Abstract
Emerging evidence suggests that increases in activated T cell populations in adipose tissue may contribute toward obesity-associated metabolic syndrome. The present study investigates three unanswered questions: 1) Do adipose-resident T cells (ARTs) from lean and obese mice have altered cytokine production in response to TCR ligation?; 2) Do the extralymphoid ARTs possess a unique TCR repertoire compared with lymphoid-resident T cells and whether obesity alters the TCR diversity in specific adipose depots?; and 3) Does short-term elimination of T cells in epididymal fat pad without disturbing the systemic T cell homeostasis regulate inflammation and insulin-action during obesity? We found that obesity reduced the frequency of naive ART cells in s.c. fat and increased the effector-memory populations in visceral fat. The ARTs from diet-induced obese (DIO) mice had a higher frequency of IFN-γ+, granzyme B+ cells, and upon TCR ligation, the ARTs from DIO mice produced increased levels of proinflammatory mediators. Importantly, compared with splenic T cells, ARTs exhibited markedly restricted TCR diversity, which was further compromised by obesity. Acute depletion of T cells from epididymal fat pads improved insulin action in young DIO mice but did not reverse obesity-associated feed forward cascade of chronic systemic inflammation and insulin resistance in middle-aged DIO mice. Collectively, these data establish that ARTs have a restricted TCR-Vβ repertoire, and T cells contribute toward the complex proinflammatory microenvironment of adipose tissue in obesity. Development of future long-term T cell depletion protocols specific to visceral fat may represent an additional strategy to manage obesity-associated comorbidities.
The remarkable capacity of adipose tissue to expand and package lipids represents a critical adaptation to chronic caloric excess during obesity. Consequently, in extreme obesity, adipose tissue becomes one of the largest organs in the human body and constitutes up to 50% of total body mass. The chronic “sterile” inflammation during obesity, in the absence of any overt infection, leads to several ancillary disease states, which include type 2 diabetes, defective immunity, and several cancers (1–3). Excessive production of proinflammatory cytokines by expanded macrophage populations within the adipose tissue is an important contributor toward insulin resistance (4, 5). In addition to macrophages, recent studies indicate that several leukocyte subsets, including mast cells and T cells that reside in adipose tissue, regulate inflammation and insulin sensitivity (6–9). However, the precise role of adipose resident leukocytes in the development of obesity-associated comorbidities is incompletely understood.
Upon release from the thymus, the naive T cells have a greater proclivity to home in on secondary lymphoid organs or inductive sites of immune response where they are primed with APCs and assume an effector phenotype (10). The effector T cells participate in immune surveillance by preferentially localizing in the nonlymphoid tissues, which are major sites of initial exposure to infection. In addition, memory T cell response can be mounted despite the absence of secondary lymphoid organs (11). The presence of several immune cell subsets, in particular T cells within adipose tissue during obesity, is an intriguing phenomenon. Typically, fat has not been considered a peripheral site of adaptive immune response. Nonetheless, considering that effector T cell cytokines are required for activation of macrophages and expanded adipose tissue is known to harbor both of these cell types, T cells are implicated in adipose-derived inflammation and insulin resistance (12).
We have recently demonstrated that obesity reduces thymopoiesis and restricts the TCR repertoire in secondary lymphoid organs that suggests reduced immune surveillance (2). Interestingly, we observed an expansion of effector-memory (E/M) T cells and a reduction in naive T cells in spleen, which is consistent with the reduced ability of T cells to respond and clear influenza virus in obesity (13, 14). During the course of our studies, several groups (7, 8) reported the presence of T cells with an effector phenotype in fat and that obesity reduced the anti-inflammatory CD4+FoxP3+ T regulatory cells (9). However, whether the T cells from specific adipose depots produce effector cytokines upon TCR ligation and whether specific cytokine production is influenced by obesity remains to be established.
Considering that the primary function of adipose tissue is to regulate energy homeostasis, the presence of T cells in adipose depots may have a direct role in regulating the local proinflammatory state and insulin sensitivity. The long-term depletion of T cells in the whole animal has recently been shown to be an effective means of reversing insulin resistance in obesity (8). However, it remains unclear whether adipose-resident T (ART) cells or general T cell lymphopenia caused by T cell depletion affects insulin sensitivity. In this study, we have identified that s.c. adipose tissue (SAT) harbors a significantly higher frequency of naive CD4 and CD8 cells, whereas visceral adipose tissue (VAT) contains predominantly E/M phenotype CD4 and CD8 cells. Interestingly, VAT-resident T cells produced excessive proinflammatory cytokines post-TCR ligation. Marked differences in the TCR diversity were identified in VAT and SAT, which were significantly restricted by obesity. Last, to distinguish the effects of ART cells and T cells present in primary and secondary lymphoid organs and extralymphoid tissues in obese mice, we achieved specific depletion of T cells in epididymal visceral fat pad via intra-adipose injections of anti-CD3 F(ab′)2 Ab. Our data demonstrate that adipose T cells participate in regulating adipose tissue inflammation. Short-term depletion of T cells in visceral fat alone can partially reverses insulin resistance in early-stage obesity but does not impact systemic glucose homeostasis in middle-aged obese mice.
Materials and Methods
Mice and surgeries
Male C57BL/6 mice were fed ad libitum a high-fat diet consisting of 60% calories from fat (D12492i; Research Diets, New Brunswick, NJ) starting at 8 wk of age, and control male mice were fed a standard chow diet consisting of 4.5% fat (5002; LabDiet, Richmond, IN). The animals were individually housed and maintained on specific diets in specific pathogen-free barrier facility with a 12-h light/12-h dark cycle with free access to food and water.
For VAT-specific T cell depletion experiments, animals were prepared for surgery and anesthetized using isoflurane vapors (5% for induction), and surgery was performed in 2% isoflurane vapors. Briefly, the animals were placed in ventral recumbency, and a 1-cm midline incision was made caudal to the rib cage over the epididymal abdominal area. After piercing the underlying muscle layer, a 1-cm incision was made lateral to the spine, and the fat pad was carefully exteriorized and injected with the sterile F(ab′)2 anti-CD3 Ab (clone145-2C11, 100 μg; BioXcell, West Lebanon, NH) solution bilaterally. The adipose tissue was then placed back into the abdominal cavity, and the incision was closed with stainless steel wound clips. Mice recovered within 30 min post-surgery and displayed normal feeding and drinking behavior. All animal use was in compliance with the Institute of Laboratory Animal Research Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Use and Care Committee of the Pennington Biomedical Research Center.
