Abstract
Little is known about the occurrence of individual variation in sexual behavior and how maternal nutrition can affect this variation. We tested the hypothesis that male offspring of female meadow voles, Microtus pennsylvanicus, that were 30% food restricted (FR) during days 1–7 of lactation (FR 1–7), days 8–14 of lactation (FR 8–14), or late days 15–21 of lactation (FR 15–21) lactation show persistent, negative effects on their sexual behavior as adults relative to male offspring of females that were not food restricted. We measured three components of sexual behavior, attractivity, proceptivity and receptivity, beginning when the males were 98 days of age. Food restriction during middle lactation (FR 8–14) but not during early (FR 1–7) and late lactation (FR 15–21) was sufficient to induce adult male voles to produce anogenital marks that were not as attractive as those produced by control males. Food restriction during lactation did not affect the proceptive behavior of male voles but did affect their receptivity. Only 4 of 12 FR 8–14 male voles mated compared to 9 of 12 FR 1–7 males, 8 of 12 FR 15–21 males, and 8 of 11 control males. However, no differences existed in their copulatory behavior among the males that did mate. The body weight of FR 1–7 and FR 8–14 males was lower than that of FR 15–21 and control males when they were between 22 days of age (weaning) and 48 days of age (puberty) but was similar when the males were 98 days of age. Food intake was similar for the FR and control males between day 22 and day 98. It remains unclear, however, if this type of maternal effect represents strategic programming of offspring behavior in response to the environment experienced by mothers or is a product of developmental processes of food restriction prior to weaning (Forstmeier et al. 2004).
Keywords: food restriction, maternal effects, phenotype, sexual behavior, voles
Introduction
Individual differences in phenotype have received much attention in the literature, however, little is known about the proximate causes of this variation (Forstmeier et al. 2004). Recently, maternal effects have been widely recognized as a mechanism for phenotypic responses to environmental heterogeneity (Bernardo 1996; Mousseau & Fox 1998a). Thus, maternal effects may influence an offspring’s fitness (Mousseau & Fox 1998a, b; Gendreau et al. 2005). Several studies have shown that the nutritional status of dams during pregnancy and lactation can alter the phenotype of their offspring in many species (Passos et al. 2000; Kerr et al. 2007). Maternal malnutrition as a result of food deprivation or restriction not only affects birth weight or growth and development of offspring (Woodall et al. 1996; Teixeira et al. 2002), but also the physiology and behavior of offspring. Among mice and rat-like hamsters, food restriction during pregnancy resulted in dams producing sons that had lower body weight and lower social status relative to those of dams that were not food restricted (Meikle & Thornton 1995; Meikle et al. 1995; Liang et al. 2004). Maternal food restriction or a diet low in protein can also affect the reproductive physiology of male offspring, usually by delaying the onset of puberty (Engelbregt et al. 2000; Da Silva et al. 2001; Leonhardt et al. 2003; Guzman et al. 2006).
Most interesting, however, is that maternal food-restriction can have persistent effects on the reproduction of male offspring when they reach adulthood. Male rat-like hamster (Cricetulus triton) dams that were food restricted during gestation had smaller gonads and lower gonadal steroid hormone titers relative to those of offspring of control dams (Liang et al. 2004). In rats (Rattus norvegicus), maternal protein restriction during pregnancy and lactation decreased sperm count and fertility in male offspring (Zambrano et al. 2005). Food restriction during pregnancy resulted in dams producing sons that had lower body weight and lower social status relative to those of dams that were not food restricted in mice (Mus musculus) (Meikle & Thornton 1995; Meikle et al. 1995) and rat-like hamsters; Liang et al. 2004). A general consensus of the literature suggests that food restriction of dams during lactation negatively impacts the morphology, physiology, and behavior of their sons, and that these long-term effects are expressed in offspring in the next generation.
A common feature of these and similar studies on the behavior of offspring raised by nutritionally-challenged females was that food availability was reduced during the entire gestation and/or lactation period. This represents an extreme condition and females facing such low food availability may not mate or become pregnant (Sabau & Ferkin 2013a). Many rodents, however, could experience acute food restriction at different times during lactation (Batzli 1985; Bronson 1989). For example, female meadow voles, Microtus pennsylvanicus, voles become relatively sedentary prior to parturition and during the first days of their 21-day lactation (Madison 1980, 1985; Keller 1985; Sabau & Ferkin 2013a) Meadow vole dams would be limited to the forage that is available in their territory and susceptible to food restriction during the three weeks it takes to wean a litter (Batzli 1985; Sabau & Ferkin 2013b).
Our recent work has shown that food restriction during early and middle lactation (but not during late lactation) reduces the amount of time that dams nurse their pups relative to that of control dams (Sabau & Ferkin 2013b). In that study, males reared by dams were food restricted during early lactation, which was considered days 1–7 of lactation (FR 1–7), middle lactation, which was considered days 8–14 of lactation (FR 8–14), and late lactation, which was considered days 15–21 of lactation (Sabau & Ferkin 2013b). Sabau and Ferkin (2013b) found that FR 1–7 males and FR 8–14 males had a lower body weight at weaning compared to FR 15–21 males and males reared by control dams. However, the body weight of FR 15–21 males and control males was similar (Sabau & Ferkin 2013b). In addition, they found that meadow vole dams that were food restricted during middle lactation spent less time licking their pups compared to control dams and dams that were food restricted during late lactation (Sabau & Ferkin 2013b). Dams that spend less time licking their pups produce adults that may not be able to interact successfully with same- and opposite-sex conspecifics (Moore 1982, 1993; Champagne et al. 2003). In addition, maternal food restriction during early and middle lactation may also affect the reproductive system of male rodents, which becomes fully developed by 10 days of age (Larsson et al. 1974), which could impact on their sexual behavior in adulthood (Govic et al. 2008).
