Abstract
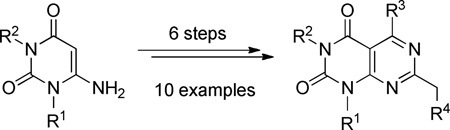
A novel synthetic route to 1,3,5,7-tetrasubstituted pyrimido[4,5-d]pyrimidine-2,4-diones, of interest for potential antitumor activity, is reported. The route uses 1,3-disubstituted 6-amino uracils as starting materials. The key step is a hydrazine-induced cyclization reaction to form the fused pyrimidine ring. By choosing different uracils, acylation reagents and alkylation reagents, substituents at N-1, N-3, C-5, and C-7 may be selectively varied to provide a structurally diverse set of compounds for biological evaluation.
Keywords: pyrimidopyrimidines, synthesis, heterocycles, antitumor, inhibitor
Graphical Abstract
Introduction
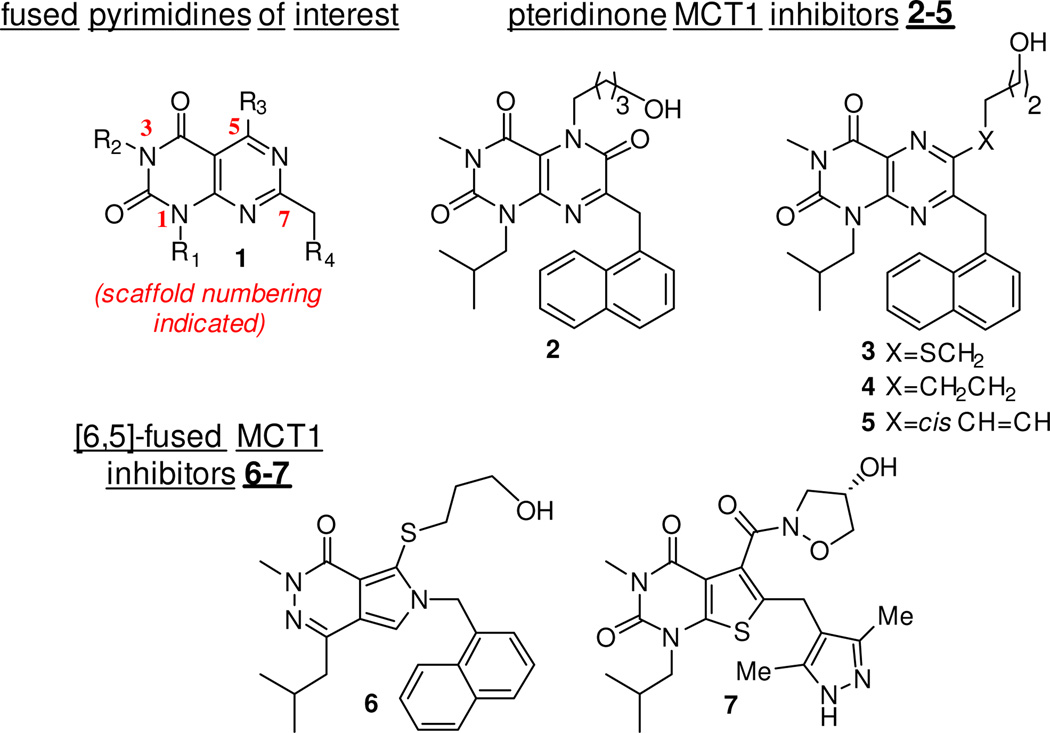
Pyrimidopyrimidines have attracted considerable attention in medicinal chemistry due to their significant and diverse biological activity,1 including antitumor,2 antiviral,3 antimicrobial,4 and anti-allergic effects.5 As part of an ongoing effort to investigate the ability of various scaffolds to act as antitumor agents by inhibiting monocarboxylate transporters (MCTs),6,7 the investigation of this scaffold (1) was of particular interest due to its structural homology with the pteridinone MCT1 inhibitor 2 (Figure 1). [6,6]-fused scaffolds such as compounds 2–5 are potent MCT1 inhibitors with antitumor activity,7 as are related [6,5]-fused ring compounds such as 6 and 7.8
Figure 1.
