Abstract
The aim of this study was to characterise expression profiles of visceral and subcutaneous adipose tissue in children. Adipose tissue samples were collected from children having elective surgery (n=71,54 boys, 6.0±4.3 years). Affymetrix microarrays (n=20) were performed to characterize the functional profile and identify genes of interest in adipose tissue. Visceral adipose tissue had an overrepresentation of Gene Ontology themes related to immune and inflammatory responses and subcutaneous adipose tissue had an overrepresentation of themes related to adipocyte growth and development. Likewise, qPCR performed in the whole cohort showed a 30-fold increase in haptoglobin (P=0.005), 7-fold increase in IL-10 (P<0.001), 8-fold decrease in VEGF (P=0.01) and a 28-fold decrease in TBOX15 (P<0.001) in visceral compared to subcutaneous adipose tissue. The inflammatory pattern in visceral adipose tissue may represent an early stage of the adverse effects of this depot, and combined with chronic obesity, may contribute to increased metabolic and cardiovascular risk.
Keywords: inflammation, visceral, adipose tissue, children
White adipose tissue from obese adults is characterised by immune cell accumulation and increased inflammatory mediators (1). Visceral and subcutaneous adipose tissue depots have distinct inflammatory profiles (2–4), with explants from visceral adipose tissue secreting increased amounts of inflammatory mediators including IL-6, IL-8 and plasminogen activator inhibitor 1 as compared to subcutaneous adipose tissue (5). The inflammatory milieu, particularly in visceral adipose tissue, may promote the pathogenesis of insulin resistance and type 2 diabetes.
Although adipose tissue gene profiles are extensively studied in adults (6–8), there have been few studies in children (9–12). Li et al found lower adiponectin mRNA levels in visceral compared to subcutaneous fat in overweight children, a difference also seen in adults (13). This is similar to findings by Sabin et al in 12 lean prepubertal children (10). Sbarbati et al reported evidence of macrophages, lymphocytes and granulocytes in subcutaneous adipose tissue in 19 obese prepubertal children (11). Together these findings suggest that inflammation exists in adipose tissue in children as early as pre-puberty. The aims of the current study were to examine inflammation gene expression in adipose tissue in 71 children, and determine potential associations between inflammation gene expression, BMI z score and age.
76 adipose tissue biopsies (63 subcutaneous, 13 visceral) were collected from 71 children (54 boys, mean age=6.1years). Participants were older than 6 months of age, had no intercurrent illness (notably inflammatory disease) and were having an elective procedure where abdominal fat could be obtained. BMI age and sex-specific z scores were calculated from the US Centers for Disease Control and Prevention 2000 reference data (14).
To identify inflammation genes of interest, Affymetrix microarray experiments (Ramaciotti Centre for Gene Function Analysis, University of New South Wales, Sydney) were performed in 15 subcutaneous and 5 visceral AT samples from children <9 years of age to minimize the potential effect of puberty on gene expression levels. Microarray and functional analyses are described in Supplementary File 1. We examined differentially expressed genes [false discovery rate (FDR)<5%] between subcutaneous and visceral depots, and chose genes that were linked with inflammation for qPCR analyses.
In the whole cohort, qPCR was performed using Taqman probes (Applied Biosystems, Darmstadt, Germany). We measured mRNA levels of inflammation genes, IL-8, IL-10, IL-18, chemokine ligand (CCL)2, CCL5, serum amyloid (SAA)-1, haptoglobin, vascular endothelial growth factor, osteopontin and neuronatin and developmental genes, T-box 15 (TBOX15) and homeobox C10 (HOXC10) and leptin. 7/13 genes (CCL5, IL-18, haptoglobin, VEGF, neuronatin, TBOX15, HOXC10) were chosen from our microarray analyses and 6/13 genes (IL-8, IL-10, CCL2, SAA1, osteopontin, leptin) were chosen from previous adult studies (6–8). The samples were run in duplicate on an ABI Prism 7900 system (Applied Biosystems, Darmstadt, Germany) with internal negative controls and a standard curve. The expression value for every sample was measured in duplicate and was normalized to β-actin expression, which was not different between fat depots (P=0.341).
