Abstract
Fibrodysplasia ossificans progressiva (FOP) leads to disabling heterotopic ossification (HO) from episodic flare-ups. However, the natural history of FOP flare-ups is poorly understood. A 78-question survey on FOP flare-ups, translated into 15 languages, was sent to 685 classically-affected patients in 45 countries (6 continents). Five-hundred patients or knowledgeable informants responded (73%; 44% males, 56% females; ages: 1-71 years; median: 23 years). The most common presenting symptoms of flare-ups were swelling (93%), pain (86%), or decreased mobility (79%). Seventy-one percent experienced a flare-up within the preceding 12 months (52% spontaneous; 48% trauma-related). Twenty-five percent of those who had received an intra-muscular injection reported an immediate flare-up at the injection site, 84% of whom developed HO. Axial flare-ups most frequently involved the back (41.6%), neck (26.4%), or jaw (19.4%). Flare-ups occurred more frequently in the upper limbs before eight years of age, but more frequently in the lower limbs thereafter. Appendicular flare-ups occurred more frequently at proximal than at distal sites without preferential sidedness. Seventy percent of patients reported functional loss from a flare-up. Thirty-two percent reported complete resolution of at least one flare-up and 12% without any functional loss (mostly in the head or back). The most disabling flare-ups occurred at the shoulders or hips. Surprisingly, 47% reported progression of FOP without obvious flare-ups. Worldwide, 198 treatments were reported; anti-inflammatory agents were most common. Seventy-five percent used short-term glucocorticoids as a treatment for flare-ups at appendicular sites. Fifty-five percent reported that glucocorticoids improved symptoms occasionally while 31% reported that they always did. Only 12% reported complete resolution of a flare-up with glucocorticoids. Forty-three percent reported rebound symptoms within 1-7 days after completing a course of glucocorticoids. This study is the first comprehensive global assessment of FOP flare-ups and establishes a critical foundation for the design and evaluation of future clinical trials.
Keywords: Fibrodysplasia ossificans progressiva (FOP), Epidemiology, Clinical Trials
Introduction
Fibrodysplasia ossificans progressiva (FOP) is a rare and disabling genetic condition of congenital skeletal malformations and progressive heterotopic ossification (HO) and the most catastrophic disorder of human HO. (1,2) A recurrent mutation in activin receptor IA/activin-like kinase 2 (ACVR1/ALK2), a bone morphogenetic protein (BMP) type I receptor, is the cause of all sporadic and familial cases of classic FOP. (3) This highly conserved target is the basis for current drug development. (4)
Comprehensive natural history studies in FOP are needed to determine outcome measures for the design of informative clinical trials of potential disease-modifying agents. These measures should be clinically meaningful milestones and predictors of outcome, such as the occurrence of HO or change in function due to HO. Clinically important differences, such as preservation of joint function, need to be understood in the context of a comprehensive, global assessment.
Previous natural history studies on FOP are more than two decades old, evaluated a limited number of subjects (approximately 40), and were conducted before the era of symptomatic treatment of exacerbations (flare-ups) with corticosteroids. (5,6) Furthermore, these early studies were neither global nor comprehensive as the FOP community was small and regional. In the past twenty years, the organized FOP community has grown tremendously largely due to the efforts of interested patients, doctors, and families and due to the explosive growth of the internet and social media.
Here we report the results of a world-wide, prospective, cross-sectional survey of flare-ups in FOP patients, episodic exacerbations that over time result in disabling HO.
Materials and Methods
Survey design
The survey was designed to provide insight into gaps of knowledge in the current understanding of FOP flare-ups. These questions were qualified with reference to any location or the following specific locations: head, jaw, neck, back, chest, abdomen, shoulders, elbows, wrists, fingers, hips, knees, ankles, and feet. The questions were developed by FSK and RJP. The 78-question survey (Supplemental Figure 1) was reviewed by the International FOP Association, as well as FOP patients and family members worldwide, for clarity and relevance. The survey was approved by the University of Pennsylvania Institutional Review Board. It is the intention of the authors to share the raw data generated by this study with pharmaceutical and academic physicians/scientists who either have investigational drugs that have completed at least phase 1 testing or who have an interest in pursuing other epidemiologic questions.
