Abstract
This “Controversies in Cardiovascular Research” article evaluates the evidence for and against the hypothesis that the circulating blood level of Growth Differentiation Factor 11 (GDF11) decreases in old age and that restoring normal GDF11 levels in old animals rejuvenates their skeletal muscle and reverses pathological cardiac hypertrophy and cardiac dysfunction. Studies supporting the original GDF11 hypothesis in skeletal and cardiac muscle have not been validated by a number of independent groups. These new studies have either found no effects of restoring normal GDF11 levels on cardiac structure and function or have shown that increasing GDF11 or its closely related family member GDF8 actually impairs skeletal muscle repair in old animals. One possible explanation for what appears to be mutually exclusive findings is that the original reagent used to measure GDF11 levels also detected many other molecules so that age dependent changes in GDF11 are still not well known. The more important issue is if increasing blood [GDF11] repairs old skeletal muscle and reverses age-related cardiac pathologies. There are substantial new and existing data showing that GDF8/11 can exacerbate rather than rejuvenate skeletal muscle injury in old animals. There is also new evidence disputing the idea that there is pathological hypertrophy in old C57bl6 mice and that GDF11 therapy can reverse cardiac pathologies. Finally, high [GDF11] causes reductions in body and heart weight in both young and old animals, suggestive of a cachexia effect. Our conclusion is that elevating blood levels of GDF11 in the aged might cause more harm than good.
Subject Terms: Hypertrophy, Growth Factors/Cytokines
Keywords: Myokines, Growth Factors, Pathological Hypertrophy, Aging
Introduction
Advanced aging is associated with alterations in cardiac muscle function and reductions in skeletal muscle mass and performance1–4. Reversal of those processes responsible for age-related functional decline is an important area of investigation and might be termed “fountain of youth research”. A recent series of studies from the Lee/Wagers laboratories5–7 suggest that age-related cardiac, skeletal muscle, and central nervous system abnormalities can be rapidly reversed if an old animal shares its circulation (parabiosis) with a young animal. These studies also suggest that an age-related reduction in growth differentiation factor 11 (GDF11), or it’s closely related family member myostatin (also known as GDF8)8, is centrally involved in these muscle abnormalities. These studies support the hypothesis that restoring youthful levels of GDF11 reverses complex age-related cardiac and skeletal muscle defects5–7.
However, there are many studies that do not support the idea that restoring GDF11 levels in aging improves muscle structure and function. Indeed, these studies show that GDF11 actually cause muscle abnormities in aging and that blocking the effects of GDF8 and GDF11 in old animals improves their muscle function9–13. These findings are clearly at odds with one another.
In this article, we will review the current controversies regarding the roles of GDF8 and GDF11 as possible causes or cures of age-related cardiac and skeletal muscle dysfunction. We will evaluate data that claim either beneficial or detrimental consequences of administering GDF8 and GDF11 in normal and aged animals.
Do GDF11 and GDF8 have distinct effects on target tissues
One important aspect of the current debate is whether or not myostatin/GDF8 and GDF11 have identical or distinct effects on target tissues. GDF8 and GDF11 are secreted proteins that are members of the Transforming Growth Factor β (TGFβ) superfamily. The expression of these two proteins has distinct tissue specificity; GDF8 is largely expressed in skeletal muscle12, 14, 15 with lower expression in heart and adipose tissue16, while GDF11 is expressed in many tissues including the pancreas, intestine, kidney, skeletal muscle, heart, developing nervous system, olfactory system, and retina17, 18. Studies with global genetic disruption demonstrates that GDF11 regulates anterior-posterior regionalization and kidney development,17–19 while GDF8 negatively regulates muscle growth14. These results suggest distinct developmental roles for GDF8 and GDF11 that may provide some insights into distinct roles within adult tissue.
GDF8 and GDF11 are highly homologous with 89% amino acid sequence identity in the mature, active, protein. Similar to other TGFβ family members, GDF8 is expressed as a 375 amino acid polypeptide, that is cleaved into an N-terminal latency associated protein and a C-terminal mature protein. Additional proteolytic processing at the RSRR site results in a 12.5 kDa C-terminal mature peptide, which forms the biologically active GDF8 dimer and the N terminus propeptide. Both forms, active dimer and propetide, have been detected in mouse and human serum20. Due to its high similarity with GDF8, GDF11 most likely follows a similar pattern of maturation, however, to our knowledge, this has never been clearly elucidated.
