Abstract
Ovarian autoimmunity is increasingly implicated in the etiology of primary ovarian insufficiency (POI), previously termed premature ovarian failure or premature menopause. Links to autoimmunity in human POI have long been noted due to the close association of POI with several autoimmune diseases and syndromes such as Addison's disease and Autoimmune polyglandular syndrome 1. However, diagnosis of autoimmune-mediated POI (aPOI) remains challenging because of the lack of sensitive or specific markers of disease. Autoimmunity can arise from the breakdown of immunological tolerance in several ways. How then may we discern what constitutes a relevant target and what represents a downstream phenomenon? The answer lies in the study of pathogenic mechanisms in translational models of disease. From examples in humans and mice, we see that ovarian autoimmunity likely arises from a limited number of antigens targeted in the ovary that are organ specific. These antigens may be conserved but not limited to those seen in animal models of autoimmune ovarian disease. Recent advances in these areas have begun to define the relevant antigens and mechanisms of immune tolerance breakdown in the ovary. Work in translational models continues to provide insight into mechanisms of disease pathogenesis that will allow more accurate diagnosis and, ultimately, improved interventions for women with aPOI.
Keywords: Primary ovarian insufficiency, ovarian autoimmunity, SCA-POI, APS1, ovarian failure
Primary ovarian insufficiency (POI), formerly known as premature menopause or premature ovarian failure, can arise from a diverse array of causes, including genetic, enzymatic, infectious, oncological, and environmental insults. Affecting 1% of women in the United States, POI is diagnosed by the presence of oligo/amenorrhea for ≥4 months with at least two serum follicle-stimulating hormone (FSH) levels in the menopausal range (separated by at least 1 month) in women <40 years of age. Clinical manifestations of the disease are quite variable, with intermittent ovarian function in 50% of women and even occasional conception after diagnosis, suggesting a continuum of impaired ovarian function more appropriately described as POI.1,2 The clinical features and biology of ovarian insufficiency are well characterized,2 but strikingly, most of the cases of POI have no clear etiology. Growing evidence suggests that ovarian autoimmunity may be a significant cause of many cases of idiopathic POI. Determination of autoimmunity in POI can impact clinical management, allowing us to intervene in a population whose ovarian function might be preserved with timely therapy. However, the challenge in ascribing an autoimmune cause for disease lies in the amount of immunological “noise” when looking for evidence of abnormal reactions to self-tissue. Many tests for autoimmunity are fraught with problems with “specificity, as exemplified by rheumatoid factor”.3 Clearly, accurate diagnosis and characterization of autoimmune disease then requires knowledge of the immune mechanisms and key antigens.
POI has long been linked to autoimmunity and specific autoimmune syndromes, but definitive diagnosis of ovarian autoimmunity has remained elusive due to the lack of understanding of the pathogenic mechanisms. Several excellent reviews have described the autoantibodies and possible antigens associated with autoimmune-mediated POI (aPOI).4–9 However, the true prevalence of aPOI remains unclear due to difficulty distinguishing true pathogenic targets from off-target effects associated with autoimmune destruction. Historically, various autoantibodies associated with aPOI have shown poor sensitivity or specificity, such that estimates of aPOI range anywhere from 7%, and 50 to 70% of women with POI.4,5 Fortunately, the development of more specific antibody assays and a growing body of work in animal models are beginning to uncover specific targets of ovarian autoimmunity.
Only a limited number of translational models for aPOI between mouse and human have been available to date, but such models hold the key to understanding disease pathogenesis. Studies in these models provide the most convincing evidence of antigen-specific and organ-specific disease, although the ovarian autoimmunity arises through several different mechanisms. Based on these examples, it seems that primary autoimmune disease of the ovary likely arises from loss of tolerance to a limited number of tissue-specific antigens targeted in the gland. Moreover, these antigens appear to be conserved but not limited to those seen in animal models of autoimmune ovarian disease. We review the immunological markers and clinical evidence for autoimmunity in women with aPOI and focus on the corresponding animal models of ovarian autoimmunity, where increasingly we are learning about the mechanisms of disease.
