Abstract
Background
The primary role of respiratory syncytial virus (RSV) in causing infant hospitalizations is well recognized, but the total burden of RSV infection among young children remains poorly defined.
Methods
We conducted prospective, population-based surveillance of acute respiratory infections among children under 5 years of age in three U.S. counties. We enrolled hospitalized children from 2000 through 2004 and children presenting as outpatients in emergency departments and pediatric offices from 2002 through 2004. RSV was detected by culture and reverse-transcriptase polymerase chain reaction. Clinical information was obtained from parents and medical records. We calculated population-based rates of hospitalization associated with RSV infection and estimated the rates of RSV-associated outpatient visits.
Results
Among 5067 children enrolled in the study, 919 (18%) had RSV infections. Overall, RSV was associated with 20% of hospitalizations, 18% of emergency department visits, and 15% of office visits for acute respiratory infections from November through April. Average annual hospitalization rates were 17 per 1000 children under 6 months of age and 3 per 1000 children under 5 years of age. Most of the children had no coexisting illnesses. Only prematurity and a young age were independent risk factors for hospitalization. Estimated rates of RSV-associated office visits among children under 5 years of age were three times those in emergency departments. Outpatients had moderately severe RSV-associated illness, but few of the illnesses (3%) were diagnosed as being caused by RSV.
Conclusions
RSV infection is associated with substantial morbidity in U.S. children in both inpatient and outpatient settings. Most children with RSV infection were previously healthy, suggesting that control strategies targeting only high-risk children will have a limited effect on the total disease burden of RSV infection.
The primary role of respiratory syncytial virus (RSV) in causing lower respiratory disease among infants has made its control a worldwide priority.1,2 However, in the United States, the total burden of RSV infection during the first 5 years of life remains poorly defined, particularly in the outpatient setting. Previous studies have been limited because they were retrospective, of short duration, lacked laboratory confirmation of RSV infection, or focused only on hospitalizations or bronchiolitis.3–8
The Centers for Disease Control and Prevention (CDC) initiated the New Vaccine Surveillance Network (NVSN), a prospective, population-based inpatient and outpatient surveillance for acute respiratory infections among children under 5 years of age in 2000 in Nashville and Rochester, New York, and in 2003 in Cincinnati. The aim of our study was to determine the population-based burden of RSV infection among hospitalized children and outpatients in emergency departments and primary care settings. We further sought to describe the effect of potential risk factors on the severity of illness.
Methods
Study Design
The design and methods of NVSN’s surveillance for acute respiratory infections have been described previously.9–16 Inpatient surveillance was conducted for 4 years, from October 2000 through September 2004, during the winter months in the counties surrounding Nashville and Rochester and during the 2003–2004 season in Cincinnati. Children were enrolled within 48 hours after admission from Sunday through Thursday. Outpatient surveillance was conducted concurrently from November through April in Nashville and Rochester from 2002 through 2004 and similarly in Cincinnati during the 2003–2004 season. Outpatients were enrolled at each site from one to four urban and suburban pediatric practices either 1 or 2 days per week and from emergency departments during daytime and nighttime shifts in Nashville and Rochester 3 or 4 days per week and in Cincinnati’s emergency department every fourth day. During the study period, surveillance hospitals cared for more than 95% of each county’s children; emergency departments cared for 30% of the children in Nashville, 60% in Rochester, and 95% in Cincinnati.
Eligible children were under 5 years of age and had received a diagnosis of acute respiratory infection, which was defined as an illness presenting with one or more of the following symptoms: fever, cough, earache, nasal congestion, rhinorrhea, sore throat, vomiting after coughing, wheezing, and labored, rapid, or shallow breathing. Excluded were children who had respiratory symptoms lasting more than 14 days, had neutropenia from chemotherapy, had been hospitalized elsewhere within 4 days, or were newborns who had been hospitalized since birth.
Written informed consent was obtained from a parent or guardian of each child. The institutional review board at each site and the CDC approved the study. The authors vouch for the completeness and accuracy of the data and the analyses presented.
Patients
We obtained children’s demographic, medical, and social histories in standardized interviews of parents or guardians.12,13 The choice of clinical management, including hospitalization, was determined by the child’s physician. Laboratory and clinical information was obtained from records from hospitals, emergency departments, and out-patient primary care settings. Recorded high-risk medical conditions included prematurity (<36 weeks of gestation); chronic pulmonary, cardiac, kidney, or immunodeficiency disease; cancer; and sickle cell anemia.