The goal of the present study was to test the hypothesis that male offspring of meadow vole dams that were food restricted for different periods of time during lactation show persistent, negative effects on their sexual behavior as adults. We predicted that these effects would be more pronounced in the FR 1–7 and FR 8–14 males than in the FR 15–21 and the control males. We tested this hypothesis and prediction by measuring the three components of sexual behavior: attractivity, proceptivity/interest in the opposite sex, and receptivity (Beach 1976). Attractivity, for meadow voles, like many other terrestrial mammals, refers to the attractiveness of one’s odors and scent marks to opposite-sex conspecifics (Pierce et al. 2005). Proceptivity includes the behaviors displayed by females and by males to show interest in and to facilitate interactions with opposite-sex conspecifics, such as investigating the scent marks of potential mates (Johnston 1979; Pierce et al. 2005; Hobbs & Ferkin 2012). Attractivity and proceptivity establish communication between potential mates, and allow them to coordinate behaviors that facilitate or inhibit direct interactions (Beach 1976; Stopka & Macdonald 1998; Ferkin 2011). Receptivity is characterized by a male’s or a female’s willingness to mate (Beach, 1976; Pierce et al. 2005). In females, lordosis is an indicator of receptivity (Gray & Dewsbury 1975; Pierce et al. 2005; Sabau & Ferkin 2013a). In males, receptivity can be scored by counting the frequency and duration of mounts, intromissions, thrusts, and ejaculations.
Methods
Animals
We used meadow voles that were 3rd – 4th generation descendants of free-living voles captured in New York, USA. The voles were born and raised under a long photoperiod (14:10 h, L: D, lights on at 0700h CST). Voles used in this study had been housed singly in clear plastic cages (27 × 16.5 × 12.5 cm, l × w × h) for 4 weeks prior to the initiation of these studies. These voles had continuous access to water, food (Harlan Teklad Rodent Diet, #8640, Madison, WI, USA), and cotton nesting material. We followed Animal Care Protocol 0647, which was approved by the IACUC at The University of Memphis. We adhered to the ‘Guidelines for the use of animals in research’ as published in Animal Behaviour (1991, 41:183–186) and the laws of the country where the research was conducted.
Male voles used in this study were offspring of dams that were either food restricted during early, middle, and late lactation or not (control group) in a recent study by Sabau and Ferkin (2013b). These males were offspring of 44 different litters. In that study, Sabau and Ferkin (2013b) randomly assigned day-1 lactating female meadow voles to one of the four groups of 11 dams each. These four groups were comprised of dams that had continuous access to food throughout lactation (control), and dams that were provided with 70% of the daily intake of the control dams between day 1 and 7 (FR 1–7), between day 8 and14 (FR 8–14), and between day 15 and 21 of lactation (FR 15–21) (Sabau & Ferkin 2013a). Dams in the FR groups had continuous access to food on days when they were not food restricted. For example, dams in treatment group FR 8–14 were provided with 70% of the daily intake of control dams between days 8–14 of lactation but had continuous access to food between days 1–7 and between days 15–21 of lactation.
On day 22 of lactation, the pups from all four groups were weaned, housed with littermates in separate cages, and thereafter, provided with continuous access to food and water. No statistical differences existed in the number of male and female pups that were weaned per litter per treatment (4.2 ± 0.5 pups per litter; Sabau & Ferkin 2013b). When the pups were 34 days-old, they were separated from littermates, and housed individually in clear polycarbonate cages (27 × 16.5 × 12.5 cm, l × w × h).
Body Weight of Male Offspring
Males from our three FR treatment groups and the control group (n = 12 males per group) were weighted to the nearest gram of 0.1 gram every 3–5 days when they were between 22 and 43 days old, and every 10 days thereafter until they were 98 days old.
Food Intake of Male Offspring
The food intake of male offspring from the treatment groups and control group was also monitored until they were 98 days old. Briefly, 30 grams of food was placed into the cage-lid hopper of each male. Twenty-four hours later, we removed the male from its cage and collected and weighed (Ohaus GT4000 Automatic Balance, Florham Park, NJ) any food that remained in the cage-lid hoppers and on the floor of the cage to determine his daily food intake.
Sexual Behaviors
We used 12 different males in each of the treatment groups (FR 1–7, FR 8–14, and FR 15–21) and 18 different males in the control group in the sexual behavior component of the study. We began testing these males for sexual behavior (attractivity, proceptivity, and receptivity) when they were between 60 and 65 days-old. The male voles underwent a single attractivity, proceptivity, and receptivity test. We used males and females that were unfamiliar and unrelated to the voles with which they were tested. We did not use more than two individuals from the same litter in any test to eliminate the potential for litter effects. We used a Latin Squares design to allow male voles to serve as scent donors in the attractivity tests and then as subjects in the proceptivity tests and receptivity tests (Pierce et al. 2005). That is, some males were subjects in the proceptivity tests first, some were first subjects in receptivity tests, and others were first used as donors in attractivity tests. A minimum of 3 days separated successive tests with the same vole.
Attractivity Component
Scent donors were 18 male voles from the control group and 12 males each from the FR 1–7, FR 8–14, and FR 15–21 groups. The males in the treatment groups were used as scent donors once; the males in the control groups were used as scent donors twice.
Subjects were 36 female voles that had continuous access to food and were 120–150 days of age, born and raised in long photoperiod, and housed singly for 30 days prior to testing. Females were randomly chosen from a pool of 68 sexually experienced voles that were unrelated to and unfamiliar with the males used in the attractivity tests. Female subjects were not currently pregnant or lactating, but were sexually experienced, having weaned a litter 30 days prior to testing. Female meadow voles do not undergo regular estrous cycles (Keller 1985) and are induced ovulators (Milligan 1982). Females used in this study will readily mate with males when housed together under a long photoperiod (Meek & Lee 1993; Pierce et al. 2005; delBarco-Trillo & Ferkin 2006).