Insulin tolerance test and glucose tolerance test
An insulin tolerance test (ITT) was conducted in mice by giving an i.p. injection of human insulin (0.8 U/kg body weight [bw]), and blood was collected using tail nicks for glucose measurement by using a glucometer at 10-, 20-, 40-, 60-, and 90-min post-insulin injections. The ITT was repeated after 4 h fasting in a total of 24 diet-induced obese (DIO) mice [n = 12 each for control and F(ab′)2 anti-CD3 Ab-treated groups] at noon. For glucose tolerance test (GTT), mice were fasted for 4 h, and sterile glucose (1.8 mg/g bw) was injected i.p., and blood glucose was measured at the indicated time points.
Preparation of stromal vascular fraction cells
The SAT (inguinal fat pad) and VAT (epididymal fat [eFat] pad) was collected to carefully exclude any lymph nodes. The adipose tissues from multiple DIO mice were pooled and enzymatically digested. After several washes with RPMI 1640 medium and hypotonic shock to lyse RBCs, the stromal vascular fraction (SVF) cell pellet was collected for FACS analysis and sorting. The s.c. fat in lean humans (body mass index [BMI] < 24) was collected from patients undergoing cosmetic liposuction surgeries. The abdominal s.c. tissue and omental fat from morbidly obese patients (BMI > 40) was collected during the bariatric surgery. The collection of human fat biopsies and analyses was performed under an approved Institutional Review Board protocol and informed consent obtained from study participants.
Flow cytometry
To identify naive and E/M T cells, the SVF cells were incubated with PerCP-conjugated anti-CD4, APC conjugated anti-CD8, PE-conjugated anti-CD62L, and FITC-conjugated anti-CD44 Abs. In addition, SVFs were used for intracellular staining of cytokines (IFN-γ–FITC and granzyme B-PE) after fixation and permeabilization according to the manufacturer’s instructions (eBioscience, San Diego, CA). All FACS Abs were purchased from eBioscience. The FACS analysis was performed on FACSCalibur, and cell sorting was conducted using FACSAria (BD Pharmingen, San Diego, CA). All the FACS data were analyzed by postcollection compensation using FlowJo (Tree Star, Ashland, OR) software.
Immunohistochemistry and confocal micoscopy
The VAT was collected and fixed in 4% buffered paraformaldehyde and paraffin embedded. After nonspecific sites were blocked, the biotinylated anti-CD3 Ab was used to perform immunohistochemistry as described previously (15, 16). For confocal microscopy, fresh adipose tissue was stained with neutral lipid dye (Bodipy from Invitrogen, Carlsbad, CA) anti-CD3– Alexa Fluor 488, and nuclei were labeled with DAPI. The images were acquired using the Zeiss 510 Meta multiphoton confocal microscope.
Real-time RT-PCR and signal-joint TCR rearrangement excision circle analysis
The real-time PCR and signal-joint TCR rearrangement excision circle (TREC) analysis was performed as described previously (2, 15, 16). The standard curves for murine TRECs were generated by using δRec ψJα TREC PCR product cloned into a pCR-XL-TOPO plasmid, a gift from Dr. G. D. Sempowski (Duke University Medical Center, Durham, NC).
Vβ TCR spectratyping analysis
A FAM-labeled nested constant β-region primer was used in combination with 24 multiplexed forward murine Vβ-specific primers to measure the CDR3 lengths in CD4 and CD8 as described previously (2, 15, 16). Each peak was analyzed and quantified with ABI PRISM GeneScan analysis software (Applied Biosystems, Foster City, CA), based on size and density. Data were used to calculate the area under the curve for each Vβ family using BioMed Immunotech software (Tampa, FL).
T cell culture and cytokine analysis
The adipose-resident CD3+ T cells were cultured at the concentration of 1 × 105 cells/well in 96-well plates and stimulated bead-bound anti-CD3 and CD28 Abs for a period of 24 h as described previously (17). The T cell supernatants and sera were analyzed for cytokine production using bead-based multiplex assay (Bioplex; Bio-Rad, Hercules, CA), according to the manufacturer’s instructions.
Statistical analyses
The results were expressed as the mean ± SEM. The differences between means and the effects of treatments were determined by one-way ANOVA using Tukey’s test (Sigma Stat, Aspire Software International, Ashburn, VA), which protects the significance (p< 0.05) of all pair combinations.
Results
ART cells in obesity
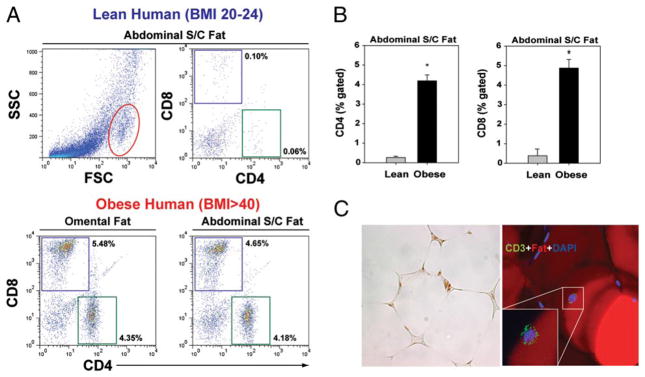
Our initial studies focused on the identification of T cell populations in human SAT derived from lean healthy females (BMI<24; n = 6) undergoing cosmetic liposuction surgery and in omental fat and abdominal s.c. fat from severely obese (BMI>40; n = 6) subjects undergoing bariatric surgery. The FACS analysis of SVF cells in enzymatically digested adipose tissue revealed that obesity significantly increases the frequency of CD4 and CD8 cells in adipose tissue (Fig. 1A, 1B) in humans. The immunohistochemical and confocal analysis of human omental fat revealed that T cells are present in close apposition with adipocytes and not simply localized in the vessels of adipose tissue (Fig. 1C).