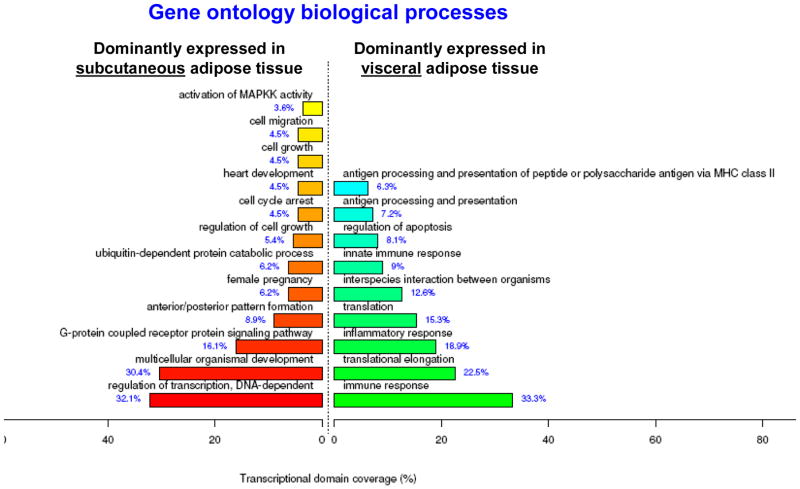
Using a threshold of FDR <5%, our microarray analyses showed that 1140 genes were differentially expressed between subcutaneous and visceral adipose depots (743 and 397 genes overexpressed in visceral and subcutaneous adipose tissue, respectively). When Gene Ontology analyses were performed, we found an over-representation of themes related to the immune response (33%) and the inflammatory response (19%) in visceral adipose tissue (Figure 1). These genes included MHC class I and II genes, Fas Interleukins 2,7,18, CD4 and TNF superligand family-member 14 and chemokines like macrophage migration inhibitory factor, CCL5, CXCL6 and CXCR4). Subcutaneous adipose tissue had an over-representation of themes related to growth and development (regulation of cell growth=5%, cell growth=5%) and regulation of transcription (32%). Differentially expressed genes included fibroblast growth factors 1 and 2, transforming growth factor; beta receptor III. From this analyses, we chose inflammatory genes IL-18, VEGF, CCL5, haptoglobin and neuronatin and development genes TBOX15 and HOXC10 to verify by PCR in the whole cohort (n=76).
Figure 1. Gene Ontology Biological Process themes enriched in subcutaneous and visceral adipose tissue in children.
1140 genes were differentially expressed (FDR<5%) in subcutaneous and visceral adipose tissue in children and were annotated by GO Biological Process themes using FunNet. This analysis is discriminant meaning that the functional profile shown is the one that best characterizes the tissue.
In the whole cohort, IL-10 and haptoglobin mRNA levels were 7- and 30-fold higher, in visceral compared to subcutaneous adipose tissue (P<0.001, 0.005), while VEGF-D, leptin and TBOX15 were 8-, 14- and 28-fold lower in visceral fat (P=0.03, 0.01, P<0.001). These differences persisted after adjustment for BMI z score. Expression of HOXC10 was low in subcutaneous AT, but could only be detected in 4 out of 13 visceral biopsies. SAA-1, osteopontin, CCL2 and CCL5, IL-8 and IL-18 and neuronatin mRNA levels were not different between adipose tissue depots. There were no significant associations between inflammation gene levels and BMI z score or age in subcutaneous or visceral adipose tissue depots.
This study shows for the first time a distinct gene expression pattern between visceral and subcutaneous adipose tissue from prepubertal children. As in adults (4), we observed that visceral adipose tissue in children has an inflammatory gene profile with increased expression of pro and anti inflammatory mediators and reduced expression of genes involved in cell development and growth. This inflammatory profile already present in healthy young children suggests that this phenotype is inherent in visceral adipose tissue early in life. In an extended group, we confirmed the increased expression of haptoglobin and IL-10 in visceral adipose tissue; these differences persisted after adjustment for BMI z score. The acute phase reactant haptoglobin plays a role in leukocyte recruitment and migration and attracts monocytes via interaction with CCR2 (15). Haptoglobin mRNA levels are raised in omental adipose tissue in morbidly obese adults (5). Although recognised as an anti-inflammatory cytokine, raised levels of IL-10 are frequently seen in inflammatory disease processes. The raised expression of IL-10 in visceral adipose tissue may indicate attempts to dampen the pro-inflammatory response and/or the early presence of inflammatory cells with an M2 or Th2 phenotype. Our findings of an inflammatory profile in visceral adipose tissue in young children are similar to findings that have previously only been reported in obese adults (6–8). Using microarray data from 10 obese men, Vohl et al showed that cytokine secretion genes (IL-6, IL1R1, C3) were higher in visceral compared to subcutaneous fat (7). Similarly, inflammation related genes CCL2 (8), CCL5 (8), CCR2 (4;8), macrophage migration inhibitory factor (4) and C3 (6), were higher in visceral compared to subcutaneous fat in obese adults.
In contrast, subcutaneous adipose tissue was mostly characterised by a developmental and growth pattern in children of varying ages. PCR analyses confirmed raised VEGF and TBOX15 in subcutaneous adipose tissue, a transcription factor from the phylogenetically conserved family of T-box genes. In contrast, adults have lower TBOX15 expression subcutaneous adipose tissue (16). In a separate study, VEGF mRNA levels were similar in subcutaneous and visceral adipose tissue but the degree of obesity was severe in that described cohort, with mean BMI 46.5 kg/m2 (17). Whether TBOX15, which is required for skin and skeletal development, is important for adipose tissue growth requires further investigation.
