Abstract
This perspective discusses the report by Pinsky and colleagues, which addresses whether noncalcified pulmonary nodules identified on CT screening carry short- and long-term risk for lung cancer. We are facing challenges related to distinguishing a large majority of benign nodules from malignant ones and among those a majority of aggressive from indolent cancers. Key questions in determining individual probabilities of disease, given their history, findings on CT, and upcoming biomarkers of risk, remain most challenging. Reducing the false positives associated with current low-dose computed tomography practices and identification of individuals who need therapy and at what time during tumor surveillance could reduce costs and morbidities associated with unnecessary interventions.
Introduction
We are facing an epidemic of indeterminate pulmonary nodules (IPN), not only those found incidentally, but also through the proliferation of CT screening programs targeting high-risk individuals for lung cancer following the encouraging results of the National Lung Screening Trial (NLST; ref. 1) and the USPSTF recommendations (2, 3). Although the large majority of IPNs are benign, current predictive tools to discriminate benign from malignant nodules are suboptimal, leading to a large number of follow-up CTs, unnecessary invasive biopsies with attendant morbidity and rare mortality, anxiety, and wasted healthcare spending. Although the optimal approach to the management of patients with IPNs is evolving as technologies develop, key questions in determining individual probabilities of disease, given their history or findings on CT, remain most challenging. IPNs are those that have some risk of cancer. They are noncalcified, 7 to 20 mm in diameter, and with a risk of malignancy between 5% and 60%. The risk is less than for suspicious nodules (>60%) and greater than for nonsuspicious ones (<5%). There is still controversy around the definition of an IPN, and all clinicians know how challenging the evaluation of an IPN can be. IPNs fall under the broader umbrella covering noncalcified nodules (NCN) as discussed in the featured manuscript (4).
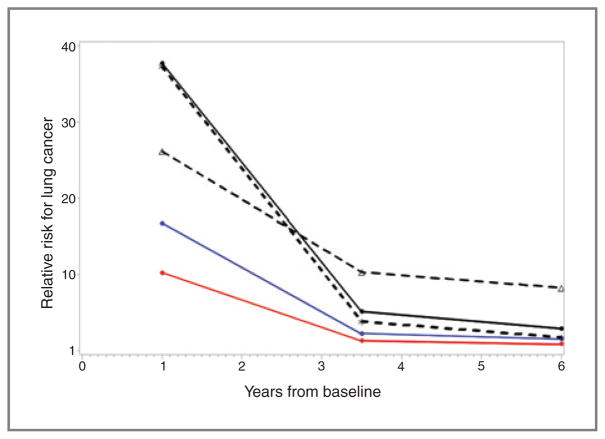
The question addressed in Pinsky and colleagues (4) is whether NCNs ≥4 mm in diameter carry a short-term (0–23 months) or long-term (60–84 months) risk for lung cancer. The featured manuscript determines that some NCNs may be cancer precursors based on the analysis of the CT screening data of the NLST. Although the majority of NCNs are not cancer precursors, NCNs are strongly associated with short-term cancer risk and weaker long-term risk (Fig. 1, courtesy of Dr. Pinsky). The presence of an NCN confers significantly elevated long-term lung cancer risk ratios (RR) of 1.8, 2.4, and 3.5 at the person, lung, and lobe levels; corresponding short-term RRs were 10.3, 16.8, and 38.0, respectively. Ground glass opacity (GGOs) were associated with long-term lung cancer risk (HR = 3.1) but inversely associated with short-term risk (HR = 0.3). This clearly signals that some NCNs and in particular some GGOs represent cancer precursor lesions that eventually behave very differently than benign ones, depending on their biologic and anatomic features. The results support that as risk biomarkers, the NCN size, attenuation, margins, and persistence (and suspected volume doubling times, although not studied here) provide different odds for cancer. As potential surrogate endpoints to follow in chemoprevention trials, the implications are that NCNs presenting as GGOs, but not as solid densities, are associated with long-term risk, with an HR of 3.1. Although this is only demonstrated after 5 years of follow-up, which is longer than most feasible chemoprevention studies, much remains to be learned from the rate of change in texture, density, and size over time as tangible surrogate endpoint biomarker.