Survey Implementation
The survey was translated into 15 languages by IFOPA members, with the help of physicians knowledgeable about FOP. The survey was distributed to 685 patients (in 45 countries) identified by the IFOPA, the IFOPA International Presidents Council (IPC), and by FSK and RJP, beginning on 17 December 2012. Countries included were Argentina, Australia, Belarus, Belgium, Bosnia-Herzegovina, Brazil, Canada, Chile, China, Colombia, Croatia, Denmark, Estonia, Finland, France, Germany, Greece, Guyana, India, Ireland, Italia, Japan, Replubic of Korea, Macedonia, Malaysia, Mexico, Norway, Panama, Peru, Poland, Portugal, Republic of San Marino, Republic of Moldova, Romania, Russia, Serbia, South Africa, Spain, Sweden, Switzerland, Turkey, United Kingdom, USA, Uruguay, and Venezuela. The survey was distributed online through Survey Gizmo or by hardcopy if a patient did not have internet access. When the survey ended on 01 July 2013, a total of 500 patients had responded (~73% response rate).
Data Analysis
To be eligible to participate in this survey, patients had to be identified as having classic FOP (known R206H mutation in activin receptor IA/activin-like kinase 2 (ACVR1/ALK2) and/or diagnosis by clinical criteria) according to the IFOPA, the IPC, and/or FSK and RJP. The duration of disease was taken to be the age of the patient, since congenital malformation of the great toes indicates presence at birth and occurred in all individuals. In addition to the total population of responders, patients were also categorized into age groups of 1 to 8 years, 9 to 14 years, and ≥15 years of age.
No data imputation methods were used for missing data. For some questions, a non-response was considered as non‐applicable due to the response from a previous question. For continuous variables, mean, standard deviations, median, minimum and maximum were calculated. For categorical variables, the number and percent of patients in each category were calculated. For some questions, the denominator used for calculation of percentages did not include patients (for example, young children) who never had a flare-up, never had a flare-up in a particular body region, responded equivocally when that was an acceptable response, or did not provide a response.
Survival for each joint was compared using Cox proportional hazards models, with p-values from pairwise comparisons between geographical regions calculated using the Tukey HSD method. Mean age was compared between geographical regions using analysis of variance followed by Tukey HSD pairwise comparisons. The proportion of each gender was compared between geographical regions using a chi-square test.
Results
Demographics and baseline characteristics
The survey recorded responses from or on behalf of patients whose ages ranged from 1.2 – 71.4 years (median age: 22.7 years). Slightly more than half were female (55.8%; 279/500). The country with the largest representation was the United States (128/500 patients; 25.6%), followed by China (54/500 patients; 10.8%]) and Brazil (42/500 patients; 8.4%). We classified geographical regions as US/Canada/Australia, Europe including Russia, Mexico/Central America/South America, Asia (mostly China, Japan, Korea), with a few patients omitted as not in any of the regions. Such regional differences in flare patterns as are statistically significant either involve Asian subjects having worse hazards or subjects from Mexico/Central America/South American having better hazards (Supplemental Table 1). Of course these could either be differences in self-reporting behavior or in actual flare-up hazards. Subjects from Asia were significantly younger than subjects from Europe/Russia and from US/Canada/Australia, and marginally younger than subjects from Mexico/Central America/South America (Supplemental Tables 2 and 3). A higher proportion of subjects from Mexico/Central America/South America are male compared to other regions (Supplementary Tables 2 and 3), although proportions do not differ significantly between regions (chi-square test P = 0.235).
Mean age at FOP diagnosis was 6.9 years (range: birth to 63 years); the median age was 5 years. Among all responders, 96.4% (479/685) reported at least one body region with partial or limited movement.
