Summary
Inflammation is tightly regulated by a vast system that is intricately interconnected with innate immunity. Aberrations in expression or signaling, such as in innate immune receptors, can create excessive inflammation and, when chronic, often promote oncogenesis. The triggering receptor expressed on myeloid cells (TREM) receptor family has been characterized as a major player in the amplification and signaling of the inflammatory response. In a number of chronic inflammatory conditions and malignancies, TREM has been implicated in disease severity and progression. In this article, the current understanding of TREM function in pre-malignant, malignant and chronic inflammatory conditions is critically reviewed. The potential for therapeutic application is also discussed.
Keywords: Triggering receptor expressed on myeloid cells, TREM-1, TREM-2, Chronic inflammation, Neoplastic disease, Inflammatory bowel disease, Colorectal cancer, Hepatocellular carcinoma, Non small cell lung cancer, Leukemia
Introduction
The inflammatory response entails an extensive and dynamic network of cellular and molecular processes, with innate immune cells playing a vital role in its control. These cells express a vast array of receptors, with equally diverse functions. Pathogen recognition receptors, such as toll-like receptors (TLR), are responsible for detection of pathogens and activation of the immune response. Other innate immune receptors, through signal amplification or dampening, function to finely modulate inflammatory and immune responses. This dynamic equilibrium of signal cascades forges a large role for innate immune receptors in inflammation and disease. The goal of this article is to critically review the available literature concerning the expression and signaling of the triggering receptor expressed on myeloid cells (TREM) receptor family and its role in inflammation and disease. Particular emphasis is placed on their role in malignancies and selected chronic inflammatory diseases that predispose patients to cancer. In addition, future directions and the potential for clinical application are discussed.
Molecular characteristics and expression of the TREM receptor family
The triggering receptor expressed on myeloid cells (TREM) gene family, clustered on human chromosome 6p21 [1], falls under the immunoglobulin superfamily with predominantly inflammatory and innate immunomodulatory function. The signaling pathways of TREM have been previously reviewed [2,3] and so will be briefly discussed in this article. TREM-1 and TREM-2, the most understood of the TREM family, are transmembrane glycoproteins of similar structure (25–30 kDa) that associate with the DNAX-activating protein of 12 kDa (DAP12) adaptor protein for signaling and function [4,5]. DAP12 contains a cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM), which after tyrosine phosphorylation recruits spleen tyrosine kinase (Syk) and Zeta-chain-associated protein kinase 70 (ZAP70) [6]. These promote downstream activation of phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ1 and extracellular signal-related kinase (ERK) 1/2 pathways, ultimately triggering intracellular Ca2+ mobilization, rearrangement of the actin cytoskeleton and activation of several transcription complexes [7].
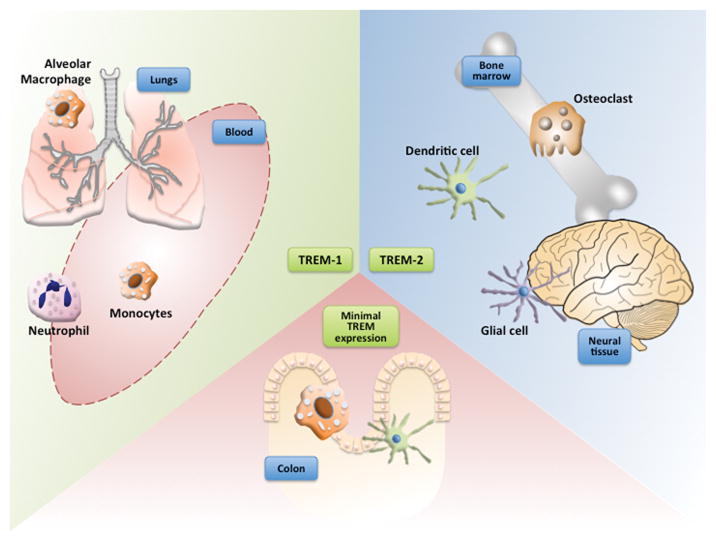
Downstream, the TREM isoforms are functionally unique. TREM-1 is highly expressed by neutrophils and some monocyte/macrophage subsets [7], particularly alveolar macrophages [8] (FIGURE 1). Initial findings have indicated TREM-1 to be an amplifier of inflammatory responses. Triggering of TREM-1 causes increases in monocyte secretion of tumor necrosis factor α (TNF-α), IL-8, and monocyte chemoattractant protein 1, and triggers neutrophil degranulation and release of myeloperoxidase and nitric oxide [4], but does not induce phagocytosis [9]. Along with these prototypic inflammatory cytokines, monocyte TREM-1 activation alternatively attenuates LPS-mediated induction of IL-12 cytokine family subunits, important signaling molecules for Th1 cell differentiation and adaptive immune response [10]. To date, the nature of the TREM-1 ligand has not been completely resolved [11]. Some evidence suggests the ligand for TREM-1 to be expressed on human platelets [12] and/or in sera from septic patients [13]. High-mobility group box 1 (HMGB1) and heat shock protein 70 (HSP70) have both been proposed as putative ligands for TREM-1. Direct interaction between TREM-1 and HMGB1 was demonstrated in a murine model through a combination of immunoprecipitation, chemical cross-linking of proteins and surface plasmon resonance techniques, indicating binding capability with TREM-1 and supporting HMGB1 as an activating ligand [14]. Peptidoglycan recognition protein 1, when complexed with bacterially derived peptidoglycan, has also been identified as a ligand for TREM-1 [15]. This pathway provides a mechanism for activation of TREM-1 by neutrophils, thereby enhancing cytokine production in the local innate immune response.
