Abstract
Background:
Perchlorate, thiocyanate, and nitrate are sodium/iodide symporter (NIS) inhibitors that block iodide uptake into the thyroid, thus affecting thyroid function. Thyroid dysfunction can adversely affect somatic growth and development in children. To our knowledge, no studies have examined effects of NIS inhibitors on body size measures.
Objective:
We investigated associations between NIS inhibitors and childhood growth in 940 girls from the Puberty Study of the Breast Cancer and Environment Research Program.
Methods:
Urine samples collected from girls 6–8 years of age at enrollment (2004–2007) from New York City, greater Cincinnati, Ohio, and the Bay Area in California were analyzed for NIS inhibitors and creatinine (C). The longitudinal association between NIS inhibitors and anthropometric measures [height, waist circumference, and body mass index (BMI)] during at least three visits was examined using mixed effects linear models, adjusted for race and site.
Results:
Compared with girls in the low-exposure group (3.6, 626, and 500 mg/gC, median perchlorate, thiocyanate, and nitrate, respectively) girls with the highest NIS inhibitor exposure (9.6, 2,343, and 955 mg/gC, median perchlorate, thiocyanate, and nitrate, respectively) had slower growth in waist circumference and BMI but not height. Significant differences in the predicted mean waist circumference and BMI between the low- and high-exposure groups were observed beginning at 11 years of age.
Conclusions:
Higher NIS inhibitor exposure biomarkers were associated with reductions in waist circumference and BMI. These findings underscore the need to assess exposure to NIS inhibitors with respect to their influence on childhood growth.
Citation:
Mervish NA, Pajak A, Teitelbaum SL, Pinney SM, Windham GC, Kushi LH, Biro FM, Valentin-Blasini L, Blount BC, Wolff MS, for the Breast Cancer and Environment Research Project (BCERP). 2016. Thyroid antagonists (perchlorate, thiocyanate, and nitrate) and childhood growth in a longitudinal study of U.S. girls. Environ Health Perspect 124:542–549; http://dx.doi.org/10.1289/ehp.1409309
Introduction
Disruption of thyroid function is one of the strongest mechanisms linking environmental exposures with adverse health outcomes (Werner et al. 2005). Perchlorate, thiocyanate, and nitrate are sodium iodide symporter (NIS) inhibitors that block iodide uptake into the thyroid and thus can affect thyroid function. As known, iodine is necessary for the synthesis of thyroid hormones. Thyroid hormones are essential for normal growth; they promote and modulate the effects of growth hormone (GH) secretion (Burstein et al. 1979), and insulin growth factor (IGF)–1 mediates many of the effects of GH (Miell et al. 1993). These NIS inhibitors are ubiquitous in the environment, leading to widespread human exposure, mainly through ingested food and water (Lau et al. 2013; Murray et al. 2008). Perchlorate is a naturally occurring anion that is formed in the atmosphere and is synthesized primarily as ammonium perchlorate for producing solid propellant for rockets, missiles, fireworks, and other explosives. It is also found in some crop fertilizers formerly used in the United States (Mendiratta et al. 1996). Thiocyanate is found in foods such as milk and vegetables (Laurberg et al. 2002; Michajlovski and Langer 1958). It is also the main metabolite of cyanide exposure coming from tobacco smoke and certain foods such as cassava and almonds (Buratti et al. 1997). Nitrates can occur naturally in food, such as green leafy vegetables, or can be added as preservative (in meat and fish).
Ecologic, experimental, and observational studies have examined relationships of perchlorate exposure with thyroid hormones in adults, pregnant women, adolescents, and infants (Brechner et al. 2000; Chang et al. 2003; Crump et al. 2000; Greer et al. 2002; Li et al. 2000) with mixed results. Associations have been observed between perchlorate and decreased levels of thyroxine (T4) and increased thyroid-stimulating hormone (TSH) (Blount 2006; Steinmaus et al. 2007), with the strongest associations in women with low iodine and high thiocyanate (Steinmaus et al. 2013). Associations have also been reported for high nitrate exposure with increased thyroid volume and thyroid disorders (Aschebrook-Kilfoy et al. 2012; Tajtáková et al. 2006; van Maanen et al. 1994) and increased TSH levels (van Maanen et al. 1994). Perchlorate, thiocyanate, and nitrate exposures are cause for concern given their potential to decrease iodide concentration in the thyroid. Iodine status may influence growth through its effect on the thyroid (Zimmermann 2007). Data from cross-sectional studies on iodine intake and childhood growth are mixed; most studies in iodine deficient (ID) areas show retarded height and decreased weight and bone maturation compared with children in nonendemic areas (Azizi et al. 1995; Bautista et al. 1982; Thurlow et al. 2006). Effects of lower-level perchlorate exposure have not been well studied in children, including effects on growth. Whether thyroid disruption will occur when iodine is adequate, and whether these chemical exposures can cause changes in growth are important questions. In vitro studies of NIS inhibitors indicate that perchlorate, thiocyanate, and nitrate act additively to inhibit iodide uptake (Tonacchera et al. 2004), thus suggesting exposures should be considered together. We a priori hypothesized that the thyroid antagonists perchlorate, thiocyanate, and nitrate would have inverse associations with growth, height, weight, waist circumference, and body mass index (BMI).
We examined whether exposure to NIS inhibitors measured at one time point were associated with height, waist circumference, and BMI trajectories during childhood in an established cohort group of young girls with adequate iodine intake.