MECHANISMS IN IMMUNE TOLERANCE: DEFINING OVARIAN AUTOIMMUNITY
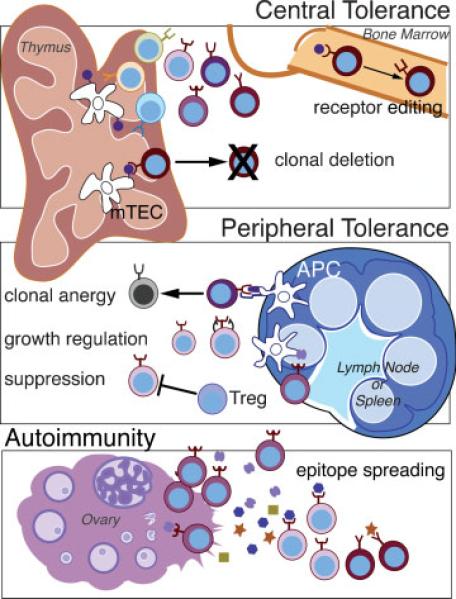
To recognize signs of autoimmunity in ovarian disease, we must first understand how tolerance to the ovary is normally established. The maintenance of nonreactivity to self-tissue is the key to immunological tolerance in a system designed to generate clonal diversity to a wide array of potential pathogens. Autoimmunity arises when the adaptive immune system loses its ability to distinguish self-tissue from nonself—such as pathogens, allergens, or tumor cells—and the normal checkpoints that maintain tolerance are broken (Fig. 1). Several central and peripheral mechanisms have evolved to establish and maintain tolerance to self during the development and daily function of the immune system.10,11 Briefly, developing T cells and B cells that display receptors to self-tissues are generally deleted, retuned, rendered inactive, or suppressed by the immune system. Central tolerance mediates the deletion of developing self-reactive T and B cells within the central lymphoid organs of the thymus and bone marrow, respectively. The binding of self-antigen to “forbidden” T-cell and B-cell receptors triggers these cells to self-edit the receptor to a less autoreactive form (primarily occurring in B cells) or to die (clonal deletion). Cells may escape this process if they express receptors with intermediate affinities for self-antigen that fall below the threshold to trigger deletion or if a self-antigen is not sufficiently abundant in the central lymphoid tissue to interact with the autoreactive cells. As another layer of regulation, several peripheral mechanisms in the secondary lymphoid organs mediate tolerance by modulating activity of mature cells. Autoreactive cells that encounter self-antigen in the periphery can be regulated by (1) the induction of signals to become functionally inactive (anergy), (2) limitation of survival factors or growth signals, and (3) active suppression by regulatory T cells. Defects in any of these tolerance mechanisms have the potential to allow autoreactive B and T cells to escape, proliferate, and induce autoimmune disease. Autoimmunity can be compounded by epitope spreading that occurs once an immune response is underway or in the setting of chronic inflammation.12 The initial destruction of tissues and release of antigens can promote further immune responses to other components of an antigen or to new self-antigens that now gain exposure to the immune system. Thus, markers and antibodies that we detect in the course of disease may reflect the primary response or events several steps removed from the initial insult. Furthermore, distinguishing between primary and secondary spread epitopes can critically impact our approach to treatment; therapy aimed at downstream spread epitopes will not prevent disease initiation and may be ineffective in halting the autoimmune response, depending on the stage of disease progression.
Figure 1.
Checkpoints in immune tolerance: Illustrated in this figure are the major mechanisms used to establish and maintain immunological tolerance, defined as nonreactivity to self-tissue. For simplicity, most of the mechanisms shown depict the regulation of T cells, although very similar mechanisms occur for B cells as well. Developing T and B cells randomly generate a diverse repertoire of receptors. Each cell expresses a single B- or T-cell receptor (BCR or TCR), of which some will recognize self-tissue (depicted as cells in various shades of brown). Regulation of these cells begins with central tolerance in the bone marrow and thymus. In the bone marrow, immature B cells that recognize self-antigens can undergo receptor editing to express BCRs with lower affinity for self. B cells that fail to rearrange and edit their BCRs to a less self-reactive form are eventually deleted. Developing T cells enter the thymus where they complete their maturation and undergo negative selection before gaining access to the rest of the body. Specialized medullary thymic epithelial cells (mTECs) function to express and present a wide array of self-antigens that are displayed to T cells. T cells that recognize self-antigen in this way are deleted (clonal deletion) in a process known as negative selection. Some autoreactive cells may escape to the periphery. Peripheral tolerance is mediated primarily in the lymph nodes and secondary lymphoid organs as well as to some extent in the target organ. Within the lymph node, antigen-presenting cells (APCs) can present self-antigens and provide signals to T cells. Autoreactive cells (shown in shades of red) that encounter self-antigen in the context of modulatory signals can be inactivated (clonal anergy). T cells may not encounter antigen (ignorance) or be deprived of growth/survival signals (growth regulation) such that they are not activated to be pathogenic. Finally, autoreactive cells may that escape all these mechanisms can go on to proliferate and cause autoimmunity in the target organ (illustrated in the ovary). Once the immune response to an antigen is initiated, other tissue antigens can be released and presented to the immune cells. This in turn leads to activation of other antigen-specific T cells in a process known as epitope spreading.
Classically, criteria for autoimmunity in a clinical disease have been defined by Witebsky's postulates with (1) demonstration of an autoantibody or cell-mediated immune response, (2) knowledge of the corresponding autoantigen, and (3) analogous response reproduced in an animal model.13,14 The hallmarks then of autoimmune conditions are the presence of self-reactive B and T cells, manifested by circulating autoantibodies and lymphocytic mononuclear cell infiltrates in affected tissues. Because definitive diagnosis of organ-based autoimmune disease requires demonstration of autoimmune infiltrates on tissue biopsies or detection of antigen-specific T-cell responses, other markers, such as autoantibodies, are frequently used as a surrogate. Ascertainment of aPOI presents a particular challenge because definitive diagnosis requires ovarian biopsy to document tissue infiltration, and the current autoantibody tests in use are plagued by poor sensitivity or specificity. Although proof of autoimmunity also requires the ability to adoptively transfer disease with autoreactive lymphocytes from an affected subject, transfer of autoimmune disease in humans is not experimentally feasible. Fortunately, transfer of many autoimmune diseases, including ovarian disease, is well documented in animal models, which provide the means to dissect the mechanisms of autoimmunity.