Each female subject underwent a single 10-minute attractivity test that followed the procedures detailed elsewhere (Pierce et al. 2005; Sabau & Ferkin 2013a). Briefly, we recorded the amount of time in seconds that females spent investigating the anogenital area scent marks of the following pairs of opposite-sex scent donors: 1) a FR 1–7 male versus a control male, 2) a FR 8–14 male versus a control male, and 3) a FR 15–21 male versus a control male.
Each female was exposed to the scent marks of a unique pair of male donors. The scent marks of these two males were placed on a clean, glass microscope slide (2.5 × 7.6 cm). Each slide was divided in three equal sections. Each section was 2.5 cm long. One end section contained a scent mark of a male donor that was reared by a food-restricted dam, while the other end section contained a scent mark of a male that was reared by a dam that had continuous access to food. The middle section contained no scent marks. Briefly, the anogenital area of a male donor was rubbed for approximately 5 seconds against the left- or right side of a clean slide. The position of the two scent marks was alternated on the left- or right-side of the slide for each test. After both scent marks had been placed on the slide, we suspended the slide on a clip and hook apparatus 1 cm above the substrate, against the wall opposite the female’s nest. During the 10-minute attractivity test, we recorded continuously the amount of time that the female subject licked or sniffed (the subject’s nose comes within approximately 1–2 cm) each scent mark and the clean section of the slide. The test began when the slide was placed into the cage of the female subject. Male voles were considered to produce more attractive scent marks if females spent significantly more time investigating their mark relative to that of another male (Pierce et al. 2005; Sabau & Ferkin 2013a).
Fresh scent marks from the anogenital area were obtained for each trial from each scent donor; anogenital area scent marks are deposited by voles in their runways (Ferkin et al. 2004). The experimenter wore disposable latex gloves to minimize human scent transfer while handling all slides. The investigator recording the behaviors was blind to the treatment conditions of the male and female voles in the attractivity tests, as well as the proceptivity and receptivity tests listed below.
Proceptivity Component
We used a scent preference test that was similar to the attractivity test to determine whether maternal food-restriction affected the proceptive behavior of male voles. Scent donors were 48 females and 48 males that had been reared by dams that were not food restricted; the male and female scent donors were between 120–150 days of age. The subjects were 48 males from the FR diet-treatment groups (n = 12 males per treatment group) and 12 males from the control group. Each male subject was tested once with a unique pair of male and female scent donors.
We followed the methods for proceptivity testing developed by Pierce et al. (2005) and are similar to the attractivity test we described above, with one notable exception. In the proceptivity test, male subjects were exposed to a glass slide that contained the scent mark of a female scent donor and the scent mark of a male scent donor. During the 10-minute proceptivity tests, we recorded the amount of time male subjects investigated the end of the slide containing the scent mark of the male scent donor and the other end of the slide containing the scent mark of the female scent donor. The position of the scent marks of the male and female donors on the left- or right-side of the slide was alternated. Male subjects were considered to display proceptive behavior if they spent significantly more time investigating the odors of the female scent donor than those of the male scent donor (Pierce et al. 2005).
Receptivity Component
We used the same methods in testing receptivity as described by Pierce et al. (2005). Briefly, a male vole was paired with a sexually experienced, 120–150 day-old unfamiliar female vole in a clear, plastic cage (37 × 21 × 15 cm; l, w, h), containing hardwood shavings, nesting material and water. At the time of pairing the females were neither pregnant nor lactating. Male voles were 12 males from each of the FR treatment-groups and 12 males from the control group; one of the males in the control group died shortly after being paired.
We allowed each pair to interact for 4 hours; meadow voles typically mate within this 4-h period (delBarco-Trillo & Ferkin 2004; Vaughn et al. 2008, 2011). We recorded each 4-hour pairing with a Sony Handycam DCR-SR68. During playback, we scored whether or not the males mated. If so, we scored the number of ejaculations by each male and his latency to first ejaculation, which was the amount of time (seconds) that elapsed between the male’s introduction into the female’s cage and his first ejaculation, which are typical measures of male copulatory behavior (Dewsbury 1972, 1975; delBarco-Trillo & Ferkin 2004, 2006, 2007). Collectively, these measures are indicators of sexual responsiveness of males and receptivity (Pierce et al. 2005). The ejaculation is characterized by a short series of rapid thrusts and intromissions followed by a noticeable relaxation of the male meadow vole’s pelvic region area and extension of his legs (Gray & Dewsbury 1975; delBarco-Trillo & Ferkin 2004, 2007; Vaughn et al. 2008).
We observed lordosis but did not measure it. Female meadow voles display lordosis after males mount them (delBarco-Trillo & Ferkin 2006). Thus, measures of mounting by males and lordosis by females are highly correlated (Dewsbury 1972, 1975).
Statistical Analyses
We used matched-paired t-tests to determine whether significant differences existed in the amount of time each subject spent investigating the scent marks of the two donors in the attractivity and proceptivity tests (Pierce et al. 2005). We used binomial tests to compare the number of males in each FR groups that mated (receptivity test) with those that mated in the control group. We used two separate one-way ANOVAs to determine if males paired with females differed among groups in their number of ejaculations and latency to first ejaculation. We used GLM repeated measures analysis of variance (ANOVA) to determine whether significant differences existed in the body weight and food intake of the offspring in the different treatment groups. Litter and not individual offspring was used as experimental unit in order to avoid pseudoreplication; we used the average mass of males in each litter to determine if the mean body weight of male pups differed between treatment groups. We conducted 1-way ANOVA’s followed by Holm-Sidák post hoc pairwise comparisons to determine the significant treatment effects. Significant differences were accepted at α < 0.05 for all statistical tests. We used SPSS 13.0 to analyze the data.