Figure 1. TREM expression in various tissue types.
TREM-1 is generally expressed in some monocyte/macrophage subtypes as well as neutrophils. In particular, alveolar macrophages have highly specific TREM-1 expression. TREM-2 regulates the development and function of dendritic cells (DCs), microglia and osteoclasts. Only a small fraction of the resident mucosal myelocyte population in the gut expresses TREM-1.
Conversely, TREM-2 regulates the development and function of dendritic cells (DCs), microglia and osteoclasts (FIGURE 1) and overall promotes an anti-inflammatory state and phagocytosis [3]. TREM-2 is suggested to play an important role in bacterial clearance. The receptor has shown pattern recognition capabilities, observed to bind specifically to several different Gram-negative and Gram-positive bacteria [16], and can induce phagocytosis [17]. TREM-2 deficiency in macrophages induces an exacerbated production of pro-inflammatory cytokines, including IL-6 and TNF-α [18], suggesting an anti-inflammatory role for TREM-2.
TREM-like transcript-1 (TLT-1), another unique TREM family member restricted to platelets and megakaryocytes, is located primarily in α-granules of resting platelets and on the surface of activated platelets [19,20]. TLT-1 and its soluble form (sTLT-1) both enhance platelet aggregation [21]. Additionally, sTLT-1 antagonizes TREM-1-induced leukocyte activation, presumably by interfering with the binding of TREM-1 ligand [22].
The TREM family has been extensively studied in acute inflammation [23]. Additionally, its role in a number of non-neoplastic inflammatory diseases has been previously reviewed [10]. Growing evidence suggests TREM involvement in chronic inflammatory diseases as well.
TREM in chronic inflammation and malignancy
Since as early as the 19th century, inflammation has been increasingly recognized as a critical constituent in cancer, playing a major role in the initiation and progression of malignancy [24,25]. This is greatly influenced by immune cell involvement in malignancy, notably tumor-associated macrophages. While not extensively studied, TREM signaling could assume a significant role in such interplay (FIGURE 2). Indeed, TREM-1 is highly expressed in colon, hepatocellular and lung carcinoma tissues [14,26–28], and likely plays some role in cancer-associated inflammation and the tumor microenvironment.
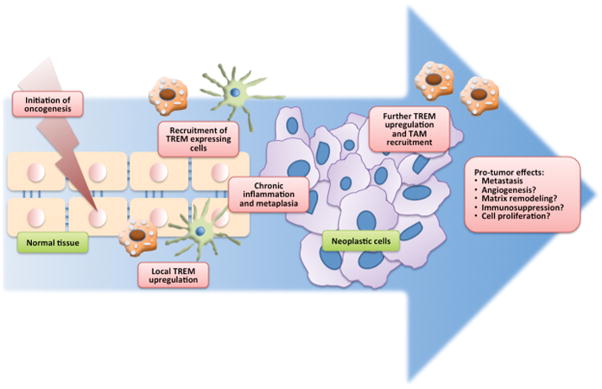
Figure 2. A generalized model of the possible roles of TREM in tumorigenesis.
Upregulation of TREM on local myelocytes as well as recruitment of TREM-expressing myelocytes will amplify the ongoing chronic inflammatory state, promoting metaplasia and oncogenesis. The tumor microenvironment could cause further upregulation of TREM in local myelocytes as well as additional recruitment of TREM-expressing macrophages, increasing the tumor-associated macrophage (TAM) population. TREM-1 is thought to be associated with metastatic ability. TAMs have been associated with pro-tumor effects including promotion of angiogenesis, matrix remodeling, immunosuppression, and tumor cell proliferation.
Colitis and colorectal cancer
The inflammatory bowel diseases (IBD), principally Crohn’s disease (CD) and ulcerative colitis (UC), are chronic gastrointestinal inflammatory diseases associated with a well-recognized increased risk of colorectal and small bowel cancers [29]. Currently, IBD is thought to be caused by a dynamic interplay between genetics and environmental factors affecting enteric microbial flora [30]. Severe and diffuse mucosal inflammation develops with production of a complex inflammatory mediator mixture and eventual superficial mucosal ulceration. Due to its inflammatory involvement, TREM has been of particular recent interest in the study of IBD pathogenesis and progression.