Scaffold 1 and potent MCT1 inhibitors 2–7
To our knowledge, general synthetic methods to prepare 1,3,5,7-tetrasubstituted pyrimido[4,5-d]pyrimidines (general structure 1) have not been extensively investigated. Hamed’s method9 has limitations in scope because it requires that the compounds produced have identical substituents at the C-5 and C-7 positions. Yoneda’s method10 also suffers from limitations of providing only products with an aryl substituent at C-7 and the requirement for relatively inaccessible starting materials. Herein we report our efforts toward developing a general route to prepare compounds in this scaffold.
Results and Discussion
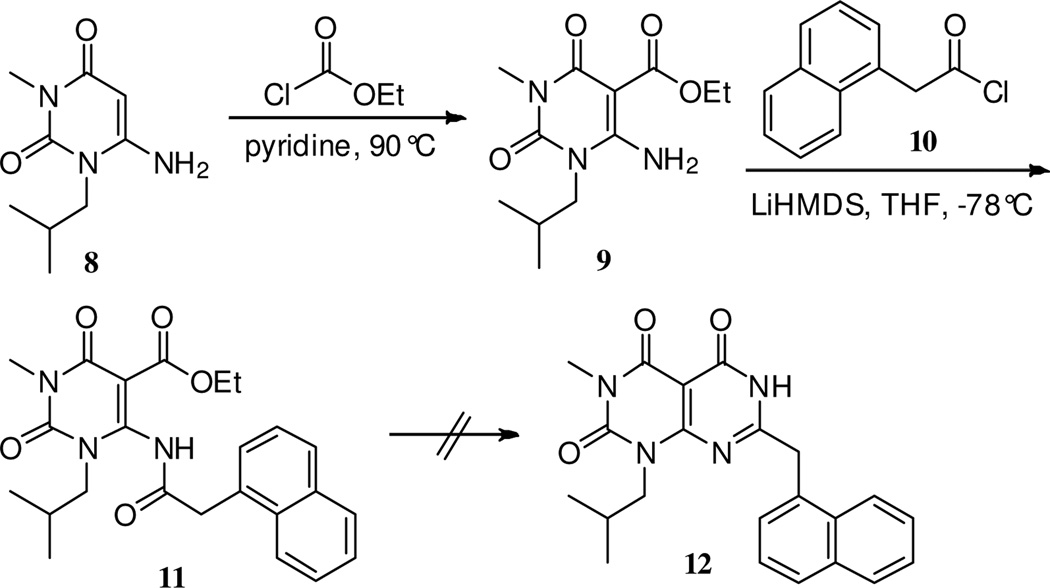
Due to its enamine character, the commercially available 6-amino uracil 8 (Scheme 1) can in principle act as a nucleophile at either a carbon or nitrogen atom. The amine group of 8 is only very weakly basic, however. For example, attempted amide coupling reactions of 8 using carboxylic acids and conventional coupling reagents, such as EDCI and HOBt, were unsuccessful and gave only recovered starting material. Reactions reported in the literature involving the amine group of 8 and its analogues are rare but include some very reactive electrophiles, including imidoyl derivatives,11 DMF-DMA12 and succinic anhydride.13
Scheme 1.
Attempts to prepare compound 12
Interestingly, even chloroformates are unreactive toward the amine group: only electrophilic attack at C-5 occurs.14 In our hands the use of ethyl chloroformate in pyridine at 90 °C cleanly gave ester 9 in 70% yield (Scheme 1). The nucleophilicity of the amine group in the ester product 9 should be even lower than that of 8, given the additional conjugation to the ester group. Thus we opted to use a strong base (LiHMDS), expecting that the anion thus provided might be acylated by an acid chloride, such as the naphthyl-substituted acetyl chloride 10. Indeed this approach was successful, giving the desired product 11 in 52% yield.15 Attempted cyclization to the [6,6]-fused pyrimidinone 12, however, proved to be problematic. Only low yields (<5%) of 12 were obtained using 7N NH3 in MeOH for 2 weeks at room temperature or under a variety of more harsh microwave reaction conditions. Amidine or amide-promoted ring closure using 2-naphthalen-1-ylacetamidine16 (neat, 170 °C, 1 h) or 2-(naphthalene-1-yl)-acetamide (DMF, reflux, 14 h, or DMF, microwave 250 °C, 30 min) were similarly unsuccessful.