Figure 1.
Relative risks are plotted at the midpoints of the three time periods. There are three curves (solid black, red, and blue lines) for the overall RR for any NCN versus no NCN at the person (red), lung (blue), and lobe (black) levels. There are two additional curves (black dotted lines), for the RR of either soft tissue or ground glass (GG) nodules compared with no NCN, at the lobe level (asterisk, soft tissue; triangle, GG).
Indeterminate Pulmonary Nodules
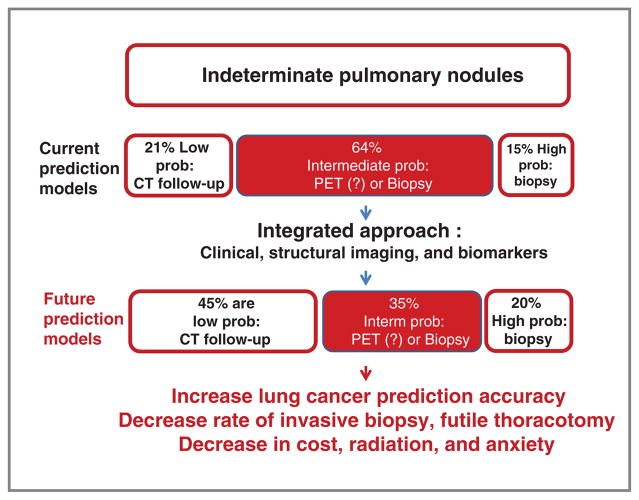
Differentiating the minority of malignant from benign IPNs represents one of the most urgent clinical problems in early detection of lung cancer, particularly on the eve of possible widespread adoption of lung cancer screening in the United States (5). When managing IPNs, the majority of diagnostic errors occur in the intermediate probability group (Fig. 2, current prediction models). This is due to a lack of deep knowledge of structural features of IPNs and the absence of validated diagnostic biomarkers for accurate disease categorization. Although most IPNs represent benign disease, significant morbidity and cost are associated with their management—up to $28 billion/year in the United States. Incorrect evaluation of IPNs causes risks that range from anxiety (6), to a high rate of unnecessary thoracotomies for benign nodules, to missed chances for cure during follow-up resulting in death. Chest CT is not capable of providing the improved diagnostic accuracy needed.
Figure 2.
IPNs and probability of cancer. Schematic representation of current prediction models compared with how improved prediction models could benefit clinical management. Probabilities of IPNs to represent lung cancer are based on their clinical presentation with recommended follow-up. Low probability is considered 0% to 5%, intermediate 6% to 60%, and high 61% to 100% (based on Wahidi et al; ref. 16) Improved predictive models could dramatically reclassify nodules into different probability groups to address the likelihood of cancer at initial discovery.
We define IPNs as noncalcified lung nodules, solid, part-solid, or ground-glass opacities, which, assuming a spherical nodule, have diameters ranging from 7 mm to 20 mm. IPNs with largest diameters above 7 mm decrease the false-positive rates to 7.2% versus 10.5% for 6 mm or 15.8% for 5 mm (7–10). IPNs may be solitary or multiple and are extremely common, with the reported prevalence between 8% and 69% depending on the clinical context (i.e., screening or prevalent disease, age, and endemic area for fungal disease; refs. 1, 11–15). Despite using a lower diameter of 4 mm for IPN in the early results of the NLST, a 20% relative reduction in lung cancer-specific mortality was found (1), though with 39.1% of the individuals having at least one positive result. The vast majority of IPNs were benign, with a false-positive (FP) rate in this high-risk cohort of 96% (1). The management of lung nodules follows the American College of Chest Physicians (ACCP; refs. 16, 17) and very similar National Comprehensive Cancer Network (NCCN) guidelines (18), which recommend that for nodules >8 mm in diameter, when the probability of lung cancer is <5%, a follow-up CT be done at 3 months. Should the probability be between 5% and 60%, the ACCP recommends a PET CT or tissue diagnosis. Should the probability be >60%, a tissue diagnosis is recommended at discovery (16). Despite attempts at bringing uniformity of IPN follow-up, controversy persists (19–23).