Onset and progression of FOP
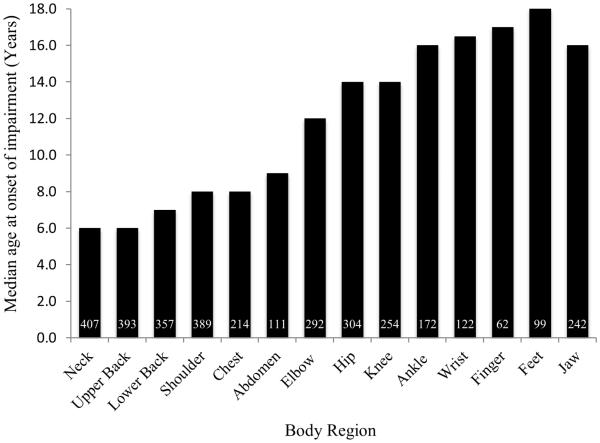
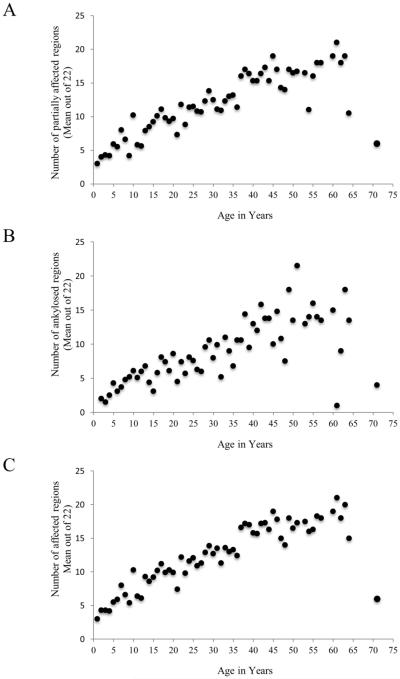
The median age of onset of impairment is shown in Figure 1 as a function of affected body region. Onset of FOP usually occurred first in the neck and upper back, and with the exception of the jaw, progressed from axial to appendicular regions (Figure 1). Progression of FOP, as quantified by the cumulative number of partially affected regions (Figure 2a), functionally ankylosed (Figure 2b) regions, or all affected regions (Figure 2c), was essentially unabated. Flare-ups that resulted in HO or subsequent joint ankylosis were distributed in a similar pattern. Most body regions were affected before the third decade of life (Figure 2c).
Figure 1.
Onset of physical impairment in FOP. The number of patients with an affected body region is shown within each bar of the histogram. Note that FOP progresses from axial to appendicular regions and within each appendicular region from proximal to distal locations.
Figure 2.
FOP is progressive through the lifespan of most patients. Shown are the number of partially affected body regions (A), ankylosed regions (B), and all affected regions (C) over time.
Characterization of flare-up symptoms
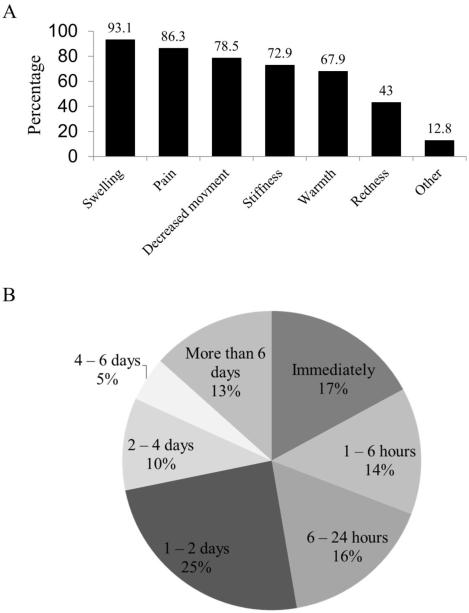
Major symptoms experienced during a flare-up included swelling (93.1%; 429/461), pain (86.3%; 398/461), decreased movement (78.5%; 362/461), stiffness (72.9%; 336/461), and warmth (15%; 313/461) [Figure 3a]. New swelling (39%; 180/461) and pain (29%; 135/461) were reported as the most reliable symptoms predictive of a flare-up. Swelling (39%; 180/461) and pain (38%; 175/461) were also the most consistent early symptoms of a flare-up. Seventy-two percent of survey responders (331/461) were able to confirm a flare-up within 48 hours of onset (Figure 3b). A majority of patients (54.4%; 251/461) had ignored an early symptom that later proved to be associated with a flare‐up. The most commonly ignored early symptoms were pain (42.2%; 106/251) and swelling (31.5%; 79/251), while 44.9% (207/461) said they also had experienced similar symptoms that did not result in a flare-up. Other symptoms reported before or during a flare-up included changes in mood or behavior (46.2%; 213/461), fever (33.8%; 156/461), loss of appetite (30.8%; 142/461), and lethargy (30.2%; 139/461).
Figure 3.
Characterization of flare-ups in FOP. (A) The majority of patients report swelling, pain, decreased movement, warmth, and redness as prominent symptoms of a flare-up. (B) Time to subjective confirmation of a flare-up. Most patients reported confirmation of a flare-up within two days of onset of symptoms.