We obtained nasal and throat swabs for viral detection for research purposes only, and the personnel caring for the patients were unaware of the results.9–16 All inpatient specimens were tested by reverse-transcriptase polymerase chain reaction (RT-PCR) and culture for RSV, influenza A, influenza B, and parainfluenza viruses 1, 2, and 3. Outpatient samples were assayed by RT-PCR for RSV and influenza. Additional viruses, including human metapneumovirus and rhinoviruses, were detected by RT-PCR among inpatient specimens from Nashville and Rochester in 2000 and 2001.9–17 Specimens were defined as positive if RSV was detected by viral isolation or by duplicate RT-PCR assays.9,10
Statistical Analysis
We determined the weighted number of hospitalizations for RSV infection and acute respiratory infections per 1000 children under 5 years of age and age-specific demographic characteristics using bootstrap methods, as described previously.12–15 Rates of visits to emergency departments and primary care offices for RSV-associated acute respiratory infections were calculated by multiplying the RSV-attributable portion from NVSN surveillance by rates of acute respiratory infections according to age group estimated from data from the National Ambulatory Medical Care Survey (NAMCS) and the National Hospital Ambulatory Medical Care Survey (NHAMCS) for the respiratory- infection seasons of November through April from 2002 through 2004.13 The confidence intervals for these rates were calculated with the use of the delta method, which accounted for variation in both the national data sets and the data from this study.13,18 Population-based rates for emergency department visits were calculated on the basis of pediatric visits to emergency departments during the winter months from 2002 through 2004 in Rochester and during the 2003–2004 season in Cincinnati, since these sites accounted for the majority of visits to emergency departments in their counties.
Chi-square tests were used for associations between categorical variables, with Yates’ correction for continuity used for analysis with dichotomous variables and Student’s t-tests for continuous variables. Three multivariable logistic-regression models were constructed for RSV-positive inpatients versus RSV-positive outpatients, for RSV-positive inpatients versus RSV-negative inpatients, and for RSV-positive outpatients versus RSV-negative outpatients. Candidate covariates in each model were sex, age group (0 to 5, 6 to 11, 12 to 23, and 24 to 59 months), the time in day care (≤4 and >4 hours per week), the presence or absence of household exposure to smoke, breastfeeding (0 to <1, 1 to 6, and ≥7 months), the presence or absence of high-risk coexisting conditions, the presence or absence of prematurity, an interaction term between high-risk conditions and prematurity, and the presence or absence of other children under 18 years of age residing in the household. Covariates were included in the multivariable model if the P value was under 0.20 when comparing RSV-positive inpatients with RSV-positive outpatients in bivariate analysis. All statistical analyses were performed with the use of Stata software, version 10.0.19
Results
Study Populations
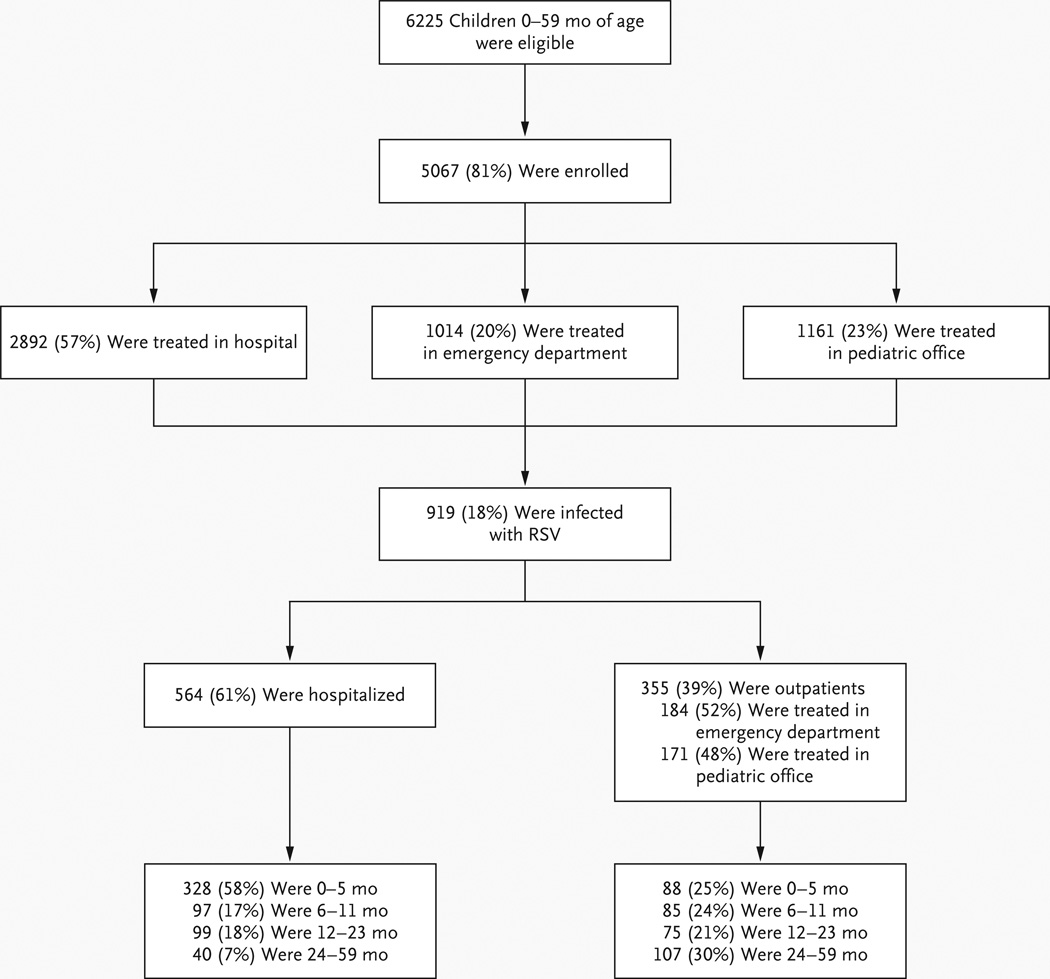
Of 6225 eligible children, 5067 (81% of inpatients and outpatients) were enrolled, and 919 had confirmed RSV infection (Fig. 1). The major reasons for nonenrollment were a lack of parental consent (65%) or unavailability of parents (20%). In only 1% of cases, the child’s physician declined participation.