GDF8 and GDF11 signal through the identical activin type II receptors and activate the canonical SMAD2/3 signaling pathway21. Indeed, Egerman et al.9 documented that both GDF8 and GDF11 equally activate this signaling pathway. These findings are entirely consistent with studies that demonstrate that both GDF11 and GDF8 inhibit skeletal myogenesis and muscle regeneration17, 20, 22. These results show that GDF8 and GDF11 signal through identical pathways. Therefore, tissue specific GDF8 or GDF11 effects are likely due to local differences in their expression. However, the current reviews are specifically discussing circulating levels of active forms of GDF8/11 rather than tissue specific expression, local protein processing, or local activation.
Genetically induced loss of GDF8 function induces dramatically increased muscle mass and reduced fat pad mass in multiple species18,14.These finding clearly demonstrate that GDF8 negatively regulates skeletal muscle growth. In contrast, loss of GDF11 results in homeotic transformations throughout the axial skeleton and posterior displacement of the hind limbs during embryonic development23. GDF11−/− mice die perinatally presumably due to developmental defects in kidney, GI tract, stomach and the palate19, 24. Analysis of mutant mice suggests that GDF11 has little involvement in skeletal muscle development19.
Collectively, these results suggest that GDF8 and GDF11 have distinct biological roles but these roles appear to be due to differences in the developmental pattern of expression or tissue specificity of expression. It does not appear that distinct GDF8 and GDF11 effects results from molecular signaling specificity, since all known data shows the two molecules bind to identical receptors and activate identical downstream signaling mechanisms.
Does GDF11 Decrease with Age and/or Disease?
One aspect of the current controversy is whether or not circulating levels of either GDF8 or GDF11 change with age. One group reports reductions of GDF11 with age5–8 and a second group reports increasing GDF11 with age9. Because GDF11 and GDF8 are 89% identical, assessing their relative levels is challenging. Also contributing to the discrepancies between the groups are differences in detection methods as well as the use of different reagents. Reliably and reproducibly documenting age-related changes in circulating GDF11 is critical to the central hypothesis of the Lee and Wagers studies5–7.
It is now well established that the antibody and SomaLogic technology used to identify and isolate GDF11 as a singular factor responsible for age-related disorders in the original Lee/Wagers report in Cell5 does not have high GDF11 specificity. This reagent binds both GDF11 and GDF89, 25, 26 and since the circulating levels of GDF8 are many fold higher than GDF1127, the original data published by Lee/Wagers are clearly inadequate to support the central hypothesis of their work. This issue has not yet been resolved, and the Lee/Wagers groups no longer differentiate between GDF11 and GDF88. This appears to be an approach taken by several labs that are exploring GDF11/GDF8 as a contributing factor to age related disorders26. Our conclusion is that data obtained using non-selective reagents is inadequate to support the initial hypothesis that GDF11 is an important factor responsible for age related cardiac, muscle, and cognitive disorders27.
These problems with reagent specificity contribute to the uncertainty regarding GDF11 concentration changes with aging and directly impact the assertion that supplemental GDF11 restores youthful functionality27. Importantly, using GDF8 null mice, Rodgers et al27 have shown that circulating GDF11 levels are 500 times lower than GDF827. If true and if circulating GDF11 and GDF8 have equal access to their common receptors in target tissues, the functional relevance of circulating levels of GDF11 is likely inconsequential.
The Glass group and the Houser lab working with Boehringer Ingelheim (BI) developed sensitive, GDF11-specific methods9, 25 to determine concentrations of free, homodimeric GDF11 in serum from both young and aged mice as well as in humans. No significant age-related differences in GDF11 for either species were found at BI (data not shown), similar to the results reported by the Glass laboratory9. Importantly, Glass’s group reported a trend for increased GDF11 levels in rats and humans. Therefore, using GDF11 specific assays, there is no evidence that GDF11 declines in serum during aging. It is clear that the specificity and sensitivity of the reagents are key factors in the discrepant findings published to date. Other contributing entities include the expression of and GDF11 binding to endogenous protein partners (e.g. GASP-1, GASP-2 and follistatin), as well as the impact of sample handling techniques on the kinetics of that binding.
We conclude that there is not yet sufficient evidence to know if the circulating levels of GDF11 (and/or GDF8) are reduced in aging. This is an area where new studies with reliable, easily repeatable approaches would help move the field forward.
Cardiac muscle
This section will address the hypothesis that restoring youthful levels of GDF11 in old mice rescues their pathological cardiac hypertrophy and restores normal cardiac performance5. The effects of GDF11 on the heart are not well known. The report published in Cell by the Lee/Wagers laboratories suggested that circulating levels of GDF11 fall in old mice, and restoring it to youthful levels reverses age-related pathological cardiac hypertrophy5. However, a subsequent study by the Houser lab, using a well-characterized lot of rGDF11, at the doses reported to be beneficial by Lee/Wagers groups was unable to validate the original findings25.