FIGURE 1.
ART cells in humans. A, Representative FACS dot plots (n = 6) show the frequency of CD4 and CD8 cells in SVF cells of adipose depots in lean (BMI<25; n = 6) and obese (BMI>40; n = 5) female subject. The representative FSC dot plot depicts the overall distribution of SVF cells derived from digested human SAT. The CD4 and CD8 cells were found to be present in the gated region (outlined in red). B, Extreme obesity in humans significantly (*p<0.05) increases the frequency of CD4 and CD8 cells in abdominal s.c. fat. C, Representative section of abdominal SAT from an obese insulin-resistant patient stained with anti-CD3 Ab, adipocytes were labeled with Bodipy, and nuclei are counterstained with DAPI (original magnification x40). FSC, forward light scatter; SSC, side scatter (of light).
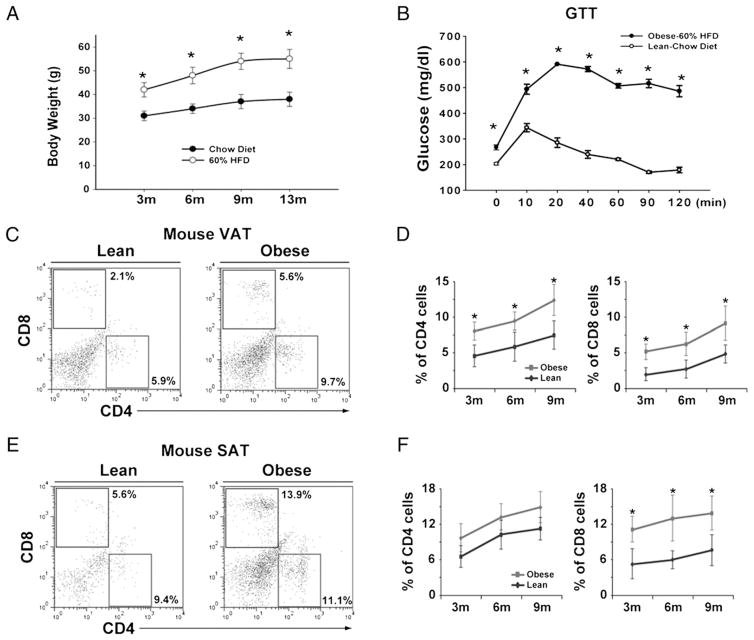
The impact of degree and duration of adiposity on T cell distribution in SAT (inguinal fat pad) and VAT (eFat pad) was further analyzed in mouse models of dietary obesity. We specifically maintained male C57BL/6 mice on 60% high-fat diet until 13 mo to mimic chronic middle-aged obesity. As expected, compared with chow-fed animals, the DIO mice at 3, 6, 9, and 13 mo had significantly higher body weight (Fig. 2A). Consistent with development of obesity, the GTT revealed that 6-mo-old DIO mice were severely insulin resistant (Fig. 2B). We observed that both CD4 and CD8 cells increased in VAT, whereas in SAT, only CD8 T cells were elevated (Fig. 2C–F).
FIGURE 2.
ART cells in DIO mice. A, The body weights of male C57BL/6 mice (n = 12–18 mice/time point) on chow and 60% HFD. B, GTT in 6-mo-old chow- and HFD-fed DIO mice (n = 6). C–F, The T cell distribution in SVF from SAT and VAT depots derived from male C57BL/6 mice fed on chow (lean) and HFD (obese). Each time point depicts percent gated CD4 and CD8 cells from adipose tissue of at least six to eight mice. All data are represented as mean (SEM); *p<0.05, statistical significance. HFD, high-fat diet.
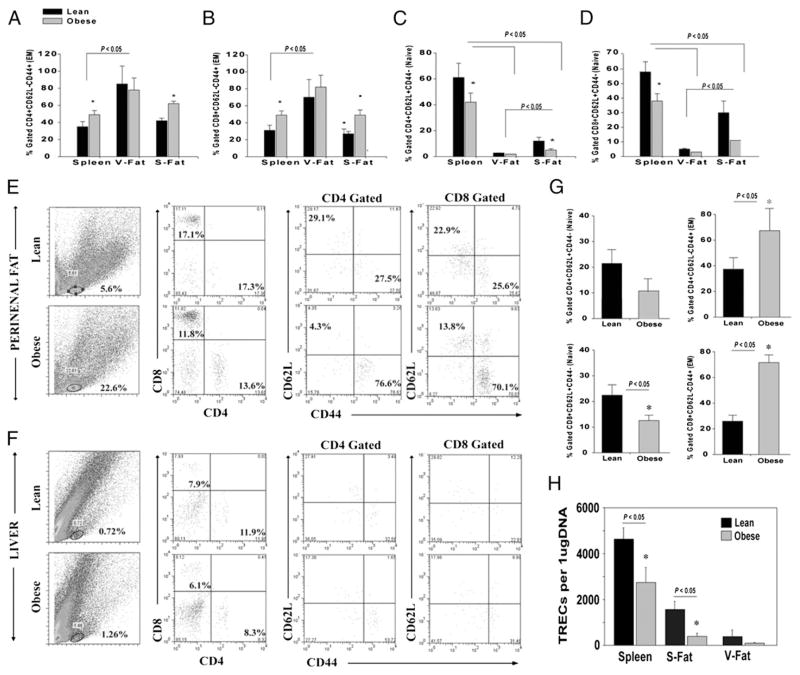
On the basis of our data that progressive adiposity increases ARTs, we next determined the impact of severe obesity (associated with insulin resistance) on the distribution of naive and E/M cells in both VAT and SAT of DIO middle-aged mice. Typically, nonlymphoid organs preferentially harbor E/M phenotype T cells, regardless of the site where they were first presented with an Ag (10, 11, 18). Localization of E/M T cells to mucosal surfaces and peripheral sites is therefore a key feature of effective immune surveillance. We found that compared with spleen, the majority of CD4 and CD8 cells in VAT (eFat pad) from 9-mo-old DIO mice were E/M cells (Fig. 3A, 3B). Furthermore, dietary obesity increased the frequency of CD4 and CD8 E/M ARTs (CD62L−CD44+) in SAT (Fig. 3A, 3B). Surprisingly, we detected a significantly higher frequency of naive CD4 and CD8 cells (CD62L+CD44−) in the SAT of lean mice, which was significantly reduced by obesity (Fig. 3C, 3D). We also examined the perirenal visceral fat pad and found that obesity significantly increased the frequency of CD4 and CD8 E/M cells, whereas liver did not display greater frequency of T cells in 5-mo-old DIO mice (Fig. 3E–G).