TRANSLATIONAL MODELS FOR AUTOIMMUNE-MEDIATED PRIMARY OVARIAN INSUFFICIENCY
To understand the significance of putative autoantigens associated with human disease requires more direct examination of the pathological immune response. To this end, animal models have been invaluable in teaching us the mechanisms by which organ-specific tolerance is broken in the ovary. Traditionally, animal models of ovarian autoimmunity have been limited by the differences in ovarian disease induction and the autoantigens targeted; however, recent advances may allow us to draw more direct parallels to human aPOI.
An Immunization Model of Autoimmune-Medicated Primary Ovarian Insufficiency: Zona Pellucida Antigen–Induced Experimental Autoimmune Oophoritis
One of the earliest models of disease was based on immunization with ovarian self-antigen to induce oophoritis in a manner similar to experimental autoimmune encephalomyelitis, the classic model system used to study multiple sclerosis. Experimental autoimmune oophoritis (EAO) is produced by immunization with zona pellucida antigen (ZP3) in combination with Freund's adjuvant.15–17 ZP3-induced disease is characterized by rapid onset of inflammatory and lymphocytic ovarian infiltrates with progressive loss of large antral follicles and a strong autoantibody response.16 Adoptive transfer of CD4+ T cells from ZP3-immunized females also induces oophoritis in wild-type recipients without a detectable autoantibody response, suggesting the primary mechanism is T-cell mediated. However, as with many other experimentally induced autoimmune diseases, ZP3-immunized mice recover from disease with regression of ovarian inflammation and return to fertility ~4 months postinoculation. Moreover, the recovered animals exhibit resistance to further oophoritis upon rechallenge with antigen, raising the question whether other mechanisms of peripheral tolerance play a role in disease modulation.17 Recently, a newer model of ZP3-mediated autoimmunity has demonstrated a requirement for B cells and ZP3 antibodies in the induction of disease.18 Mice infected with a recombinant murine-cytomegalovirus expressing murine-ZP3 (rMCMV-mZP3) develop similar disease on ovarian histology with permanent infertility.19 Immunoglobulin-deficient mice are protected from rMCMV-mZP3 induced disease, and passive transfer of hyperimmune sera or a ZP3-specific antibody can induce transient infertility in naive female recipients. Thus a requirement for B cell responses in liberating antigen or antigen presentation may be necessary for achieving durable autoimmunity in this model. Indeed, autoantibodies have been demonstrated to be necessary to redirect pathogenic T-cell responses leading to severe oophoritis and ovarian atrophy in ZP3-induced EAO.20
The rationale for targeting ZP3 arose from observations that antibodies to this molecule were implicated in women with infertility.21 However, an increased incidence of ZP3 autoantibodies has not been conclusively demonstrated in women with POI.4,6 The significance of ZP3 antibodies in human aPOI remains to be determined, although their usefulness as a contraceptive measure in animals remains an area of active interest.
Steroidal-Cell Antibody-Primary Ovarian Insufficiency
The first suggestion of an autoimmune etiology in human POI came from observations in women with Addison's disease, where association of the two conditions is highly prevalent.22,23 Antibodies to steroid cells were first noted in Addison's disease patients24 and linked to clinical ovarian deficiency.22 These steroidal-cell antibodies (SCAs) were detected based on indirect immunofluorescence staining of adrenal, ovary, placental, or testis tissue sections. Depending on the method of detection, estimates of SCAs range from 73% to 100% of women with both POI and Addison's disease.25–27 SCA-POI is highly linked to adrenal autoimmunity, both for women with clinical evidence for adrenal insufficiency as well as those who are asymptomatic in this regard yet test positive for adrenal cortex antibodies by indirect immunofluoresence.27–29 It is now known that the major autoantigen that reacts with adrenal cortical antibodies (ACAs) detected by indirect immunofluorescence is the enzyme 21-hydroxylase (21-OH),30,31 and 21-OH autoantibodies are the best predictor of Addison's disease.28,32 Notably, detection of ACAs and SCAs is not concordant in all cases of isolated Addison's disease,25 perhaps due to differences in assay methods or because the 21-hydroxylase enzyme is not expressed in the ovary. Nevertheless, because the association of SCA-POI and Addison's disease is very strong and onset of ovarian disease frequently precedes clinical adrenal insufficiency, screening of all women with SCA-POI for adrenal function and counseling regarding the symptoms of adrenal insufficiency is recommended.33–35
The presence of SCAs correlates well with an autoimmune etiology for ovarian disease. In a small series of ovarian biopsies performed in women with spontaneous POI, 100% of women with SCAs were found to have CD3+ lymphocytic infiltrates on ovarian biopsy, indicating the presence of active, T-cell–mediated autoimmune oophoritis.29 All of the biopsies in SCA-negative women did not demonstrate any ovarian infiltrates. Furthermore, review of the reported cases of POI with histological evidence of oophoritis found evidence of positive SCAs in all instances. Closer examination of the pattern of SCA immunofluorescence revealed a cytoplasmic pattern of staining in ovarian theca cells among many women with positive SCAs, suggesting that common steroidogenic cell enzymes might be targeted between the adrenal and ovary.25 Further investigation has demonstrated that SCAs are composed in part of antibodies to the 17-α hydroxylase (17OHAb) and side-chain cleavage enzymes (p450sccAb) in women with SCA-POI,26,27,33,36 that they are known mediators of thecal cell androgen synthesis, and that they consist primarily of immunoglobin G1 isotypes that are associated with autoimmune T-cell responses.37
Clinical observations support a model of selective thecal cell destruction suggested by the autoantibody data. Typically, women with POI exhibit low serum concentrations of estradiol, inhibin A, and inhibin B due to decreased numbers of mature or developing follicles.38–40 However, women with SCA-POI demonstrate elevated inhibin A and B levels with multiple antral follicles, low estradiol, and relatively elevated FSH,40,41 as well as, in some cases, normal anti-Müllerian hormone levels.35 Interestingly, in multiple histological reports on this condition, primordial follicles are spared any lymphocytic infiltration.4,29 These observations are consistent with a model of selective thecal cell loss in which granulosa cells are preserved but exhibit impaired estradiol synthesis due to lack of androgen substrates. Thus, some women with SCA-POI are known to maintain a pool of primordial follicles early in the disease process; from this perspective, with proper immunosuppressive therapy, it might be possible to restore fertility for these women.