Frequency and resolution of flare-ups
Nearly 70% of responders (323/461) reported a flare-up within the days to months preceding their completion of the survey. These results were similar across the age categories. About 71% (341/460) reported at least one flare-up in the previous 12 months. Overall, patients reported an average of 1.9 ± 0.1 flare-ups in the preceding 12 months (median 1.0; range of 0 to 7), although a higher percentage (13.1%; 8/61) of younger patients (i.e., those in the 1-8 year old category) reported having more than six flare-ups in the preceding 12 months than those in the other age categories (2.9% or 2/68 in the 9-14 year old group; 4.2% or 14/331 in the ≥ 15 year old group). Almost 26% (119/460) experienced no flare-ups in the previous 12 months, a finding that was similar across the age categories. In the year prior to survey participation, flare-ups of the head, neck, back, chest, and abdomen were most common in the 1-8 year old age group; flare-ups of the elbows, wrists, fingers, hips, ankles and feet were most common in the ≥ 15 year old age group (Table 1). Flare-ups of the shoulders were most frequent in those who were 14 years of age or younger, while flare-ups of the jaws and knees were most frequently reported in those greater than age nine years of age (Table 1). There was no preferential sidedness to flare-ups.
Table 1.
Flare-ups in the past 12 months by body region
| 1-8 Years (N=76) n (%) |
9-14 Years (N=73) n (%) |
≥15 Years (N=351) n (%) |
Total (N=500) n (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Head | 23 | (46.9) | 4 | (7.7) | 17 | (7.1) | 44 | (12.9) |
| Jaw | 6 | (12.2) | 12 | (23.1) | 48 | (20.0) | 66 | (19.4) |
| Neck | 22 | (44.9) | 19 | (36.5) | 49 | (20.4) | 90 | (26.4) |
| Back | 36 | (73.5) | 25 | (48.1) | 81 | (33.8) | 142 | (41.6) |
| Chest | 10 | (20.4) | 7 | (13.5) | 30 | (12.5) | 47 | (13.8) |
| Abdomen | 11 | (22.4) | 2 | (3.8) | 19 | (7.9) | 32 | (9.4) |
| Shoulders | 17 | (34.7) | 18 | (34.6) | 59 | (23.3) | 91 | (26.7) |
| Elbows | 7 | (14.3) | 4 | (7.7) | 62 | (25.8) | 73 | (21.4) |
| Wrists | 1 | (2.0) | 2 | (3.8) | 28 | (11.7) | 31 | (9.1) |
| Fingers | 2 | (4.1) | 1 | (1.9) | 17 | (7.1) | 20 | (5.9) |
| Hips | 6 | (12.2) | 14 | (26.9) | 77 | (32.1) | 97 | (28.4) |
| Knees | 6 | (12.2) | 14 | (26.9) | 67 | (27.9) | 87 | (25.5) |
| Ankles | 1 | (2.0) | 4 | (7.7) | 38 | (15.8) | 43 | (12.6) |
| Feet | 1 | (2.0) | 4 | (7.7) | 32 | (13.3) | 37 | (10.9) |
Shading indicates the body regions where flare-ups occur most frequently, stratified by age. For any single body region only, the age group for which the highest percentage of flare-ups occurred over the preceding 12 months is highlighted. For the same body region, if two age groups had similarly high occurrences of flare-ups, both were highlighted if the difference between the highest percentages was arbitrarily < 4.
With the exception of the back and hips, most flare-ups resolved within eight weeks of onset (Table 2). In the back, 50.2% of flare-ups took four to eight weeks or longer to resolve. In the hips, 55.1% of flare-ups took four to eight weeks or longer to resolve. Although 32% of patients reported having a flare-up that completely resolved (148/460), only 12% (18/148) reported that a high proportion (81-100%) resolved completely without loss of function. The most common body regions where resolution occurred were in the head (28.3%; 39/138) and the back (26.1%; 36/138). Most patients (58.1%; 86/148) reported that flare-ups resolved completely (without functional loss) ≤ 20% of the time.
Table 2.