FIGURE 3.
Obesity reduces naive and expands E/M T cells in adipose depots. The frequency of CD4 (A) and CD8 E/M (CD62L−CD44+) (B) cells in spleen, eFat (V-Fat), and inguinal fat depot (S-Fat) of 9-mo-old lean (chow fed) and DIO mice (n = 6–9). The frequency of CD4 (C) and CD8 (D) naive (CD62L+CD44−) cells in spleen and adipose depots of 9-mo chow and DIO mice. E, Distribution of CD4 and CD8 naive and E/M T cells in perirenal fat of 5- to 6-mo-old chow and DIO male mice (n = 6). The forward light scatter/side scatter (of light) gate represents location of T cells in SVF cells in collagenase-digested murine adipose tissue. F, The representative FACS plot depicting the frequency of T cells in the liver of 5-mo-old chow and DIO male mice. Because of the few T cells in liver, CD4- and CD8-gated T cells did not present sufficient events to discriminate naive and E/M populations. G, The naive and E/M T cell distribution in perirenal fat. All data are represented as mean (SEM); *p<0.05, statistical significance. H, The CD4+ ARTs from epididymal (V-Fat), inguinal fat (S-Fat), and spleen of 9-mo-old lean and DIO mice were purified by FACS sorting and used to prepare DNA and signal-joint TREC levels were analyzed using quantitative PCR analysis. S-Fat, s.c.-fat; V-Fat, visceral-fat.
Considering that nonlymphoid tissues lacks high endothelial venules and specific APCs, it was initially hypothesized that naive T cells do not typically reside or traffic to peripheral nonlymphoid sites (18). Because the CD62L+CD44− T cells are not the only markers of naivety, it was necessary to confirm that ARTs in SAT of lean mice are truly naive. Considering that the naive T cells of recent thymic origin can be determined by quantitation of signal-joint episomal DNA called TRECs, a product of VDJ recombination in thymus (19, 20), we FACS sorted the ART cells from SVF and analyzed the TREC levels. These analyses determined that compared with VAT, the SAT from lean mice contains higher numbers of naive T cells that exhibited a significant decline during obesity (Fig. 3H).
Obesity restricts Vβ-TCR diversity of ART cells
Central to the function of T cells is their ability to recognize and respond to a broad array of Ags via the expression of a diverse TCR repertoire. The sequence diversity of the TCR, CDR1, and CDR2 loops is encoded by the V sequence of the gene, which is germline encoded. In contrast, the CDR3 region of TCR is assembled through the rearrangement of α- and β-chains by recombination of noncontiguous V, D, and J gene segments in thymus. Thus, the measurement of length and distribution of CDR3 hypervariable region of Vβ-chains allows a comprehensive analysis of TCR diversity (21–23). It is currently not known whether adipose T cells in s.c. or visceral fat have a diverse TCR-Vβ repertoire.
We conducted TCR spectratyping by using 24 Vβ primers that span almost the entire TCR b CDR3 region, allowing a broader estimate of TCR diversity of ARTs and their overall complexity. This procedure is based on PCR amplification of individual Vβ alleles using primers that span Cβ region and others that are specific to individual Vβ segments. Subsequently, this PCR product is amplified with runoff reaction by Jβ-specific FAM-labeled primer. Thus, the profile of CDR3 lengths for each Vβ-Jβ combinations in ARTs can be determined. The diversity of TCR is dependent on the rejoining of the VDJ genes and the random insertion of nucleotides coding for 6–14 aa. In newly generated naive T cells, the relative frequencies of insertions follow a Gaussian distribution, and expansion of activated T cells results in skewing of the repertoire and a departure from the Gaussian pattern and oligoclonal T cell expansions are reflected by the emergence of distinct peaks (22).
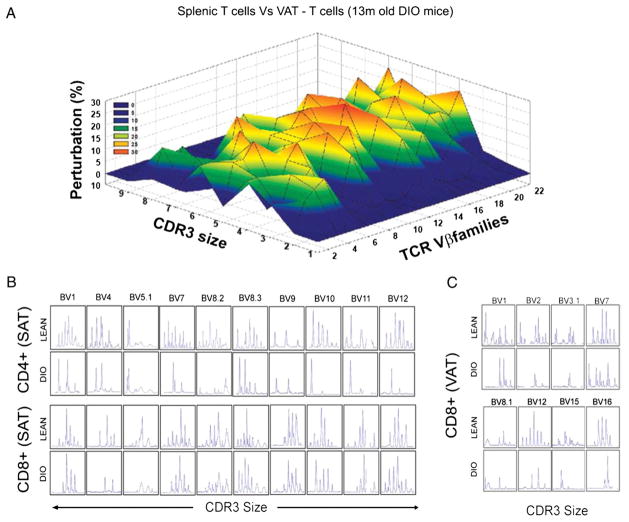
We performed the CDR3 polymorphism analysis in middle-aged 13-mo-old DIO and lean mice and compared the TCR diversity of FACS-sorted CD4 and CD8 cells in spleen, VAT, and SAT. The Gaussian distribution profiles of CDR3 lengths of CD4 cell were translated into probability distributions as functions of the area under the curve as described previously (2, 16, 23). Our data revealed that compared with splenic CD4 cells, the CD4 ART cells from VAT had marked perturbations and restriction of all TCR Vβ families (Fig. 4A).