In healthy adult animals the heart weight (HW) changes in proportion to changes in body weight (BW) 25. Pathological cardiac hypertrophy (PCH) on the other hand is an increase in HW that is disproportionate to the BW. PCH usually results from acquired diseases such as chronic hypertension, ischemic heart disease or from genetic defects28–32. PCH characteristics include increased HW/BW, increased cardiomyocyte size, altered myocyte Ca2+ handling properties, decreased cardiomyocyte number due to myocyte death, and increased fibrosis29, 33. Some of these features can be present in the aged heart34, but there is little conclusive evidence that PCH develops as part of the normal aging process. In fact, age- related cardiomyopathy is often secondary to the acquisition of cardiovascular disease35, 36. Molecular causes of true age-dependent cardiomyopathy, in the absence of disease, are not well defined. There is a need for studies that better explain the mechanisms of aging-related cardiomyopathy to better define strategies to prevent or reverse these defects.
The remainder of this section will discuss the idea that restoring youthful levels of GDF11 reverses age-related pathological cardiac hypertrophy5. This area of investigation began with a parabiosis experiment in which old mice shared a circulation with young mice. The heart weight/tibial length (HW/TL) of old mice was rapidly reduced (within a month) while in the control animals (old mice sharing a circulation with another old mouse), both the HW/TL and myocyte size were unchanged. The authors reported GDF11 as a circulating factor that was reduced in the blood of old animals that returned to normal levels in old mice sharing a circulation with young mice. In the second portion of this study, old animals received daily injections of what turned out to be poorly characterized rGDF11 (0.1 mg/Kg)8, for one month. This amount of GDF11 therapy reduced HW/TL and myocyte size without changing the body weight5. The Lee/Wagers labs suggested that GDF11 could be a novel therapeutic to reverse the adverse cardiovascular consequences of aging.
Needless to say, others should have been able to validate these results, since the relevant translational approaches were simple; daily injection of rGDF11 at the dose of 0.1 mg/kg. The Houser laboratory, collaborating with investigators from Boehringer-Ingelheim (BI), set out to validate and then extend these provocative findings. BI first performed extensive in-vitro and then in-vivo testing of rGDF11 obtained from R&D Systems. BI documented the stability of the recombinant protein in solution at body temperature and ensured it maintained its bioactivity at 37° C for up to four weeks.
Given the importance of using well characterized recombinant protein, we next discuss some of the studies that were performed to characterize rGDF11 for the study published by the Houser lab25. Like many TGFβ family members, the active form of GDF11 is not soluble at physiological pH. Therefore, before injection of rGDF11 into animals, an extensive biophysical analysis was performed on purified rGDF11 from R&D Systems. These studies showed that rGDF11 exists as a disulfide linked dimer in solution but only when the pH is 5.0 or lower. Aggregation and precipitation was observed in rGDF11 samples neutralized from pH 5.0 to pH 7.0 with transient nanoparticles about 50 nm (200 million Daltons) and larger species observed. At pH 7.0, residual smaller species were undetectable using UV absorption at 280 or 230 nm. These control studies showed that, because of variability in the efficiency of reconstitution from lyophilized powders, the best practice was to solubilize rGDF11 in an acidic buffer and measure the absorbance at 280 nm to document protein amount. These studies showed that assuming that the lyophilized powder is 100% protein (or even a certain percentage was protein by weight) generates incorrect data and is not a recommended way to ensure correct protein concentration.