Results
Body Weight of Male Offspring After Weaning
The body weight of male offspring was affected by food restriction during lactation (F3, 35=2.7, p < 0.001) and whether food restriction occurred during early, middle or late lactation (F3.1, 110.1= 359.5, p < 0.001). A significant interaction existed between these variables (F9.4, 110.1= 2.0, p < 0.05). After weaning (day 21) and until day 48, control males weighed significantly more than males reared by FR 1–7 dams and those reared by FR 8–14 dams (Holm Sidák, p < 0.05; Fig. 1). With the exception of day 21, no significant difference was found in the body weight of males reared by FR 15–21 dams and those reared by dams that were not food restricted (p > 0.05; Fig. 1). Between 26 and 48 days of age, males reared by FR 1–7 and FR 8–14 dams had lower body weight than did males reared by FR 15–21 and control dams (Fig. 1), However, the body weight of males raised by FR dams and control dams was similar when they were between 49 and 98 days of age (Fig. 1). There was no difference among the dams in birth weights and number of offspring at weaning on day 21. The mean litter size at weaning was 4.2 + 0.69 pups and was similar for litters of control and FR dams (F3,43 = 0.89, P = 0.45).
Figure 1.
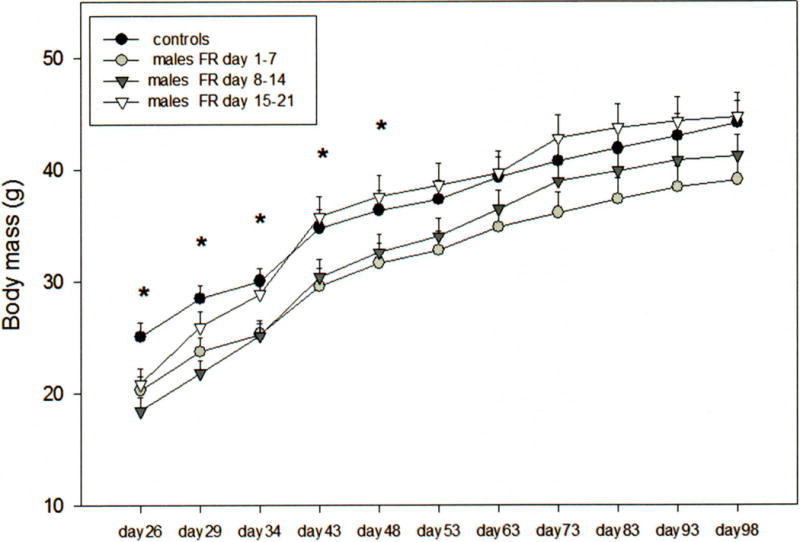
Mean ± SEM body weight (g), of FR 1–7 males, FR 8–14 males, FR 15–21 males, and control males. An asterisk (*) denotes significant differences between groups (p < 0.05).
Food Intake of Male Offspring After Weaning
All the male voles increased their food intake between days and 98 (F7, 238= 23.15, p < 0.01). However, there was no interaction between treatment (control or FR) whether food restriction occurred during early, middle or late lactation (F21, 238= 1.48, p = 0.085). The amount of food consumed by the males was similar for FR males and control males between day 34 and day 98 (F3, 34 =2.35, p > 0.05).
Attractivity
All female subjects investigated both scent marks, spending more time investigating the two scent marks of the two males than the middle portion of the slide. Maternal food-restriction during lactation affected the attractivity of scent marks of male offspring to female conspecifics (Fig. 2). Female voles spent significantly more time investigating the scent mark of control males than those of FR 8–14 males (t11 = 3.51, p = 0.007; Fig. 2). However, female voles spent similar amounts of time investigating the scent mark of a FR 1–7 male and that of a control male (t11= 0.78, p = 0.505; Fig. 2) as well as the scent mark of a FR 15–21 male and that of a control male (t11 = 1.82, p = 0.092; Fig. 2).
Figure 2.
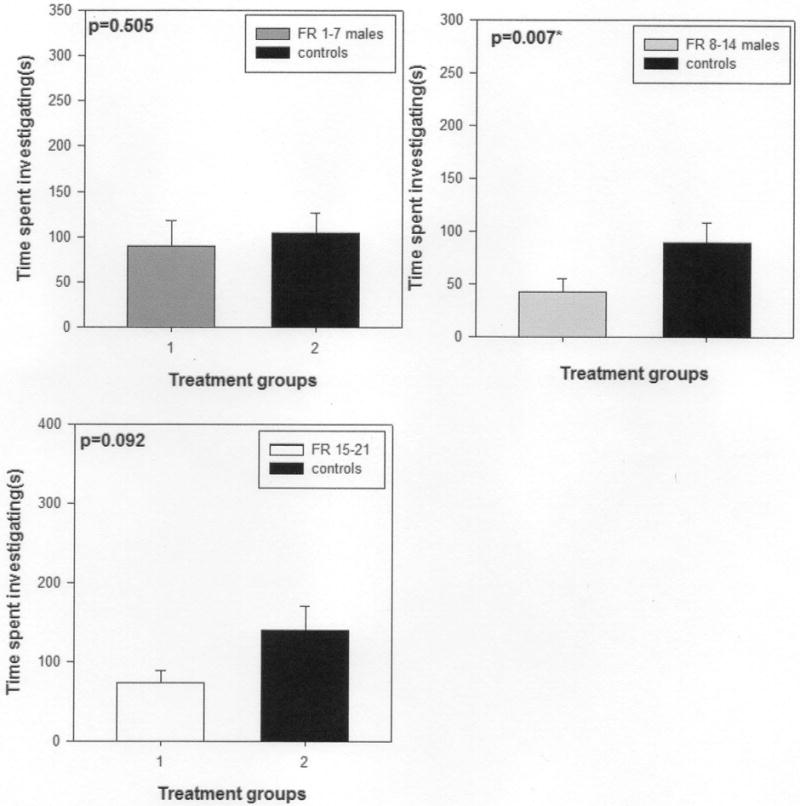
Mean ± SEM time (s) spent by female voles during a 10-minute test investigating the anogenital scent marks of in the following paired comparisons:a control male and a FR 1–7 male; a control male and a FR 9–14 male; and a control male and a FR 15–21 male. An asterisk (*) indicates significant differences between groups (p < 0.05).