The discrimination of benign from malignant IPNs is based on the nodule density, size, shape, and changes in these characteristics over time, particularly in growth rate demonstrated by nodule volume doubling time (VDT; ref. 24). Volumetric analysis of nodules has improved significantly with the rates of change of the volumes (growth rates) being some of the best predictors of malignancy (23). Yet growth rate cannot provide the answer the patients want at the time of discovery because calculation of growth rate requires follow-up studies. Automated approaches for volumetric analysis are being aggressively pursued (25, 26) and are facing the challenges of nodules of low density, those abutting the pleura or the mediastinum, those in direct relationship with the vasculature, and, very importantly, the challenge of addressing methods to determine accurately and reproducibly the smallest measurable change. We expect very rapid and significant improvement in this field as we also preconize acquisition of thin sections CT for lung nodule evaluation (NCCN guidelines; refs. 27, 28; ACR guidelines). Accurate volumetric analysis is likely soon to be part of the arsenal of quantifiable features, image-based biomarkers to be used in clinical practice as molecular markers have successfully entered our clinical practice. Tumor volume estimation should lead to improving current guidelines of the management of IPNs.
Risk for Having or Risk for Developing Lung Cancer?
A separate discussion of short-term risk (risk of having lung cancer) and long-term risk (risk of developing lung cancer) is necessary as the variables predicting disease vary dramatically depending on the clinical setting. Associated with these risks are probabilities. At baseline, the size of an IPN on CT confers the most valuable estimate of malignancy, with nodule diameter related to likelihood of malignancy, respectively, of: <5 mm: 0%–1%; 5–10 mm: 6%–28%; 11–20 mm: 37%–64%; >20 mm: 64%–82% (16, 29). However, growth rate is most closely associated with malignancy (30). The proportions of IPNs falling into high, intermediate, or low risk for cancer vary with clinical setting, that is, screening, diagnostic, or preoperative, but the largest proportion (50%–76%) falls in the intermediate-risk group. Nodules in this group account for the largest number of invasive biopsies for benign disease. A critical goal is to improve the proper classification of these intermediate probability nodules, at discovery, into the low- or high-risk groups (Fig. 2). Diagnostic models have been proposed by Gurney and colleagues (31), Gould and colleagues (32), and Swensen and colleagues (33) but, as we have shown, only provide 69% accuracy in IPNs (34, 35). These models include age, smoking history, and size/location of the nodule and are improved by McWilliams and colleagues (36) but do not yet integrate automated quantitative imaging analysis, including assessment of volume growth rate, or molecular biomarkers.
Predicting the risk of developing lung cancer is a difficult task. As good as the models are currently, they are imperfect. The study from Pinsky and colleagues has the merit of demonstrating risk of cancer even at a long-term point, in this case 6.5 years. Bringing NCNs to the attention of risk modelers is essential as there are few other variables used thus far, including age, smoking history, asbestos exposure, and Chronic Obstructive Pulmonary Disease (COPD). In a population that is screened with low-dose helical CT scans, incorporation of nodule characteristics, particularly non-solid nodules, into the risk assessment further identifies individuals at greater risk (37). Not surprisingly, the field of diagnostics is improving much faster than our ability to predict cancer development.
Intermediate Endpoint Biomarkers in Chemoprevention Studies
Chemoprevention efforts are most compelling given the morbidity and the costs associated with managing patients with lung cancer. The goal is to prevent cancer or to treat the field effect or identifiable precancerous lesions in high-risk individuals. Safety and efficacy of chemopreventive strategies are difficult to establish over a short period of treatment (38, 39). Six months to a year typically does not have a major impact on cancer risk. Although phase III trials will assess clinical utility of these interventions, trials that test lung cancer as outcome are long and expensive. In contrast, phase II efficacy cancer prevention trials rely on intermediate endpoints that are predictive of patient outcomes, such as cancer incidence. Importantly, surrogate endpoint biomarkers would have tremendous impact for phase III trials. Yet, intermediate endpoint biomarkers in lung cancer chemoprevention trials have never been proven against hard outcomes such as cancer diagnosis (39). Ideally, these biomarkers are involved in tumorigenesis and modulated by the intervention. Because bronchial airway preinvasive lesions are more prevalent in the cancer population (40, 41), and because GGOs are also more prevalent among patients with known lung cancer (42), precursor lesions and in particular GGOs may represent a surrogate intermediate endpoint biomarker of risk. Some NCNs may fit this description (43).