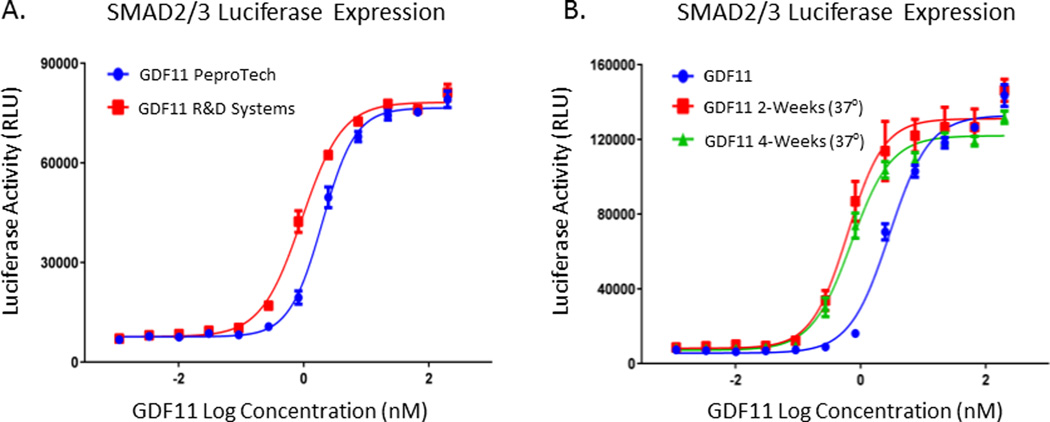
When handled carefully using acidic buffers to avoid precipitation, rGDF11 was a stable and well-behaved homogenous solution of disulfide linked dimers with unremarkable hydrodynamic properties. Analytical ultracentrifugation was used to characterize two samples of rGDF11 (from PeproTech and R&D Systems) before and after incubation at 37° C for one month. The purity and hydrodynamic sedimentation coefficient were examined using analytical ultracentrifugation showing minor loss of purity and total protein, and essentially identical shape and weight for the main species. Figure 1 demonstrates the biological activity of rGDF11 from PeproTech vs R&D Systems in the SMAD2/3 cell based assay. No difference in activity was observed between proteins from these two vendors. Additionally, activity of rGDF11 (R&D) was maintained for up to 4 weeks when stored in NaAcetate Buffer, pH 4.5. Collectively, these data document that the rGDF11 protein used in the study by the Houser lab was well characterized and stable for the dosing interval of 1-month. The Lee/Wagers group cautions others8 to be careful when preparing rGDF11. However, they do not describe precautions taken in their studies to ensure reliable dosing or rGDF11 bioactivity. Their published reports suggest that they weigh the recombinant protein supplied by the vendor, place it in saline, and inject it into animals5, 8.
Figure 1.
Functional activity of rGDF11 was determined by measuring GDF11 dose-dependent activation of Smad2/3 signaling in HepG2 reporter cells using a luciferase assay as reported in Smith et al. A. Similar activity was observed for both PeproTech and R&D Systems rGDF11. B. Biological activity of rGDF11 (R&D) was maintained for up to 4 weeks when stored in NaAcetate Buffer at 37°, pH 4.5 at a concentration of 1mg/ml.
Once fully characterized, rGDF11 (0.1 mg/Kg) or vehicle was injected daily into old mice for a month25. This blinded study25 showed that rGDF11 injections raised circulating GDF11 to detectable levels. In this regard, BI developed an assay that could discriminate between GDF8 and GDF11, rather than the reagent used by the Lee/Wagers group that we and other showed readily detects both GDF11 and GDF89, 25, 26
The first important finding of the Houser lab/BI study was that there is no evidence for the existence of pathological cardiac hypertrophy or deranged cardiac function in 23-month-old C57BL6 mice. The putative pathological hypertrophy reported in both reports by the Lee/Wagers laboratories is simply an artifact of using HW/TL rather than HW/BW to document pathological hypertrophy in mature adult mammals. A recent editorial by McNally37 regarding the new GDF8/GDF11 study by the Lee/Wagers groups also identifies this concern8. The Houser lab found that daily injections of rGDF11 (0.1 mg/kg) had no significant effect on HW, BW, cardiac structure or cardiac function25.
The Lee/Wagers groups have recently published a follow up study in which they perform a rGDF11 dose-response experiment in young and old mice8. In these experiments they suggest that the rGDF11 dose (0.1 mg/Kg) used in their original report is actually too low to cause decreases in heart size. Since they documented a reduction in heart and myocyte size with a dose of rGDF11 in the original report5, we assume this means that they do not know the rGDF11 concentration used in their original study. Given that they now caution others to properly reconstitute the recombinant protein, it is likely that their original methodology was flawed. It is unclear when they realized they did not know the actual rGDF11 dose used in the original studies. It is unfortunate that this problem was not promptly reported to the scientific community. As discussed above, the Houser and BI labs properly characterized rGDF11 25 and we are confident that a rGDF11 dose of 0.1mg/Kg was used in our validation study. The unknown GDF11 dosage used in the original study from the Lee/Wagers laboratory was reported to cause a decrease in heart weight and myocyte size with no change in body weight in their old mice5.
So what are the key differences between these studies and how might they be explained? First, is there any pathological cardiac hypertrophy in old (23–24 months of age) C57Bl6 mice? In both GDF11 reports from the Lee/Wagers groups5, 8 they start with the idea that C57Bl6 mice have aging-induced pathological cardiac hypertrophy. We were unable to document any pathological cardiac hypertrophy in our old mice25. As discussed above and in a recent editorial by McNally37, the putative pathological hypertrophy is an artifact of using HW/TL as a measure of cardiac pathology. Disease-free mature adult humans often gain and lose weight and their heart weight changes proportionally with body weight. This is just the normal biology of adult mammals and does not reflect any pathological process.