Methods
This project was carried out as part of the Breast Cancer and the Environment Research Project (BCERP), a longitudinal study that is investigating early life risk factors for pubertal maturation that may be related to later breast cancer risk. Subjects include 1,239 females age 6–8 years at baseline enrollment (2004–2007) with no underlying endocrine medical condition affecting metabolism or growth. The study was conducted at three sites: a) Icahn School of Medicine at Mount Sinai Medical Center (NYC), which recruited only black or Hispanic girls in East Harlem, New York, with girls seen annually; b) Cincinnati Children’s Hospital/University of Cincinnati (Cincinnati), which recruited in the Cincinnati metropolitan area and through the Breast Cancer Registry of Greater Cincinnati, with girls seen semiannually for the first 5 years and then annually thereafter; and c) Kaiser Permanente Northern California (KPNC), which recruited KPNC Health Plan members in the San Francisco Bay Area, with girls seen annually. All sites obtained written informed consent from a parent or guardian and were approved by the institutional review board at each site as well as by the Centers for Disease Control and Prevention (CDC). Parents or guardians of the participants identified girls’ race or ethnicity as black, white, Asian, or Hispanic. Standardized anthropometry and pubertal staging were done at each visit (Biro et al. 2010a). Body size characteristics we looked at were height, weight, umbilical waist circumference, and BMI (weight in kilograms divided by squared height in meters). For this analysis we included 940 girls who had anthropometric, dietary, and demographic data with at least three anthropometric measures over the total of 7 years of follow-up (age range, 11–16 years at their last follow-up visit). Height was measured to the nearest 0.1 cm, weight to 0.1 kg, and waist circumference to 0.1 cm. A spot urine sample was collected at baseline for NYC and KPNC and for Cincinnati, at approximately 6 months after baseline and was used to measure the biomarker analytes.
Laboratory analysis. Perchlorate, thiocyanate, nitrate, and iodide concentrations in urine were determined by isotope dilution and ion chromatography/tandem mass spectroscopy (IC/MS/MS) as reported previously (Valentin-Blasini et al. 2007). We measured iodide, which is a form of total iodine. Iodide is an inorganic ion used to measure iodine intake, the micronutrient important for thyroid function. Total iodine methods mathematically convert all forms into a single form and report the sum. Therefore, a total iodine measurement includes iodide and a number of organic forms of iodine. Iodide anion accounts for > 90% of total iodine in urine and is the biologically available form that is transported into the thyroid to thyroid hormones (CDC 2008). A total of 940 urine samples were analyzed (312 from NYC, 247 from Cincinnati, and 381 from KPNC). In addition to the internal CDC quality control procedures, we incorporated approximately 10% masked quality control specimens (n = 89) from a single urine pool for which the coefficients of variation results were acceptable. Normalization for urine dilution was done by using creatinine-corrected values for perchlorate, thiocyanate, and iodide [micrograms per gram creatinine (gC)] and for nitrate (milligrams per gC). We used this method of correcting for urine dilution to be consistent with others examining NIS inhibitors (Blount 2006). To examine whether results were affected by creatinine adjustment, we analyzed biomarkers uncorrected (micrograms per liter and milligrams per liter) as well as removing extreme creatinine concentrations (< 50 mg/dL and > 300 mg/dL) (Alessio et al. 1985), and we determined that low creatinine values did not produce extreme biomarker concentrations (data not shown). Results based on creatinine-uncorrected values and after excluding samples with extreme values (data not shown) were similar to results from models of creatinine-corrected (reported).
Additional analyses on a subset of 111 girls from the NYC site were performed to assess the intraindividual variation in urinary biomarkers over three time points—at baseline and at 1 and 3 years—using three statistical methods described for a previous analysis of variation over 6 months (Mervish et al. 2012; Teitelbaum et al. 2008). Specifically, we estimated intraclass correlation coefficient (ICC) (Rosner 2000) and Spearman correlation coefficients (SCC), and performed a surrogate category analysis to assess how well tertile ranking by a single biomarker measurement represented average concentrations over 3 years (Hauser et al. 2004; Willett 1998).
Statistical analyses. All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC). Geometric means and parametric tests of the continuous analyte concentrations were done on natural log–transformed values to achieve a near-normal distribution. Linear mixed-effects models (Proc Mixed in SAS) were used to evaluate associations of one time-point perchlorate, thiocyanate, and nitrate measures with repeated outcome anthropometric measurements (height, waist circumference, weight, and BMI) measured during 3–13 visits. This approach allowed random intercepts and took into account the intrasubject correlation as well as unequal timing of anthropometric measurements. A quadratic term for age was included in the final model because it improved the fit of the model assessed by the Akaike information criterion (AIC). Estimates were compared by testing the difference of the least-square means of the fixed-effects exposure group at each age using the LSMEANS function in Proc Mixed. Statistical significance was defined as p < 0.05. We compared the mixed-effects model to a Gompertz model, which has been shown to achieve a reasonably good fit for adolescent human growth (Deming 1957). Results from the Gompertz and mixed-effects model were similar in directionality and effect size (data not shown), which suggests the mixed-effects model adequately described height in our study population.
Covariates considered as potential confounders were selected on a biologic basis or if they were related to both growth and urinary biomarker concentrations [chi-square test, t-test, analysis of variance (ANOVA) p < 0.05]. Vegetable, dairy, and water consumption are considered the major sources of perchlorate, thiocyanate, and nitrate intake in children (Lau et al. 2013; Murray et al. 2008) and have the potential to be related to growth. In this study population, vegetable, dairy, and water consumption were associated with concentrations of perchlorate and thiocyanate (and nitrate for water) but not with any of the growth outcomes and were therefore were not considered confounders. Additional confounders considered were secondhand smoke exposure (no smoker in the home, smoker in the home, and ≤ or > 35/cigarettes/week) and age at pubertal development (defined as breast Tanner stage 2 and dichotomized at the median of 9.4 years). To investigate differences in growth by site, we included an interaction variable for site by age. Models with and without this interaction term were similar with respect to the AIC; therefore, we present models without the interaction term for ease of interpretation. Because of the complexity of the model, variables that did not alter the coefficients for the association between the exposures categories and the growth outcomes by > 15% or improve the fit of the model by a comparison of the AIC were excluded from the models in a forward selection approach. All final models were adjusted for site and race/ethnicity (indicator terms for black, Hispanic, white, and Asian).