Using the large NLST dataset, Pinsky and colleagues (4) determined the short- and long-term lung cancer risk of noncalcified lung nodules. The study confirms previous findings. NCNs presenting as GGO on chest CT have a lower risk of developing into cancer; therefore, guidelines suggest that GGOs be monitored longer than solid nodules for progression to invasive lung cancer. Solid or part solid, and especially lobulated and/or spiculated nodules, however, have known greater risk for being lung cancer. Short-term follow-up CT can identify fast-growing intermediate-size lung nodules, and yet most fast-growing nodules on short-term follow-up CT still prove to be benign. Use of an optimized NCN diameter may decrease false-positive case referrals for lung cancer (8). Similarly, lowering the VDT threshold (VDT cutoff) from 400 days to 232 days lowers the false-positive referrals while maintaining sensitivity for lung cancer diagnosis (44). In the NLST dataset, there is no certain answer to whether given NCNs end up being lung cancers because there are only 2 years of imaging follow-up beyond baseline. All of the long-term cancers, by definition, occurred at least 5 years from baseline. Therefore, at the time of cancer diagnosis, we do not have images available to examine whether a NCN evolved into the cancer.
The fundamental assumption of chemopreventive strategies is that treatment of a premalignant lesion prevents the development of an invasive cancer. If this assumption is correct, and if some NCNs represent precursor lesions, a successful therapeutic intervention would prevent these lesions from developing into cancer. In fact, the majority of lung nodules do not represent precursor lesions, few of the premalignant lesions progress (40, 45–47), and it is unclear whether chemoprevention strategies do prevent lung cancer development or allow regression of preinvasive lesions. Moreover, most of these lesions are not biopsied so it remains quite challenging to work with the lack of proven, histologic diagnosis for these lesions. Should an intervention be tested against such NCN as surrogate endpoint, the results could be confounded by the fact that most nodules will regress (because most are inflammatory and contained by an appropriate immune response) with their response being true but unrelated to the effect of the chemopreventive strategy. The challenge is to identify with accuracy and noninvasively those precursor lesions that will not behave indolently, which at the moment we cannot reliably do. The limitation of using CT scan-detected lung nodules as an intermediate end point is the lack of confirmation of the underlying pathology. Some strategies are promising however. Nodule growth, changes in CT attenuation (density), nodule enhancement with CT contrast agents, and circulating biomarkers may represent candidate surrogate bio-markers of an aggressive behavior.
Many chemoprevention endpoint biomarkers in lung cancer have been proposed, including changes in histologic grade, proliferation index (Ki-67 or PCNA), blood biomarkers, or urinary metabolites (PGEM; refs. 38, 39). None have been tested against an ultimate outcome, such as cancer development. Noninvasive surrogate biomarkers would be ideal as tissue requirements are usually quite limiting for feasibility reasons. Blood biomarkers have been very difficult to use, and none of them have been validated in chemoprevention studies. Molecular markers may allow us to select individuals at greater risk, but markers of response to chemoprevention strategies have been most difficult to establish (38, 39). Chemoprevention studies where the evaluation by nodule type was considered revealed a nonsignificant trend toward regression of non-solid and subsolid lesions after budesonide treatment (43, 48, 49). However, resolution of CT-detected nodules was not originally an endpoint in the study protocols, and, therefore, there were only a small number of subjects with nodules. Consequently, NCNs should be investigated further as potential intermediate endpoints for early-phase chemoprevention trials, akin to the use of colon polyps in colon cancer prevention studies.