The parabiosis experiments in the original Lee and Wagers study are consistent with our reinterpretation of their data5. The data required for us to reach this conclusion can be found in Supplementary Table 3 that is in the version of this report uploaded to the NIH after the manuscript was accepted for publication (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3677132/). These critical data are not found in the version of the report published in Cell5. The data in Supplemental Table 3 show that the body weights of old animals were 25–30% greater than the young animals before parabiosis. After a month of parabiosis, the old animals lost almost 25–30% of their body weight, and now were almost the same size as their youthful counterparts. These data strongly suggest that there was no change in the HW/BW of old animals after parabiosis, however the raw data needed to determine this were not in the published studies. The reduction in HW/TL reported in these animals, in our view, is adequately explained by the parabiosis-induced reduction in BW. However, an unexplained aspect of this study5 is that when an old animal shared a circulation with another old mouse (the obvious control experiment), both old mice lost about 25% of their body weight, but no reduction in HW/TL was reported. We have no scientific explanation for how an animal can lose 25–30% of its body weight without having a corresponding reduction in heart mass?
In our GDF11 study, we used heart weight to body weight ratios (HW/BW) to define the presence or absence of pathological hypertrophy25; we reported heart weights and body weights along with the HW/BW ratios. We found no age-related changes in HW/BW ratio and daily rGDF11 injections (0.1 mg/Kg) had no effect on HW/BW or myocyte size. In addition, we showed that standard markers of pathological hypertrophy were not different in young and old mice or in old mice before and after GDF11 treatment25.
In a new follow up study from the Lee and Wagers groups8, a rGDF11 dose response experiment was performed in both young and old animals. The authors again state that rGDF11 reversed pathological cardiac hypertrophy in old animals, and surprisingly even in young animals. In this report, the critical heart and body weight data are included in Online Table 18. In this new study, the influence of age and rGDF11 dose was tested in both young and old animals. The significance of these new data is difficult to evaluate because of the statistical approaches used. In our view, an ANOVA was needed to document age and treatment effects and if this is established, then the appropriate post hoc analyses could be performed. Instead the authors performed an unpaired t-test, within each age group, between saline treatment and each of the rGDF11 doses tested. Another concern is that there is an apparent mistake in the reported “delta body weight %” in the old saline treated mice (the comparator group for the unpaired t-test statistical analysis) that appears to invalidate the major conclusions of these experiments. Readers are referred to Online Table 1 where the saline induced body weights decrease (average body weight reported), but the % change is listed as an increase. Once this mistake is corrected there are no apparent rGDF11-induced changes in BW. Regardless of the questionable statistical approach and the discordance of raw body weight and % body weight changes in the old saline treated mice, there was clearly no effect of rGDF11, at any dose, on HW/BW, documenting that rGDF11 does not reverse pathological hypertrophy.
It is worth noting that the new data from the Lee/Wagers groups8 shows that daily injections of high levels of rGDF11 in young mice, that should raise circulating GDF11 well above normal levels, causes weight loss and reduced heart weight. These young, lean animals have no cardiac pathologies. In our view, a rapid (9 days) reduction in heart and body weight in young, lean mice suggests toxic effects of GDF11– certainly not a desired effect in young, healthy animals. Finally, the dose-dependent GDF11 effects reported were identical in young and old animals. These findings appear to invalidate the original hypothesis since there is clearly no pathological hypertrophy or cardiac dysfunction in young mice and an identical dose response relationship would not be expected if old animals truly have reduced circulating GDF11 levels.
Our conclusion is that the data from the Lee/Wagers5, 8 and the Houser lab studies25 do not support the hypotheses that 1) there is PCH in old C57Bl6 mice, 2) that reduced circulating levels of GDF11 are responsible for this PCH and 3) that raising GDF11 levels with daily injections of rGDF11 rescues cardiac pathologies. Additional work with carefully characterized animal models and rGDF11 should help the field determine if raising circulating GDF11 levels in old age repairs or damages the heart.