While the resident mucosal macrophage population in the gut is quite extensive, normal human small and large intestine contains only a small fraction (<10%) of macrophages expressing TREM-1 [31]. The local IL-10 and TGF-β are thought to synergistically down-regulate TREM-1, inducing a refractory state in the intestinal macrophages. Likely, this diminished TREM-1 presence is to prevent excessive inflammation and subsequent intestinal tissue damage. In the mucosa of IBD patients (both CD and UC), however, the presence of TREM-1 expressing macrophages (with a minority of TREM-1 expressing neutrophils) is markedly increased [32]. Additionally, serum/plasma sTREM-1 is elevated in patients with either CD or UC [33–36], though sTREM-1 level appears to have stronger correlation with clinical disease activity in UC than in CD [37]. However, serum sTREM-1 may not be sufficiently accurate as a marker for disease activity. IBD patients with active disease appear to have no substantial difference in serum sTREM-1 level compared to patients with quiescent disease [35]. Additionally, levels of TREM-2 are increased in the inflamed mucosa of colon samples from IBD patients, compared to those with cancer (controls) [38]. This study suggests a pro-inflammatory role of TREM-2 in the gut, rather than negatively regulate inflammatory responses, as previously described.
The role of TREM-1 has been explored in the murine model of DSS-induced colitis and colitis-associated tumorigenesis using C57BL/6 mice [39]. Treatment with the TREM-1 antagonist LP17 attenuated colitis and colitis-associated tumorigenesis: LP17-treated mice developed less histopathological alterations, with fewer and smaller colon tumors. Concomitantly, levels of inflammatory cytokines, including TNF-α, IL-6, and IL-1β, were substantially reduced in treated mice. Together, these studies reveal a pathogenic role for TREM-1 in promoting inflammatory disease. Additionally, the potential for TREM-1 to have tumorigenic activity necessitates further investigation.
Pulmonary diseases and malignancy
The immense pulmonary surface area and resultant antigenic load offer a major challenge for the immune system to maintain local homeostasis. In normal tissue, TREM-1 is selectively expressed in alveolar macrophages, which specialize in pathogen clearance and removal of macromolecules and apoptotic cells [8]. In murine model of allergen-induced pulmonary inflammation [18], resident alveolar macrophages are induced to express TREM-2, which in turn attenuates macrophage cytokine production, though the mechanism remains unclear. However, such an interplay suggests a possible TREM-1 and TREM-2 paradigm that could serve to fine-tune inflammatory response in the respiratory and elsewhere. Interestingly, the active form of vitamin D, 1,25(OH)2D3, can induce TREM-1 mRNA in normal human bronchial epithelial cells studied in vitro [40]. This induction, while likely not the primary TREM-1 activator, occurs through a vitamin D response element and further expands the complex and dynamic TREM signaling network.
Growing evidence suggests a role for TREM-1 in chronic inflammation in pulmonary disease, as well. Cigarette smoking is by far the leading cause of lung cancer, with associated pulmonary pro-inflammatory changes having a large role in tumor promotion [41,42]. In a murine model of chronic second hand smoke exposure [43], increasing doses of second hand smoke exposure induced alveolar macrophage recruitment and activation, consistent with changes seen in human chronic cigarette smoke exposure. These activated macrophages expressed elevated TREM-2 levels and were observed in conjunction with increases in monocyte chemotactic protein 1 and TNF-α, both of which have been previously associated with the development of chronic obstructive pulmonary disease (COPD). Characterized as a progressive lung disease with chronically poor and obstructed airflow, COPD has increasingly been recognized as a persistent systemic inflammatory disease [44]. Levels of serum sTREM-1 are elevated in COPD patients and show a significant negative correlation with lung function impairment [45].
Cystic fibrosis is another pulmonary disease characterized by a vicious cycle of airway obstruction, infection, and heightened inflammatory state [46]. Cancer risk in cystic fibrosis patients is increased, notably cancer of the digestive tract, testicle, and lymphoid leukemia [47]. Interestingly, circulating monocytes isolated from cystic fibrosis patients share characteristics with monocytes in endotoxin tolerance, a transient refractory state following LPS exposure in which monocytes exhibit decreased cytokine production in response to further pro-inflammatory stimulus [48]. These tolerant monocytes were characterized by increased TREM-1 expression and exhibited potent phagocytic activity with an impaired capability for antigen presentation. This indicates a multi-faceted role of TREM-1. Additionally, the upregulation of TREM could perhaps act as a source for sTREM-1, which has been reported to be produced via proteolytic cleavage of the membrane anchored form [49] and is thought to be anti-inflammatory. Further investigation is warranted to elicit this unknown pathway.