A clear and immediately apparent concern is that screening chest CTs offered at the onset of chemoprevention studies will detect NCNs, most of which will be benign. A great proportion of the precursor lesions detected may in fact be precursors of rather indolent cancers and represent risk for overdiagnosis bias, thus defeating the purpose of any chemoprevention trial. Yet, imaging biomarkers predicting precursor lesion behavior would be invaluable in this regard. Because thin-slice (e.g., sub-2 mm) low-dose chest CTs are being deployed across the nation following the growing evidence that screening for lung cancer by low-dose chest CT saves lives, these studies will bring more individuals to these chemoprevention trials with high-quality imaging data available. In phase 0 trials, one could refine a biomarker assay using human tissue or imaging data. The follow-up of these GGOs over time, and their “degree of attenuation” (Hounsfield densities) and growth or regression, may have major implications as candidate surrogate endpoints. Unfortunately, volumetric analysis of the NLST nodules is not available, and this hypothesis could not be tested in the Pinsky manuscript.
Challenges: Precursor Lesions, Overdiagnosis
NCNs can be categorized as solid or subsolid nodules. The subsolid nodules are subcategorized as part-solid and non-solid (GGOs). Approximately 19% of IPNs are sub-solid nodules (14, 36, 50), which include GGOs (~14%) and part-solid nodules (~5%). Because there is strong correlation between the size of the solid component on CT and the invasive component on pathology (51), features are expected to be readily analyzable by structural imaging. Although subsolid nodules are particularly difficult to segment with automated software, novel segmentations that both identify the solid component and encompass the semisolid component within a sphere of fixed diameter are showing promise (52). This approach allows for quantitative features describing size, shape, density, and texture to be extracted from solid nodules and density and texture features from semisolid components.
Overdiagnosis is a serious problem in screening detected lung cancers. Overdiagnosis may account for up to 18.5% of screening detected lung cancers (53). In the COSMOS trial, slow-growing or indolent cancer comprised approximately 25% of incident cases, many of which may have been overdiagnosed (54). We hope to eventually identify indolent cancers by careful surveillance and provide new management strategies, considering intervention when the nodule progresses. There is a great association between GGOs and atypical adenomatous hyperplasia (AAH). Yet, the rate of true AAH progressing to invasive malignancy is unclear. The reported incidence of AAH in resections of ground-glass opacities varies widely in the peer-reviewed literature from 6% to 58% (48). We must further study the natural history of preinvasive lesions. We should find new ways of studying GGOs and part-solid nodules that are better than cross-sectional CT studies followed by lengthy prospective cohorts, which are difficult, expensive, and do not provide the necessary tissue for detailed study. We must study the biology of GGOs and part-solid nodules in greater details, and go beyond their characterization by means of EGFR and KRAS mutation status. We need to improve our understanding of their metabolism and heterogeneity, as well as improve our imaging and molecular probes to distinguish those that are malignant from those that are not. Molecular markers predictive to tumor progression would be particularly important in selecting those patients in need for intervention.
Conclusions
Many areas of uncertainty remain and need further investigation of our understanding of IPNs, especially part-solid nodules and GGOs. To cite a few examples, regular exponential growth of a nodule cannot be assumed from GGOs as it is for solid nodules (55, 56). The slow growth and metastatic behavior of this understudied group of IPNs are poorly understood. Is there a role of COPD in presentation of nodules and/or their evolution to invasive cancers? How can we improve the quantitative, noninvasive assessment of these nodules by low-dose CT, specifically the accurate measurement of growth rate/VDT and detection of early development of solid (invasive) components in previously purely nonsolid nodules? Addressing these challenges will help in the study of these IPNs as intermediate endpoint biomarkers in chemoprevention. The natural history of precursor lesions remains unclear and depends on many transforming factors caused by repeated injury with smoking, environment, possibly new infectious stresses, or others yet unidentified. Therefore, linking GGOs to precursor lesions suffers the lack of histologic evaluation and the lack of predictive ability for progression to cancer. These uncertainties can be addressed by phenotypic characterization of GGOs and distinguishing them from infectious etiologies, and at the molecular level where we hypothesize that some GGOs carry significant alterations predictive of an aggressive behavior. Whether CT scan-detected ground-glass opacities can serve as intermediate endpoints for lung cancer chemoprevention trials requires additional study.