The main limitation of this study is that we were unable to collect paired subcutaneous and visceral adipose tissue biopsies from the majority of children. Nevertheless, our data are one of the few studies that examine gene expression in adipose tissue in children, extending previous adult findings to a pediatric age group. In addition, our findings add to the already extensive literature linking serum inflammatory markers, especially raised CRP and decreased adiponectin in overweight children (18). Together with early inflammation gene expression in the visceral depot, these features may be indicative of early pathological changes associated with overweight in children.
In conclusion, this is the first study to demonstrate that visceral adipose tissue has a distinct inflammatory profile even in young children. Coupled with chronic obesity, the inflammatory nature of visceral adipose tissue may contribute to the known increased metabolic and cardiovascular risk in later life.
Supplementary Material
Acknowledgments
Funding
This study was funded by a 2007 Diabetes Australia Research Trust Grant; Australian National Health and Medical Research Council (NHMRC)/National Heart Foundation of Australia Postgraduate Biomedical Scholarship to CST (#457224) and NHMRC Travelling Award for Research Training to CST (#571180); NHMRC Fellowship (#481354) to LKH; and the European Community Seventh Framework Program, Adipokines as Drugs to Combat Adverse Effects of Excess Adipose Tissue Project (ADAPT Grant Agreement No 201100) to KC.
We would like to gratefully acknowledge all the patients that participated in this study, the surgeons and anaesthetists in the Department of Surgery and Anaesthesia at The Children’s Hospital at Westmead and Drs Roy and Tony Brancatisano at the Institute of Weight Control for their assistance with sample collection, the Department of Histopathology at The Children’s Hospital at Westmead for their assistance with sample preparation and Dr Eric Ravussin for critical reading of the manuscript.
Reference List
- 1.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010 Mar 17;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 2.Clement K, Langin D. Regulation of inflammation-related genes in human adipose tissue. J Intern Med. 2007 Oct;262(4):422–30. doi: 10.1111/j.1365-2796.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu A, McLaughlin T, Liu T, Sherman A, Yee G, Abbasi F, et al. Differential Intra-abdominal Adipose Tissue Profiling in Obese, Insulin-resistant Women. Obes Surg. 2009 Nov;19(11):1564–73. doi: 10.1007/s11695-009-9949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 2010 May;18(5):879–83. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 5.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and Visceral Adipose Tissue Gene Expression of Serum Adipokines That Predict Type 2 Diabetes. Obesity (Silver Spring) 2010;18(5):884–9. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- 7.Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004 Aug;12(8):1217–22. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 8.Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008 May 20; doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Lindquist S, Angsten G, Yi J, Olsson T, Hernell O. Adiponectin and peroxisome proliferator-activated receptor gamma expression in subcutaneous and omental adipose tissue in children. Acta Paediatr. 2008 May;97(5):630–5. doi: 10.1111/j.1651-2227.2008.00715.x. [DOI] [PubMed] [Google Scholar]
- 10.Sabin MA, Holly JM, Shield JP, Turner SJ, Grohmann MJ, Stewart CE, et al. Mature subcutaneous and visceral adipocyte concentrations of adiponectin are highly correlated in prepubertal children and inversely related to body mass index standard deviation score. J Clin Endocrinol Metab. 2006 Jan;91(1):332–5. doi: 10.1210/jc.2005-1571. [DOI] [PubMed] [Google Scholar]
- 11.Sbarbati A, Osculati F, Silvagni D, Benati D, Galie M, Camoglio FS, et al. Obesity and inflammation: evidence for an elementary lesion. Pediatrics. 2006 Jan;117(1):220–3. doi: 10.1542/peds.2004-2854. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Lindquist S, Chen R, Myrnas T, Angsten G, Olsson T, et al. Depot-specific messenger RNA expression of 11 beta-hydroxysteroid dehydrogenase type 1 and leptin in adipose tissue of children and adults. Int J Obes (Lond) 2007 May;31(5):820–8. doi: 10.1038/sj.ijo.0803470. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Morante JJ, Milagro FI, Larque E, Lujan J, Martinez JA, Zamora S, et al. Relationship among adiponectin, adiponectin gene expression and fatty acids composition in morbidly obese patients. Obes Surg. 2007 Apr;17(4):516–24. doi: 10.1007/s11695-007-9090-6. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed January 2009]; http://www.cdc.gov/growthcharts/
- 15.Maffei M, Funicello M, Vottari T, Gamucci O, Costa M, Lisi S, et al. The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-C motif) receptor 2 (CCR2) BMC Biol. 2009;7:87. doi: 10.1186/1741-7007-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006 Apr 25;103(17):6676–81. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledoux S, Queguiner I, Msika S, Calderari S, Rufat P, Gasc JM, et al. Angiogenesis associated with visceral and subcutaneous adipose tissue in severe human obesity. Diabetes. 2008 Dec;57(12):3247–57. doi: 10.2337/db07-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam CS, Clement K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obes Rev. 2010 Feb;11(2):118–26. doi: 10.1111/j.1467-789X.2009.00674.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.