Skeletal Muscle
The extensive repair capacity of adult skeletal muscle becomes progressively reduced during aging in large part from effects on the muscle myofibers, losses of satellite cells (SCs) and losses of SC function38. A number of laboratories have demonstrated that aged satellite cell function and muscle repair can be improved by exposure to a young systemic environment or the inhibition of pro-geronic factors39, 40. Novel therapeutics to restore skeletal muscle repair mechanisms could improve the quality of life in old individuals.41
A recent study from the Lee/Wagers laboratories suggested that reduced circulating levels of GDF11 are responsible in part for the age related decline in skeletal muscle function and restoring normal GDF11 levels rejuvenates skeletal muscle6. Recombinant GDF11 [rGDF11: 0.1mg/kg, Peprotech] was administered daily over 4 weeks to restore normal GDF11 levels in old mice. Multiple assays including muscle regeneration, transplantation and in vitro SC cultures were used to examine whether restoring normal GDF11 levels rescues the defective “regenerative capacity of satellite cells”6. The authors reported a significant improvement in skeletal muscle structure and function in GDF11-treated aged mice. GDF11-treated mice had increased mean muscle fiber size in regeneration assays; SCs had improved engraftment capability in transplantation assays and increased myogenic clonogenic capacity in cell culture assays. Adult mice were ‘unresponsive’ to rGDF11 therapy. No mechanistic studies were performed to address how GDF11 could function in a manner completely opposite to its highly-conserved ortholog GDF85, 6
A subsequent study from the Glass group9 was designed to reevaluate the idea that GDF11 can restore the reparative/regenerative properties of aged muscle tissue. They instead found that exogenously administered rGDF11 protein, delivered at same dose and duration (0.1mg/kg, R&D Systems) as reported by the Lee/Wagers groups6 did not improve aged muscle regeneration, or increase the number of satellite cells in vivo. Moreover, Egerman et al.,9 showed that administration of higher levels of GDF11 (0.3 mg/kg, RnD) to adult mice (daily over 28 days) actually inhibited muscle repair. In addition, signaling studies and gene expression analysis confirmed that GDF11 and GDF8 were virtually identical in their activity.
The two reports appear to reach mutually exclusive conclusions, with one study showing a beneficial anti-aging effect of exogenously administered GDF11, and the other showing that increasing GDF11 actually enhances age-related skeletal muscle damage. These findings are difficult to reconcile. While the experiments involving muscle injury and satellite cell cultures are similar they are not identical. Differences in the form of muscle injury, source of recombinant protein and culture media conditions in vitro, are potential sources of variability. Below we address each of those potential factors.
Types of muscle injury
Different forms of muscle injury (thermal6 versus cardiotoxin9) were used in the two conflicting studies discussed above6, 9. Both injury models are commonly used methods to induce muscle repair. Cardiotoxin is usually administered via injection into the targeted muscle, whereas thermal injury is conducted following surgery to expose the muscle. The extent of injury can vary significantly between laboratories due to the type and amount of cardiotoxin used, and the method used to apply the thermal injury. The different injury paradigms result in somewhat different rates of muscle repair possibly due to differences in the inflammatory response and extent of cellular death42. The Lee/Wagers groups reported improved regeneration of rGDF11-treated muscle following thermal injury, and improved engraftment of adult satellite cells into rGDF11-treated muscle following cardiotoxin injury6. Thus, it does not appear that the mode of injury is likely to explain the difference in findings. However, this idea can be directly tested.
Individual muscle fiber size varies considerably within a muscle tissue during the repair process. This variability is compounded if pre-existing i.e. non-injured muscle fibers, are factored into the analysis. Egerman et al.9 provided a detailed description of the rigorous methods used to quantify fiber size and accounted for uninjured fibers in the analysis. They did not see any enhancement in myofiber size no matter how the data were analyzed. Unfortunately, Sinha and co-workers6 did not fully describe the methods for myofiber size quantification, whether uninjured fibers were excluded, whether the analysis was blinded, or if samples were excluded. In addition, it is also impossible to determine if appropriate statistical analyses were performed because the raw data are not reported.
Satellite cell cultures
The Glass group9 showed that rGDF11 causes a dose dependent reduction in the expansion of both normal adult and aged satellite cells grown in culture, or on single fibers (the niche). The Lee/Wagers groups6 showed a rGDF11 dose-dependent increase in aged satellite cell expansion and differentiation with no effect on adult satellite cells. These disparate findings are also difficult to reconcile since one group found that rGDF11 reduced aged satellite cell expansion at all rGDF11 doses while the other found increased expansion as the exclusive effects.