Acknowledgments
Grant Support
This work was supported by federal funds from the National Cancer Institute (UO1 CA152662).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the authors.
Authors’ Contributions
Conception and design: P.P. Massion
Development of methodology: P.P. Massion
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): P.P. Massion
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): P.P. Massion, R.C. Walker
Writing, review, and/or revision of the manuscript: P.P. Massion, R.C. Walker
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): P.P. Massion
Study supervision: P.P. Massion
References
- 1.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;5:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boiselle PM, Chiles C, Patz E, Tammemagi M, Wood DE. Expert opinion: United States preventive services task force recommendation on screening for lung cancer. J Thorac Imaging. 2014;4:197. doi: 10.1097/RTI.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 3.de Koning HJ, Meza R, Plevritis SK, ten Haaf K, Munshi VN, Jeon J, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;5:311–20. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinsky P, Nath H, Gierada D, Sonavane S, Szabo E. Short- and long-term lung cancer risk associated with non-calcified nodules observed on low-dose CT. Cancer Prev Res. 2014;7:1179–85. doi: 10.1158/1940-6207.CAPR-13-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagler R, Savulescu D, Krayzler E, Leschiner S, Veenman L, Gavish M. Cigarette smoke decreases salivary 18 kDa translocator protein binding affinity – in association with oxidative stress. Curr Med Chem. 2010;23:2539–46. doi: 10.2174/092986710791556050. [DOI] [PubMed] [Google Scholar]
- 6.Gareen IF, Duan F, Greco EM, Snyder BS, Boiselle PM, Park ER, et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer. 2014;20:3401–9. doi: 10.1002/cncr.28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJ, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;8:848–54. doi: 10.1164/rccm.201209-1651OC. [DOI] [PubMed] [Google Scholar]
- 8.Henschke CI, Yip R, Yankelevitz DF, Smith JP. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Inter Med. 2013;4:246–52. doi: 10.7326/0003-4819-158-4-201302190-00004. [DOI] [PubMed] [Google Scholar]
- 9.Yip R, Henschke CI, Yankelevitz DF, Smith JP. CT screening for lung cancer: alternative definitions of positive test result based on the national lung screening trial and international early lung cancer action program databases. Radiology. 2014;273:591–6. doi: 10.1148/radiol.14132950. [DOI] [PubMed] [Google Scholar]
- 10.Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med. 2012;4:363–72. doi: 10.1164/rccm.201104-0679CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson DO, Weissfeld JL, Fuhrman CR, Fisher SN, Balogh P, Landreneau RJ, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med. 2008;9:956–61. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rampinelli C, De Fiori E, Raimondi S, Veronesi G, Bellomi M. In vivo repeatability of automated volume calculations of small pulmonary nodules with CT. AJR Am J Roentgenol. 2009;6:1657–61. doi: 10.2214/AJR.08.1825. [DOI] [PubMed] [Google Scholar]
- 13.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;9173:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 14.Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol. 2002;5:1053–7. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 15.Henschke CI, Yankelevitz DF, Libby DM, McCauley D, Pasmantier M, Altorki NK, et al. Early lung cancer action project: annual screening using single-slice helical CT. Ann N Y Acad Sci. 2001;952:124–34. doi: 10.1111/j.1749-6632.2001.tb02733.x. [DOI] [PubMed] [Google Scholar]
- 16.Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):94S–107S. doi: 10.1378/chest.07-1352. [DOI] [PubMed] [Google Scholar]