Involvement of TREM in lung cancer is indeed supported by current literature. In murine Lewis lung cancer cells, the TLR4 signaling pathway has been shown to promote the expression of TGF-β1 and IL-10 and tumor cell migration [50]. Considering its ability to cross talk with and amplify TLR4, TREM-1 has definite potential to promote tumor escape and progression. When co-cultured with human lung cancer cells, macrophages exhibit an increased expression of TREM-1 that is dependent on COX-2 signaling [51]. In the same study, investigation of tumor tissue from non-small cell lung cancer (NSCLC) patients demonstrated increased expression of TREM-1 on CD68 positive tumor-associated macrophages (TAM). Separately, increased TREM-1-positive TAMs in tumor tissue of NSCLC patients was found to be associated with reduced disease-free and overall survival [27]. Further analysis indicated that TREM-1 was an independent predictor of patient survival (hazard ratio (HR) = 2.72; 95% CI = 1.33–5.57; P = 0.006). Additionally, the study found TREM-1 and pro-inflammatory cytokine (TNF-α and IL-1β) expression in primary isolated peripheral blood macrophages to be directly upregulated by co-cultured lung cancer cells. Conversely, Karapanagiotou et al. [52] investigated the value of serum sTREM-1 level as a prognostic marker for solid malignancy metastasis to the lung. Higher concentrations of sTREM-1 correlated with absence of lung metastases, supporting the role of sTREM-1 as a decoy receptor and protective factor.
The effects of TREM suppression were explored in a recent study [53] involving NSCLC. Using the signaling chain homo-oligomerization (SCHOOL) model, a ligand-independent peptide inhibitor specific for TREM-1 was developed. Administration to mice xenografted with human lung carcinoma tumors significantly suppressed tumor growth at a specific dose compared to the vehicle negative control (with paclitaxel as positive control). Together, these results suggest TREM-1 to be a possible target for adjunctive therapy in lung cancer treatment. Further studies unraveling the role of the TREM family in the lung cancer microenvironment are needed, particularly in the molecular pathways underlying initiation and promotion of lung cancer.
Hepatic inflammation and carcinoma
Granulomas, characterized by inflammatory monocyte (predominantly macrophage) accumulations, present in a wide range conditions. Nonetheless, by their nature, granulomatous lesions offer an interesting niche for the study of TREM in chronic inflammation. Murine zymosin A-elicited hepatic granuloma serves as a chronic inflammatory granuloma model in the liver. Nochi and colleagues [54], via adenoviral gene transfer, introduced FLAG/DAP12 (Ad-FDAP12) and a combination of the extracellular domain of mouse TREM-1 and the Fc portion of human IgG1 (Ad-TREM-1 Ig) into the mouse model to observe DAP12 and TREM-1 modulatory effects in chronic hepatic granulomatous inflammation. The Ad-TREM-1 Ig construct effectively acted as a blocker of DAP12/TREM-1 signaling when expressed in mice, similar to sTREM-1 function. Consequently, Ad-TREM-1 Ig-treated mice had consistently lower levels of granuloma formation throughout the study period, compared to DAP12 transgene mice and even control mice. This offers strong implications for the role TREM-1 in not only the exacerbation of inflammatory states, but also a pathogenic potential for TREM-1.
Hepatocellular carcinoma (HCC) is a well-known type of inflammation-related cancer. While not fully understood, liver Kupffer cells (resident inflammatory cells), in conjunction with recruited monocytes and neutrophils, have been implicated in HCC pathogenesis. Following hepatocyte death in the setting of chronic inflammation, most commonly hepatitis or cirrhosis, Kupffer and other inflammatory cells are activated to produce cytokines that drive the compensatory proliferation of remaining hepatocytes, ultimately evolving to HCC [14,55–57]. Demonstrated using murine diethylnitrosamine (DEN)-induced HCC model, TREM-1 is suggested to be a pivotal determinant of Kupffer cell activation in liver carcinogenesis [14]. Deletion of the murine homolog Trem1 attenuated HCC development in DEN-treated mice: Trem1-deficient mice were tumor-free at 8 months, with only 4% developing small HCC tumors at 14 months. In contrast, exposed WT mice developed large numbers of typical HCC with maximal diameters. Compared to the controls, DEN administration in Trem1-deficient mice resulted in significantly reduced hepatocyte death and less hepatic injury, marked by reduced liver enzyme alanine aminotransferase release. Finally, adoptive transfer of WT Kupffer cells to Trem1-deficient mice reversed the unresponsiveness to DEN-induced liver injury. These results indicate a critical role for TREM-1 in Kupffer cell activation and development of HCC.
A separate study has confirmed elevated TREM-1 expression in human HCC cells [26]. High TREM-1 expression in tissue from HCC patients significantly correlates with poorer survival and serves as an independent prognostic factor for recurrence (HR = 1.58; 95% CI = 1.12– 2.24; P = 0.009). Interestingly, TREM-1 affects the migratory ability of cells in culture, suggesting a role in cancer metastasis. Administration of an anti-TREM-1 agonistic mAb significantly increased migratory ability of HCC cells in transwell migration assays. These data further supports the TREM-1 relationship to aggressive tumor behavior, including cancer spread.