Proceptivity
The preference for the scent marks of a female vole over the scent marks of a male vole was not affected by whether the males were reared by dams that were not food restricted or that were food restricted during early, middle, or late lactation (Fig. 3). FR 1–7 males (t11 = 4.12, p = 0.001), FR 8–14 males (t11 = 2.83, p = 0.01), FR 15–21 males (t11 = 4.09, p = 0.002) and control males (t11 = 3.541, p = 0.005) spent more time investigating the scent mark of female conspecifics compared to that of male conspecifics (Fig. 3).
Figure 3.
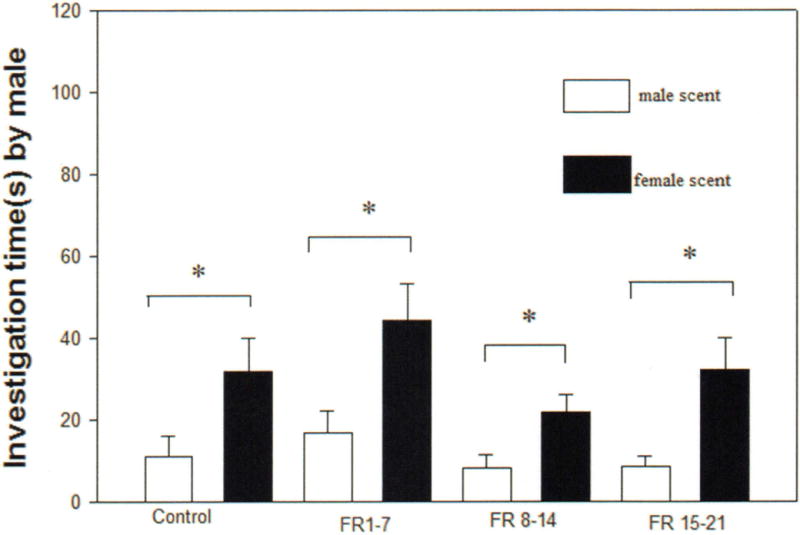
Mean ± SEM time (s) spent by control males, FR 1–7 males, FR 8–14 males, and FR 15–21 males during a 10-minute test investigating the anogenital scent marks of a male conspecific and a female conspecific. An asterisk (*) indicates significant differences between groups (p < 0.05).
Receptivity
We found that 9 of 12 FR 1–7 males, 4 of 12 FR 8–14 males, 8 of 12 FR 15–21, and 8 of 11 control males copulated with the females. FR 8–14 males had lower mating success relative to control (Binomial Critical Value test, p = 0.006; Fig. 4a). However, the mating success was similar for control males and FR 1–7 males (Binomial test, p=0.555) and controls and males FR 15–21(Binomial test, p=0.445; Fig. 4a). Thus, the number of males that copulated with a female vole was affected by whether the males were reared by dams that were food restricted during early, middle, or late lactation. There were also no significant differences in the latency to first mount (F3, 25 = 0.7, p= 0.27) and first ejaculation (F3, 25= 0.10, p= 0.85; Fig. 4b) between FR males and control males or the number of ejaculations control and FR males had when paired with a female (F3, 25 = 0.21, p= 0.93; Fig. 4c).
Figure 4.
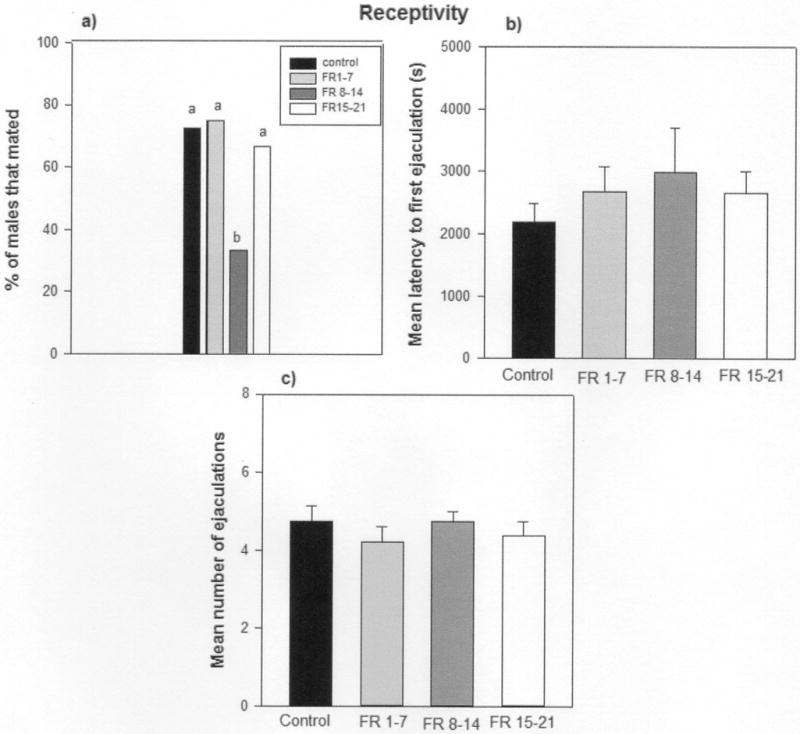
a) Percentage of control males, FR 1–7 males, FR 8–14 males, and FR 15–21 males that mated when paired with a sexually receptive female vole for 4 hours. b) The latency to first ejaculation of of these control males, FR 1–7 males, FR 8–14 males, and FR 15–21 males. c) The mean ± SEM number of ejaculations of these control males and the FR 1–7, FR 8–14, and FR 15–21 males. In Figure 4a the histograms capped with different letters indicate significant differences between groups (p < 0.05). In Figure 4b and 4c there were no significant differences between groups (p > 0.1).