Importantly, the Lee/Wagers groups reported that myostatin (GDF8) a known inhibitor of SC expansion decreased expansion of adult and aged satellite cell cultures. As discussed above, a major issue in this debate is the fact that GDF11 and GDF8 share significant homology and activate the same signaling mechanism through the same ActRIIb receptors. Tissue specific effects of GDF11 versus GDF8 could come about by tissue specific processing of inactive forms of these molecules. However, this point is irrelevant to the current controversy since the debate centers on circulating levels of mature, active forms of these molecules that were obtained from commercial providers.
The difficulty the Lee/Wagers groups have had in defining the actual GDF11 concentrations/activity in their studies5, 6 also do not easily explain the disparate results in the literature. The Glass9 and Houser25 laboratories carefully characterized different lots of GDF11 from different suppliers and only used well characterized and well solubilized recombinant protein. Both groups9, 25 showed dose dependent GDF11 signaling through SMAD2/3 reporter assays and then used the characterized protein in in-vivo studies. The Glass group clearly shows that all rGDF11 doses restricted expansion of SCs from both normal and aged animals9.
Egerman et al.9 used two complimentary assays to examine SC function, one that purifies SCs based on enzymatic digestion of muscle tissue, cell surface marker expression and flow cytometry, the other that isolated single muscle fibers which retains the SCs within their own niche. Sinha et al.6 assessed SCs in isolation only. Both groups show that the effects of GDF11 on isolated SCs are relatively modest. Egerman et al.9 reported a more robust phenotype using single muscle fiber assays, suggesting that the muscle fiber or a component of the niche is important for the inhibitory action of GDF11 on SC function. Somewhat counter intuitively, Sinha et al.6 reported numerous beneficial effects of GDF11 treatment of aged muscle including reduced DNA damage, rejuvenated mitochondrial function, and muscle force production. Again, no mechanistic studies were performed to elucidate the rejuvenating effects of rGDF11.
Media composition
The notion that different media and serum composition could influence GDF11 activity in cell culture experiments is a possibility. Indeed, serum contains proteases that could impact processing and thus activity of GDF11. However, the recombinant protein used in all of the experiments by both groups was in the mature form, and therefore, it is hard to imagine that protease processing was an issue. GDF11 signaling could be altered if soluble inhibitors such as follistatin and activin were present at different quantities within the media used in the different laboratories. While this cannot be excluded, in vitro experiments performed in the labs of Dr. Glass and Dr. Brack9 produced the same in vitro phenotypes with different media. This suggests that GDF11 induces a robust inhibitory response in muscle cells in vitro.
Conclusions
The idea that normal skeletal and cardiac muscle properties can be restored in old animals by daily injections of rGDF11 is not well supported by the studies that have been published to date. This field would benefit from studies with well-described, easily repeatable approaches, well-characterized reagents, and the presentation of and appropriate analysis of all data needed to form conclusions.
Acknowledgments
The authors would like to thank David Hayes, Ph.D. who performed the GDF11 biophysical analyses and Ashraf Khalil who developed and performed the smad2/3 assays that are discussed in this article. This work was performed at Boehringer-Ingelheim.
Sources of Funding: None
Nonsandard Abbreviations and Acronyms
- GDF11
Growth differentiation factor 11
- PCH
Pathological cardiac hypertrophy
- HW
Heart weight
- BW
Body weight
- TL
Tibial Length
- GDF8
Growth differentiation factor 8 (myostatin)
- TGFβ
Transforming Growth Factor β
- NVRM
Neonatal rat ventricular myocytes
Footnotes
Disclosures: None
References
- 1.Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiol Rev. 1993;73:725–764. doi: 10.1152/physrev.1993.73.4.725. [DOI] [PubMed] [Google Scholar]
- 2.Waller BF. The old-age heart: normal aging changes which can produce or mimic cardiac disease. Clin Cardiol. 1988;11:513–517. doi: 10.1002/clc.4960110802. [DOI] [PubMed] [Google Scholar]