Leukemia
Leukemia constitutes a heterogeneous group of cancers affecting hematopoietic tissue. The pathology of leukemia, distinct from that of traditional solid tumors, creates a unique paradigm for the study of oncogenesis and development of therapy. Primary chronic lymphocytic leukemia (CLL) cells in vitro exhibit prolonged survival when co-cultured with stromal or non-malignant leukocytes due to cross-talk with the microenvironment [58]. Gene expression in these survival-supportive culture conditions, determined by microarray-based analysis, exhibited involvement of TLR signaling, nuclear respiratory factor-2-mediated oxidative stress response, and TREM-1 signaling. These pathways correlated with upregulation of inflammatory cytokines, most notably chemokine (C-C motif) ligand 2 (CCL2) in vitro and in serum of patients with CLL. This adds further evidence to the role of TREM in malignant cell survival. Interestingly, flow cytometric study of myelogenous leukemia demonstrated a decreased TREM-1 surface expression on leukemia cells in both acute (AML) and chronic (CML) myelogenous leukemia compared with mean fluorescence intensity in healthy controls and patients in complete remission [59]. This lies in stark contrast to the increased expression of TREM-1 seen previously in solid tumors. In patients with AML and CML, leukemia cell surface TREM-1 expression was inversely related with serum sTREM-1 levels. As previously discussed, sTREM-1 is predominantly produced by metalloproteinase cleavage of surface TREM-1 [49]. Some metalloproteinases have been implicated in tumor promotion, particularly angiogenesis and invasion in leukemias [60]. It is possible; therefore, that the decreased surface TREM-1 found in AML and CML could be due to metalloproteinase activity, as opposed to actual differences in protein expression.
Expert Commentary and Five-year View
While TREM-1 and TREM-2 activity is most likely to be controlled by ligand binding, the identity of the ligands remains unknown. HMGB1 has been shown to bind with TREM-1, along with a multiple other surface receptors including TLR2, TLR4, and RAGE to modulate inflammation [61]. The activity of HMGB1 in TREM signaling, whether an agonist or antagonist, remains unresolved. Additionally, a complex between peptidoglycan recognition protein 1 and bacterially derived peptidoglycan has been identified as a potent ligand capable of binding and activating TREM-1 [15]. This offers a mechanism for activation of TREM-1 by neutrophils in sepsis. However, the functionality of this pathway relies on peptidoglycan presence and complex formation. The mechanism of TREM upregulation in aseptic pathologies, such as chronic inflammation and cancer, requires further elucidation.
The most commonly accepted model for the TREM receptors implicates TREM-1 as a pro-inflammatory amplifier, particularly in TLR-initiated responses to microbial challenges [7]. Conversely, TREM-2 functions to control myeloid cell activity, including DCs, osteoclasts and microglia, and promote an anti-inflammatory state. These, while likely to be the primary roles or perhaps an oversimplification due in large to the need for further investigation, are likely not the full extent of TREM activity. In a TREM-1/3-deficient murine model (Trem3 is a homologous murine gene adjacent to Trem1 but is a pseudogene in humans with no functional overlap. As such, TREM-1/3 deficient mice serve to model TREM-1 blocking in humans), mice challenged with Pseudomonas pulmonary infection revealed ineffective neutrophil migration across primary airway epithelia [62]. This suggests a new function for TREM-1 in neutrophil migration, though its specific role, whether via indirect signaling or directly participating in neutrophil migration, must be discerned. Furthermore, in various models, TREM-1 blockade or deficiency has exhibited diminished epithelial proliferation [14,35], warranting further investigation in this and, broadly, other potential roles for TREM-1.
The most well studied member of the TREM family is TREM-1, by far. Investigation of TREM-2, especially regarding involvement in cancer, is severely lacking. While TREM-2 is generally thought of as an anti-inflammatory receptor, some diverging evidence has emerged. TREM-2 was shown to be required for efficient mucosal repair in a murine model for wound healing [63]. The implicated TREM-2+ infiltrating macrophages showed higher expression of IL-4 and IL-13, mediators of inflammation and epithelial wound repair, whereas TREM-2 KO mice exhibited diminished wound healing and reduced epithelial proliferation. These findings highlight the need for further investigation to uncover the complete paradigm of TREM signaling and function.
Tumor-associated macrophages (TAMs) have been extensively studied as players in the tumor microenvironment and regarding cancer promotion and progression. In particular, polarization of macrophages to M1/M2 subtypes holds implications in cancer prognosis and therapy [64,65]. Certain TAMs are associated with pro-tumor functions including immune suppression, angiogenesis, matrix remodeling, and tumor proliferation or metastasis. TREM-2 has demonstrated some association with M1 macrophage infiltration in a murine wound healing model [63]. However, involvement of TREM in the M1/M2 paradigm has yet to be investigated in cancer, and may further elucidate our understanding of the tumor microenvironment.
There has been much discussion of TREM as a biomarker for diagnosis and disease progression. Most notably, levels of sTREM-1 have been extensively studied in diagnosis of lower respiratory tract [66] and pleural [67] infections, and in the prognosis, severity [68] and detection of bacteremia [69] in sepsis. In these extensive infectious diseases, sTREM-1 may have some potential value as a biomarker. As discussed above, based on the few studies available, TREM as a biomarker for neoplastic disease may have less value. Further research is needed to confirm the clinical value of TREM as a biomarker.