Discussion
Food restriction of female meadow vole dams during middle lactation induced persistent, long-term negative effects on the attractivity component of the sexual behavior of their male offspring. FR 8–14 males produced anogenital marks that were not as attractive as those produced by control males and those of FR 1–7 males and FR 15–21 males. The scent marks of FR 1–7, FR 15–21 and control males were similar to one another in the attractiveness to female conspecifics. Estrus female house mice and rat like hamsters were also more attracted to the scent marks of male conspecifics reared by dams that were not food restricted during gestation than to those of males reared by dams that were food restricted (Meikle et al. 1995; Liang et al. 2004). Males that produce scent marks that are more attractive to females would be more likely to signal their presence in an area to potential mates. Our data suggest that compared to their counterparts, FR 8–14 male voles less likely than control males, FR 1–7 males, and FR 15–21 males to compete for mates. It is not clear why maternal food-restriction during early or late lactation had no effect on the attractiveness of scent marks produced by males but food-restriction during middle lactation had a negative and persistent effect on the attractiveness of the scent marks of male meadows to female conspecifics. We suggest that this impairment is due to a deficit associated with major development of meadow vole during middle lactation. During middle lactation, pups open their eyes, spend less time nursing, and begin to eat solid food (McGuire & Novak 1984; Nadeau 1985). Thus, it is possibility that the FR 8–14 males were also food-restricted at a time when they were beginning to eat solid food and receiving less milk from their mothers (McGuire & Novak 1984), and this nutritional challenge was sufficient to trigger changes in the odor-producing tissues of males. The decrease in the attractiveness of the scent marks of FR 8–14 males may reflect a tradeoff between developing the tissues that support growth and development, which will increase the pup’s likelihood of surviving and those needed for production of sexually discriminable scent marks, which will increase the pup’s likelihood of attracting a mate as an adult.
Food restriction during lactation did not affect a male vole’s interest in female conspecifics. Male voles, independent of whether they were food restricted or not, spent more time investigating the scent mark of a male donor to that of a female donor. Similarly, Hobbs and Ferkin (2011) reported that food availability did not affect the scent marking and over-marking behavior of male meadow voles when they encountered the scent marks of female conspecifics. Male rodents show interest in particular females by scent marking, self-grooming or investigating their scent marks (Hobbs & Ferkin 2011, 2012). These proceptive-like behaviors facilitate further interactions with potential mates (Beach 1976; Stopka & Macdonald 1998). Our findings suggest that male meadow voles do not show reductions in the behaviors that indicate their interest in females. Thus, male voles that experienced nutritional challenges during lactation do not reduce their likelihood of finding potential mates.
We discovered that only 33.3% of the FR 8–14 males copulated during the receptivity test, whereas 75% of the FR 1–7 males, 66.6% of the FR 15–21 males, and 72.7% of the control males copulated. The relatively low mating success of FR 1–7 male voles may be associated with the pronounced reduction the amount of care they received relative to controls early in lactation. Sabau and Ferkin (2013b) found that FR 1–7 dams spent little time compared to control dams, FR 8–14 dams, and FR 15–21 dams nursing and licking their pups. Similarly, lactating rats that were food restricted to 50% of the intake of food of control females during the first 10 days of lactation spent less time licking their young than did control dams (Smart & Preece 1973; Smart 1976). In rats, reductions in maternal licking caused offspring to become less likely to form social affiliations and mate (Moore 1984, 1992). We cannot rule out, the possibility that the amount of nutrition FR 1–7 males received was not sufficient to support the development of neuroendocrine substrates that mediate sexual receptivity. Although we are not aware of any studies that have examined the effects of maternal-food restriction during different periods of lactation on the receptivity of male offspring, our results are similar to most studies that showed dams that were food-restricted during gestation gave birth to sons with deficits in some aspect of their mating behavior and reproductive physiology (Larsson et al. 1974; Rhees & Fleming 1981; Zambrano et al. 2005). Our results do not follow the same pattern as other those reporting that maternal-food restriction increased aspects of the sexual behavior of male offspring among rats (Tonkiss et al. 1984; Govic et al. 2008) or had no effect on sexual behavior of male offspring among sheep (Ovis aries)(Rae et al. 2002).
The body weight of FR 1–7 and FR 8–14 males was lower than that of control males and FR 15–21 males at 48 days of age; puberty occurs around this time for male meadow voles (Nadeau 1985). This result was similar to that of other studies reporting that male offspring of mice and rats reared by dams that were under nutritional stress weighed less at weaning compared to male offspring that were reared by dams that were not under nutritional stress (Teixeira et al., 2002). The low body weight at puberty for FR 1–7 and FR 8–14 males may represent a tradeoff between size and survival as juveniles (Gendreau et al. 2005; Fairbanks & Hinde 2013; Holekamp et al. 2013). It is also possible that the low body of FR 1–7 and FR 8–14 males at puberty were associated with deficits in their attractivity and their receptivity, respectively. The fact that by 98 days of age the FR 1–7 and FR 8–14 males weighed the same as FR 15–21 males and control males suggest the former voles have experienced some type of compensatory weight gain, but that this weight gain was not sufficient to reverse the negative effects of maternal-food restriction on aspects of their sexual behavior.
Why does food restriction during early or middle lactation affect components of the sexual behavior and body weight of male offspring? It is possible that maternal-food restriction, food restriction experienced by the pups, or changes in the amount of maternal care provided by the dams singly or together are sufficient to induce epigenetic effects that can trigger persistent, impairments on the sexual behavior and weight of male meadow voles. Several studies have shown that nutirional challenges during lactation may affect the phenotype of offspring (McGowan et al. 2011; Fairbanks & Hinde 2013), their sexual behaviorand reproductive physiology (Forstmeier et al. 2004) and ability to form attachments with opposite-sex conspecifics (Francis et al. 1999; Cameron et al. 2008), which can transcend generations (Champagne et al. 2003). For a male meadow voles facing nutritional challenges during lactation could affect their survival (Sabau & Ferkin 2013b) as well as their lifetime mating and reproductive success (Larsson et al. 1974; Boonstra et al. 1993; Berteaux et al. 1999).
Acknowledgments
We thank Adam Ferkin, Lyndsey Pierson, and Dr. Javier delBarco-Trillo and an anonymous reviewer for reading earlier drafts of this manuscript. This research was supported by NIH grants HD 049525 and an ARRA supplement, and NSF grant IOB 0444553 to MHF.