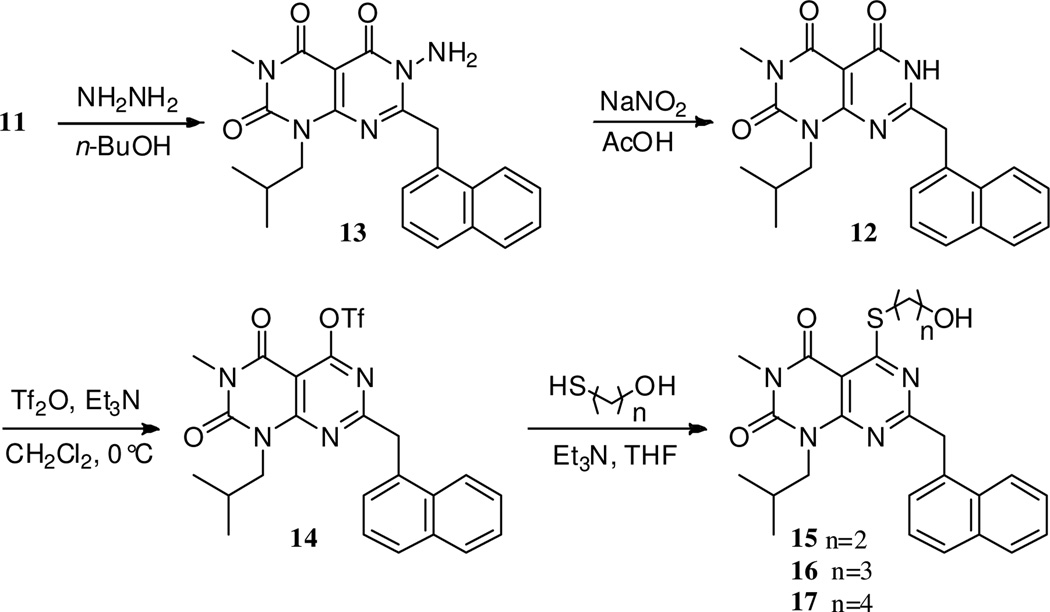
The desired cyclization was observed when the more nucleophilic reagent hydrazine was used in place of ammonia (Scheme 2).17 Thus, treatment of 11 with hydrazine gave product 13, which required cleavage of the N-N bond to give compound 12. This was achieved under diazotization conditions (NaNO2, AcOH) followed by heating to reflux to give 12 in 38% yield for the two step procedure.18 While efforts to improve the yield for this conversion were unsuccessful, compound 12 was readily converted to the triflate 14 (triflic anhydride, triethylamine) in 64% yield.19 Access to triflate 14 permitted the facile preparation of the desired thioethers 15–17, analogues of pteridinone 2, in 67–77% yield by treatment with ω-mercaptoalcohols.20
Scheme 2.
Successful cyclization and conversion to test compounds
We investigated a number of alternative strategies to access the fused pyrimidinone 12, given the modest yield of the two step conversion from ester 11. This included the reaction of acyl cyanates21 with enamine 8. Such alternative strategies were unfortunately ill-suited for suitable for efficient and scalable preparation of this compound.