We analyzed the three NIS inhibitors in combination as categorical variables. To investigate possible combined effects of three thyroid disrupting chemicals we created three exposure categories ranked by perchlorate, thiocyanate, and nitrate urinary concentrations We dichotomized at the median perchlorate urinary concentration (5.87 μg/gC), urinary thiocyanate (1,180 μg/gC), and urinary nitrate (676 mg/gC) concentrations. The high-exposure category included girls who had all values above the median for all three NIS inhibitors, the low-exposure category included girls with all values below the median, and the medium-exposure group included the rest of the girls.
Results
Our sample included 940 girls with baseline urinary biomarkers (median age, 7.3 years) and at least three (range, 3–13) longitudinal anthropometric measures over 7 years of observation. The covariate distributions (urine donation age, race/ethnicity, type of water, vegetable, and dairy consumption) in the full cohort (n = 1,239) and in the subsample included in these analyses (n = 940) were similar. Table 1 shows urinary exposure biomarker concentrations corrected for creatinine by selected covariates and overall means compared to 6- to 9-year-old girls included in 2007–2008 NHANES (National Health and Nutrition Examination Surveys; http://www.cdc.gov/nchs/nhanes.htm). Concentrations of all NIS biomarkers decreased with age of the girl at urine collection. Urine concentrations of perchlorate, thiocyanate, and nitrate differed significantly among the three sites, with the lowest mean values among NYC participants. Perchlorate and nitrate concentrations decreased with increased BMI percentile, and all NIS inhibitors increased with greater than high school education.
Table 1.
Geometric means (95% CIs) of creatinine-corrected urine NIS concentrations according to characteristics at baseline among girls in the BCERP cohort (2004–2007, n = 940).
| Characteristic | n | Perchlorate (μg/gC) | Thiocyanate (μg/gC) | Nitrate (mg/gC) | Iodide (μg/gC) |
|---|---|---|---|---|---|
| Race/ethnicity | |||||
| Black | 275 | 4.76 (4.37, 5.18) | 1,140 (1,040, 1,260) | 604 (567, 645) | 179 (164, 194) |
| Hispanic | 285 | 5.83 (5.36, 6.33) | 900 (820, 989) | 662 (621, 705) | 286 (264, 311) |
| Asian | 53 | 7.12 (5.87, 8.62) | 1,610 (1,290, 2,000) | 931 (803, 1E3) | 260 (215, 314) |
| White | 327 | 7.68 (7.11, 8.30)* | 1,410 (1,290, 1,540)* | 847 (798, 899)* | 243 (225, 262)* |
| Age at urine donation (years) | |||||
| 6–6.99 | 290 | 6.44 (5.93, 7.01) | 1,150 (1,050, 1,270) | 726 (680, 774) | 258 (237, 280) |
| 7–7.99 | 473 | 6.53 (6.11, 6.97) | 1,260 (1,170, 1,360) | 753 (716, 793) | 237 (222, 253) |
| ≥ 8 | 177 | 4.71 (4.23, 5.25)* | 964 (853, 1,090)* | 610 (562, 663)* | 195 (175, 217)* |
| BMI percentile at urine donation | |||||
| < 50th | 644 | 6.37 (6.02, 6.74) | 1,170 (1,100, 1,250) | 751 (719, 784) | 237 (224, 251) |
| 50–85th | 142 | 6.31 (5.59, 7.12) | 1,280 (1,110, 1,460) | 718 (655, 788) | 234 (208, 264) |
| > 85th | 154 | 5.02 (4.47, 5.64)* | 1,050 (924, 1,200) | 584 (534, 638)* | 224 (200, 251) |
| Caregiver education | |||||
| ≤ High school | 272 | 5.16 (4.73, 5.62) | 965 (875, 1,060) | 648 (606, 694) | 245 (225, 267) |
| > High school | 648 | 6.66 (6.30, 7.05)* | 1,260 (1,180, 1,340)* | 746 (714, 779)* | 233 (220, 246) |
| Not reported | 20 | ||||
| Site | |||||
| NYC | 312 | 4.48 (4.14, 4.84) | 742 (682, 808) | 572 (538, 607) | 245 (226, 265) |
| Cincinnati | 247 | 6.62 (6.07, 7.23) | 1,370 (1,240, 1,500) | 847 (792, 907) | 207 (189, 227) |
| KPNC | 381 | 7.49 (6.98, 8.04)* | 1,520 (1,410, 1,650)* | 771 (730, 815)* | 245 (228, 264)* |
| Environmental smoking exposure | |||||