There exists much interest to find therapeutic applications for TREM. In murine models, a number of TREM inhibitors have been studied, as discussed above. The TREM-1 peptide antagonist, LP17, decreased disease severity in IBD, even when administered after the establishment of colitis [32]. Separately, a ligand-independent peptide-based TREM-1 inhibitor was designed using the signaling chain homo-oligomerization (SCHOOL) model of immune signaling [53]. Administration delayed tumor growth in a mouse xenograft model of human NSCLC. Such studies support the potential for TREM as a therapeutic target. However, caution must be exercised with regard to altering TREM activity. Numerous animal studies demonstrate a fine line between alleviating excessive inflammation and rendering the immune response completely ineffective. In addition, patients with mutations in either TREM-2 or DAP12 develop polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL or Nasu-Hakola disease), while unexpectedly having no apparent defects in cell-mediated immunity [10]. Ultimately, with further understanding of its signaling paradigm, TREM receptor modulation could be a useful therapeutic modality for treatment of acute and chronic inflammation and perhaps adjuvant therapy for some neoplastic disease.
Key issues.
TREM receptors play important roles in the inflammatory response and innate immune signaling.
High-mobility group box 1 has been shown to bind TREM-1 along with other inflammatory receptors, including TLR and RAGE. The peptidoglycan recognition protein 1, when complexed with bacterially derived peptidoglycan, has also been identified as a ligand for TREM-1. However, other TREM ligands have yet to be characterized.
Growing evidence implicates TREM involvement in chronic inflammatory diseases, rather than solely pathogen-induced acute inflammation.
Solid tumors are associated with upregulation of TREM-1 on infiltrating myelocytes, whereas leukemia cells unexpectedly demonstrate lower surface TREM-1 levels.
Functions in addition to the traditional pro-/anti-inflammatory paradigm may exist for TREM-1 and TREM-2, including immune cell migration and non-immune cell proliferation, respectively.
Evidence in animal models suggests modulation of TREM activity could attenuate excessive inflammatory disorders. This represents significant therapeutic potential for TREM as a pharmacological target.
Footnotes
Financial and competing interest’s disclosure
This work was supported by research grants R01 HL106042, R01 HL112597, and R01 HL120659 to DK Agrawal from the Office of the Director of National Institutes of Health and the National Heart Lung and Blood Institute, NIH, USA. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Allcock RJN, Barrow AD, Forbes S, et al. The human TREM gene cluster at 6p21. 1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol. 2003;33:567–77. doi: 10.1002/immu.200310033. [DOI] [PubMed] [Google Scholar]
- 2.Arts RJW, Joosten LaB, van der Meer JWM, et al. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. 2013;93:209–15. doi: 10.1189/jlb.0312145. [DOI] [PubMed] [Google Scholar]
- 3.Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213:701–13. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 4**.Bouchon A, Dietrich J, Colonna M. Cutting Edge: Inflammatory Responses Can Be Triggered by TREM-1, a Novel Receptor Expressed on Neutrophils and Monocytes. J Immunol. 2000;164:4991–5. doi: 10.4049/jimmunol.164.10.4991. The original study to identify TREM-1 and TREM-2 receptors and characterize signaling through DAP12. [DOI] [PubMed] [Google Scholar]
- 5.Bouchon A, Hernández-munain C, Cella M, et al. A DAP12-mediated Pathway Regulates Expression of CC Chemokine Receptor 7 and Maturation of Human Dendritic Cells. J Exp Med. 2001;194:1111–22. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227:150–60. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–53. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 8.Colonna M, Facchetti F. TREM-1 ( Triggering Receptor Expressed on Myeloid Cells ): A New Player in Acute Inflammatory Responses. J Infect Dis. 2003;1:397–401. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- 9.Bleharski JR, Kiessler V, Buonsanti C, et al. A Role for Triggering Receptor Expressed on Myeloid Cells-1 in Host Defense During the Early-Induced and Adaptive Phases of the Immune Response. J Immunol. 2003;170:3812–8. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 10*.Pelham C, Agrawal D. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert Rev Clin Immunol. 2014;10:243–56. doi: 10.1586/1744666X.2014.866519. A review exploring the role of TREM in a number of non-neoplastic inflammatory diseases, including those affecting metabolism and the cardiovascular and respiratory systems. [DOI] [PubMed] [Google Scholar]
- 11*.Pelham CJ, Pandya AN, Agrawal DK. Triggering receptor expressed on myeloid cells receptor family modulators_: a patent review. Expert Opin Ther Pat. 2014;24:1383–95. doi: 10.1517/13543776.2014.977865. In this article, new roles for TREM-1, TREM-2, and TREM-like transcripts are discussed together with the putative endogenous ligands and novel synthetic peptide blockers of TREM receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haselmayer P. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood. 2007;110:1029–36. doi: 10.1182/blood-2007-01-069195. [DOI] [PubMed] [Google Scholar]