Despite the clear autoimmune markers seen in SCA-POI, there is evidence to suggest that the antibodies themselves are not pathogenic. It may be that SCAs arise through epitope spreading, that other yet undetermined antigens initiate the autoimmune destruction in SCA-POI, or that autoantibodies alone are not sufficient to precipitate disease. Indeed, women with SCA-POI have been shown to have antibodies to the granulosa cells and ovum25 with multiple autoreactive targets within the oocyte.42 Although the pathological significance of SCAs remains ambiguous, SCAs represent a highly useful clinical marker for histological autoimmune oophoritis.
The Neonatal Thymectomy Model
One long-standing example of ovarian autoimmunity that arises from disruption of both central and peripheral tolerance is the neonatal thymectomy model of disease. In various strains of inbred mice, thymectomy at day 3 after birth induces a wide array of organ-specific autoimmune diseases, including autoimmune ovarian disease. Most notably, oophoritis and ovarian failure can be seen in 90% of C57Bl/6xA/J F1 mice.43–45 Immunological mechanisms of disease in this model have been extensively studied (reviewed by Tung et al),46 with some of the earliest observations leading to the discovery of regulatory T cells (Tregs).47,48 Neonatal thymectomy (NTx) results in the relative depletion of CD4+CD25+ regulatory T cells (Tregs), which emigrate from the thymus after day 3, and autoimmunity ensues in part from an imbalance between regulatory and effector T cells. Replacement of Tregs in NTx mice prevents autoimmune disease.49 Like women with SCA-POI, female mice that undergo NTx develop lymphocytic infiltrates and autoantibodies to several ovarian targets. Similar to a subset of women with autoimmune POI,25 NTx-treated female mice demonstrate an early robust antibody response predominantly to several oocyte proteins. The common targeting of oocyte proteins suggests a possible equivalent mechanism for in these women in what might be termed oocyte-autoimmunity POI.
Further characterization of the ovarian autoantibodies observed in NTx mice allowed the identification of a novel ovarian antigen known as MATER (Maternal Antigen That Embryos Require).50 Mater encodes a 125-kDa protein that is highly expressed in oocytes. Inactivation of the mouse Mater gene causes embryos from Mater knockout mothers to arrest at the two-cell stage and eventually degenerate, indicating that Mater is required for embryonic development after fertilization.51 Similarly, the human homolog for mouse Mater also shows largely ovarian-specific expression.52
Many studies in the NTx model have examined the mechanisms by which Tregs modulate disease. Suppression of ovarian disease by adoptive transfer of polyclonal Tregs depends on exposure to endogenous autoantigen in the periphery and is disease specific.45 Expression of self-antigen in the context of major histocompatability complex (MHC) class II in the thymus and the periphery has been shown to mediate immune tolerance in several models of autoimmunity.53 Indeed, transgenic expression of MATER driven by an MHC class II promoter mediates a significant reduction in autoimmune oophoritis in NTx-treated mice.54 In those cases where oophoritis persists, autoantibodies to other ovarian proteins are detected, suggesting that multiple antigens may play a role in the pathogenesis of autoimmune oophoritis in this model. Thus, MATER represents a significant pathogenic antigen in NTx-induced ovarian autoimmunity and an attractive candidate for the development of diagnostic testing as well as for induction of tolerance in human autoimmune oophoritis.
APS1 and the Aire Knockout Mouse Model of Autoimmune-Mediated Primary Ovarian Insufficiency
Rare genetic disorders frequently provide us the ability to gain unique insights into fundamental aspects of biology. Autoimmune polyglandular syndrome type 1 (APS1) is one such example. APS1 is a rare monogenic disorder that manifests as a pleomorphic array of autoimmune diseases that primarily target endocrine organs, including the ovary. Before the discovery of the causative gene, the Autoimmune Regulator (AIRE), diagnosis was based on the development of hallmark clinical conditions of autoimmune hypoparathyroidism, Addison's disease, and mucocutaneous candidiasis.55,56 The subsequent burst of investigation has uncovered much about the cellular and molecular mechanisms of disease and enhanced our knowledge about the thymus and basic immunology.57,58 Notably, although variable involvement of other target organs is seen in this syndrome, ovarian failure is highly prevalent with up to 72% of women affected by age 40.55
Mutations in APS1 are not only a known genetic cause of disease but also give insight into a confirmed autoimmune mechanism. Inactivation of the Aire gene in mouse models has allowed the elucidation of the molecular mechanism for the pleomorphic organ-based autoimmunity seen in APS1 disease.59–61 Aire acts primarily in the thymus to regulate the expression of an array of tissue-specific self-antigens (TSAs) within specialized medullary thymic epithelial cells (mTECs), whose role is to educate developing T cells. Normally, autoreactive T cells that encounter self-antigen presented by these mTECs are deleted through thymic negative selection (Fig. 1). In the absence of Aire, these mTECs do not express many TSAs, and autoreactive T cells can escape into the periphery to cause disease.