| No smokers | 695 | 6.40 (6.06, 6.76) | 1,160 (1,090, 1,240) | 739 (709, 771) | 246 (233, 259) |
| ≥ 1 smoker, 0 cigs | 126 | 5.91 (5.20, 6.72) | 1,250 (1,080, 1,440) | 716 (649, 791) | 215 (189, 243) |
| ≥ 1 smoker, 1–35 cigs/week | 48 | 5.23 (4.25, 6.44) | 915 (724, 1,160) | 561 (478, 658) | 243 (198, 298) |
| ≥ 1 smoker, > 35 cigs/week | 65 | 4.68 (3.91, 5.59)* | 1,210 (993, 1,480) | 589 (514, 676)* | 174 (146, 208)* |
| Not reported | 6 | ||||
| Season of urine donation | |||||
| Fall | 176 | 5.35 (4.80, 5.96) | 1,180 (1,040, 1,330) | 668 (614, 726) | 213 (191, 238) |
| Spring | 310 | 6.73 (6.20, 7.31) | 1,070 (975, 1,170) | 744 (699, 792) | 246 (227, 267) |
| Winter | 181 | 5.67 (5.10, 6.32) | 1,030 (917, 1,170) | 662 (610, 719) | 234 (211, 260) |
| Summer | 273 | 6.28 (5.75, 6.85)* | 1,380 (1,260, 1,530)* | 754 (705, 807)* | 236 (216, 257) |
| Primary drinking-water source | |||||
| 50/50 bottle/spring-tap | 132 | 6.24 (5.50, 7.07) | 1,260 (1,100, 1,450) | 715 (650, 788) | 227 (201, 257) |
| Primarily bottle/spring | 263 | 5.21 (4.77, 5.69) | 1,020 (926, 1,130) | 626 (585, 670) | 222 (203, 243) |
| Primarily tap | 533 | 6.61 (6.21, 7.04)* | 1,210 (1,130, 1,300)* | 761 (725, 798)* | 244 (229, 260) |
| Not reported | 12 | ||||
| Vegetable consumption tertiles | |||||
| 1 (0.00–0.741 veg/day) | 288 | 5.38 (4.93, 5.86) | 1,040 (946, 1,150) | 687 (643, 735) | 222 (204, 242) |
| 2 (0.741–1.447 veg/day) | 289 | 6.16 (5.65, 6.72) | 1,160 (1,050, 1,270) | 692 (648, 740) | 257 (236, 280) |
| 3 (1.448–5.18 veg/day) | 289 | 6.83 (6.27, 7.45)* | 1,330 (1,210, 1,470)* | 748 (700, 800) | 232 (213, 252) |
| Not reported | 74 | ||||
| Dairy consumption tertiles | |||||
| 1 (0.00–1.576 dairy/day) | 288 | 5.66 (5.18, 6.17) | 995 (905, 1,090) | 702 (657, 751) | 208 (191, 226) |
| 2 (1.577–2.316 dairy/day) | 289 | 6.25 (5.73, 6.82) | 1,170 (1,060, 1,280) | 717 (671, 766) | 244 (224, 265) |
| 3 (2.317–6.85 dairy/day) | 289 | 6.40 (5.87, 6.98) | 1,380 (1,260, 1,520)* | 707 (662, 756) | 261 (240, 284)* |
| Not reported | 74 | ||||
| Age at breast Tanner stage 2 (years) | |||||
| ≤ 9.4 | 473 | 5.80 (5.43, 6.20) | 1,130 (1,050, 1,220) | 707 (671, 744) | 226 (211, 241) |
| > 9.4 | 433 | 6.34 (5.91, 6.80) | 1,190 (1,100, 1,280) | 722 (685, 762) | 239 (223, 256) |
| Not available | 34 | ||||
| ALL | 940 | 6.11 (5.83, 6.41) | 1,170 (1,110, 1,230) | 716 (690, 742) | 234 (224, 245) |
| NHANESa | 284 | 7.27 (6.56, 8.05) | 1,540 (1,370, 1,730) | 813 (756, 875) | NA |
| Abbreviations: cig, cigarettes; NA, not available; veg, vegetables. aNHANES 2007–2008, 6- to 9-year-old girls; creatinine-corrected geometric mean (http://www.cdc.gov/nchs/nhanes.html). *ANOVA p < 0.05 | |||||
It is worth noting that there were no differences in thiocyanate concentrations according to secondhand smoke exposure. There were significant differences in perchlorate and nitrate levels by primary drinking-water source. Perchlorate and thiocyanate significantly differed by vegetable consumption, whereas dairy consumption was only significantly associated with thiocyanate. There were no significant differences in the analyte concentrations by age of pubertal onset. Concentrations among girls in our study were lower than observed among girls ages 6–9 years in 2007–2008 NHANES survey data (http://www.cdc.gov/nchs/nhanes.htm) (Table 1). Geometric means and medians of urinary NIS inhibitors by the three combined NIS exposure categories (low, medium, and high) are shown in Table 2.
Table 2.
Creatinine-corrected urine iodine and creatinine-corrected urine NIS inhibitor concentrations according to categories of combined NIS exposures (low, medium, and high) in 940 girls (BCERP cohort 2004–2007).