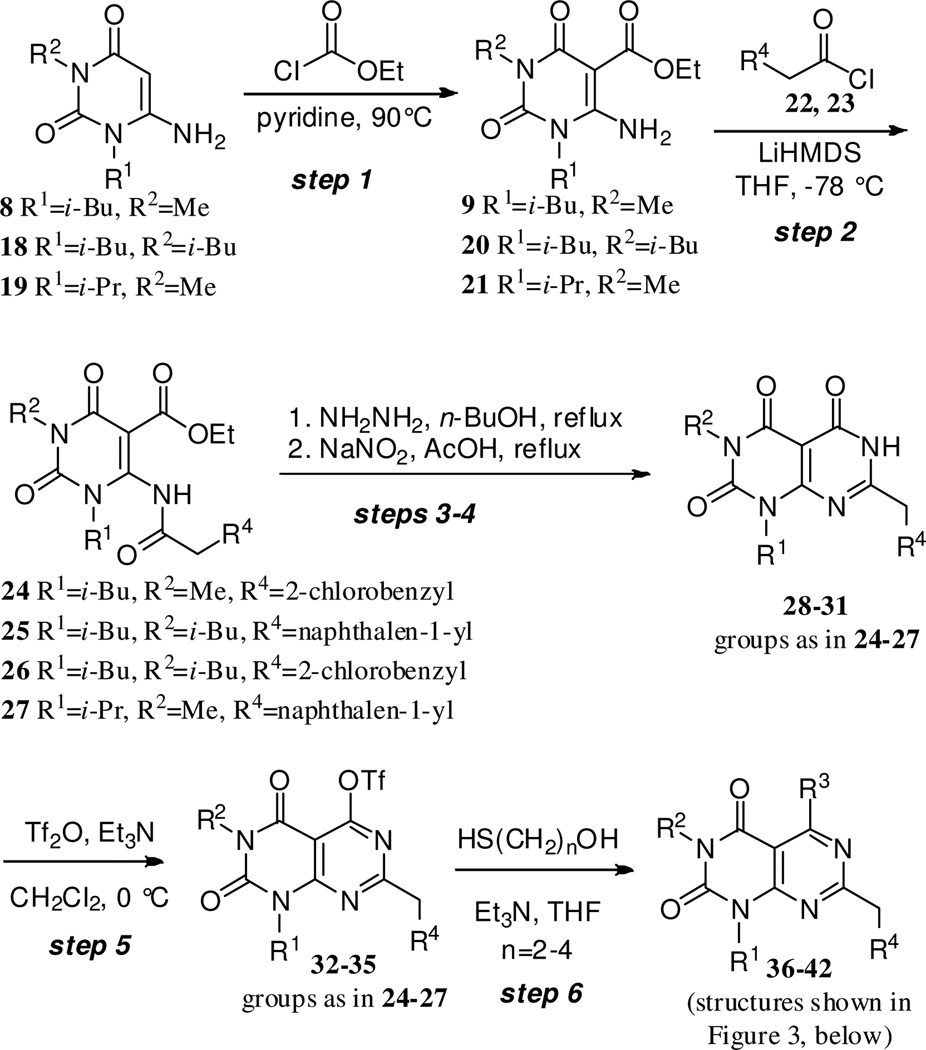
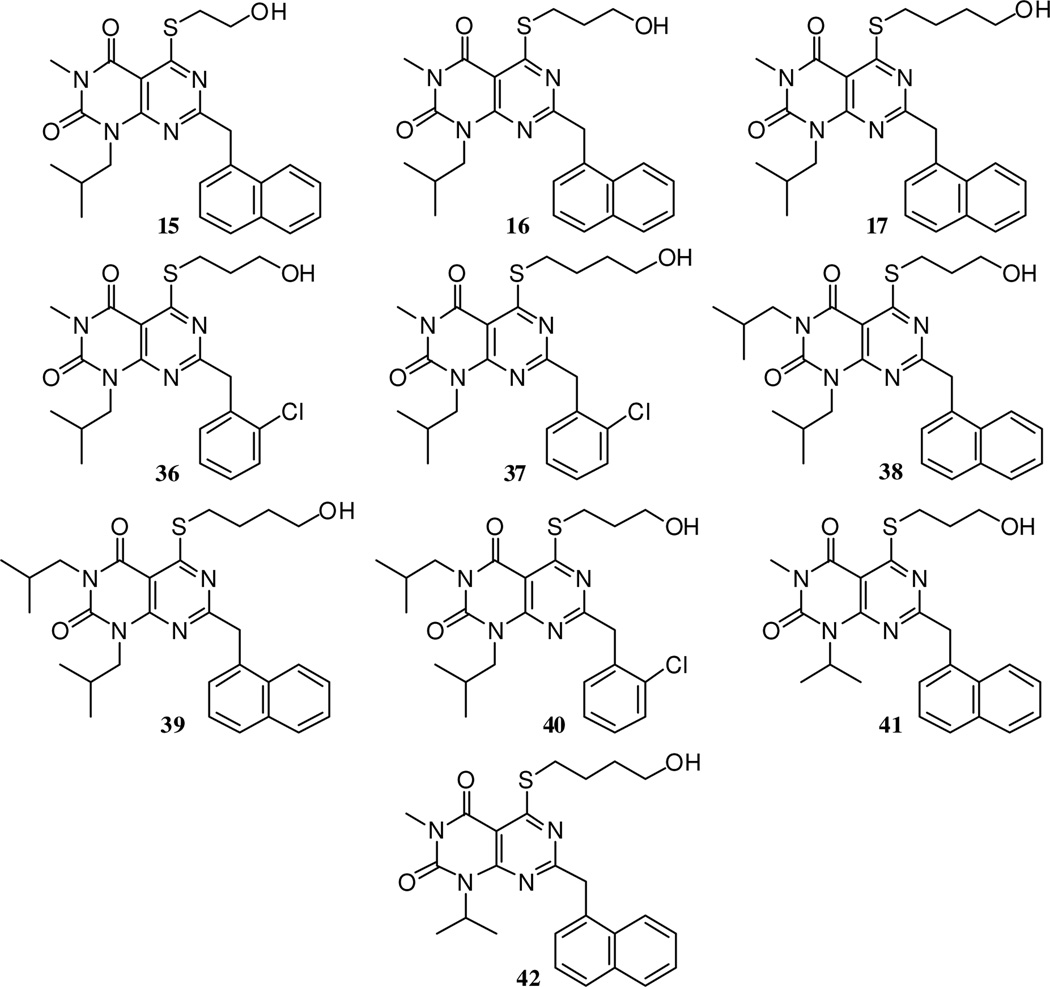
Encouraged by the ability of the Scheme 1–2 route to give the desired products, albeit in modest yields, the generality of the approach was then investigated (Scheme 3). Using three different 6-aminouracil starting materials (8, 18 and 1922), the same six step sequence was followed. The acid chloride used to install the R4 substituent was varied, as was the thiol nucleophile used to install the R3 group in the final step. With these modifications, seven additional analogues of compounds 15–17 were synthesized (Figure 2, compounds 36–42). Because structural analogues of pteridinone 2 were desired, only aryl acetyl chlorides 22 and 23 were used, but a variety of other acid chlorides would likely be well-suited for use in this process. Similarly, triflates 32–35 would likely be quite useful substrates for transition metal-mediated conversions. Thus diverse structures in the scaffold are accessible using these methods.
Scheme 3.
Generalization of the six step sequence.
Figure 2.
The isolated yields for material obtained in each step during the synthesis of compounds 15–17 and 36–42 was fairly consistent. Step 1 yields ranged from 60–70%, step 2 from 47–52%, steps 3–4 (combined) from 31–54%, step 5 from 52–83%, and step 6 from 45–82%. Thus while the overall yield for the final products made by this six-step process was low (4–7%), the method gave access to densely-substituted heterocycles, compounds that to our knowledge are inaccessible using previously-reported methods.