Like women with APS1, Aire KO female mice develop a high incidence of ovarian autoimmunity with histological oophoritis and infertility in up to 100% of BALB/c.58,61 Knockout (KO) mice develop lymphocytic infiltrates in the ovary and autoantibodies that recognize the oocyte.58 In contrast to previous models, disease in the Aire KO mouse arises spontaneously and, as such, represents a significant advance in our ability to study aPOI as well to clarify molecular mechanisms for tolerance breakdown. Moreover, the link to a human syndrome of ovarian autoimmunity suggests that antigens identified in this model will have direct relevance to clinical disease. Indeed, characterization of a novel lung autoantigen in Aire KO mice showed striking similarity to the lung autoreactivity seen in an APS1 patient with lung disease.62 Alimohammadi et al reported the discovery of NALP5 as a putative parathyroid autoantigen in patients with APS1. Strikingly, NALP5, also known as MATER, is the self-same autoantigen identified in NTx-induced aPOI.50 Autoantibodies to NALP5/MATER are specific for patients with APS1 who are affected with autoimmune parathyroid disease; none of the APS1 patients without parathyroid disease had detectable NALP5/MATER autoantibodies.63 Notably, many patients with APS1 and NALP5/MATER autoantibodies also had POI. Although these antibodies were not specific for women with APS1 and POI, 53% of antibody-positive individuals also had hypogonadism (most of these representing women with aPOI), suggesting that NALP5/MATER could represent a common target of autoimmunity in both the parathyroid and the ovary. It is unknown whether Aire regulates expression of Nalp5/Mater within the thymus or if Nalp5/Mater autoreactivity initiates the ovarian autoimmunity in Aire KO female mice. Also whether or not the presence of NALP5/MATER antibodies defines a specific autoimmune mechanism in women with idiopathic POI is not known. Nevertheless, the identification of NALP5 in two models of aPOI arising from different immune defects suggests it represents a putative common target of ovarian disease in women with autoimmune-mediated POI.
CONCLUSIONS
Review of the immunopathology and antigens targeted in both mouse and human models indicates that several forms of autoimmunity may ultimately lead to POI (Table 1). However, despite disease induction via different methods of tolerance breakdown, similar antigens are targeted in the ovary, as in the example of NALP5/MATER. It remains to be determined whether the various antibodies that have been described with the clinical disease represent pathophysiological targets or markers associated with the autoimmune condition. However, the identification of the same target in both mouse and human disease models speaks to the importance of these studies.
Table 1.
Translational Models for Autoimmune Primary Ovarian Insufficiency
| Model System | Mechanism of Disease | Autoantibodies and Antigens | Notable Features |
|---|---|---|---|
| Human | |||
| SCA-POI | Unknown | SCAs 17-OH SCC |
• Close association with Addison's disease • Frequent association with other autoimmune diseases • CD3+ lymphocytic infiltrates • Affects antral follicles more than primary follicles |
| APS1 |
AIRE gene defect Impaired negative selection |
SCAs NALP5 (MATER) |
• 72% incidence of POI • 68% of women with POI also positive for NALP5/MATER antibodies |
| Mouse | |||
| ZP3-induced | Immunization with ZP3 | ZP3 | • Initial oophoritis with resolution of disease and return to fertility |
| EAO | Infection with rCMV-mZP3 | • Durable disease in recent models (rCMV-mZP3) • CD4+CD8+ lymphocytic infiltrates of ovary • Autoantibody response required for disease induction |
|
| NTx | Thymectomy induced Reduction in Tregs vs Teff |
Mater/Nalp5 Other oocyte proteins |
• Antibodies to multiple oocyte proteins with a predominant response to Mater/Nalp5; other ovarian autoantibodies also seen • Lymphocytic infiltrates seen with antral follicles affected more than primary follicles; eventual ovarian atrophy • Ovarian disease transferable by CD4 + T cells |
| Aire knockout |
Aire gene defect Decreased negative selection of autoreactive T cells |
Oocyte proteins | • 100% incidence of ovarian failure in certain strains • Lymphocytic infiltrates seen in ovaries • Autoantibodies to oocyte proteins seen in serum of affected mice • Autoimmune disease transferable by CD4 + T cells |
SCA-POI, steroidal-cell antibody primary ovarian insufficiency; SCA, steroid cell antibodies; 17-OH, 17α-hydroylase enzyme; SCC, p450 side-chain cleavage enzyme; APS1, Autoimmune polyglandular syndrome type 1; AIRE, Autoimmune regulator; ZP3, zona pellucida 3; EAO, experimental autoimmune oophoritis; NALP5, NACHT leucine-rich-repeat protein 5; MATER, maternal antigen that embryos require; NTx, neonatal thymectomy; Treg, regulatory T cell; Teff, effector T cell.