- 13.Wong-Baeza I, González-Roldán N, Ferat-Osorio E, et al. Triggering receptor expressed on myeloid cells (TREM-1) is regulated post-transcriptionally and its ligand is present in the sera of some septic patients. Clin Exp Immunol. 2006;145:448–55. doi: 10.1111/j.1365-2249.2006.03158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Wu J, Li J, Salcedo R, et al. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 2012;72:3977–86. doi: 10.1158/0008-5472.CAN-12-0938. Report demonstrating Trem1 deficient mice exhibited attenuated Kupffer cell activation and hepatocellular carcinogenesis after DEN exposure. Adoptive transfer of WT Kupffer cells reversed this unresponsiveness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read CB, Kuijper JL, Hjorth Sa, et al. Cutting Edge: Identification of Neutrophil PGLYRP1 as a Ligand for TREM-1. J Immunol. 2015;194:1417–21. doi: 10.4049/jimmunol.1402303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daws MR, Sullam PM, Niemi EC, et al. Pattern Recognition by TREM-2: Binding of Anionic Ligands. J Immunol. 2014;171:594–9. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- 17.N’Diaye E-N, Branda CS, Branda SS, et al. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184:215–23. doi: 10.1083/jcb.200808080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbull IR, Gilfillan S, Cella M, et al. Cutting Edge: TREM-2 Attenuates Macrophage Activation. J Immunol. 2006;177:3520–4. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 19.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl):S13–33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 20.Washington AV, Schubert RL, Quigley L, et al. A TREM family member, TLT-1, is found exclusively in the a-granules of megakaryocytes and platelets. Blood. 2004;104:1042–8. doi: 10.1182/blood-2004-01-0315. [DOI] [PubMed] [Google Scholar]
- 21.Washington AV, Gibot S, Acevedo I, et al. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest. 2009;119:1489–501. doi: 10.1172/JCI36175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derive M, Bouazza Y, Sennoun N, et al. Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis. J Immunol. 2012;188:5585–92. doi: 10.4049/jimmunol.1102674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 26.Liao R, Sun T-W, Yi Y, et al. Expression of TREM-1 in hepatic stellate cells and prognostic value in hepatitis B-related hepatocellular carcinoma. Cancer Sci. 2012;103:984–92. doi: 10.1111/j.1349-7006.2012.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho C-C, Liao W-Y, Wang C-Y, et al. TREM-1 expression in tumor-associated macrophages and clinical outcome in lung cancer. Am J Respir Crit Care Med. 2008;177:763–70. doi: 10.1164/rccm.200704-641OC. [DOI] [PubMed] [Google Scholar]
- 28.Zhu W, Fang C, Gramatikoff K, et al. Proteins and an inflammatory network expresed in colon tumors. J Proteome Res. 2012;10:2129–39. doi: 10.1021/pr101190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. doi: 10.3389/fimmu.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 31.Schenk M, Bouchon a, Birrer S, et al. Macrophages Expressing Triggering Receptor Expressed on Myeloid Cells-1 Are Underrepresented in the Human Intestine. J Immunol. 2004;174:517–24. doi: 10.4049/jimmunol.174.1.517. [DOI] [PubMed] [Google Scholar]
- 32.Schenk M, Bouchon A, Seibold F, et al. TREM-1 – expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117:3097–106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billioud V, Gibot S, Massin F, et al. Plasma soluble triggering receptor expressed on myeloid cells-1 in Crohn’s disease. Dig Liver Dis. 2012;44:466–70. doi: 10.1016/j.dld.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Park JJ, Cheon JH, Kim BY, et al. Correlation of serum-soluble triggering receptor expressed on myeloid cells-1 with clinical disease activity in inflammatory bowel disease. Dig Dis Sci. 2009;54:1525–31. doi: 10.1007/s10620-008-0514-5. [DOI] [PubMed] [Google Scholar]
- 35.Saurer L, Rihs S, Birrer M, et al. Elevated levels of serum-soluble triggering receptor expressed on myeloid cells-1 in patients with IBD do not correlate with intestinal TREM-1 mRNA expression and endoscopic disease activity. J Crohns Colitis. 2012;6:913–23. doi: 10.1016/j.crohns.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Tzivras M, Koussoulas V, Giamarellos-Bourboulis E, et al. Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease. World J Gastroenterol. 2006;12:3416–9. doi: 10.3748/wjg.v12.i21.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung YS, Park JJ, Kim SW, et al. Correlation between soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) expression and endoscopic activity in inflammatory bowel diseases. Dig Liver Dis. 2012;44:897–903. doi: 10.1016/j.dld.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Correale C, Genua M, Vetrano S, et al. Bacterial sensor triggering receptor expressed on myeloid cells-2 regulates the mucosal inflammatory response. Gastroenterology. 2013;144:346–56. e3. doi: 10.1053/j.gastro.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 39*.Zhou J, Chai F, Lu G, et al. TREM-1 inhibition attenuates inflammation and tumor within the colon. Int Immunopharmacol. 2013;17:155–61. doi: 10.1016/j.intimp.2013.06.009. Report demonstrating treatment with the TREM-1 antagonist LP17 reduced severity of DSS-induced colitis and colitis-associated tumorigenesis in murine model. [DOI] [PubMed] [Google Scholar]