Conclusion
In this study a six-step sequence was devised to prepare ten 1,3,5,7-tetrasubstituted pyrimido[4,5-d]pyrimidine-2,4-diones. By choosing different starting materials and reagents, substituents were varied at N-1, N-3, C-5, and C-7. Efforts to optimize these synthetic procedures and the evaluation of the biological properties of the final products will be the subject of future reports from our laboratory.
Supplementary Material
Acknowledgments
Financial support was provided by NIH grants (R01CA154739 and U54 MH084512-05-21755). We thank Dr. Xiangming Kong, NMR facility manager at TSRI, for the help with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data
1H NMR spectra for all new compounds are provided in supporting information.
References and Notes
- 1.Abdel-Rahman R, El-Mahdy K. Heterocycles. 2012;85:2391–2414. [Google Scholar]
- 2.(a) Tong Y, Penning TD, Florjancic AS, Miyashiro J, Woods KW. 0220572. U.S. Pat. Appl. 2012; (b) El-Moghazy SM, Ibrahim DA, Abdelgawad NM, Farag NAH, El-Khouly AS. Scientia Pharma. 2011;79:429–447. doi: 10.3797/scipharm.1103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Delia TJ, Baumann M, Bunker A. Heterocycles. 1993;35:1397–1410. [Google Scholar]
- 3.(a) Iadonato SP, Bedard K, Imanaka MW, Fowler KW. WO 2013049352 A2 20130404. PCT Int. Appl. 2013; (b) Krueger AC, Madigan DL, Beno DW, Betebenner DA, Carrick R, Green BE, He W, Liu D, Maring CJ, McDaniel KF, Mo H, Molla A, Motter CE, Pilot-Matias TJ, Tufano MD, Kempf DJ. Bioorg. Med. Chem. Lett. 2012;22:2212–2215. doi: 10.1016/j.bmcl.2012.01.096. [DOI] [PubMed] [Google Scholar]
- 4.(a) Sharma P, Rane N, Pandey P. Archiv der Pharmazie. 2006;339:572–578. doi: 10.1002/ardp.200600067. [DOI] [PubMed] [Google Scholar]; (b) Sharma P, Rane N, Gurram VK. Bioorg. Med. Chem. Lett. 2004;14:4185–4190. doi: 10.1016/j.bmcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura N, Ohnishi A. EP 0 163 599. Eur. Patent Appl. 1985
- 6.Doherty JR, Yang C, Scott K, Cameron MD, Fallahi M, Li W, Hall MA, Amelio AL, Mishra JK, Li F, Tortosa M, Genau HM, Rounbehler RJ, Yungi L, Dang CV, Kumar KG, Butler AA, Bannister TD, Hooper AT, Unsal-Kacmaz K, Roush WR, Cleveland JL. Cancer Res. 2014;74:908–920. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Yang C, Doherty JR, Roush WR, Cleveland JL, Bannister TD. J. Med. Chem. 2014;57:7317–7324. doi: 10.1021/jm500640x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Guile SD, Bantick JR, Cheshire DR, Cooper ME, Davis AM, Donald DK, Evans R, Eyssade C, Ferguson DD, Hill S, Hutchinson R, Ingall AH, Kingston LP, Marin I, Martin BP, Mohammed RT, Murry C, Perry MWD, Reynolds RH, Thorne PV, Wilkinson DJ, Withnall J. Bioorg. Med. Chem. Lett. 