Increasingly, reports have been made of autoimmune oophoritis in idiopathic POI in cases of “occult ovarian failure” or unexplained infertility,5,64 and autoimmunity has been invoked in the pathology of PCOS and endometriosis based on the detection of various autoantibodies. However, direct demonstration of lymphocytic oophoritis on ovarian biopsy has been very limited in cases not associated with known autoimmunity (reviewed by Hoek et al).4 Autoantibodies in human serum can be difficult to measure due to high levels of background from other serum proteins as well as the often relatively low levels of circulating antigen-specific antibody. The insight we gain from translational models of aPOI allows us to interpret autoantibody markers with knowledge of the pathogenic mechanisms and relevant disease targets to determine more accurately the contribution of autoimmunity in these conditions.
Given the variable clinical course of aPOI, correct diagnosis of this subset of POI patients can be critical because some patients might conceive with appropriate immunomodulatory therapy. Furthermore, detection and prediction of aPOI in patients with known autoimmune disease or unexplained infertility would be valuable prognostic information, allowing changes in family planning or assisted reproductive interventions. To date, treatment is limited to general immunosuppression with glucocorticoids and is being studied in an ongoing prospective clinical trial based at the National Institutes of Health (Trial ID NCT00001306). As illustrated by the model systems in this review, knowledge of pathogenic targets can be gained through the examination of relevant antibody responses and is essential in enabling the further mechanistic study of both of tolerance breakdown and tolerance induction. Future research in the identification of ovarian antigens will allow improved diagnostic and prognostic testing in aPOI. With the characterization and discovery of novel ovarian antigens, targeted immunotherapy and antigen-specific treatment will be possible, moving the care of patients with aPOI into a new era.
ACKNOWLEDGMENTS
Supported in part by the Intramural Research Program, National Institute of Child Health and Human Development, National Institutes of Health. LMN is a commissioned officer in the U.S. Public Health Service. MHC is supported by the National Institute of Child Health and Human Development, National Institutes of Health (K08HD058599).
REFERENCES
- 1.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shmerling RH, Delbanco TL. How useful is the rheumatoid factor? An analysis of sensitivity, specificity, and predictive value. Arch Intern Med. 1992;152(12):2417–2420. [PubMed] [Google Scholar]
- 4.Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18(1):107–134. doi: 10.1210/edrv.18.1.0291. [DOI] [PubMed] [Google Scholar]
- 5.Luborsky J. Ovarian autoimmune disease and ovarian autoanti-bodies. J Womens Health Gend Based Med. 2002;11(7):585–599. doi: 10.1089/152460902760360540. [DOI] [PubMed] [Google Scholar]
- 6.Forges T, Monnier-Barbarino P, Faure GC, Béné MC. Autoimmunity and antigenic targets in ovarian pathology. Hum Reprod Update. 2004;10(2):163–175. doi: 10.1093/humupd/dmh014. [DOI] [PubMed] [Google Scholar]
- 7.Monnier-Barbarino P, Forges T, Faure GC, Béné MC. Gonadal antibodies interfering with female reproduction. Best Pract Res Clin Endocrinol Metab. 2005;19(1):135–148. doi: 10.1016/j.beem.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Tuohy VK, Altuntas CZ. Autoimmunity and premature ovarian failure. Curr Opin Obstet Gynecol. 2007;19(4):366–369. doi: 10.1097/GCO.0b013e328220e90c. [DOI] [PubMed] [Google Scholar]
- 9.La Marca A, Brozzetti A, Sighinolfi G, Marzotti S, Volpe A, Falorni A. Primary ovarian insufficiency: autoimmune causes. Curr Opin Obstet Gynecol. 2010;22(4):277–282. doi: 10.1097/GCO.0b013e32833b6c70. [DOI] [PubMed] [Google Scholar]
- 10.Goodnow CC, Sprent J. Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435(7042):590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 11.Walker LSK, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2(1):11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 12.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2(2):85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 13.Witebsky E, Rose NR, Terplan K, Paine JR, Egan RW. Chronic thyroiditis and autoimmunization. J Am Med Assoc. 1957;164(13):1439–1447. doi: 10.1001/jama.1957.02980130015004. [DOI] [PubMed] [Google Scholar]
- 14.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol Today. 1993;14(9):426–430. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 15.Skinner SM, Mills T, Kirchick HJ, Dunbar BS. Immunization with zona pellucida proteins results in abnormal ovarian follicular differentiation and inhibition of gonadotropin-induced steroid secretion. Endocrinology. 1984;115(6):2418–2432. doi: 10.1210/endo-115-6-2418. [DOI] [PubMed] [Google Scholar]
- 16.Rhim SH, Millar SE, Robey F, et al. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J Clin Invest. 1992;89(1):28–35. doi: 10.1172/JCI115572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lou YH, McElveen F, Adams S, Tung KS. Altered target organ. A mechanism of postrecovery resistance to murine autoimmune oophoritis. J Immunol. 1995;155(7):3667–3673. [PubMed] [Google Scholar]