FIGURE 4.
Obesity restricts TCR diversity of ART cells. A, The CD4 cells were sorted from spleen and VAT of chow-fed and high-fat fed DIO mice (13 mo old) to prepare cDNA that was used for TCR spectratyping. The three-dimensional graph depicts perturbation in CD4 ARTs in VAT compared with spleen. Each line crossing on the y-axis of the landscape denotes change from splenic CD4 cell-specific CDR3 length or size (x-axis) of a particular Vβ family (z-axis). The perturbations in TCR diversity are shown as landscape surfaces, in which smooth (blue) landscapes show an unchanged TCR diversity. The mountains (in green, yellow, and orange) depict area under the curve of amplified peaks of CDR3 lengths. B and C, Effect of obesity on the CDR3 lengths of altered Vβ families of CD4 and CD8 ARTs in SAT and VAT. The data are representative of pooled ARTs from a total of 12 mice.
Given the significantly high frequency of naive ARTs in SAT, we also investigated the CD4 and CD8 TCR Vβ repertoire in T cells derived from SAT inguinal fat pads of 13-mo-old obese insulin-resistant mice. Consistent with higher naive phenotype of ARTs, we found that SAT-derived CD4 and CD8 ARTs exhibited a greater polyclonal repertoire than VAT (Fig. 4B). Interestingly, obesity caused a striking loss of SAT-ART cell diversity in Vβ1, -4, -5.1, -7, -8.2, -8.3, -9, -10, -11, and -12 (Fig. 4C, 4D). In addition, we observed that in 13-mo-old lean mice, CD8 ARTs from VAT exhibited polyclonal TCR repertoire for Vβ1, -2, -3.1, -7, -8.1, -12, -15, and -16, which was significantly compromised in age-matched obese animals (Fig. 4C). Taken together, these data demonstrate that compared with “conventional CD4” T cells in the spleen, the ARTs are distinct and possess a unique TCR profile that is severely restricted in obesity.
Obesity increases proinflammatory cytokine production from ARTs
Considering that successful adaptive immune response is a highly energy intensive event, the metabolic and immune function are functionally linked and reciprocally regulated to impart an advantage to the host (24). However, the chronic energy excess during obesity and the resultant metabolic overload leads to inflammation of adipose tissue that is coupled with lipotoxicity, insulin resistance, and type 2 diabetes mellitus (1). Recent studies suggest adipose inflammation in obesity is associated with an increase in T cells in fat (7–9). However, it remains unclear whether obesity alters the TCR-mediated cytokine production from CD4 and CD8 cells derived from SAT and VAT.
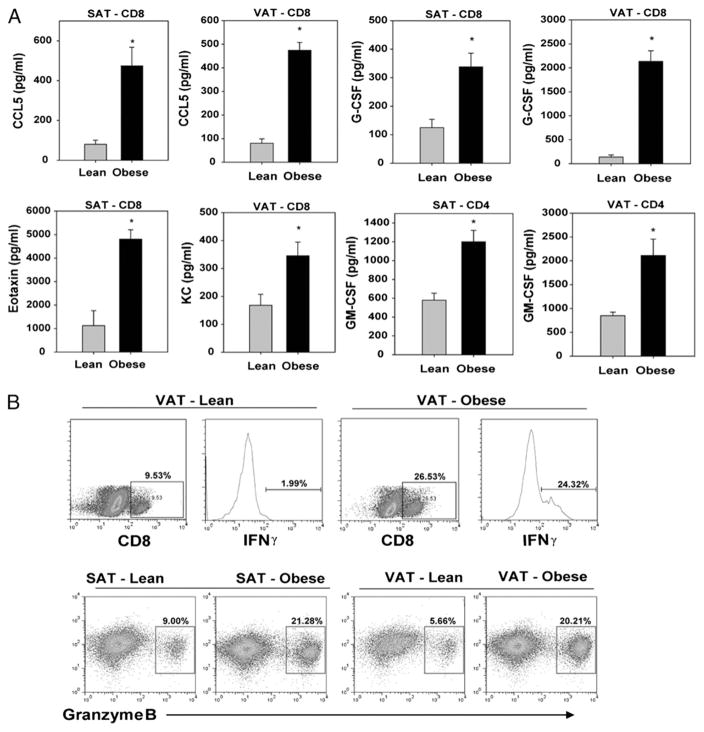
We sorted the CD4 and CD8 cells (>95% purity) from 13-mo-old lean and obese mice and stimulated the ARTs via anti-CD3 and anti-CD28 bead-bound Abs to mimic TCR ligation and costimulatory signals. We found that during obesity, the CD4 ARTs from both the SAT and VAT produced significantly greater levels of GM-CSF (Fig. 5A). Interestingly, we detected that obesity induced marked alterations in the proinflammatory cytokine production from CD8 ARTs. We found that CD8 ARTs produced higher levels of CCL5/RANTES and G-CSF in SAT and VAT of obese mice (Fig. 5A). Furthermore, although SAT-derived CD8 T cells from obese mice specifically produced higher eotaxin, the VAT-origin CD8 T cells secreted higher KC (Fig. 5A). The levels of IL-2, IL-17, and Th2 cytokines, IL-4, IL-5, and IL-10 were below the sensitivity of the assay (data not shown).
FIGURE 5.
Obesity induces production of proinflammatory mediators from ART cells. A, The CD4 and CD8 cells from 13-mo-old mice were sorted using FACSAria to 95% purity and stimulated via TCR ligation using bead bound anti-CD3 and anti-CD28 Ab for 24 h. The CCL5/RANTES, G-CSF, GM-CSF, eotaxin/CCL11, and KC/IL-8 were analyzed in culture supernatants using bead-based multiplex assays. B, The dot plots and histogram show representative examples of intracellular staining for IFN-γ and granzyme B on CD8-gated ARTs derived VAT and SAT of lean and DIO mice. The FACS data are representative of pooled ARTs from four mice each (13 mo old). The cytokine assays were performed on ARTs sorted from pooled adipose tissue of 12 mice each, and the experiment was repeated three times. All data are represented as mean (SEM); *p<0.05, denotes statistical significance.