Figure 1. Enrollment and Outcomes.
The number of children who were hospitalized includes 78 children who were initially enrolled as outpatients (74 from emergency departments and 4 from pediatric practices).
Laboratory Analyses
Of 564 RSV-positive inpatient specimens, 547 (97%) were identified by RT-PCR and 171 (30%) by tissue culture. Of 500 specimens tested by both methods, 329 (66%) were deemed to be positive only by RT-PCR and 17 (3%) by culture.
Epidemiologic Analysis
RSV outbreaks at each site occurred yearly between November and April. The median onset was December 1. RSV was detected for a median of 18 weeks in Nashville and 20 weeks in Rochester and Cincinnati.
RSV was identified in 919 of 5067 specimens (18%) and was associated with 20% of annual hospitalizations, 18% of emergency department visits, and 15% of office visits for acute respiratory infections from November through April. In 58 of the samples (6%), coinfecting viruses were identified, including 26 samples with influenza virus, 8 with parainfluenza virus, 11 with rhinovirus, 6 with adenovirus, 5 with cytomegalovirus, and 4 with other viruses. The circulation of strain groups was similar among sites, with RSV A predominating during 3 of 4 years. Of 753 RSV isolates that were typed, 602 (80%) were group A, 137 (18%) were group B, and 14 (2%) were determined to be both A and B by RT-PCR. RSV A strains comprised 81% of inpatient isolates and 78% of outpatient isolates (P = 0.32).
Demographic Characteristics
Significantly more inpatients than outpatients with RSV infection were non-Hispanic white, were under the age of 6 months, and had private insurance (Table 1, and Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Inpatients with RSV infection were significantly more likely than inpatients who did not have RSV infection to be non-Hispanic white and under the age of 6 months.
Table 1.
Characteristics of Children under 5 Years of Age with or without Respiratory Syncytial Virus (RSV) Infection.*
| Variable | Hospitalized Patients | Outpatients | P Value for Inpatients vs. Outpatients with RSV Infection |
||||
|---|---|---|---|---|---|---|---|
| RSV-Positive (N = 564) |
RSV-Negative (N = 2328) |
P Value | RSV-Positive (N = 355) |
RSV-Negative (N = 1820) |
P Value | ||
| no. (%) | no. (%) | ||||||
| Sex | 0.54 | 0.58 | 0.63 | ||||
| Male | 245 (43) | 1045 (45) | 160 (45) | 856 (47) | |||
| Female | 319 (57) | 1283 (55) | 195 (55) | 964 (53) | |||
| Race or ethnic group† | <0.001 | 0.42 | <0.001 | ||||
| White | 313 (55) | 1064 (46) | 127 (36) | 702 (39) | |||
| Black | 165 (29) | 825 (35) | 140 (39) | 718 (39) | |||
| Hispanic | 59 (10) | 290 (12) | 57 (16) | 235 (13) | |||
| Other or unknown | 27 (5) | 149 (6) | 31 (9) | 165 (9) | |||
| Age (mo) | <0.001 | <0.001 | <0.001 | ||||
| 0–5 | 328 (58) | 1042 (45) | 88 (25) | 257 (14) | |||
| 6–11 | 97 (17) | 306 (13) | 85 (24) | 354 (19) | |||
| 12–23 | 99 (18) | 441 (19) | 75 (21) | 513 (28) | |||
| 24–59 | 40 (7) | 539 (23) | 107 (30) | 696 (38) | |||
| Type of health insurance | 0.15 | 0.95 | 0.009 | ||||
| Public | 295 (52) | 1237 (53) | 220 (62) | 1147 (63) | |||
| Private | 225 (40) | 860 (37) | 119 (34) | 597 (33) | |||
| None | 39 (7) | 214 (9) | 14 (4) | 70 (4) | |||
| Unknown | 5 (1) | 17 (1) | 2 (1) | 6 (<1) | |||
Inpatient surveillance was conducted for 4 years, from October 2000 through September 2004, in the counties surrounding Nashville and Rochester and during the 2003–2004 season in Cincinnati. Outpatient surveillance was conducted concurrently from November through April in Nashville and Rochester from 2002 through 2004 and similarly in Cincinnati in the 2003–2004 season. Percentages may not total 100 because of rounding.
Race or ethnic group was reported by parents or guardians.