- 18.Lloyd ML, Papadimitriou JM, O'Leary S, Robertson SA, Shellam GR. Immunoglobulin to zona pellucida 3 mediates ovarian damage and infertility after contraceptive vaccination in mice. J Autoimmun. 2010;35(1):77–85. doi: 10.1016/j.jaut.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary S, Lloyd ML, Shellam GR, Robertson SA. Immunization with recombinant murine cytomegalovirus expressing murine zona pellucida 3 causes permanent infertility in BALB/c mice due to follicle depletion and ovulation failure. Biol Reprod. 2008;79(5):849–860. doi: 10.1095/biolreprod.108.067884. [DOI] [PubMed] [Google Scholar]
- 20.Lou YH, Park KK, Agersborg S, Alard P, Tung KS. Retargeting T cell-mediated inflammation: a new perspective on autoantibody action. J Immunol. 2000;164(10):5251–5257. doi: 10.4049/jimmunol.164.10.5251. [DOI] [PubMed] [Google Scholar]
- 21.Shivers CA, Dunbar BS. Autoantibodies to zona pellucida: a possible cause for infertility in women. Science. 1977;197(4308):1082–1084. doi: 10.1126/science.70076. [DOI] [PubMed] [Google Scholar]
- 22.Irvine WJ, Chan MM, Scarth L, et al. Immunological aspects of premature ovarian failure associated with idiopathic Addison's disease. Lancet. 1968;2(7574):883–887. doi: 10.1016/s0140-6736(68)91053-2. [DOI] [PubMed] [Google Scholar]
- 23.de Moraes Ruehsen M, Blizzard RM, Garcia-Bunuel R, Jones GS. Autoimmunity and ovarian failure. Am J Obstet Gynecol. 1972;112(5):693–703. doi: 10.1016/0002-9378(72)90797-1. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JR, Goudie RB, Gray K, Stuart-Smith DA. Immunological features of idiopathic Addison's disease: an antibody to cells producing steroid hormones. Clin Exp Immunol. 1968;3(2):107–117. [PMC free article] [PubMed] [Google Scholar]
- 25.Sotsiou F, Bottazzo GF, Doniach D. Immunofluorescence studies on autoantibodies to steroid-producing cells, and to germline cells in endocrine disease and infertility. Clin Exp Immunol. 1980;39(1):97–111. [PMC free article] [PubMed] [Google Scholar]
- 26.Falorni A, Laureti S, Candeloro P, et al. Steroid-cell autoantibodies are preferentially expressed in women with premature ovarian failure who have adrenal autoimmunity. Fertil Steril. 2002;78(2):270–279. doi: 10.1016/s0015-0282(02)03205-3. [DOI] [PubMed] [Google Scholar]
- 27.Dal Pra C, Chen S, Furmaniak J, et al. Autoantibodies to steroidogenic enzymes in patients with premature ovarian failure with and without Addison's disease. Eur J Endocrinol. 2003;148(5):565–570. doi: 10.1530/eje.0.1480565. [DOI] [PubMed] [Google Scholar]
- 28.Betterle C, Coco G, Zanchetta R. Adrenal cortex autoanti-bodies in subjects with normal adrenal function. Best Pract Res Clin Endocrinol Metab. 2005;19(1):85–99. doi: 10.1016/j.beem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Bakalov VK, Anasti JN, Calis KA, et al. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46,XX spontaneous premature ovarian failure. Fertil Steril. 2005;84(4):958–965. doi: 10.1016/j.fertnstert.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 30.Winqvist O, Gebre-Medhin G, Gustafsson J, et al. Identification of the main gonadal autoantigens in patients with adrenal insufficiency and associated ovarian failure. J Clin Endocrinol Metab. 1995;80(5):1717–1723. doi: 10.1210/jcem.80.5.7745025. [DOI] [PubMed] [Google Scholar]
- 31.Baumann-Antczak A, Wedlock N, Bednarek J, et al. Autoimmune Addison's disease and 21-hydroxylase. Lancet. 1992;340(8816):429–430. doi: 10.1016/0140-6736(92)91513-8. [DOI] [PubMed] [Google Scholar]
- 32.Falorni A, Nikoshkov A, Laureti S, et al. High diagnostic accuracy for idiopathic Addison's disease with a sensitive radiobinding assay for autoantibodies against recombinant human 21-hydroxylase. J Clin Endocrinol Metab. 1995;80(9):2752–2755. doi: 10.1210/jcem.80.9.7673419. [DOI] [PubMed] [Google Scholar]
- 33.Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf) 1993;39(1):35–43. doi: 10.1111/j.1365-2265.1993.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 34.Betterle C, Dal Pra C, Mantero F, Zanchetta R. Auto-immune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23(3):327–364. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- 35.La Marca A, Marzotti S, Brozzetti A, et al. Italian Addison Network. Primary ovarian insufficiency due to steroidogenic cell autoimmunity is associated with a preserved pool of functioning follicles. J Clin Endocrinol Metab. 2009;94(10):3816–3823. doi: 10.1210/jc.2009-0817. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Sawicka J, Betterle C, et al. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison's disease, and premature ovarian failure. J Clin Endocrinol Metab. 1996;81(5):1871–1876. doi: 10.1210/jcem.81.5.8626850. [DOI] [PubMed] [Google Scholar]
- 37.Brozzetti A, Marzotti S, La Torre D, et al. Italian Addison Network. Autoantibody responses in autoimmune ovarian insufficiency and in Addison's disease are IgG1 dominated and suggest a predominant, but not exclusive, Th1 type of response. Eur J Endocrinol. 2010;163(2):309–317. doi: 10.1530/EJE-10-0257. [DOI] [PubMed] [Google Scholar]
- 38.Buckler HM, Evans CA, Mamtora H, Burger HG, Anderson DC. Gonadotropin, steroid, and inhibin levels in women with incipient ovarian failure during anovulatory and ovulatory rebound cycles. J Clin Endocrinol Metab. 1991;72(1):116–124. doi: 10.1210/jcem-72-1-116. [DOI] [PubMed] [Google Scholar]