- 3.Williams GN, Higgins MJ, Lewek MD. Aging skeletal muscle: physiologic changes and the effects of training. Phys Ther. 2002;82:62–68. doi: 10.1093/ptj/82.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 5.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poggioli T, Vujic A, Yang P, Macias-Trevino C, Uygur AN, Loffredo FS, Pancoast JR, Cho M, Goldstein J, Tandias RM, Gonzalez E, Walker RG, Thompson TB, Wagers AJ, Fong YW, Lee RT. Circulating Growth Differentiation Factor 11/8 Levels Decline with Age. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.307521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O'Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965–971. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ. Sprinting without myostatin: a genetic determinant of athletic prowess. Trends Genet. 2007;23:475–477. doi: 10.1016/j.tig.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argiles JM, Orpi M, Busquets S, Lopez-Soriano FJ. Myostatin: more than just a regulator of muscle mass. Drug Discov Today. 2012;17:702–709. doi: 10.1016/j.drudis.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 14.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 15.Ji S, Losinski RL, Cornelius SG, Frank GR, Willis GM, Gerrard DE, Depreux FF, Spurlock ME. Myostatin expression in porcine tissues: tissue specificity and developmental and postnatal regulation. Am J Physiol. 1998;275:R1265–R1273. doi: 10.1152/ajpregu.1998.275.4.R1265. [DOI] [PubMed] [Google Scholar]
- 16.Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180:1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Gamer LW, Cox KA, Small C, Rosen V. Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Dev Biol. 2001;229:407–420. doi: 10.1006/dbio.2000.9981. [DOI] [PubMed] [Google Scholar]
- 18.McPherron AC. Metabolic Functions of Myostatin and Gdf11. Immunol Endocr Metab Agents Med Chem. 2010;10:217–231. doi: 10.2174/187152210793663810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McPherron AC, Huynh TV, Lee SJ. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev Biol. 2009;9:24. doi: 10.1186/1471-213X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souza TA, Chen X, Guo Y, Sava P, Zhang J, Hill JJ, Yaworsky PJ, Qiu Y. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol Endocrinol. 2008;22:2689–2702. doi: 10.1210/me.2008-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 22.Lee YS, Lee SJ. Regulation of GDF-11 and myostatin activity by GASP-1 and GASP-2. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1309907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- 24.Harmon EB, Apelqvist AA, Smart NG, Gu X, Osborne DH, Kim SK. GDF11 modulates NGN3+ islet progenitor cell number and promotes beta-cell differentiation in pancreas development. Development. 2004;131:6163–6174. doi: 10.1242/dev.01535. [DOI] [PubMed] [Google Scholar]
- 25.Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR. GDF11 Does Not Rescue Aging-Related Pathological Hypertrophy. Circ Res. 2015;117:926–932. doi: 10.1161/CIRCRESAHA.115.307527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson KA, Beatty AL, Heidecker B, Regan MC, Brody EN, Foreman T, Kato S, Mehler RE, Singer BS, Hveem K, Dalen H, Sterling DG, Lawn RM, Schiller NB, Williams SA, Whooley MA, Ganz P. Association of growth differentiation factor 11/8, putative anti-ageing factor, with cardiovascular outcomes and overall mortality in humans: analysis of the Heart and Soul and HUNT3 cohorts. Eur Heart J. 2015;36:3426–3434. doi: 10.1093/eurheartj/ehv385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodgers BD, Eldridge JA. Reduced Circulating GDF11 Is Unlikely Responsible for Age-Dependent Changes in Mouse Heart, Muscle, and Brain. Endocrinology. 2015;156:3885–3888. doi: 10.1210/en.2015-1628. [DOI] [PubMed] [Google Scholar]
- 28.Tanijiri H. Cardiac hypertrophy in spontaneously hypertensive rats. Jpn Heart J. 1975;16:174–188. doi: 10.1536/ihj.16.174. [DOI] [PubMed] [Google Scholar]
- 29.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 31.Orenstein TL, Parker TG, Butany JW, Goodman JM, Dawood F, Wen WH, Wee L, Martino T, McLaughlin PR, Liu PP. Favorable left ventricular remodeling following large myocardial infarction by exercise training. Effect on ventricular morphology and gene expression. J Clin Invest. 1995;96:858–866. doi: 10.1172/JCI118132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard P, Villard E, Charron P, Isnard R. The genetic bases of cardiomyopathies. Journal of the American College of Cardiology. 2006;48:A79–A89. [Google Scholar]
- 33.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–262. doi: 10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 34.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 35.Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 37.McNally EM. Questions and Answers About Myostatin, GDF11, and the Aging Heart. Circ Res. 2016:6–8. doi: 10.1161/CIRCRESAHA.115.307861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brack AS, Rando TA. Age-Dependent Changes in Skeletal Muscle Regeneration. In: Schiaffino S, Partridge T, editors. Skeletal Muscle Repair and Regeneration. 3. Springer Netherlands; 2008. pp. 359–374. [Google Scholar]
- 39.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 40.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 41.Brack AS, Munoz-Canoves P. The ins and outs of muscle stem cell aging. Skelet Muscle. 2015;6:1. doi: 10.1186/s13395-016-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gayraud-Morel B, Chretien F, Flamant P, Gomes D, Zammit PS, Tajbakhsh S. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev Biol. 2007;312:13–28. doi: 10.1016/j.ydbio.2007.08.059. [DOI] [PubMed] [Google Scholar]