| Exposure | n | Geo mean | Median | Minimum | Maximum | p95th |
|---|---|---|---|---|---|---|
| Low perchlorate, thiocyanate, and nitratea | ||||||
| Perchlorate (μg/gC) | 196 | 3.26 | 3.59 | 0.51 | 5.86 | 5.5 |
| Thiocyanate (μg/gC) | 196 | 554.61 | 625.84 | 75.77 | 1176.47 | 1103.4 |
| Nitrate (mg/gC) | 196 | 452.35 | 500.1 | 5.92 | 673.12 | 644.1 |
| Iodide (μg/gC) | 196 | 176.74 | 167.7 | 29.23 | 2111.11 | 691.59 |
| Medium perchlorate, thiocyanate, and nitrate | ||||||
| Perchlorate (μg/gC) | 555 | 6.16 | 5.92 | 0.25 | 193.14 | 18.72 |
| Thiocyanate (μg/gC) | 555 | 1179.71 | 1201.72 | 62.34 | 12756.26 | 3709.45 |
| Nitrate (mg/gC) | 555 | 734.83 | 680.25 | 95.74 | 129662.5 | 1895.77 |
| Iodide (μg/gC) | 555 | 235.69 | 230.26 | 28.6 | 4194.92 | 732.05 |
| High perchlorate, thiocyanate, and nitrateb | ||||||
| Perchlorate (μg/gC) | 189 | 11.48 | 9.58 | 5.87 | 136.84 | 35.96 |
| Thiocyanate (μg/gC) | 189 | 2438.13 | 2343.28 | 1180.23 | 24807.69 | 5675.68 |
| Nitrate (mg/gC) | 189 | 1066.49 | 955.22 | 676.47 | 6647.73 | 2423.08 |
| Iodide (μg/gC) | 189 | 308.37 | 294.12 | 31.71 | 2636.36 | 1060.22 |
| Abbreviations: Geo, geometric; p, percentile. aBelow the median concentration of all three NIS biomarkers. bAbove the median concentration of all three NIS biomarkers. | ||||||
Intraindividual variability. The covariate distributions (urine donation age, race/ethnicity, BMI, type of water, vegetable, and dairy consumption) in this subsample (n = 111) and in the NYC sample used in the main analyses (n = 312) were similar.
However, the girls’ caregivers had lower education compared with the full sample (data not shown). The ICC measure of reproducibility was poor (0.13) for perchlorate, and fair for thiocyanate (0.24), nitrate (0.35), and iodide (0.25) based on creatinine-corrected urine concentrations. However, when average concentrations over all three samples (used as a surrogate indicator of their “true” concentrations over time) were categorized into tertiles according to the distribution of concentrations at each time point, the median value of each tertile increased monotonically from the lowest to the highest tertile (see Supplemental Material, Table S1), thus supporting the use of a single spot urine sample to classify girls into low, medium, and high exposure categories.
Growth models. Height. Girls exposed to combined low concentrations of perchlorate, thiocyanate, and nitrate were consistently taller than the girls with high perchlorate, thiocyanate, and nitrate (Table 3). However, differences in height began to converge by 12 years of age, and heights were similar between the high- and low-exposure groups by 13 years of age (Figures 1 and 2).
Table 3.
Height, waist circumference, and BMI by age in low, medium, and high combined NIS inhibitor biomarker exposure categoriesa: predicted means and differences (95% CIs) based on mixed effects modelsb in 940 girls, BCERP 2004–2007.
| Age (years)c | Low-exposure category | Medium-exposure category | High-exposure category | Difference between low and high (95% CI) | p-Valued |
|---|---|---|---|---|---|
| Height (cm) | |||||
| 7 | 121.2 (120.1, 122.2) | 120.6 (120.0, 121.3) | 120.1 (119.1, 121.2) | 1.0 (–0.4, 2.5) | 0.172 |
| 8 | 129.0 (127.9, 130.0) | 127.8 (127.2, 128.5) | 126.9 (125.9, 127.9) | 2.1 (0.6, 3.5) | 0.004 |
| 9 | 136.0 (135.0, 137.1) | 134.5 (133.9, 135.2) | 133.4 (132.3, 134.4) | 2.7 (1.3, 4.1) | < 0.001 |
| 10 | 142.4 (141.3, 143.4) | 140.8 (140.1, 141.4) | 139.5 (138.5, 140.6) | 2.8 (1.4, 4.3) | < 0.001 |
| 11 | 148.0 (146.9, 149.0) | 146.5 (145.9, 147.2) | 145.4 (144.4, 146.4) | 2.6 (1.1, 4) | < 0.001 |
| 12 | 152.8 (151.8, 153.9) | 151.8 (151.1, 152.5) | 151.0 (150.0, 152.0) | 1.8 (0.4, 3.3) | 0.011 |
| 13 | 157.0 (155.9, 158.0) | 156.6 (155.9, 157.3) | 156.3 (155.2, 157.3) | 0.7 (–0.8, 2.1) | 0.356 |
| Waist circumference (cm) | |||||
| 7 | 57.1 (55.5, 58.8) | 58.7 (57.7, 59.8) | 58.0 (56.4, 59.7) | –0.9 (–3.2, 1.4) | 0.444 |
| 8 | 61.3 (59.7, 62.9) | 62.2 (61.2, 63.2) | 61.1 (59.5, 62.7) | 0.2 (–2, 2.4) | 0.830 |
| 9 | 65.2 (63.6, 66.8) | 65.6 (64.6, 66.6) | 64.1 (62.5, 65.7) | 1.2 (–1, 3.3) | 0.295 |
| 10 | 68.8 (67.2, 70.4) | 68.8 (67.8, 69.8) | 67.0 (65.4, 68.5) | 1.9 (–0.3, 4.1) | 0.091 |
| 11 | 72.1 (70.5, 73.8) | 71.9 (70.9, 72.9) | 69.7 (68.2, 71.3) | 2.4 (0.2, 4.6) | 0.032 |
| 12 | 75.2 (73.5, 76.8) | 74.9 (73.9, 75.9) | 72.4 (70.9, 74.0) | 2.7 (0.5, 4.9) | 0.016 |