- 39.Petraglia F, Hartmann B, Luisi S, et al. Low levels of serum inhibin A and inhibin B in women with hypergonadotropic amenorrhea and evidence of high levels of activin A in women with hypothalamic amenorrhea. Fertil Steril. 1998;70(5):907–912. doi: 10.1016/s0015-0282(98)00283-0. [DOI] [PubMed] [Google Scholar]
- 40.Welt CK, Falorni A, Taylor AE, Martin KA, Hall JE. Selective theca cell dysfunction in autoimmune oophoritis results in multifollicular development, decreased estradiol, and elevated inhibin B levels. J Clin Endocrinol Metab. 2005;90(5):3069–3076. doi: 10.1210/jc.2004-1985. [DOI] [PubMed] [Google Scholar]
- 41.Tsigkou A, Marzotti S, Borges L, et al. High serum inhibin concentration discriminates autoimmune oophoritis from other forms of primary ovarian insufficiency. J Clin Endocrinol Metab. 2008;93(4):1263–1269. doi: 10.1210/jc.2007-1675. [DOI] [PubMed] [Google Scholar]
- 42.Pires ES, Meherji PK, Vaidya RR, Parikh FR, Ghosalkar MN, Khole VV. Specific and sensitive immunoassays detect multiple anti-ovarian antibodies in women with infertility. J Histochem Cytochem. 2007;55(12):1181–1190. doi: 10.1369/jhc.7A7259.2007. [DOI] [PubMed] [Google Scholar]
- 43.Kojima A, Tanaka-Kojima Y, Sakakura T, Nishizuka Y. Spontaneous development of autoimmune thyroiditis in neonatally thymectomized mice. Lab Invest. 1976;34(6):550–557. [PubMed] [Google Scholar]
- 44.Taguchi O, Nishizuka Y, Sakakura T, Kojima A. Auto-immune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol. 1980;40(3):540–553. [PMC free article] [PubMed] [Google Scholar]
- 45.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+ CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005;202(6):771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tung KSK, Setiady YY, Samy ET, Lewis J, Teuscher C. Autoimmune ovarian disease in day 3-thymectomized mice: the neonatal time window, antigen specificity of disease suppression, and genetic control. Curr Top Microbiol Immunol. 2005;293:209–247. doi: 10.1007/3-540-27702-1_10. [DOI] [PubMed] [Google Scholar]
- 47.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 48.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+ CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160(3):1212–1218. [PubMed] [Google Scholar]
- 49.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Auto-immune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184(2):387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong ZB, Nelson LM. A mouse gene encoding an oocyte antigen associated with autoimmune premature ovarian failure. Endocrinology. 1999;140(8):3720–3726. doi: 10.1210/endo.140.8.6911. [DOI] [PubMed] [Google Scholar]
- 51.Tong ZB, Gold L, Pfeifer KE, et al. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet. 2000;26(3):267–268. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- 52.Tong Z-B, Bondy CA, Zhou J, Nelson LM. A human homologue of mouse Mater, a maternal effect gene essential for early embryonic development. Hum Reprod. 2002;17(4):903–911. doi: 10.1093/humrep/17.4.903. [DOI] [PubMed] [Google Scholar]
- 53.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11(1):21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 54.Otsuka N, Tong ZB, Vanevski K, et al. Autoimmune oophoritis with multiple molecular targets mitigated by transgenic expression of Mater. Endocrinology. 2011;152(6):2465–2473. doi: 10.1210/en.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perheentupa J. APS-I/APECED: the clinical disease and therapy. Endocrinol Metab Clin North Am. 2002;31(2):295–320. vi. doi: 10.1016/s0889-8529(01)00013-5. [DOI] [PubMed] [Google Scholar]
- 56.Husebye ES, Perheentupa J, Rautemaa R, Kämpe O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J Intern Med. 2009;265(5):514–529. doi: 10.1111/j.1365-2796.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 57.Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7(8):645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- 58.Cheng MH, Shum AK, Anderson MS. What's new in the Aire?. Trends Immunol. 2007;28(7):321–327. doi: 10.1016/j.it.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 60.Ramsey C, Winqvist O, Puhakka L, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11(4):397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 61.Kuroda N, Mitani T, Takeda N, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174(4):1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 62.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202(6):805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shum AK, DeVoss J, Tan CL, et al. Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Sci Transl Med. 2009;1(9):9ra20. doi: 10.1126/scitranslmed.3000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alimohammadi M, Björklund P, Hallgren A, et al. Auto-immune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med. 2008;358(10):1018–1028. doi: 10.1056/NEJMoa0706487. [DOI] [PubMed] [Google Scholar]
- 65.Cameron IT, O'Shea FC, Rolland JM, Hughes EG, de Kretser DM, Healy DL. Occult ovarian failure: a syndrome of infertility, regular menses, and elevated follicle-stimulating hormone concentrations. J Clin Endocrinol Metab. 1988;67(6):1190–1194. doi: 10.1210/jcem-67-6-1190. [DOI] [PubMed] [Google Scholar]