- 17.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: ACCP evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood DE, Eapen GA, Ettinger DS, Hou L, Jackman D, Kazerooni E, et al. Lung cancer screening. J Natl Compr Canc Netw. 2012;2:240–65. doi: 10.6004/jnccn.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu DM, Gietema H, de Koning H, Vernhout R, Nackaerts K, Prokop M, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer. 2006;2:177–84. doi: 10.1016/j.lungcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer? : ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):108S–30S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 21.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2007;2:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 22.Tammemagi MC, Freedman MT, Pinsky PF, Oken MM, Hu P, Riley TL, et al. Prediction of true positive lung cancers in individuals with abnormal suspicious chest radiographs: a prostate, lung, colorectal, and ovarian cancer screening trial study. J Thorac Oncol. 2009;6:710–21. doi: 10.1097/JTO.0b013e31819e77ce. [DOI] [PubMed] [Google Scholar]
- 23.van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;23:2221–9. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
- 24.Xu DM, van der Zaag-Loonen HJ, Oudkerk M, Wang Y, Vliegenthart R, Scholten ET, et al. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the NELSON study: cancer risk during 1 year of follow-up. Radiology. 2009;1:264–72. doi: 10.1148/radiol.2493070847. [DOI] [PubMed] [Google Scholar]
- 25.Gavrielides MA, Kinnard LM, Myers KJ, Petrick N. Noncalcified lung nodules: volumetric assessment with thoracic CT. Radiology. 2009;1:26–37. doi: 10.1148/radiol.2511071897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, de Bock GH, van Klaveren RJ, van Ooyen P, Tukker W, Zhao Y, et al. Volumetric measurement of pulmonary nodules at low-dose chest CT: effect of reconstruction setting on measurement variability. Eur Radiol. 2010;5:1180–7. doi: 10.1007/s00330-009-1634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood DE. Lung cancer screening: the last 10 years. J Natl Compr Canc Netw. 2012;11:1323–5. doi: 10.6004/jnccn.2012.0138. [DOI] [PubMed] [Google Scholar]
- 28.Oda S, Awai K, Murao K, Ozawa A, Yanaga Y, Kawanaka K, et al. Computer-aided volumetry of pulmonary nodules exhibiting ground-glass opacity at MDCT. AJR Am J Roentgenol. 2010;2:398–406. doi: 10.2214/AJR.09.2583. [DOI] [PubMed] [Google Scholar]
- 29.Gould MK. Evaluation of screening-detected lung nodules: minimising the risk of unnecessary biopsy and surgery. Thorax. 2011;4:277–9. doi: 10.1136/thx.2010.142067. [DOI] [PubMed] [Google Scholar]
- 30.Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology. 2000;1:251–6. doi: 10.1148/radiology.217.1.r00oc33251. [DOI] [PubMed] [Google Scholar]
- 31.Gurney JW, Lyddon DM, McKay JA. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part II. Application Radiology. 1993;2:415–22. doi: 10.1148/radiology.186.2.8421744. [DOI] [PubMed] [Google Scholar]
- 32.Gould MK, Ananth L, Barnett PG. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;2:383–8. doi: 10.1378/chest.06-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Int Med. 1997;8:849–55. [PubMed] [Google Scholar]
- 34.Pecot CV, Li M, Zhang XJ, Rajanbabu R, Calitri C, Bungum A, et al. Added value of a serum proteomic signature in the diagnostic evaluation of lung nodules. Cancer Epidemiol Biomarkers Prev. 2012;5:786–92. doi: 10.1158/1055-9965.EPI-11-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isbell JM, Deppen S, Putnam JB, Jr, Nesbitt JC, Lambright ES, Dawes A, et al. Existing general population models inaccurately predict lung cancer risk in patients referred for surgical evaluation. Ann Thorac Surg. 2011;1:227–33. doi: 10.1016/j.athoracsur.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;10:910–9. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maisonneuve P, Bagnardi V, Bellomi M, Spaggiari L, Pelosi G, Rampinelli C, et al. Lung cancer risk prediction to select smokers for screening CT–a model based on the Italian COSMOS trial. Cancer Prev Res. 2011;11:1778–89. doi: 10.1158/1940-6207.CAPR-11-0026. [DOI] [PubMed] [Google Scholar]