Literature Cited
- Batzli GO. Nutrition. In: Tamarin RH, editor. Biology of new world Microtus. Vol. 8. Amer Soc Mammal Sp Publ; 1985. pp. 779–811. [Google Scholar]
- Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Bernardo J. Maternal effects in animal ecology. Amer Zool. 1996;36:83–105. [Google Scholar]
- Berteaux D, Bety J, Rengifo E, Bergeron J. Multiple paternity in meadow voles (Microtus pennsylvanicus): investigating the role of the female. Behav Ecol Sociobiol. 1999;45:283–291. [Google Scholar]
- Boonstra R, Xia XH, Pavone L. Mating system of the meadow vole, Microtus pennsylvanicus. Behav Ecol. 1993;4:83–89. [Google Scholar]
- Bronson FH. Mammalian Reproductive Biology. University of Chicago Press; Chicago: 1989. [Google Scholar]
- Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. Epigenetic programming of phenotypic variation in reproductive strategies in the rat through maternal care. J Neuroendocrinol. 2008;20:795–801. doi: 10.1111/j.1365-2826.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Da Silva P, Aitken RP, Rhind SM, Racey PA, Wallace JM. Influence of placentally mediated fetal growth restriction on the onset of puberty in male and female lambs. Reproduction. 2001;122:375–383. doi: 10.1530/rep.0.1220375. [DOI] [PubMed] [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature. 2004;431:446–449. doi: 10.1038/nature02845. [DOI] [PubMed] [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Female meadow voles cause external ejaculations in male meadow voles. Behaviour. 2006;143:1425–1437. [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Risk of sperm competition does not influence copulatory behavior in the promiscuous meadow vole (Microtus pennsylvanicus) J Ethol. 2007;25:139–145. [Google Scholar]
- Dewsbury DA. Patterns of copulatory behavior in male mammals. Q Rev Biol. 1972;47:1–33. doi: 10.1086/407097. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Diversity and adaptation in rodent copulatory behavior. Science. 1975;190:947–954. doi: 10.1126/science.1188377. [DOI] [PubMed] [Google Scholar]
- Engelbregt MJ, Houdijk ME, Popp-Snijders C, Delemarre-van de Waal HA. The effects of intra-uterine growth retardation and postnatal undernutrition on onset of puberty in male and female rats. Pediatr Res. 2000;48:803–807. doi: 10.1203/00006450-200012000-00017. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Hinde K. Behavioral response of mothers and infants to variation in maternal condition: adaptation, compensation and resilience. In: Clancy KBH, Hinde K, Rutherford JN, editors. Primate developmental trajectories in proximate and ultimate perspectives. Springer; New York: 2013. pp. 281–302. [Google Scholar]
- Ferkin MH. Odor-related behavior and cognition in meadow voles, Microtus pennsylvanicus (Arvicolidae, Rodentia) Folia Zool. 2011;60:262–276. [Google Scholar]
- Forstmeier W, Coltman DW, Birkhead TR. Maternal effects influence the sexual behavior of sons and daughters in the zebra finch. Evolution. 2004;58:2574–2583. doi: 10.1111/j.0014-3820.2004.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations in maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Gendreau Y, Cote SD, Festa-Bianchet M. Maternal effects on post-weaning physical and social development in juvenile mountain goats (Oreamnos americanus) Behav Ecol Sociobiol. 2005;58:237–246. [Google Scholar]
- Govic A, Kent S, Levay EA, Hazi A, Penman J, Paolini AG. Testosterone, social and sexual behavior of perinatally and lifelong calorie restricted offspring. Physiol Behav. 2008;94:516–522. doi: 10.1016/j.physbeh.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW, Zambrano E. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol. 2006;572:97–108. doi: 10.1113/jphysiol.2005.103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GD, Dewsbury DA. A quantitative description of the copulation behavior of meadow voles (Microtus pennsylvanicus) Anim Behav. 1975;23:261–267. [Google Scholar]
- Hobbs NJ, Ferkin MH. Effects of protein content of the diet on scent marking and over-marking behavior in meadow voles, Microtus pennsylvanicus. Behaviour. 2011;148:1027–1044. [Google Scholar]
- Hobbs NJ, Ferkin MH. Effects of food availability on proceptivity: a test of the reproduction at all costs and metabolic fuels hypotheses. Behav Proc. 2012;91:192–197. doi: 10.1016/j.beproc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Swanson EW, Van Meter PE. Developmental constraints on behavioural flexibility. Philos Trans Roy Soc B. 2013;368(1618):20120350. doi: 10.1098/rstb.2012.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE. Olfactory preferences, scent marking and “proceptivity” in female hamsters. Horm Behav. 1979;13:21–39. doi: 10.1016/0018-506x(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Keller BL. Reproductive patterns. In: Tamarin RH, editor. Biology of new world Microtus. Vol. 8. Amer Soc Mammal Sp Publ; 1985. pp. 725–778. [Google Scholar]
- Kerr TD, Boutin S, LaMontagne JM, McAdam AG, Humphries MM. Persistent maternal effects on juvenile survival in North American red squirrels. Biol Lett. 2007;3:289–291. doi: 10.1098/rsbl.2006.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K, Carlsson SG, Sourander P, Forsstrom B, Hansen S, Henriksson B, Lindquist A. Delayed onset of sexual activity of male rats subjected to pre- and postnatal undernutrition. Physiol Behav. 1974;13:307–311. doi: 10.1016/0031-9384(74)90049-3. [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Lesage J, Croix D, Dutriez- Casteloot I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod. 2003;68:390–400. doi: 10.1095/biolreprod.102.003269. [DOI] [PubMed] [Google Scholar]