In addition, the intracellular cytokine staining and FACS analysis revealed that similar to our in vitro data, obesity increased the in vivo frequency of IFN-γ–expressing CD8 ARTs in VAT (Fig. 5B). We also analyzed the expression of granzyme B, a neutral serine protease that is typically stored in lytic granules of cytotoxic CD8 T cells and is directly implicated in inducing apoptosis of target cells during an active immune or autoimmune response (25). Interestingly, we found that obesity markedly increased the frequency of granzyme B+ CD8 ARTs in both SAT and VAT (Fig. 5B). Our findings of increased expression of granzyme B in ARTs and higher proinflammatory cytokine/chemokines production during obesity are consistent with an ongoing inflammatory insult, bystander tissue damage, and adipocyte death in the adipose organ.
Elimination of ARTs in visceral fat enhances insulin sensitivity during early-stage obesity
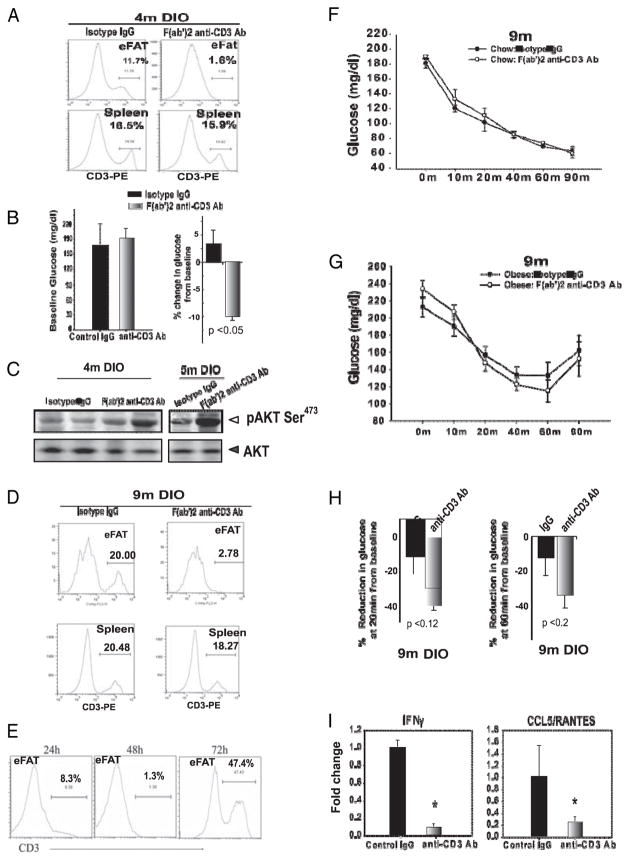
Recent studies found that systemic elimination of T cells in obese mice can result in improvement of insulin sensitivity (7, 8). However, it remains unclear whether the ART cells in visceral fat contribute toward the obesity-associated peripheral inflammation and insulin resistance. The eFat pads were surgically exteriorized and intra-adipose, injection of F(ab′)2 anti-CD3 Ab was performed to eliminate T cells without the induction of a cytokine storm (26). In 4-mo-old DIO mice, our protocol achieved specific and efficient T cell depletion in e-fat within 48 h and did not disturb T cell homeostasis in the spleen (Fig. 6A) or thymus (data not shown). No significant differences in fasting glucose levels of e-fat T cell-deficient and control obese mice were detected. Interestingly, 10 min after insulin injection, the e-fat T cell-deficient obese mice had a significantly (p<0.05) greater reduction in blood glucose levels (Fig. 6B). The metabolic effects of insulin action are dependent on P13K-AKT signaling (27). Considering that obesity-induced inflammation is known to impair insulin sensitivity, we investigated the Ser473 phosphorylation of AKT in adipose tissue of e-fat T cell-deficient obese mice. Consistent with improvement of insulin action in T cell-depleted mice, insulin-induced AKT phosphorylation was improved in e-fat T cell-depleted 4- to 5-mo-old DIO mice (Fig. 6C).
FIGURE 6.
Short-term depletion of ARTs in epididymal adipose tissue (VAT) partially reverse insulin resistance. A, The abdominal fat pads in 4-mo-old DIO mice were exteriorized and bilaterally injected with control IgG or anti-CD3 F(ab′)2 Ab (100 μg), and representative histogram plot of T cell depletions 48 after Ab injection in eFat, thymus, and spleen are depicted. B, Fasting blood glucose values at baseline and percent reduction in blood glucose after i.p. insulin injection (0.8 mU/g bw). C, The immunoblot analysis for AKT 10 min after insulin injection in 4- to 5-mo-old DIO mice. D, eFat-specific elimination of T cells in after 48 h of Ab treatment. E, Time course of T cell depletion within VAT. ITT in 9-mo-old chow-fed (F) and 9-mo-old DIO (G) mice treated with isotype control or anti-CD3 F(ab′)2 Ab. H, Percent reduction in blood glucose at 20 and 40 min after i.p. insulin injection in 9-mo-old DIO mice. I, Real-time PCR analysis of IFN-γ and CCL5/RANTES in VAT 48 h post–intra-adipose T cell depletion. Data are represented as mean (SEM) from 10–12 mice/group.
We next examined whether elimination of T cells in 9-mo-old middle-aged nonobese and DIO mice impacts insulin sensitivity. Consistent with our earlier data, intra-adipose delivery of F(ab′)2 anti-CD3 Ab in e-ffat caused efficient elimination of T cells without decreasing T cell numbers in spleen (Fig. 6D) and thymus (data not shown). After the single injection of F(ab′)2 anti-CD3 Ab in the eFat, the kinetics and efficacy of T cell depletion was evaluated at 24, 48, and 72. Our protocol eliminated T cells in the VAT within 48 h; however, 3–4 d post–intra-VAT Ab delivery, the ARTs homeostatically expand with a dramatic increase in T cell numbers in e-fat (Fig. 6E). Similar kinetics of T cell depletions were achieved in 9-mo-old chow-fed mice (data not shown). The ITT revealed that short-term elimination of e-fat ART cells in 9-mo-old nonobese (Fig. 6F) and DIO mice did not impact systemic glucose homeostasis (Fig. 6G). In addition, 9-mo-old DIO e-fat T cell-deficient mice did not show statistically significant differences in the degree of drop of blood glucose in response to insulin (Fig. 6H). Importantly, the ART depletion reduced the VAT expression of T cell-derived IFN-γ and CCL5/RANTES. These data provide the first conclusive evidence that although the ARTs contribute toward a proinflammatory milieu in adipose tissue, the short-term elimination of ARTs in epididymal visceral pad alone is not sufficient to reverse the feed forward cascade of metabolic syndrome in chronic advanced obesity.