- 40.Rigo I, McMahon L, Dhawan P, et al. Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(OH)2 vitamin D3. Innate Immunol. 2012;18:250–7. doi: 10.1177/1753425911399796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res. 2004;567:447–74. doi: 10.1016/j.mrrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Woodruff PG, Ellwanger A, Solon M, et al. Alveolar macrophage recruitment and activation by chronic second hand smoke exposure in mice. COPD. 2009;6:86–94. doi: 10.1080/15412550902751738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gan WQ. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radsak MP, Taube C, Haselmayer P, et al. Soluble triggering receptor expressed on myeloid cells 1 is released in patients with stable chronic obstructive pulmonary disease. Clin Dev Immunol. 2007:1–7. doi: 10.1155/2007/52040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol. 2008;34:146–62. doi: 10.1007/s12016-007-8039-9. [DOI] [PubMed] [Google Scholar]
- 47.Maisonneuve P, Marshall BC, Knapp Ea, et al. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst. 2013;105:122–9. doi: 10.1093/jnci/djs481. [DOI] [PubMed] [Google Scholar]
- 48.Del Fresno C, García-Rio F, Gómez-Piña V, et al. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol. 2009;182:6494–507. doi: 10.4049/jimmunol.0803350. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Pina V, Soares-Schanoski a, Rodriguez-Rojas a, et al. Metalloproteinases Shed TREM-1 Ectodomain from Lipopolysaccharide-Stimulated Human Monocytes. J Immunol. 2007;179:4065–73. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 50.Li C, Li H, Jiang K, et al. TLR4 signaling pathway in mouse Lewis lung cancer cells promotes the expression of TGF-β1 and IL-10 and tumor cells migration. Biomed Mater Eng. 2014;24:869–75. doi: 10.3233/BME-130879. [DOI] [PubMed] [Google Scholar]
- 51.Yuan Z, Mehta HJ, Mohammed K, et al. TREM-1 is induced in tumor associated macrophages by cyclo-oxygenase pathway in human non-small cell lung cancer. PLoS One. 2014;9:e94241. doi: 10.1371/journal.pone.0094241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Karapanagiotou EM, Pelekanou E, Charpidou A, et al. Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) detection in cancer patients: a prognostic marker for lung metastases from solid malignancies. Anticancer Res. 2008;28:1411–5. Report using the signaling chain homo-oligomerization (SCHOOL) model of immune signaling. Administration delayed tumor growth in a mouse xenograft model of human NSCLC. [PubMed] [Google Scholar]
- 53.Sigalov AB. A novel ligand-independent peptide inhibitor of TREM-1 suppresses tumor growth in human lung cancer xenografts and prolongs survival of mice with lipopolysaccharide-induced septic shock. Int Immunopharmacol. 2014;21:208–19. doi: 10.1016/j.intimp.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nochi H, Aoki N, Oikawa K, et al. Modulation of hepatic granulomatous responses by transgene expression of DAP12 or TREM-1-Ig molecules. Am J Pathol. 2003;162:1191–201. doi: 10.1016/S0002-9440(10)63915-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pikarsky E, Porat RM, Stein I, et al. NF- k B functions as a tumour promoter in inflammation-associated cancer. Lett to Nat. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 56.Maeda S, Kamata H, Luo J-L, et al. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–90. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Sakurai T, Maeda S, Chang L, et al. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006;103:10544–51. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulz A, Toedt G, Zenz T, et al. Inflammatory cytokines and signaling pathways are associated with survival of primary chronic lymphocytic leukemia cells in vitro: a dominant role of CCL2. Haematologica. 2011;96:408–16. doi: 10.3324/haematol.2010.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li W, Yi X, Huang S, et al. Differential Expression Of TREM-1 In Myelogenous Leukemia Cells. Li Li HJ, ed Blood. 2013;122:4951. [Google Scholar]
- 60.Klein G, Vellenga E, Fraaije MW, et al. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 62.Klesney-tait J, Keck K, Li X, et al. Transepithelial migration of neutrophils into the lung requires TREM-1. J Clin Invest. 2013;123:138–49. doi: 10.1172/JCI64181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seno H, Miyoshi H, Brown SL, et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci. 2008;106:256–61. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen AH, Berim IG, Agrawal DK. Cellular and molecular immunology of lung cancer: therapeutic implications. Expert Rev Clin Immunol. 2014;10:1711–30. doi: 10.1586/1744666X.2014.975692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allavena P, Mantovani a. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167:195–205. doi: 10.1111/j.1365-2249.2011.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye W, Hu Y, Zhang R, et al. Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in lower respiratory tract infections: a meta-analysis. Respirology. 2014;19:501–7. doi: 10.1111/resp.12270. [DOI] [PubMed] [Google Scholar]
- 67.Summah H, Tao L-L, Zhu Y-G, et al. Pleural fluid soluble triggering receptor expressed on myeloid cells-1 as a marker of bacterial infection: a meta-analysis. BMC Infect Dis. 2011;11:280. doi: 10.1186/1471-2334-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, She D, Feng D, et al. Dynamic changes of serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) reflect sepsis severity and can predict prognosis: a prospective study. BMC Infect Dis. 2011;11:53. doi: 10.1186/1471-2334-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su L, Han B, Liu C, et al. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis. 2012;12:157. doi: 10.1186/1471-2334-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]