- 38.Keith RL, Miller YE. Lung cancer chemoprevention: current status and future prospects. Nature reviews Clin Oncol. 2013;6:334–43. doi: 10.1038/nrclinonc.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szabo E, Mao JT, Lam S, Reid ME, Keith RL. Chemoprevention of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e40S–60S. doi: 10.1378/chest.12-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishizumi T, McWilliams A, MacAulay C, Gazdar A, Lam S. Natural history of bronchial preinvasive lesions. Cancer Metastasis Rev. 2010;1:5–14. doi: 10.1007/s10555-010-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirsch FR, Prindiville SA, Miller YE, Franklin WA, Dempsey EC, Murphy JR, et al. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst. 2001;18:1385–91. doi: 10.1093/jnci/93.18.1385. [DOI] [PubMed] [Google Scholar]
- 42.Yokose T, Ito Y, Ochiai A. High prevalence of atypical adenomatous hyperplasia of the lung in autopsy specimens from elderly patients with malignant neoplasms. Lung Cancer. 2000;2:125–30. doi: 10.1016/s0169-5002(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 43.Veronesi G, Szabo E, Decensi A, Guerrieri-Gonzaga A, Bellomi M, Radice D, et al. Randomized phase II trial of inhaled budesonide versus placebo in high-risk individuals with CT screen-detected lung nodules. Cancer Prev Res. 2011;1:34–42. doi: 10.1158/1940-6207.CAPR-10-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heuvelmans MA, Oudkerk M, de Bock GH, de Koning HJ, Xie X, van Ooijen PM, et al. Optimisation of volume-doubling time cutoff for fast-growing lung nodules in CT lung cancer screening reduces false-positive referrals. Eur Radiol. 2013;7:1836–45. doi: 10.1007/s00330-013-2799-9. [DOI] [PubMed] [Google Scholar]
- 45.Coldren CD, Miller YE. Progressive endobronchial premalignancy: marked by original CIN. Am J Respir Crit Care Med. 2011;8:869–70. doi: 10.1164/rccm.201108-1476ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breuer RH, Pasic A, Smit EF, van Vliet E, Vonk Noordegraaf A, Risse EJ, et al. The natural course of preneoplastic lesions in bronchial epithelium. Clin Cancer Res. 2005;11(2 Pt 1):537–43. [PubMed] [Google Scholar]
- 47.Jeremy George P, Banerjee AK, Read CA, O’Sullivan C, Falzon M, Pezzella F, et al. Surveillance for the detection of early lung cancer in patients with bronchial dysplasia. Thorax. 2007;1:43–50. doi: 10.1136/thx.2005.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam S, leRiche JC, McWilliams A, Macaulay C, Dyachkova Y, Szabo E, et al. A randomized phase IIb trial of pulmicort turbuhaler (budesonide) in people with dysplasia of the bronchial epithelium. Clin Cancer Res. 2004;19:6502–11. doi: 10.1158/1078-0432.CCR-04-0686. [DOI] [PubMed] [Google Scholar]
- 49.van den Berg RM, Teertstra HJ, van Zandwijk N, van Tinteren H, Visser C, Pasic A, et al. CT detected indeterminate pulmonary nodules in a chemoprevention trial of fluticasone. Lung Cancer. 2008;1:57–61. doi: 10.1016/j.lungcan.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Raad RA, Suh J, Harari S, Naidich DP, Shiau M, Ko JP. Nodule characterization: subsolid nodules. Radiol Clin North Am. 2014;1:47–67. doi: 10.1016/j.rcl.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Lee KH, Goo JM, Park SJ, Wi JY, Chung DH, Go H, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Onc. 2014;1:74–82. doi: 10.1097/JTO.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 52.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Cavalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Comm. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patz EF, Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemagi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Int Med. 2014;2:269–74. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veronesi G, Maisonneuve P, Bellomi M, Rampinelli C, Durli I, Bertolotti R, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Int Med. 2012;11:776–84. doi: 10.7326/0003-4819-157-11-201212040-00005. [DOI] [PubMed] [Google Scholar]
- 55.Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Tazelaar HD, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007;2:555–62. doi: 10.1148/radiol.2422052090. [DOI] [PubMed] [Google Scholar]
- 56.Infante M, Berghmans T, Heuvelmans MA, Hillerdal G, Oudkerk M. Slow-growing lung cancer as an emerging entity: from screening to clinical management. Eur Respir J. 2013;6:1706–22. doi: 10.1183/09031936.00186212. [DOI] [PubMed] [Google Scholar]