Hospitalized Patients
Rates of RSV infection
From 2000 through 2004, the average annual rate of RSV-associated hospitalization was 3 per 1000 children under the age of 5 years and 17 per 1000 children under the age of 6 months (Table 2). Hospitalization rates varied yearly and regionally. During the 2003–2004 season, among children under the age of 5 years, the rates were 5.7 per 1000 in Cincinnati, 2.3 per 1000 in Nashville, and 2.4 per 1000 children in Rochester. Among children under the age of 6 months, rates were 33.9 per 1000 in Cincinnati, 12.8 per 1000 in Nashville, and 14.2 per 1000 in Rochester.
Table 2.
Rates of Inpatient and Outpatient Treatment for Children under 5 Years of Age with Confirmed Respiratory Syncytial Virus (RSV) Infection per 1000 Children, According to Year.*
| Treatment Site and Year | Age, in Months | ||||
|---|---|---|---|---|---|
| 0–5 | 6–11 | 12–23 | 24–59 | 0–59 | |
| rate/1000 patients (95% CI) | |||||
| Inpatient | |||||
| Hospital | |||||
| 2000–2001 | 18.5 (14.4–22.9) | 7.4 (5.1–9.9) | 3.2 (1.9–4.8) | 0.4 (0.2–0.7) | 3.5 (2.9–4.1) |
| 2001–2002 | 11.7 (9.1–14.7) | 4.1 (2.4–5.8) | 2.5 (1.5–3.6) | 0.2 (0.0–0.4) | 2.2 (1.8–2.7) |
| 2002–2003 | 12.4 (9.4–15.2) | 3.4 (1.9–5.0) | 1.9 (1.1–2.8) | 0.2 (0.0–0.4) | 2.1 (1.7–2.5) |
| 2003–2004 | 21.7 (18.8–24.6) | 5.4 (3.8–7.0) | 3.1 (2.3–3.9) | 0.5 (0.3–0.8) | 3.7 (3.3– 4.1) |
| 2000–2004 | 16.9 (15.3–18.5) | 5.1 (4.6–5.5) | 2.7 (2.3–2.7) | 0.4 (0.3–0.4) | 3.0 (2.8–3.4) |
| Outpatient† | |||||
| Emergency department | |||||
| 2002–2003 | 39 (12–124) | 45 (13–157) | 24 (7–87) | 15 (5–44) | 22 (10–49) |
| 2003–2004 | 69 (34–143) | 68 (27–175) | 38 (15–102) | 11 (3–39) | 32 (19–54) |
| 2002–2004 | 55 (24–126) | 57 (20–161) | 32 (11–92) | 13 (4–41) | 28 (15–50) |
| Pediatric practice | |||||
| 2002–2003 | 108 (33–346) | 194 (77–492) | 53 (13–222) | 31 (9–100) | 61 (24–154) |
| 2003–2004 | 157 (54–462) | 160 (45–576) | 80 (22–282) | 77 (26–230) | 99 (44–219) |
| 2002–2004 | 132 (46–383) | 177 (61–511) | 66 (18–245) | 57 (19–167) | 80 (36–179) |
The presence of RSV infection was confirmed by reverse-transcriptase polymerase chain reaction (RT-PCR) and culture for inpatients and by RT-PCR for outpatients. Inpatient surveillance was conducted for 4 years, from October 2000 through September 2004, in the counties surrounding Nashville and Rochester and during the 2003–2004 season in Cincinnati. Outpatient surveillance was conducted concurrently in Nashville and Rochester from 2002 through 2004 and similarly in Cincinnati during the 2003–2004 season. Rates of infection are listed for November through April of each annual season.
Rates of infection for outpatients were calculated by multiplying the national rate of outpatient visits for acute respiratory infections from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey by the proportion of visits for acute respiratory infections in this study attributable to RSV for November through April 2002–2003 and 2003–2004.
Clinical Characteristics
Discharge diagnoses were based on clinical and laboratory information; clinicians were unaware of NVSN laboratory results. Among 564 inpatients with RSV infection, bronchiolitis was diagnosed in 397 (70%) and RSV-associated illness in 252 (45%). Among RSV-positive inpatients under the age of 12 months, bronchiolitis was diagnosed in 85% and RSV-associated illness in 53%, as compared with rates of 31% and 18% in children between the ages of 24 and 59 months, respectively (P<0.001). The major diagnoses among this older age group were pneumonia (51%) and asthma (60%). Among all RSV-positive inpatients, 95% had labored respirations and required supplemental oxygen, 78% had wheezing, 69% were febrile, and 1% had apnea. There were no deaths. Hospitalizations lasted for a median of 2 days and lasted from less than 1 day to 26 days.
Outpatients
Rates of RSV Infection
Overall, the estimated rates of outpatient office visits for acute respiratory infections among children under 5 years of age were higher than the estimated rates of emergency department visits by a factor of 3, but the difference ranged from a factor of 2 to a factor of 11, depending on the calendar year and the age group (Table 2). For children under 5 years of age, population-based rates of RSV-associated emergency department visits in Rochester were 19 per 1000 for the 2002–2003 season and 36 per 1000 for the 2003–2004 season; in Cincinnati, the rate was 44 per 1000 children during the 2003–2004 season. These population-based rates corresponded closely to emergency department rates estimated from national databases (NAMCS and NHAMCS) for 2002–2003 and 2003–2004 (22 and 32 per 1000, respectively).