2006;16:2260–2265. doi: 10.1016/j.bmcl.2006.01.024. [DOI] [PubMed] [Google Scholar]; (b) Guile SD, Bantick JR, Cooper ME, Donald DK, Eyssade C, Ingall AH, Lewis RJ, Martin BP, Mohammed RT, Potter TJ, Reynolds RH, St-Gallay SA, Wright AD. J. Med. Chem. 2007;50:254–263. doi: 10.1021/jm060995h. [DOI] [PubMed] [Google Scholar]; (c) Bueno V, Binet I, Steger U, Bundick R, Ferguson D, Murray C, Donald D, Wood K. Transplantation. 2007;84:1204–1207. doi: 10.1097/01.tp.0000287543.91765.41. [DOI] [PubMed] [Google Scholar]; (d) Ovens MJ, Davies AJ, Wilson MC, Murray CM, Halestrap AP. Biochem. J. 2010;425:523–530. doi: 10.1042/BJ20091515. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Critchlow SE, Tate L. 089580. WO Patent Appl. 2010
- 9.Hamed A. Synthesis. 1992:591–595. [Google Scholar]
- 10.Yoneda F, Higuchi M. Chem. Pharm. Bull. 1972;20:2076–2078. [Google Scholar]
- 11.Liubchak K, Tolmachev A, Grygorenko OO, Nazarenko K. Tetrahedron. 2012;68:8564–8571. [Google Scholar]
- 12.Prajapati D, Thakur AJ. Tetrahedron Lett. 2005;46:1433–1436. [Google Scholar]
- 13.Kolotova NV, Dolzhenko AV, Kozminykh VO, Kotegov VP, Godina AT. Pharma. Chem. J. 1999;33:635–637. [Google Scholar]
- 14.(a) Yoneda F, Higuchi M. Bull. Chem. Soc. Jpn. 1973;46:3849–3853. [Google Scholar]; (b) Marumoto R, Furukawa Y. Chem. Pharma. Bull. 1977;25:2974–2982. [Google Scholar]
- 15.Synthesis of ethyl 1-isobutyl-3-methyl-6-(2-(naphthalene-1-yl)acetamido)-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylate (11): A solution of 9 (757 mg, 2.81 mmol) in THF (42 mL) was cooled to −78 °C under N2. A solution of LiHMDS in THF (3.23 mL, 3.23 mmol, 1 M in THF) was added dropwise. The reaction mixture was stirred at −78 °C for 10 min, 0 °C for 30 min, and cooled to −78 °C. Acid chloride 10 (748 mg, 3.66 mmol) was added in one portion. The resultant mixture was slowly warmed up to room temperature and stirred overnight. The reaction was quenched with saturated NH4Cl, extracted with ethyl acetate (EA). The combined organic extracts were washed with brine and dried over Na2SO4. The crude product was concentrated and purified by flash chromatography on silica gel (hexanes:ethyl acetate=2:3) to afford 633 mg (52%) of 11 as a yellow solid. Rf= 0.34 (hexanes:ethyl acetate=1:1); LC-MS (ESI): m/z 438 [M+1]+; 1H NMR (400 MHz, CDCl3) δ (ppm) 0.14 (d, J=6.8 Hz, 6H), 1.15 (t, J=7.2 Hz, 3H), 1.44 (sep, J=6.8 Hz, 1H), 3.11 (s, 3H), 3.24 (d, J=7.6 Hz, 2H), 3.99 (s, 2H), 4.03 (q, J=7.2 Hz, 2H), 7.08 (s, 1H), 7.33–7.43 (m, 3H), 7.70–7.83 (m, 3H), 9.36 (s, 1H); 13C NMR (100 MHz, CDCl3) δ (ppm) 14.1, 19.1, 27.4, 28.5, 43.3, 53.3, 61.9, 97.2, 123.2, 125.9, 126.4, 127.3, 128.7, 129.1, 129.2, 129.3, 131.9, 134.1, 150.0, 151.8, 159.1, 166.1, 170.5.