Duration of flare-ups
| Body Region |
Time (weeks) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <2 n (%) |
2 -4 n (%) |
4 - 8 n (%) |
8 - 12 n (%) |
>12 n (%) |
||||||
| Head | 65 | (40.6) | 41 | (25.6) | 17 | (10.6) | 13 | (8.1) | 24 | (15.0) |
| Jaw | 71 | (32.4) | 56 | (25.6) | 36 | (16.4) | 16 | (7.3) | 40 | (18.3) |
| Neck | 87 | (29.3) | 87 | (29.3) | 45 | (15.2) | 32 | (10.8) | 46 | (15.5) |
| Back | 85 | (27.5) | 69 | (22.3) | 55 | (17.8) | 30 | (9.7) | 70 | (22.7) |
| Chest | 53 | (30.5) | 46 | (26.4) | 29 | (16.7) | 20 | (11.5) | 26 | (14.9) |
| Abdomen | 38 | (33.9) | 31 | (27.7) | 14 | (12.5) | 15 | (13.4) | 14 | (12.5) |
| Shoulders | 73 | (24.6) | 89 | (30.0) | 54 | (18.2) | 26 | (8.8) | 55 | (18.5) |
| Elbows | 65 | (27.3) | 66 | (27.7) | 41 | (17.2) | 21 | (8.8) | 45 | (18.9) |
| Wrists | 41 | (36.6) | 30 | (26.8) | 19 | (17.0) | 5 | (4.5) | 17 | (15.2) |
| Fingers | 30 | (39.5) | 17 | (22.4) | 11 | (14.5) | 5 | (6.6) | 13 | (17.1) |
| Hips | 49 | (19.8) | 62 | (25.1) | 44 | (17.8) | 31 | (12.6) | 61 | (24.7) |
| Knees | 62 | (26.6) | 65 | (27.9) | 33 | (14.2) | 24 | (10.3) | 49 | (21.0) |
| Ankles | 41 | (30.6) | 31 | (23.1) | 20 | (14.9) | 14 | (10.4) | 28 | (20.9) |
| Feet | 33 | (34.4) | 24 | (25.0) | 9 | (9.4) | 10 | (10.4) | 20 | (20.8) |
Shading indicates body regions where a majority of patients indicated that flare-ups lasted longer than four weeks.
Causes of flare-ups
Approximately 88% (406/461) reported that at least some of their flare-ups occurred as a result of injury, viral infection, or overuse. Intramuscular immunization was the cause of a flare-up in 25.1% (115/459), which led to bone formation in 84.3% (97/115) of these patients. As many as 61% of all flare-ups occurred spontaneously.
Disability associated with flare-ups
On a 0-10 pain scale (0 = no pain; 10 = worst pain), hip flare-ups were associated with the greatest pain (6.9 ± 0.19), followed by flare-ups of the knees (6.2 ± 0.19) and back (5.7 ± 0.17). On a 0-10 soft-tissue swelling scale, back flare-ups were associated with the most swelling (6.9 ± 0.16), followed by flare-ups of the knees (5.8 ± 0.2) and neck (5.7 ± 0.17). The most disabling flare-ups were those of the shoulders (49.5%), hips (47.1), neck (43%), and back (38.6%). Independent of location, approximately 70% of survey responders reported that movement (318/460) and overall functioning (320/4600 was affected by their most recent flare-up (Table 3). The ≥ 15 years of age group reported greater losses of movement and function compared to younger groups.
Table 3.
Effects of the most recent flare-up on function (all responders*)
| Percent loss of function |
Movement (% responders) |
Overall functioning (% responders) |
|---|---|---|
| 0 | 30.9 | 30.4 |
| 25 | 25.4 | 30.4 |
| 50 | 13.3 | 14.3 |
| 75 | 15.9 | 16.1 |
| 100 | 14.6 | 8.7 |
Total number of responders = 460.
Currently-used symptomatic treatment for flare-ups
Glucocorticoids such as prednisone are the standard of care for flare-ups in the appendicular skeleton, anterior neck, and jaw. About 75% (344/460) of participants reported that they had used prednisone (or its equivalent). Nearly 55% (188/344) reported that prednisone sometimes improved symptoms associated with flare-ups, and 30.5% (105/344] endorsed that prednisone always improved symptoms. Approximately 82% (240/293) reported that prednisone decreased swelling associated with flare-ups and 66.9% (196/293) reported that prednisone decreased pain. The locations where participants reported that prednisone gave the greatest symptomatic relief were the back [30.4% (89/293)], jaw [30.0% (88/293)], and neck [30.0% (88/293)]. However, only 11.6% (34/293) of responders reported complete resolution of a flare-up after taking prednisone. That prednisone always prevented flare-ups after injury was supported by 20.9% (39/187) of survey participants, with 39.6% (74/187) reporting that it sometimes prevented injury-induced flare-ups. Forty-three percent (126/293) of participants confirmed a rebound effect after completion of a course of steroids, with 65.1% (82/126) reporting the time to rebound being within one to seven days.