Clinical Characteristics
The most frequent clinical findings among 355 RSV-positive outpatients were cough (98%), fever (75%), labored respirations (73%, including 85% and 73% of patients in emergency departments and pediatric offices, respectively; P<0.001), and wheezing (65%). Discharge diagnoses included upper respiratory tract infection (32%), bronchiolitis (20%), asthma (13%), and pneumonia (8%). Only 3% of outpatients with RSV infection received the diagnosis of RSV-associated illness, as compared with 45% of inpatients (P<0.001).
Risk Factors for Hospitalization
To assess the effects that environmental and host factors may have had on severe illness, we compared the results for RSV-positive inpatients with those of RSV-positive outpatients. To assess the effects of these factors on the development of an acute respiratory infection specifically from RSV, as compared with other causes, we compared the results of children with respiratory infections who were RSV-positive with those who were RSV-negative (Table 3).
Table 3.
Proportion of Inpatients and Outpatients with and without Respiratory Syncytial Virus (RSV) with Factors Potentially Associated with the Risk of RSV Infection and Severity of Illness.*
| Variable | All Patients (N = 5067) |
Children with RSV Infection (N = 919) |
Children without RSV Infection (N = 4148) |
||||
|---|---|---|---|---|---|---|---|
| Hospitalized (N = 564) |
Outpatient (N = 355) |
P Value | Hospitalized (N = 2328) |
Outpatient (N = 1820) |
P Value | ||
| no./total no. (%) | no./total no. (%) | ||||||
| Underlying condition | |||||||
| Any high-risk condition | 1765 (35) | 189 (34)† | 96 (27) | 0.05 | 984 (42) | 495 (27) | <0.001 |
| Prematurity | 657 (13) | 91 (16) | 30 (8) | 0.001 | 355 (15) | 181 (10) | <0.001 |
| Prophylaxis with palivizumab | |||||||
| High-risk children (% of all high-risk children) | 174 (10) | 17 (9) | 4 (4) | 0.22 | 117 (12) | 36 (7) | 0.009 |
| Premature birth (% of all premature births) | 140 (21) | 16 (18) | 3 (10) | 0.48 | 93 (26) | 28 (15) | 0.008 |
| Day-care conditions | |||||||
| >4 hrs/wk | 1868 (37) | 160 (28) | 140 (39)† | <0.001 | 683 (29) | 885 (49) | <0.001 |
| Attendees‡ | |||||||
| ≥6 | 1024/3826 (27) | 55/353 (16) | 85/352 (24)† | 0.006 | 256/1321 (19) | 628/1800 (35) | <0.001 |
| >12 | 266/3826 (7) | 6/353 (2)§ | 26/352 (7) | <0.001 | 53/1321 (4) | 181/1800 (10) | <0.001 |
| Age of other children in home | |||||||
| All | 3616 (71) | 427 (76) | 242 (68) | 0.02 | 1723 (74) | 1224 (67) | <0.001 |
| <5 yr | 2373 (47) | 305 (54) | 137 (39) | <0.001 | 1171 (50) | 760 (42) | <0.001 |
| 5 to <18 yr | 2204 (43) | 235 (42) | 152 (43) | 0.78 | 1020 (44) | 797 (44) | 0.97 |
| Smoking exposure in home | |||||||
| Any | 2081 (41) | 239 (42) | 141 (40) | 0.43 | 978 (42) | 723 (40) | 0.12 |
| Mother‡ | 764/3090 (25) | 83/307 (27) | 80/355 (23) | 0.21 | 156/608 (26) | 445/1820 (24) | 0.59 |
| Breast-feeding history | |||||||
| Any duration | 2811 (55) | 320 (57) | 210 (59) | 0.55 | 1283 (55) | 998 (55) | 0.89 |
| None or <1 mo | 3078 (61) | 360 (64) | 186 (52) | <0.001 | 1491 (64) | 1041 (57) | <0.001 |
| 1–6 mo | 1437 (28) | 166 (29) | 120 (34) | 0.21 | 637 (27) | 514 (28) | <0.53 |
| ≥7 mo | 515 (10) | 32 (6) | 48 (14) | <0.001 | 185 (8) | 250 (14) | <0.001 |
| Maternal education of high school or less‡ | 2129/3700 (58) | 189/347 (54) | 197/352 (56) | 0.75 | 716/1198 (60) | 1027/1803 (57) | 0.14 |
Inpatient surveillance was conducted for 4 years, from October 2000 through September 2004, in the winter months in the counties surrounding Nashville and Rochester and during the 2003–2004 season in Cincinnati. Outpatient surveillance was conducted concurrently from November through April in Nashville and Rochester from 2002 through 2004 and similarly in Cincinnati during the 2003–2004 season.
P<0.001 for the comparison with patients in the same category without RSV.
Data were obtained only from 2002 through 2004.
P = 0.04 for the comparison with patients in the same category without RSV.