| 13 | 77.9 (76.2, 79.5) | 77.7 (76.7, 78.7) | 75.0 (73.4, 76.6) | 2.8 (0.6, 5) | 0.013 |
| BMI (kg/m2) | |||||
| 7 | 16.3 (15.6, 16.9) | 16.7 (16.3, 17.1) | 16.4 (15.7, 17.0) | –0.1 (–1, 0.8) | 0.871 |
| 8 | 17.3 (16.6, 17.9) | 17.5 (17.1, 17.9) | 17.0 (16.4, 17.6) | 0.2 (–0.6, 1.1) | 0.604 |
| 9 | 18.2 (17.6, 18.8) | 18.3 (17.9, 18.7) | 17.7 (17.1, 18.3) | 0.5 (–0.4, 1.3) | 0.259 |
| 10 | 19.1 (18.5, 19.7) | 19.1 (18.7, 19.5) | 18.4 (17.8, 19.0) | 0.7 (–0.1, 1.6) | 0.100 |
| 11 | 20.0 (19.4, 20.6) | 20.0 (19.6, 20.4) | 19.1 (18.5, 19.7) | 0.9 (0.1, 1.8) | 0.038 |
| 12 | 20.9 (20.2, 21.5) | 20.8 (20.4, 21.2) | 19.8 (19.2, 20.4) | 1.1 (0.2, 1.9) | 0.015 |
| 13 | 21.7 (21.1, 22.3) | 21.7 (21.3, 22.1) | 20.5 (19.9, 21.2) | 1.2 (0.3, 2) | 0.008 |
| aLow group is below the median concentration of all three NIS biomarkers; high group is above the median concentration of all three NIS biomarkers; medium group includes all the others. bPredicted means and differences were computed from the final model (adjusted for race/ethnicity and site) using the LSMEANS function in Proc Mixed SAS version 9.4. cNo estimate was provided for age 6 years due to small sample of girls who were exactly 6 years old at enrollment, truncated at age 13. dSignificance of the difference between the low- and high-exposure category. | |||||
Figure 1.
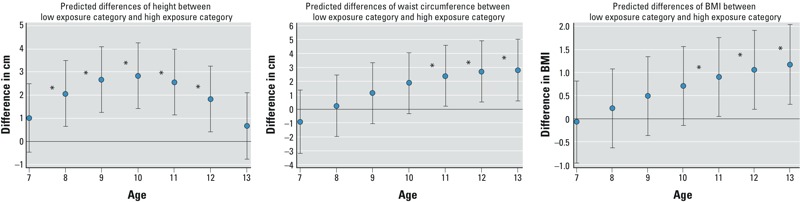
Estimated differences (95% CI) in height, waist circumference, and BMI, by age between the low and high combined NIS biomarker categories: predicted differences using mixed-effects models in 940 girls, BCERP 2004–2007. Low group is below the median concentration of all three NIS biomarkers; high group is above the median concentration of all three NIS biomarkers. Medium group not shown. Final mixed-effects model was adjusted for race/ethnicity and site. *p < 0.05 for the predicted differences between the low- and high-exposure category.
Figure 2.
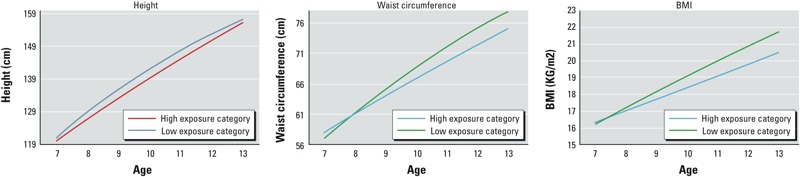
Height, waist circumference, and BMI growth trajectories by age in the low and high combined NIS inhibitor biomarker exposure categories: predicted means using mixed-effects models in 940 girls, BCERP 2004–2007. Low group is below the median concentration of all three NIS biomarkers; high group is above the median concentration of all three NIS biomarkers. Medium group not shown. Growth curves were derived from the LSMEANS function in SAS 9.4 by each exposure category at each age from the final mixed-effects model (adjusted for race/ethnicity and site).
Waist circumference and BMI. Waist circumference and BMI models showed similar patterns, such that after age 7 years, girls with low exposures had larger values than girls in the medium- and high-exposure groups, respectively, though differences between the low- and high-exposure groups were not statistically significant until 11 years of age (Table 3). In addition, differences between the low- and high-exposure groups increased with age (Figures 1 and 2).
Discussion
To our knowledge, this is the first study to examine either individual or combined effects of NIS inhibitors on body size and growth during childhood. High exposure to all three NIS inhibitors combined (perchlorate, thiocyanate, and nitrate urinary concentrations) showed significant differences in growth outcomes at various ages compared with low exposure to all three NIS inhibitors. Our results are consistent with these chemicals as thyroid antagonists, in that higher exposure to the three NIS inhibitors was associated with smaller mean body size measurements at each age and slower growth in girls. Beginning at 11 years of age, the predicted difference was significant for mean waist circumference and BMI between the low- and high-exposure groups. Known sources of exposure and factors associated with growth in children (vegetable, dairy, and water consumption) were examined as potential confounders but did not alter associations (data not shown). Chronically impaired iodide uptake is associated with alterations in thyroid hormone levels (Blount et al. 2006; Steinmaus et al. 2007, 2013). However in our population, iodide levels were generally adequate so that observed associations between exposures and body size measures may be through another mechanism. It is also possible that the various chemicals compete for iodide, but do not necessarily deplete iodide. Greer et al. (2002) suggested that intermittent periods of low iodide intake may not affect thyroid hormone production.