- 16.Yoneda F, Higuchi M. J. Chem. Soc. Chem. Comm. 1972:402. [Google Scholar]
- 17.Skibo EB, Gilchrist JH. J. Org. Chem. 1988;53:4209–4218. [Google Scholar]
- 18.Synthesis of 1-isobutyl-3-methyl-7-(naphthalen-1-ylmethyl) pyrimido[4,5-d]pyrimidine-2,4,5(1H,3H,6H)-trione (12): A suspension of 11 (224 mg, 0.512 mmol) in n-BuOH (40 mL) was treated with NH2NH2•H2O (0.77 mL) under N2. The resultant mixture was refluxed for 30 min. The reaction was concentrated to afford crude 6-amino-1-isobutyl-3-methyl-7-(naphthalen-1-ylmethyl)pyrimido[4,5-d]pyrimidine-2,4,5(1H,3H,6H)-trione (13), which was used directly in the next step. LC-MS (ESI): m/z 406 [M+1]+. A solution of crude 13 in acetic acid (30 mL) was treated with NaNO2 (283 mg, 4.10 mmol). The resultant mixture was refluxed for 5 min and cooled to room temperature. Additional NaNO2 (283 mg, 4.10 mmol) was added and the solution was refluxed for 5 min. The reaction mixture was concentrated in vacuo. The residue was dissolved in dichloromethane (DCM), washed with H2O, and concentrated in vacuo. The crude product was purified by flash chromatography on silica gel (DCM:MeOH=19:1 to 9:1 gradient) to afford 75 mg (38% for two steps) of 12 as a yellow solid. Rf= 0.07 (DCM:MeOH=19:1); LC-MS (ESI): m/z 391 [M+1]+; 1H NMR (400 MHz, DMSO-d6) δ (ppm) 0.34 (d, J=6.8 Hz, 6H), 1.50 (sep, J=6.8 Hz, 1H), 3.10 (s, 3H), 3.46 (d, J=7.6 Hz, 2H), 4.44 (s, 2H), 7.46–7.54 (m, 4H), 7.86–8.10 (m, 3H) 13.05 (brs, 1H); 13C NMR (100 MHz, DMSO-d6) δ (ppm) 19.2, 26.5, 27.5, 37.4, 48.8, 94.2, 124.3, 125.4, 125.6, 126.1, 127.8, 128.2, 128.4, 131.4, 131.9, 133.4, 150.7, 155.1, 158.2, 158.3, 164.6.
- 19.Synthesis of 8-isobutyl-6-methyl-2-(naphthalen-1-ylmethyl)-5,7-dioxo-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidin-4-yl trifluoromethanesulfonate (14): A solution of 12 (20 mg, 0.051 mmol) in DCM (3 mL) was cooled to 0 °C. Et3N (15 mg, 0.15 mmol) was added followed by Tf2O (26 mg, 0.092 mmol). The reaction was stirred at 0 °C for 30 min, quenched with saturated NH4Cl and extracted with DCM. The combined organic extracts were washed with brine and dried over Na2SO4. The crude product was purified by flash chromatography on silica gel (hexanes:ethyl acetate=9:1) to afford 17 mg (64%) of triflate 14 as a white solid. Rf= 0.34 (hexanes:ethyl acetate=4:1); LC-MS (ESI): m/z 523 [M+1]+; 1H NMR (400 MHz, CDCl3) δ (ppm) 0.63 (d, J=6.8 Hz, 6H), 1.77 (sep, J=6.8 Hz, 1H), 3.39 (s, 3H), 3.83 (d, J=7.2 Hz, 2H), 4.70 (s, 2H), 7.44–7.51 (m, 4H), 7.79–8.08 (m, 3H); 19F NMR (400 MHz, CDCl3) δ (ppm) −73.3.
- 20.General procedure for synthesis of thioethers 15–17: A solution of triflate 14 (1.0 eq) in THF was treated with a ω-mercaptoalcohol (1.5 eq.) and Et3N (2.0 eq.). The reaction was stirred at room temperature for 12–16 hours. The solvent was removed in vacuo and the residue was purified by column chromatography.
- 21.(a) Borzilleri R, Chen Z, Huynh T, Vaccaro W, Chen X-T, Kim K, Cai Z. 0288290. Hui: U.S. Patent Appl. 2005; (b) Speziale AJ, Smith LR. J. Org. Chem. 1963;28:1805–1811. [Google Scholar]
- 22.(a) Merlos M, Gomez L, Vericat ML, Bartroli J, Garcia-Rafanell J, Forn J. Eur. J. Med. Chem. 1990;25:653–658. [Google Scholar]; (b) Papesch V, Schroeder EF. J. Org. Chem. 1951;16:1879–1890. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.