One or more high-risk conditions were present in 189 of 564 of RSV-infected inpatients (34%) and in 96 of 355 outpatients (27%) (Table 3). The most frequent conditions among high-risk RSV-positive children were previous wheezing, which occurred in 104 inpatients (55%) and 70 outpatients (73%) (P = 0.005), and prematurity, which occurred in 91 inpatients (48%) and 30 outpatients (31%) (P = 0.009). Among children with acute respiratory infections that were either RSV-positive or RSV-negative, the presence of any high-risk condition significantly correlated with hospitalization and increasing age (P<0.001). However, more RSV-negative inpatients than RSV-positive inpatients had high-risk conditions (42% vs. 34%, P = 0.001).
Prophylaxis with palivizumab was administered to 174 of 1765 of high-risk children (10%) and to 140 of 657 children with a history of prematurity (21%) (Table 3). The proportion of high-risk patients with RSV infection who received palivizumab was smaller than the proportion of patients without RSV infection, but the difference was not significant (21 of 285 patients [7%] vs. 153 of 1479 patients [10%], P = 0.15). The rates of prophylaxis with palivizumab differed significantly between high-risk inpatients and high-risk outpatients only among children with RSV-negative acute respiratory infections (P = 0.009).
Factors indicative of a child’s environment and care, including day-care attendance, exposure to tobacco smoke, and maternal educational level, were also examined (Table 3). None of these factors correlated with illness requiring hospitalization among children with RSV-positive or RSV-negative acute respiratory infections. Only breast-feeding of less than 1 month’s duration and the presence of other children under the age of 5 years in the home significantly correlated with hospitalization.
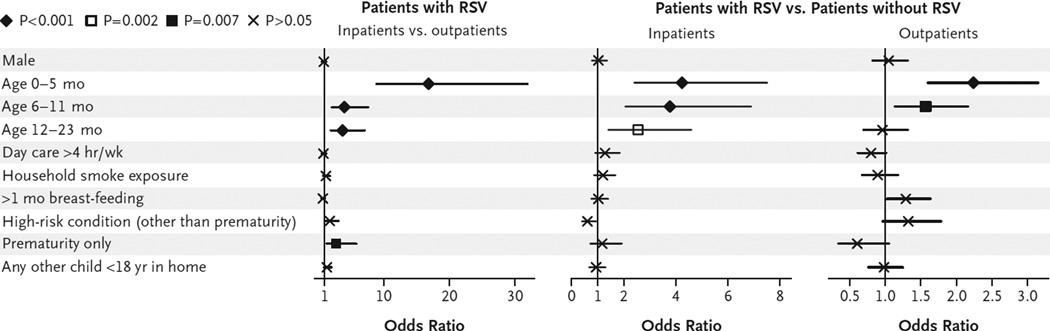
However, multiple logistic-regression analyses revealed that none of these potential risk factors, including sex, independently correlated with more severe illness among patients with RSV infection after adjustment for other covariates (Fig. 2). Only a younger age and prematurity were independently associated with RSV illness requiring hospitalization. In comparison with RSV-negative patients, RSV-positive inpatients and outpatients were significantly younger (P<0.001) but were not more likely to have been premature, indicating that a young age imposed a greater risk for RSV-positive illness than for RSV-negative illness.
Figure 2. Odds Ratios for Potential Risk Factors in Patients with and Those without Respiratory Syncytial Virus (RSV) Infection, According to Treatment Site.
According to multiple logistic-regression analyses, the only risk factors associated with RSV illness requiring hospitalization were an age of less than 2 years (especially under 6 months) and a history of prematurity. For age groups, the reference group is patients between the ages of 24 months and 59 months. Horizontal lines indicate 95% confidence intervals.
Discussion
Our findings from three geographically diverse populations provide a comprehensive view of the RSV burden among children within the first 5 years of life. In our population, rates of hospitalization for RSV-associated illness were three times as high as those associated with influenza or parainfluenza viruses, and the proportion of children receiving influenza immunization was low (18%).13 The estimated rates of outpatient visits associated with RSV infection were similar to those associated with influenza for all children under 5 years of age but were markedly greater for children under the age of 6 months.12–14 However, prospective population-based information concerning RSV infection among older children and outpatients is lacking.3–5,8,20–22 By concurrently addressing these populations, our study demonstrates a previously unrecognized size and spectrum of burden of RSV infection among all children under 5 years of age.
If we extrapolate from our population-based data to the entire U.S. population, an estimated 2.1 million children under 5 years of age with RSV infection would require medical attention each year. Among these children with RSV-related illnesses, approximately 57,527 (3%) are hospitalized, 517,747 (25%) are treated in emergency departments, and 1,534,064 (73%) are treated by pediatric practices; of these outpatients, 1,256,014 (61%) are between 2 and 5 years of age.
These rates of visits for RSV-associated acute respiratory infections indicate that a major proportion of RSV’s burden results in outpatient visits among children beyond infancy. Furthermore, the estimated outpatient rates are probably close to the actual national rates, since our directly determined population-based emergency department rates in Rochester and Cincinnati were very similar and well within the confidence intervals of the estimated rates.