Chemicals may affect different growth indices during distinct periods of a child’s life. Our study examined the prepubertal and pubertal time periods. Early-maturing girls have significantly higher BMI and percent body fat than on-time and later-maturing girls (Biro et al. 2010b). When we included the mean age at pubertal development in our models, the association remained the same (data not shown). The trajectories of waist circumference (WC) and BMI are consistent with growth during puberty; as heavier girls grow older, there is also a greater increase in adiposity compared with lighter girls (Biro and Wein 2010). WC and BMI have been used as a means of identifying children at risk for hypertension, type 2 diabetes, and cardiovascular disease in both childhood and later as adults (Czernichow et al. 2011b; Freedman et al. 2007). More recently, WC, a measure of visceral fat deposits and used for defining abdominal obesity, is shown to be better than BMI for predicting those conditions in children, including metabolic syndrome and all-cause mortality (Czernichow et al. 2011a; Xi et al. 2014). In this population, WC, BMI, and weight are all highly correlated with each other (r > 0.90).
Studies show elevated thyroid hormone levels [TSH, T4, and triiodothyronine (T3)] in obese children and adults compared with normal weight persons (Chomard et al. 1985; Gussekloo et al. 2004; Knudsen et al. 2005; Matzen et al. 1989; Reinehr and Andler 2002; Shalitin et al. 2009). An association between thyroid function and BMI may be attributable to alterations in energy expenditure or leptin produced by adipocyte tissue, although the mechanism is unclear (Reinehr 2010). Controversy exists as to whether the changes in TSH or other thyroid hormones are causes or consequences of weight status.
Perchlorate associations with thyroid hormone production have been studied in infants and children with mixed results (Brechner et al. 2000; Crump et al. 2000; Lamm 2003; Li et al. 2001; Téllez Téllez et al. 2005). Thyroid deficiency is of particular concern during development because these hormones regulate brain development (Haddow et al. 1999). Several studies of NIS inhibitors on thyroid hormones have estimated exposure based on perchlorate levels measured in water supplies. Most have not reported associations with thyroid hormone concentrations on either the pregnant mother or neonate (Amitai et al. 2007; Crump et al. 2000; Li et al. 2001; Téllez Téllez et al. 2005); however, one study reported elevated TSH levels in newborns whose drinking-water supply was perchlorate-contaminated compared with those supplied with drinking water that had no perchlorate contamination (Brechner et al. 2000). Several experimental and observational studies using urinary perchlorate biomarkers in adults have reported that perchlorate was not associated with T4 or TSH levels at exposure levels orders of magnitude higher than the median levels found in our study (Braverman et al. 2005, 2006; Lamm et al. 1999; Lamm 2003). One study found perchlorate negatively associated with T4 only in women with iodine < 100 μg/L (Blount et al. 2006), suggesting that iodine levels must be sufficiently low for environmental levels of perchlorate and thiocyanate to overcome compensatory mechanisms that maintain thyroid hormone (Steinmaus et al. 2007), at least in adults.
Previous research on perchlorate and possible thyroid-related health effects has paid little attention to the other common environmental NIS inhibitors, thiocyanate and nitrate. The focus on perchlorate arises in part because its relative potency as an NIS inhibitor is 10–200 times that of thiocyanate and nitrate on a molar basis (Greer et al. 1966; Tonacchera et al. 2004). However, based on average daily intake of percholorate equivalents of nitrates and thiocyanates, a person’s exposure to both thiocyanate and nitrate from drinking water and food account for a larger proportion of iodine uptake inhibition than does perchlorate exposure (De Groef et al. 2006). Moreover, in vitro studies of NIS indicate that perchlorate, nitrate, and thiocyanate act additively to inhibit iodide uptake (Tonacchera et al. 2004). Therefore, it seems important to study the combination of chemicals.
Nitrate is a natural common chemical contaminant from both food and drinking-water intake, and vegetables account for between 30% and 80% of the total nitrate intake (Du et al. 2007). High levels of nitrate (> 200 mg/L) in drinking water have been associated with goiter incidence (Gatseva and Argirova 2008), increased thyroid volume (van Maanen et al. 1994), and subclinical thyroid disorders (Tajtáková et al. 2006). A study with lower levels than the above studies and ours—mean, 53 mg/L—found no association with thyroid volume (Below et al. 2008). Although nitrate itself has no toxicity, its metabolites, nitrite and N-nitroso compounds (NOCs), can be toxic. On the other hand, NOCs, which can be produced in the stomach, have multiple physiological roles, some of which are positive (Du et al. 2007). Thiocyanate has a low toxicity and can be found as both a metabolite of cyanide, which enters the body mainly from tobacco smoke, and free thiocyanate, as found in vegetables. The latter form has antibacterial properties (Youso et al. 2012). The fact that nitrates and thiocyanate are capable of both deleterious and beneficial effects calls for a better understanding of factors associated with their metabolism. Moreover, because vegetable, dairy, and water consumption are major sources of NIS inhibitors (De Groef et al. 2006; Lau et al. 2013; Murray et al. 2008), the risks and benefits of exposure to NIS inhibitors need to be carefully considered.
There is increasing evidence linking environmental toxicants to thyroid dysfunction. Studies have reported associations between thyroid hormone levels and exposure to polychlorinated biphenyls (PCBs) (Schell et al. 2008), phthalates (Meeker et al. 2007), perchlorate (NRC 2005), and bisphenol A (BPA) (Meeker et al. 2010). Mechanisms underlying thyroid interference by these chemicals are diverse, and include increased inhibition of TH synthesis or increased metabolism of THs via induction of deiodinases (Zoeller 2007). These same chemicals have also been associated with body size, including phthalates (Hatch et al. 2008; Stahlhut et al. 2007) and BPA (Rubin and Soto 2009), potentially through the thyroid mechanism (Newbold et al. 2008).