- Liang H, Zhang J, Zhang Z. Food restriction in pregnant rat-like hamsters (Cricetulus triton) affects endocrine, immune function and odor attractiveness of male offspring. Physiol Behav. 2004;82:453–458. doi: 10.1016/j.physbeh.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Madison DM. An integrated view of the social biology of Microtus pennsylvanicus. The Biologist. 1980;62:20–33. [Google Scholar]
- Madison DM. Activity rhythms and spacing. In: Tamarin RH, editor. Biology of new world Microtus. Vol. 8. Amer Soc Mammal Sp Publ; 1985. pp. 373–419. [Google Scholar]
- McGowan PO, Meaney MJ, Szyf M. Epigenetics, phenotype, diet, and behavior. In: Preedy VR, Watson RR, Martin CR, editors. Handbook of behavior, food and nutrition. Springer Science; France: 2011. pp. 17–31. [Google Scholar]
- McGuire B, Novak MA. A comparison of maternal behavior in the meadow vole (Microtus pennsylvanicus), prairie vole (M. ochrogaster), and pine vole (M. pinetorum) Anim Behav. 1984;32:1132–1141. [Google Scholar]
- Meek LR, Lee TM. Prediction of fertility by mating latency and photoperiod in nulliparous and primiparous meadow voles Microtus pennsylvanicus. J Reprod Fert. 1993;97:353–357. doi: 10.1530/jrf.0.0970353. [DOI] [PubMed] [Google Scholar]
- Meikle DB, Kruper JH, Browning CR. Adult male house mice born to undernourished mothers are unattractive to oestrous females. Anim Behav. 1995;50:753–758. [Google Scholar]
- Meikle DB, Thornton MW. Premating and gestational effects of maternal nutrition on secondary sex ratio in house mice. J Reprod Fertil. 1995;105:193–196. doi: 10.1530/jrf.0.1050193. [DOI] [PubMed] [Google Scholar]
- Milligan SR. Induced ovulation in mammals. Oxford Rev Reprod Biol. 1982;4:1–46. [Google Scholar]
- Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev Psychobiol. 1984;17:347–356. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- Moore CL. The role of maternal stimulation in the development of sexual behavior and its neural basis. Ann NY Acad Sci. 1992;662:160–177. doi: 10.1111/j.1749-6632.1992.tb22859.x. [DOI] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. Maternal effects as adaptations. NewYork: Oxford University Press; 1998a. [Google Scholar]
- Mousseau TA, Fox CW. The adaptive significance of maternal effects. Trends Ecol Evol. 1998b;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Nadeau JH. Ontogeny. In: Tamarin RH, editor. Biology of new world Microtus. Vol. 8. Amer Soc Mammal Sp Publ; 1985. pp. 254–285. [Google Scholar]
- Passos MCF, Ramos CF, Moura EG. Short and long term effects of malnutrition in rats during lactation on the body weight of offspring. Nutr Res. 2000;20:1603–1612. [Google Scholar]
- Pierce AA, Ferkin MH, Williams TK. Food-deprivation-induced changes in sexual behavior of meadow voles, Microtus pennsylvanicus. Anim Behav. 2005;70:339–348. [Google Scholar]
- Rae MT, Kyle CE, Miller DW, Hammond AJ, Brooks AN, Rhind SM. The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim Reprod Sci. 2002;72:63–71. doi: 10.1016/s0378-4320(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Fleming DE. Effects of malnutrition, maternal stress, or ACTH injections during pregnancy on sexual behavior of male offspring. Physiol Behav. 1981;27:879–882. doi: 10.1016/0031-9384(81)90057-3. [DOI] [PubMed] [Google Scholar]
- Sabau RM, Ferkin MH. Food deprivation and restriction during late gestation affect the sexual behavior of postpartum female meadow voles, Microtus pennsylvanicus. Ethology. 2013a;119:29–38. doi: 10.1111/eth.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabau RM, Ferkin MH. Food restriction affects the mass of dams and pups and the amount of maternal behavior provided by female meadow voles, Microtus pennsylvanicus. J Mammal. 2013b;94:1068–1076. [Google Scholar]
- Smart JL. Maternal behavior of undernourished mother rats toward well-fed and underfed young. Physiol Behav. 1976;16:147–149. doi: 10.1016/0031-9384(76)90298-5. [DOI] [PubMed] [Google Scholar]
- Smart JL, Preece J. Maternal behavior of undernourished mother rats. Animal Behaviour. 1973;21:613–619. doi: 10.1016/s0003-3472(73)80024-7. [DOI] [PubMed] [Google Scholar]
- Stopka P, Macdonald DW. Signal interchange during mating in the wood mouse Apodemus sylvaticus: The concept of active and passive signaling. Behaviour. 1998;135:231–249. [Google Scholar]
- Teixeira CV, Passos MCF, Ramos CF, Dutra SCP, Moura EG. Leptin serum concentration, food intake and body weight in rats whose mothers were exposed to malnutrition during lactation. J Nutri Biochem. 2002;13:493–498. doi: 10.1016/s0955-2863(02)00197-3. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Smart JL, Griffiths EC. Mating behaviour of male rats following pre- and early postnatal undernutrition: a comparison of two outbred stocks. Physiol Behav. 1984;32:397–401. doi: 10.1016/0031-9384(84)90253-1. [DOI] [PubMed] [Google Scholar]
- Vaughn AA, delBarco-Trillo J, Ferkin MH. Sperm investment in male meadow voles is affected by the condition of the nearby male conspecifics. Behav Ecol. 2008;19:1159–1164. doi: 10.1093/beheco/arn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AA, delBarco-Trillo J, Ferkin MH. The duration and occurrence of sociosexual behaviors in male meadow voles, Microtus pennsylvanicus, varies before, during, and after copulation. Curr Zool. 2011;57:4–349. [Google Scholar]
- Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatric Res. 1996;40:438–443. doi: 10.1203/00006450-199609000-00012. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Rodriguez-Gonzales GL, Guzman C, Garcia-Beccera R, Boeck L, Diaz L, Menjivar M, Larrea F, Nathanielsz PW. A maternal low protein during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]