Among non-steroid interventions, temperature modalities were most frequently employed for relief of symptoms associated with flare-ups. Ice was used by 48% of study participants (219/456) and heat used by 30% (137/456). As a pharmacologic class, non-steroidal anti-inflammatory agents, including COX-2 inhibitors, were most frequently used, followed by bisphosphonates. The mostly commonly used non-steroid mediation was ibuprofen (32.8%; 164/500) followed by the leukotriene receptor antagonist montelukast (23.2%; 116/500).
Progression of FOP in the absence of flare-ups
Almost half of all patients (46.9%; 230/490) reported disease progression without flare-up symptoms. The frequency of body regions where such progression occurred is shown in Table 4.
Table 4.
FOP progression without flare-up symptoms
| Location | n | (%) |
|---|---|---|
| Neck | 97 | (42.2) |
| Back | 94 | (40.9) |
| Shoulders | 80 | (34.8) |
| Hips | 61 | (26.5) |
| Jaw | 54 | (23.5) |
| Elbows | 54 | (23.5) |
| Chest | 49 | (21.3) |
| Knees | 49 | (21.3) |
Total number of responders who reported FOP progression without flare-ups = 230.
Attitudes toward investigational treatments
About 80% of survey participants (413/485) indicated that they would be open to participating in a clinical trial to test an investigational drug, with or without prednisone. Almost 59% of responders (58.8%; 157/267) indicated that they would be willing to undergo a major operation to surgically unlock a joint and test whether an investigational drug could prevent re-ankylosis if there were at least a 50% chance of success. The joints that patients would preferentially choose to salvage through such a procedure were hips (25.4%; 68/267) and shoulders (24.3%; 65/267). Most patients have internet access (95.9%; 465/485) and would be able to obtain an x-ray within the first three days of the beginning of a new flare-up (89.9%; 436/485).
Discussion
A major reason why clinical trials fail is the lack of valid natural history studies. (7) Without such studies, inappropriate outcome measures may be employed and genotypic or phenotypic diversity may not be represented in clinical trials. The main objective of our survey was to obtain a comprehensive and contemporary understanding of the natural history of FOP by soliciting a wide range of information from patients regarding exacerbations or flare-ups. The results were intended to support the design of meaningful and interpretable clinical trials that will explore pharmacologic interventions to alter the natural history of FOP.
Natural history studies can take the form of simple literature reviews or retrospective chart reviews, but these approaches may not always be adequate. The “gold-standard” remains the prospective, longitudinal natural history study, which is time-consuming and often difficult to obtain with ultra-rare diseases. Our study was a prospective, cross-sectional assessment designed to assess flare-up characteristics and contributions to disability in FOP.
Although the total population of patients with FOP is unknown, estimates support the prevalence to be 1 in 1.6-2.0 million individuals. (8,9) Given this relatively low prevalence, the sample size in this study reflects a significant proportion of the total FOP population worldwide. Therefore, the descriptive statistics presented here are likely to be a good approximation of the true population-wide parameters that these statistics are estimating, and extrapolation to the world-wide population is a reasonable assumption. We acknowledge that despite the respectable response rate there still may be some component of non-representativeness on the basis of those who did not respond to the survey.
Previous natural history studies characterized the anatomic and temporal patterns of HO seen in patients with FOP. (5,6) These studies were limited by small sample size, analysis before standard treatment of flare-ups with steroids, and lack of a comprehensive or global perspective. This current, global study demonstrates large differences in the anatomic distribution of flare-ups when stratified by age, confirms general HO patterns seen with onset and progression of FOP, and expands upon many other characteristics of flare-ups that have never been rigorously evaluated and that are necessary to inform the design of meaningful clinical trials. Although the patterns of HO initiation and progression are generally consistent, it is striking that a few patients deviate greatly from the typical patterns and represent minimally affected individuals worthy of future study.
