showed the lack of a significant relationship between serum level of IL-17 and colorectal cancer [34]. Kwon et al. also found no significant difference between IL-6 in controls and colorectal cancer patients [35]. However, Ravishankaran et al. proved the presence of a high serum level of IL-6 in breast cancer. Their study showed that preoperative high IL-6 levels were proposed as a poor prognostic factor for disease recurrence and overall survival in patients with breast cancer [36]. These findings suggest that both IL-6 and 17 are multi-functional cytokines that can promote growth of Th17 tumours.
There is a reciprocal effect between Th17 and Treg cells in regulation of the immune response [37]. The performance of each cell should be evaluated together, especially in growth or regression of the tumours [38]. According to our study, serum levels of IL-6, IL-17, and TGF-β in early stages of breast cancer were significantly decreased in comparison with the control group, which can be interpreted as a reflection of reduced Th17 responses. Lower Th17-induced inflammation can be associated with a lack of angiogenesis. As explained, while cancer progresses particularly in late stages, with an increase in Th17 development, cancer may deteriorate.
Transforming growth factor β is known to be released both by tumour and T regulatory cells during the late stages of most solid cancers [39]. Decreased serum levels of TGF-β in the present study may be due to the fact that chemotherapy agents suppress tumour and Treg cells.
According to the presented data, it can be concluded that reduced serum levels of IL-6, IL-17, and TGF-β in patients with the early stage of cancer indicates probable chemotherapy and radiotherapy suppressing effects on IL-17-producing Th17 cells and TGF-β-producing tumour cells in the early stage; however, with tumour progression, elevated IL-17-producing Th17 is expected.
Furthermore, in the late stages of cancer, which is associated with increased TGF-β, angiogenesis factors may worsen the cancer. Since cytokine serum levels in tumour tissue and peripheral blood are associated with each other, their concentration in serum can be a representation of immune responses in the tumour. Therefore, the cytokine profile in peripheral blood can be listed as an indicator following malignancy progress and immune response to cancer development. Also, using monoclonal antibodies against Th17-related cytokines can be considered as a suitable target for immune therapy of breast cancer in the final stages when the immune system is not competent against cancer. Finally, being aware of the precise balance between Th17 and Treg cells can help to provide new therapies against cancer, especially breast cancer.
Acknowledgements
The authors would like to thank the patients with breast cancer as well as the healthy individuals for their kind participation in this project. This study was financially supported by a grant from the Shiraz Institute for Cancer Research.
The authors declare no conflict of interest.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. Definition according to profiles of lymphokine activities and secreted. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 3.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peck A, Mellins ED. Breaking old paradigms: Th17 cells in autoimmune arthritis. Clin Immunol 2009. 2009;132:295–304. doi: 10.1016/j.clim.2009.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 7.Zambrano-Zaragoza JF, Romo-Martínez EJ, Durán-Avelar Mde J, et al. Th17 cells in autoimmune and infectious diseases. Int J Inflam. 2014;2014:651503. doi: 10.1155/2014/651503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam JS, Terabe M, Kang MJ, et al. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–3923. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shime H, Yabu M, Akazawa T, et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 11.Lebrun JJ. The dual role of TGF in human cancer: from tumor suppression to cancer metastasis. ISRN Mol Biol. 2012;2012:1–28. doi: 10.5402/2012/381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z, Sun W, Liang Y, et al. Fenofibrate inhibited the differentiation of T helper 17 cells in vitro. PPAR Res. 2012;2012:145654. doi: 10.1155/2012/145654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 14.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 16.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 17.Passos ST, Silver JS, O'Hara AC. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol 2010. 2010;184:1776–1783. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mucida D, Cheroutre H. TGFbeta and retinoic acid intersect in immune-regulation. Cell Adh Migr. 2007;1:142–144. doi: 10.4161/cam.1.3.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Yi T, Kortylewski M, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato T, Furumoto H, Ogura T, et al. Expression of IL-17 mRNA in ovarian cancer. Biochem Biophys Res Commun. 2001;282:735–738. doi: 10.1006/bbrc.2001.4618. [DOI] [PubMed] [Google Scholar]
- 22.Radosavljevic G, Ljujic B, Jovanovic I, et al. Interleukin-17 may be a valuable serum tumor marker in patients with colorectal carcinoma. Neoplasma. 2010;57:135–144. doi: 10.4149/neo_2010_02_135. [DOI] [PubMed] [Google Scholar]
- 23.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovanovic DV, Di Battista JA, Martel-Pelletier J, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 25.Antonysamy MA, Fanslow WC, Fu F, et al. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- 26.Mousavi SM, Montazeri A, Mohagheghi MA, et al. Breast cancer in Iran: an epidemiological review. Breast J. 2007;13:383–391. doi: 10.1111/j.1524-4741.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu ZJ, Yadav PK, Su JL, et al. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2009;15:5784–5788. doi: 10.3748/wjg.15.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Z, Sun W, Liang Y, et al. Fenofibrate inhibited the differentiation of T helper 17 cells in vitro. PPAR Res. 2012;2012:145654. doi: 10.1155/2012/145654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baharlou R, Ahmadi Vasmehjani A, Dehghani A, et al. Reduced interleukin-17 and transforming growth factor beta levels in peripheral blood as indicators for following the course of bladder cancer. Immune Netw. 2014;14:156–163. doi: 10.4110/in.2014.14.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Yi T, Zhang W, et al. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70:10112–10120. doi: 10.1158/0008-5472.CAN-10-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radosavljevic G, Ljujic B, Jovanovic I, et al. Interleukin-17 may be a valuable serum tumor marker in patients with colorectal carcinoma. Neoplasma. 2009;57:135–144. doi: 10.4149/neo_2010_02_135. [DOI] [PubMed] [Google Scholar]
- 35.Kwon KA, Kim SH, Oh SY, et al. Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC Cancer. 2010;10:203. doi: 10.1186/1471-2407-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravishankaran P, Karunanithi R. Clinical significance of preoperative serum interleukin-6 and C-reactive protein level in breast cancer patients. World J Surg Oncol. 2011;9:18. doi: 10.1186/1477-7819-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-) evolutionary perspective. Nat Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 38.Hsu HC, Yang P, Wang J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 39.Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-beta targeted cancer therapy. Int J Biol Sci. 2012;8:964–978. doi: 10.7150/ijbs.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]