Discussion
Although a clear role of adipose tissue in immune surveillance has not been identified, expanded adipose tissue in obesity harbors several activated immune cell subsets, including T cells and macrophages. It is established that obesity-associated chronic inflammation causes insulin resistance. However, the provenance of adipose tissue-derived inflammation and biological relevance of ARTs in pathophysiology of obesity is not fully understood. In this paper, we demonstrate that although the majority of T cells in fat are of E/M phenotype, the SAT contains significant numbers of naive T cells. Obesity was found to increase the frequency of E/M and to reduce the naive T cells in adipose depots. Furthermore, compared with the ARTs of nonobese animals, the T cells derived from s.c. and visceral adipose depots of obese mice produced excessive proinflammatory cytokines and chemokines and displayed a restricted TCR diversity. Interestingly, in early stage of obesity, removal of T cells in e-fat enhanced insulin sensitivity. However, when ARTs were transiently depleted in eFat without affecting peripheral lymphoid organs, the chronic middle-aged obesity-associated peripheral inflammation and insulin resistance could not be reversed.
Several studies have demonstrated the localization of E/M T cells adipose tissue of obese mice (7–9). However, our findings that naive T cells are present in SAT and their number is reduced in obesity are intriguing and unexpected. This previously unrecognized localization of naive T cells specifically to SAT but not VAT may suggest that naive ARTs participate in immune surveillance in adipose depots and may come in contact with as-of-yet undetermined adipose-specific or developmentally associated Ags. Presence of naive T cell in SAT is counterintuitive because these cells require Ag presentation to function, and unlike dendritic cells, tissue-resident macrophages are not thought to be potent Ag presenters. Interestingly, it has been shown that even 81 d after a vesicular stomatitis virus infection, tetramer-bound Ag-specific T cells are present in high frequency in fat pads (28). The restricted TCR repertoire and oligoclonal profiles of ARTs in obese mice are suggestive of ongoing Ag exposure (unavoidable exposure to environmental Ags in specific pathogen-free mouse colonies) and T cell activation. Consistent with active adipose-immune interactions, recent data demonstrate that adipose tissue also harbor Lin−Sca1+Kit+ lymphoid progenitors cells and can participate in extramedullary hematopoiesis (29). Although speculative, taken together with our data, these findings raise the possibility that adipose depots may participate in certain aspects of adaptive immune response or may even serve as a potential reservoir of clonally expanded Ag-specific E/M T cells. Considering that obesity alters T cell homeostasis (2) and also increases the risk and severity of several infections (2, 3, 13, 14), restriction of ART cell repertoire diversity in extralymphoid tissues may also contribute to deficits in immune surveillance. Furthermore, although no organ or adipose tissue-specific autoantibodies have so far been detected in fat, the likelihood that autoimmune process contributes toward restricted Vβ TCR repertoire remains to be determined.
Our data that ARTs are key cellular source of eotaxin in obesity are consistent with previous findings showing that obesity increases the adipose tissue expression of this chemokine in humans (30). Furthermore, high expression of granzyme B in ARTs is indicative of a direct role of this neutral serine protease in inducing adipocyte apoptosis and tissue damage that is typically observed in advanced obesity (31). In addition, it is known that in vitro, an activated type 1 macrophage phenotype can be induced by GM-CSF (32). Taken together with our data that purified ARTs in obesity produce elevated levels of CCL5, IFN-γ, GM-CSF, G-CSF, and IL-8 provides a mechanistic insight into the origin of adipose tissue inflammation. These findings suggest an autoinflammatory loop where ART cells derived cytokines may lead to M1 macrophage polarization/activation and contribute toward chronic inflammation in obesity.
Consistent with our findings, a recent study demonstrates that epididymal adipose tissue explant cultures derived from obese mice produce higher levels of IFN-γ (33). Accordingly, the obese IFN-γ−1− mice are protected from obesity-induced adipose tissue inflammation and insulin resistance (34). Considering that chronic obesity is a complex multisystem disorder, several neuroendocrine and metabolic factors can affect ART cells (24). Notably, leptin, a predominant adipokine, is known to directly affect the T cells through the functional long form of leptin receptor and induce proinflammatory and Th1 response (17, 35–37). It is likely that ART cells are exposed to several-fold higher concentrations of adipocyte-derived leptin than lymphoid-resident T cells. Therefore, it is plausible that obesity-associated leptin may promote proinflammatory Th1 phenotype of ARTs. However, typically obese mice and humans develop leptin resistance, which is reflected in an inability of higher leptin levels to reduce food intake or affect energy balance or thermogenesis (38). Although possible, it is unclear whether T cells and immune compartment during obesity are sensitive to leptin’s proinflammatory action. Importantly, and consistent with our findings, clinical evidence suggests that obesity-induced inflammation is associated with poor outcomes from solid organ transplantation, bone marrow transplantation, and increased severity of grafts versus host disease (39, 40), suggesting an important role for adipose-immune interactions.