The outpatient burden of RSV on health care resources is probably not fully recognized by health care providers and by public health officials, since only 3% of outpatients with confirmed RSV infection received the specific diagnosis of RSV infection; bronchiolitis was diagnosed in 20% of such children. Significantly more RSV outpatient visits occurred among primary care practices than among emergency departments, and the illnesses of patients from pediatric practices were similar to those of patients in emergency departments and reflected moderately severe disease. This suggests that outpatient visits contribute greatly to RSV’s burden by both their frequency and the severity of illness.
The rates of RSV infections requiring medical attention are high not only during infancy but throughout the first 5 years of life. This factor underscores the as-yet-unmet need for an effective vaccine.1,23–26 Of the estimated 2 million children under the age of 5 years who require care for RSV infections annually, 78% are over the age of 1 year. Meanwhile, much effort has been appropriately focused on children who are at highest risk for severe RSV disease and who most need prophylaxis and health care resources.24,27–29 However, our study suggests that since RSV infection among previously healthy children imposes a much larger burden than was previously recognized, the need to address this issue may be even greater.
Characteristics that were most frequently associated with RSV illness requiring hospitalization included male sex, chronic coexisting medical conditions, lower socioeconomic status, smoke exposure, lack of breast-feeding, and contact with other children.3,6,8,29–36 However, none of these factors in our population independently correlated with illness severity, except for young age and prematurity, and the risk of prematurity was not specific for RSV disease. Only young age imposed a significantly greater risk of severe illness among children with RSV infection than among children with acute respiratory infections caused by other organisms.
Our findings may differ from those of previous studies partly because our study was designed to evaluate the role of risk factors in acquiring RSV infection and the development of more severe disease specifically associated with RSV. We assessed the first factor by comparing children with acute respiratory infections who were RSV-positive with those who were RSV-negative. We assessed the second factor by comparing children with RSV infection who were hospitalized with those who were treated as outpatients. Greater use of palivizumab prophylaxis also may have affected the rates and characteristics of recently hospitalized high-risk children.37 This explanation is supported by our findings, which suggest that the proportion of high-risk children receiving prophylaxis who were hospitalized with RSV-negative infection was significantly greater than the proportion of those with RSV-positive infection.
Most children with RSV infection, both those who were hospitalized and those who were treated as outpatients, had no coexisting medical conditions or characteristics that significantly identified them as being at greater risk for severe RSV disease, except for being under 2 years of age. RSV’s major burden, therefore, occurs among previously healthy children whose risk of severe illness cannot be predicted.
On the basis of our findings, we estimate that among children under the age of 5 years, RSV infection results in approximately 1 of 334 hospitalizations, 1 of 38 visits to an emergency department, and 1 of 13 visits to a primary care office each year in the United States.
Supplementary Material
Acknowledgments
Supported through cooperative agreements with the CDC.
Dr. Hall reports receiving grant support and consulting fees from MedImmune; Dr. Weinberg, research support from Astellas and MedImmune and consulting fees from MedImmune; Dr. Staat, consulting fees or fees for serving on paid advisory boards from GlaxoSmithKline, Merck, and MedImmune, lecture fees from Merck, and research support from MedImmune; Dr. Griffin, grant support from MedImmune and Pfizer; and Dr. Grijalva, lecture fees from Wyeth. No other potential conflict of interest relevant to this article was reported.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the CDC.
We thank the children and their parents who participated in this study; all the members of the New Vaccine Surveillance Network, including Geraldine Lofthus, Kenneth Schnabel, Andrea Marino, Lynne Shelley, Jennifer Carnahan, Linda Anderson, Gladys Lathan, Charlene Freundlich, Christina Albertin, and Rebecca Martinez at the University of Rochester; John Williams, Diane Kent, Ann Clay, Ayesha Khan, Yi Wei Tang, Haijing Li, Jennifer Doersam, Amondrea Blackman, Nayleen Whitehead, Erin Keckley, and Jody Peters at Vanderbilt University; Linda Jamison, David Witte, David Bernstein, Emilie Grube, Vanessa Florian, Pamela Groen, and Joel Mortensen at the Cincinnati Children’s Medical Center; and Ranee Seither, Aaron Curns, Larry Anderson, Carolyn B. Bridges, Benjamin Schwartz, Frances Walker, John Copeland, Alicia Fry, Jennifer Reuer, and John Zhang at the CDC.