Evaluation of potential medications for the episodic treatment of FOP flare-ups must necessarily require clinical criteria that define the flare-up event. Our study suggests that major criteria for identification of a new flare-up should include at least the presence of swelling or pain for up to two days in duration. Other supporting criteria should include decreased movement, stiffness, warmth, and redness. The presence of decreased movement may be secondary to, and a surrogate for, swelling and pain. Distinguishing flare-ups on the basis of assessment of the degree of pain and swelling is probably less important, although scales that measure these parameters may provide important information as part of patient-reported outcomes.
Dosing of medications that might be used to treat FOP episodically must also take into consideration the duration and frequency of flare-ups, which vary according to location and age. Here we show that most flare-ups resolved within 8 weeks; however, flare-ups of the hips and back tended to last 12 weeks or longer. Nearly 70% of patients reported a flare-up within days to months prior to completing the survey, with a mean frequency of about two flare-ups per year for patients aged nine years or more and a mean frequency of 2.6 flare-ups per year in those younger than age nine. These data also have implications for clinical trial recruitment, including feasibility of appropriate sample size and timeliness to recruit the desired number of subjects.
Clinical trials that test drugs to be used either episodically or preventatively (chronically) must not only demonstrate efficacy in minimizing heterotopic ossification, but must also provide evidence that functional status is maintained or that loss of activity is comparatively reduced. The survey showed that a majority of patients lose movement about a joint with flare-ups, and that flare-ups also reduce overall functioning. Almost half of survey participants reported that their last flare-up resulted in at least a 50% reduction of mobility around a joint, with 15% reporting a complete loss of movement. These results will serve as functional benchmarks for future clinical trials.
A potential episodic treatment for FOP must resolve flare-ups more frequently than the rate at which flare-ups resolve spontaneously, with or without currently recommended interventions. Based on survey results, an estimate of the rate of spontaneous resolution is between 12 and 20 percent. This range represents the percentage of survey responders who reported a complete resolution of a flare-up in response to prednisone and the percentage of the time that most patients reported spontaneous resolution (without loss of movement or HO), respectively. This estimate will vary according to patient age and location of the flare-up.
This survey confirms our clinical experience regarding the major precipitating factors associated with flare-ups. The majority of survey participants reported that at least some flare-ups are the result of injury, viral infection, or overuse. About one quarter of participants reported that intramuscular immunizations were a cause of flare-ups and in this setting a very high proportion of these flare-ups resulted in HO. These findings are supported by the previous reports that HO occurs at the site of injection after diphtheria-tetanus-pertussis immunizations and that influenza-like viral illnesses are associated with flare-ups. (10,11)
Most survey participants reported use of prednisone or one of its equivalents for the treatment of flare-ups. It is clear that prednisone improved symptoms but infrequently resulted in complete resolution of FOP exacerbations (i.e., preservation of joint function and inhibition of HO). Many other pharmacologic and non-pharmacologic interventions are employed by patients, which are mostly consistent with current guidelines for the medical management of FOP; (12) however, the intention of this survey was not to evaluate potential efficacy of these many and varied agents and modalities but to survey their use among the worldwide population of FOP patients,
Although flare-ups are the sine qua non of FOP, they may not be the exclusive clinical manifestation of disease progression. Almost half of survey participants reported FOP progression without flare-ups. One may postulate that disease progression is related to other aspects of functional decline known to occur in FOP, such as accelerated arthritis, but also leaves open the possibility that successful episodic treatment of flare-ups (or their prevention) may not be sufficient to completely prevent long term complications of the disease.
In summary, the results from this first world-wide survey of flare-ups in patients with FOP provide a comprehensive perspective on the presentation, initiating factors, anatomic location, and resolution of flare-ups. These results will form the basis for design of future clinical trials of drugs for episodic and chronic treatment of FOP flare-ups.
Supplementary Material
Acknowledgements
This work was supported in part by the International Fibrodysplasia Ossificans Progressiva Association (IFOPA), the Center for Research in FOP and Related Disorders, the Ian Cali Endowment for FOP Research, the Whitney Weldon Endowment for FOP Research, the Isaac and Rose Nassau Professorship of Orthopaedic Molecular Medicine (to FSK), the Cali-Weldon Research Professorship in FOP (to EMS), and the National Institutes of Health (NIH R01-AR41916).