The visceral fat harbors E/M ARTs and is known to be a key organ that plays an important role in obesity-associated inflammation and insulin resistance. To determine a cause-effect relationship between adipose T cell in visceral fat and insulin resistance, we performed eFat depot-specific T cell depletions without inducing general lymphopenia. Our data demonstrate that in early-stage obesity, depletion of adipose tissue T cells in visceral fat can partially reverse insulin resistance. However, short-term ablation of T cells in visceral fat is not sufficient to reverse systemic inflammation and insulin resistance in chronically obese middle-aged mice. Importantly, recent studies have demonstrated that systemic depletion of T cells via F(ab′)2 anti-CD3 lasting for 9 wk can effectively reverse insulin resistance in 14-wk-old high-fat–fed obese mice (7, 8). Our data further underscore that in chronic obesity, long-term T cell depletion in adipose tissue may be necessary to reverse inflammation and insulin resistance. However, it is well recognized that lymphopenia induced by Ab-mediated T cell depletion causes a homeostatic proliferation of lymphocytes that is primarily driven by self–MHC-peptide interactions and induction of proliferative γ-chain cytokines such as IL-7 and IL-15 (41). Furthermore, the T cells emerging after the aftermath of generalized lymphodepletions exhibit an E/M phenotype and contribute toward resistance to tolerance induction in autoimmune diseases and exhibit proinflammatory phenotype (42). Consistent with this, 72 h after a single injection of F(ab′)2 anti-CD3 in visceral fat, we observed a dramatic increase in ARTs in Ab-treated mice. Given that E/M T cells are relatively resistant to apoptosis and have a greater homeostatic proliferation rate than the naive T cells (43), the lymphodepletion protocols, unless sustained and targeted, may have limited clinical use over existing approaches for management of inflammation and treatment of insulin resistance in a chronic disorder such as obesity.
In conclusion, we provide evidence that obesity reduces naive and expands the E/M phenotype of ARTs that contributes toward adipose tissue inflammation and insulin resistance. We determined that oligoclonal ART expansion and adipose depot-specific restriction of TCR Vβ diversity during obesity is reflective of reduced peripheral immune surveillance. Specific elimination of T cells within e-fat partially reverses insulin resistance in early-stage obesity. Considering a complex immunological profile of adipose tissue in chronic obesity, our findings suggest that in addition to T cells, a multipronged adipose tissue leukocyte targeting approach may lead to reduced adipose inflammation and improvement of related comorbidities.
Acknowledgments
This work was supported by a Two-Year Pilot and Feasibility grant from the Clinical Nutrition Research Unit (National Institutes of Health Grant P30 DK072476 to V.D.D.). The research in the Laboratory of Neuroendocrine-Immunology is supported in part by the Coypu Foundation and National Institutes of Health Grant AG031797R01. The present work used the Genomics and Computational Biology and Bioinformatics Core facilities supported by National Institutes of Health Grant 1 P20 RR02/1945 and the Cell Biology and Bioimaging Core Facility of the Pennington Center of Biomedical Research Excellence (National Institutes of Health Grant P20 RR-021945) and the Clinical Nutrition Research Unit (National Institutes of Health Grant P30 DK072476).
We thank Dr. David Hildeman in the Department of Immunobiology, Cincinnati Children’s Hospital Medical Center (Cincinnati, OH), and Dr. Eric Ravussin at the Pennington Biomedical Research Center for thoughtful discussions and presubmission review of the manuscript. We also thank Steven Bond and Gang Yu for expert technical assistance, Dr. Barry Robert for mouse surgeries, and Marilyn Dietrich for FACS sorting.
Abbreviations used in this paper
- ART
adipose-resident T cell
- BMI
body mass index
- bw
body weight
- DIO
diet-induced obese
- e-fat
epididymal fat
- E/M
effector-memory
- FSC
forward light scatter
- GTT
glucose tolerance test
- HFD
high-fat diet
- ITT
insulin tolerance test
- SAT
s.c. adipose tissue
- S-Fat
s.c.-fat
- SSC
side scatter (of light)
- SVF
stromal vascular fraction
- TREC
TCR rearrangement excision circle
- VAT
visceral adipose tissue
- V-Fat
visceral-fat
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. J Am Med Assoc. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 4.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARg controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 8.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 11.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Lefrançois L, Cauley LS, Harmsen AG, Lund FE, Randall TD. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15:846–847. doi: 10.1038/nm0809-846. [DOI] [PubMed] [Google Scholar]
- 13.Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci USA. 2007;104:20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 15.Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol. 2009;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefrançois L, Masopust D. T cell immunity in lymphoid and non-lymphoid tissues. Curr Opin Immunol. 2002;14:503–508. doi: 10.1016/s0952-7915(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 19.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 20.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 21.Pannetier C, Cochet M, Darche S, Casrouge A, Zöller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor b chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 23.Gorochov G, Neumann AU, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debré P. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 24.Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol. 2008;84:882–892. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Koning PJ, Kummer JA, Bovenschen N. Biology of granzyme M: a serine protease with unique features. Crit Rev Immunol. 2009;29:307–315. doi: 10.1615/critrevimmunol.v29.i4.20. [DOI] [PubMed] [Google Scholar]
- 26.Renders L, Valerius T. Engineered CD3 antibodies for immunosuppression. Clin Exp Immunol. 2003;133:307–309. doi: 10.1046/j.1365-2249.2003.02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virkamäki A, Ueki K, Kahn CR. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 29.Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, Kim I, Koh GY. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115:957–964. doi: 10.1182/blood-2009-05-219923. [DOI] [PubMed] [Google Scholar]
- 30.Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, Corry DB, Ballantyne CM. Eotaxin and obesity. J Clin Endocrinol Metab. 2006;91:256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- 31.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 32.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strissel KJ, Defuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity. doi: 10.1038/oby.2010.1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-g, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 36.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 37.Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- 38.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 39.Fuji S, Kim SW, Yoshimura K, Akiyama H, Okamoto S, Sao H, Takita J, Kobayashi N, Mori S Japan Marrow Donor Program. Possible association between obesity and posttransplantation complications including infectious diseases and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:73–82. doi: 10.1016/j.bbmt.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Olarte IG, Hawasli A. Kidney transplant complications and obesity. Am J Surg. 2009;197:424–426. doi: 10.1016/j.amjsurg.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 41.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neujahr D, Turka LA. Lymphocyte depletion as a barrier to immunological tolerance. Contrib Nephrol. 2005;146:65–72. doi: 10.1159/000082066. [DOI] [PubMed] [Google Scholar]
- 43.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]