References
- 1.Bethesda, MD: National Institute of Allergy and Infectious Diseases; 2002. [Accessed January 12, 2009]. The Jordan Report: accelerated development of vaccines 2002. at http://www3.niaid.nih.gov/topics/Malaria/PDF/jordan20_2002.pdf. [Google Scholar]
- 2.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 3.Boyce TG, Mellen BG, Mitchel EF, Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 4.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 5.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(Suppl):S127–S132. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 6.Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 7.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Peck AJ, Holman RC, Curns AT, et al. Lower respiratory tract infections among American Indian and Alaska Native children and the general population of U.S. children. Pediatr Infect Dis J. 2005;24:342–351. doi: 10.1097/01.inf.0000157250.95880.91. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg GA, Erdman DD, Edwards KM, et al. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189:706–710. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
- 10.Erdman DD, Weinberg AG, Edwards KM, et al. GeneScan reverse transcription-PCR assay for detection of six common respiratory viruses in young children hospitalized with acute respiratory illness. J Clin Microbiol. 2003;41:4298–4303. doi: 10.1128/JCM.41.9.4298-4303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullins JA, Erdman DD, Weinberg GA, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 13.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 14.Miller EK, Griffin MR, Edwards KM, et al. Influenza burden for children with asthma. Pediatrics. 2008;121:1–8. doi: 10.1542/peds.2007-1053. [DOI] [PubMed] [Google Scholar]
- 15.Miller EK, Lu X, Erdman DD, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD. Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. Pediatr Infect Dis J. 2004;23(Suppl):S188–S192. doi: 10.1097/01.inf.0000144660.53024.64. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. doi: 10.1016/j.jpeds.2008.11.034. (in press) [DOI] [PubMed] [Google Scholar]
- 18.Casella G, Berger RL, editors. Statistical inference. 2nd. Pacific Grove, CA: Duxbury/Thomson Learning; 2002. Properties of a random sample; pp. 240–245. [Google Scholar]
- 19.StataCorp. Stata statistical software: release 10. College Station, TX: StataCorp; 2007. [Google Scholar]
- 20.Schanzer DL, Langley JM, Tam TW. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr Infect Dis J. 2006;25:795–800. doi: 10.1097/01.inf.0000232632.86800.8c. [DOI] [PubMed] [Google Scholar]
- 21.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson KG, McNally T, Silverman M, Simons P, Stockton JD, Zambon MC. Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine. 2006;24:102–108. doi: 10.1016/j.vaccine.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clin Infect Dis. 2008;46:50–57. doi: 10.1086/524019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins PL, Murphy BR. New generation live vaccines against human respiratory syncytial virus designed by reverse genetics. Proc Am Thorac Soc. 2005;2:166–173. doi: 10.1513/pats.200501-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Englund J. In search of a vaccine for respiratory syncytial virus: the saga continues. J Infect Dis. 2005;191:1036–1039. doi: 10.1086/427998. [DOI] [PubMed] [Google Scholar]
- 26.van Drunen Littel-van den Hurk S, Mapletoft JW, Arsic N, Kovacs-Nolan J. Immunopathology of RSV infection: prospects for developing vaccines without this complication. Rev Med Virol. 2007;17:5–34. doi: 10.1002/rmv.518. [DOI] [PubMed] [Google Scholar]
- 27.Welliver RC. Respiratory syncytial virus infection: therapy and prevention. Paediatr Respir Rev. 2004;(Suppl):S127–S133. doi: 10.1016/s1526-0542(04)90024-3. [DOI] [PubMed] [Google Scholar]
- 28.Meissner HC, Rennels MB, Pickering LK, Hall CB. Risk of severe respiratory syncytial virus disease, identification of high risk infants and recommendations for prophylaxis with palivizumab. Pediatr Infect Dis J. 2004;23:284–285. doi: 10.1097/01.inf.0000121203.33560.f9. [DOI] [PubMed] [Google Scholar]
- 29.Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143(Suppl):S118–S126. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen HE, Siersma V, Andersen S, et al. Respiratory syncytial virus infection — risk factors for hospital admission: a case-control study. Acta Paediatr. 2003;92:1314–1321. [PubMed] [Google Scholar]
- 31.Reeve CA, Whitehall JS, Buettner PG, Norton R, Reeve DM, Francis F. Predicting respiratory syncytial virus hospitalisation in Australian children. J Paediatr Child Health. 2006;42:248–252. doi: 10.1111/j.1440-1754.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 32.Bradley JP, Bacharier LB, Bonfiglio J, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115(1):e7–e14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 33.von Linstow ML, Høgh M, Nordbø SA, Eugen-Olsen J, Koch A, Høgh B. A community study of clinical traits and risk factors for human metapneumovirus and respiratory syncytial virus infection during the first year of life. Eur J Pediatr. 2008;167:1125–1133. doi: 10.1007/s00431-007-0643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbonell-Estrany X, Figueras-Aloy J, Law BJ. Identifying risk factors for severe respiratory syncytial virus among infants born after 33 through 35 completed weeks of gestation: different methodologies yield consistent findings. Pediatr Infect Dis J. 2004;23(Suppl):S193–S201. doi: 10.1097/01.inf.0000144664.31888.53. [DOI] [PubMed] [Google Scholar]
- 35.Law BJ, Langley JM, Allen U, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J. 2004;23:806–814. doi: 10.1097/01.inf.0000137568.71589.bd. [DOI] [PubMed] [Google Scholar]
- 36.Rossi GA, Medici MC, Arcangeletti MC, et al. Risk factors for severe RSV-induced lower respiratory tract infection over four consecutive epidemics. Eur J Pediatr. 2007;166:1267–1272. doi: 10.1007/s00431-007-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero JR. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: results from four years of palivizumab usage. Pediatr Infect Dis J. 2003;22(Suppl):S46–S54. doi: 10.1097/01.inf.0000053885.34703.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.