Conception and design of the work were by RJP and FSK. Collection and/or assembly of data were predominately by CBG and ML. The manuscript was written by RJP and FSK with revisions by EMS, DMR, and BD-J. Data analysis and interpretation were performed by all authors. The manuscript was approved by all authors.
We gratefully acknowledge the many patients, family members, and FOP physicians and advocates around the world who contributed to the development, review, translations, online testing, invitations to participate, and hardcopy distribution of the survey. We are greatly indebted to the following contributors, without whom this study would not have been possible: Massimo Alfieri, Renata Bocciardi, Betsy Bogard, Jud Bogard, Amanda Cali, Ian Cali, Tae-Joon Cho, Inho Choi, Julie Collins, Carrie Connell, Enrico Cristoforetti, Lori Danzer, Carmen De Cunto, Patricia Delai, Marie Hallbert Fahlberg, Mark Gambaiana, Nobushiko Haga, Julie Hopwood, Joe Kitterman, Martine Lemerrer, Juliana Louise, Victoria Mandracken, Jelena Milosevic, Rolf Morhart, Karen Munro, Malcolm Munro, Coen Netelenbos, Surinder Singh Oberoi, Jeannie Peeper, Antonio Morales Piga, Thomas Przybysz, Kamlesh Rai, Manuel Roberts, Roberto Ravazzolo, Astrid Schulze, Christiaan Scott, Irene Snijder, Jennifer Snow, Stephanie Snow, Denise Vietti, Keqin Zhang, Katarzyna Ziaja, Heidi zum Felde and Roger zum Felde. Most importantly, we are grateful to each participating FOP patient, family member and personal physician worldwide for their support in the implementation and completion of this study.
Footnotes
Disclosures:
The authors indicate no potential conflicts of interest.
References
- 1.Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011;6:80. doi: 10.1186/1750-1172-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: diagnosis, management, and therapeutic horizons. Pediatr Endocrinol Rev. 2013 Jun;10(Suppl 2):437–48. [PMC free article] [PubMed] [Google Scholar]
- 3.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006 May;38(5):525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan FS, Pignolo RJ, Shore EM. From mysteries to medicines: drug development for fibrodysplasia ossificans progressive. Expert Opin Orphan Drugs. 2013 Aug;1(8):637–649. doi: 10.1517/21678707.2013.825208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen RB, Hahn GV, Tabas JA, Peeper J, Levitz CL, Sando A, Sando N, Zasloff M, Kaplan FS. The natural history of heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. A study of forty-four patients. J Bone Joint Surg Am. 1993 Feb;75(2):215–9. doi: 10.2106/00004623-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Rocke DM, Zasloff M, Peeper J, Cohen RB, Kaplan FS. Age- and joint-specific risk of initial heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1994 Apr;(301):243–8. [PubMed] [Google Scholar]
- 7.Groft SC, Rao G, Austin C, Gallin J. Workshop summary. Workshop on natural history studies of rare diseases. 2012:1–41. [Google Scholar]
- 8.Connor JM, Evans DA. Genetic aspects of fibrodysplasia ossificans progressiva. J Med Genet. 1982 Feb;19(1):35–9. doi: 10.1136/jmg.19.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales-Piga A, Kaplan FS. Osteochondral diseases and fibrodysplasia ossificans progressiva. Adv Exp Med Biol. 2010;686:335–48. doi: 10.1007/978-90-481-9485-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanchoney TF, Cohen RB, Rocke DM, Zasloff MA, Kaplan FS. Permanent heterotopic ossification at the injection site after diphtheria-tetanus-pertussis immunizations in children who have fibrodysplasia ossificans progressiva. J Pediatr. 1995 May;126(5):762–4. doi: 10.1016/s0022-3476(95)70408-6. Pt 1. [DOI] [PubMed] [Google Scholar]
- 11.Scarlett RF, Rocke DM, Kantanie S, Patel JB, Shore EM, Kaplan FS. Influenza-like viral illnesses and flare-ups of fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 2004 Jun;(423):275–9. doi: 10.1097/01.blo.0000129557.38803.26. [DOI] [PubMed] [Google Scholar]
- 12.Pignolo RJ. Kaplan FS, Shore EM, Pignolo RJ, editors. International Clinical Consortium on F 2011 The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. Clin Proc Intl Clin Consort FOP. 4:1–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.