A limitation of our study is using one spot urine sample, because these chemicals have short half-lives in the body (< 1 day, except thiocyanate, which is about 6 days) and may not represent one’s long-term exposure. This study and an earlier investigation (Mervish et al. 2012) examined repeatability of measures over 3 years and 6 months, respectively. Here, we showed poor to fair agreement (ICCs between 0.13–0.35) of NIS concentrations in samples over 3 years, comparable with results from the 6-month interval (Mervish et al. 2012). The use of a single spot urine sample would most likely lead to nondifferential misclassification. We also had no information on maternal BMI, birth weight, and other maternal indices associated with their offspring’s childhood growth (Laitinen et al. 2001; Terry et al. 2007). An additional limitation is our inability to directly investigate the proposed pathway involving the thyroid because we do not have thyroid hormone measures.
Strengths of this prospective study include longitudinal standardized measures of several parameters of growth. We have at least 3 and as many as 13 anthropometric measurements to examine longitudinal growth. We used mixed-effects growth curve modeling, which allows examination of timing of differences in growth. We were able to investigate the combined effects of three thyroid-disrupting agents—animal and laboratory studies have shown that they act in combination to affect thyroid hormone production (De Groef et al. 2006).
Although growth was associated with exposure to NIS inhibitors in our study, growth is a result of a complex interaction between genetic and environmental factors and not attributable solely to any one factor. Given the limitations of having one spot urine sample, no thyroid measures, and low exposures, these findings need to be further investigated. Nonetheless, changes in growth due to these NIS inhibitors are biologically plausible because normal somatic growth requires the thyroid hormone axis be intact, and alterations in thyroid hormones can potentially affect body size changes (Zimmermann 2007). Studying potential influences of childhood growth is important because childhood growth is predictive of adult disease and mortality (Freedman et al. 2007). However, it is unclear whether the differences in growth associated with exposure in our study population have implications for future growth and health.
Supplemental Material
Acknowledgments
We gratefully acknowledge our many collaborators at the three medical centers involved in this research, including J. Montana, R. Osborne, L. Boguski, J. Forman, P. Monroe, B. Bornschein, R. Hiatt, L. Greenspan, and J. Deardorff. We also thank our community partners, including B. Brenner, J. Barlowe, and G. Greenberg.
Footnotes
This research was supported by the Breast Cancer and the Environment Research Program (BCERP; award nos. U01ES012770, U01ES012771, U01ES012800, U01ES012801, U01ES019435, U01ES019453, U01ES019454, U01ES019457, and R827039), the National Institute of Environmental Health Sciences, the National Institutes of Health, the Department of Health and Human Services (NIEHS/NIH/DHHS; P01ES009584 and P30ES006096), the National Cancer Institute (NCI/NIH, DHHS), the U.S. Environmental Protection Agency, the NIH/DHHS (CSTA-UL1RR029887), the New York State Empire Clinical Research Investigator Program, the Pediatric Environmental Health Fellowship (HD049311), and the Avon Foundation. L.H.K. is employed by Kaiser Permanente, Oakland, California.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, the NIH, or the California Department of Public Health.
The authors declare they have no actual or potential competing financial interests.
References
- Alessio L, Berlin A, Dell’Orto A, Toffoletto F, Ghezzi I. Reliability of urinary creatinine as a parameter used to adjust values. Int Arch Occup Environ Health. 1985;55:99–106. doi: 10.1007/BF00378371. [DOI] [PubMed] [Google Scholar]
- Amitai Y, Winston G, Sack J, Wasser J, Lewis M, Blount BC, et al. Gestational exposure to high perchlorate concentrations in drinking water and neonatal thyroxine levels. Thyroid. 2007;17:843–850. doi: 10.1089/thy.2006.0336. [DOI] [PubMed] [Google Scholar]
- Aschebrook-Kilfoy B, Heltshe SL, Nuckols JR, Sabra MM, Shuldiner AR, Mitchell BD, et al. 2012. Modeled nitrate levels in well water supplies and prevalence of abnormal thyroid conditions among the Old Order Amish in Pennsylvania. Environ Health 11 6; doi: 10.1186/1476-069X-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi F, Kalani H, Kimiagar M, Ghazi A, Sarshar A, Nafarabadi M, et al. Physical, neuromotor and intellectual impairment in non-cretinous schoolchildren with iodine deficiency. Int J Vitam Nutr Res. 1995;65:199–205. [PubMed] [Google Scholar]
- Bautista A, Barker PA, Dunn JT, Sanchez M, Kaiser DL. The effects of oral iodized oil on intelligence, thyroid status, and somatic growth in school age children from an area of endemic goiter. Am J Clin Nutr. 1982;35:127–134. doi: 10.1093/ajcn/35.1.127. [DOI] [PubMed] [Google Scholar]
- Below H, Zöllner H, Völzke H, Kramer A. Evaluation of nitrate influence on thyroid volume of adults in a previously iodine-deficient area. Int J Hyg Environ Health. 2008;211:186–191. doi: 10.1016/j.ijheh.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, et al. Pubertal assessment method and baseline characteristics in a mixed study of girls. Pediatrics. 2010a;126:e583–e590. doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Huang B, Morrison JA, Horn PS, Daniels SR. Body mass index and waist-to-height changes during teen years in girls are influenced by childhood body mass index. J Adolesc Health. 2010b;46:245–250. doi: 10.1016/j.jadohealth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Wein M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91:1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Pirkle JL, Osterloh JD, Valentin-Blasini L, Caldwell KL. 2006. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect 114 1865 1871; doi: 10.1289/ehp.9466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman LE, He X, Pino S, Cross M, Magnani B, Lamm SH, et al. The effect of perchlorate, thiocyanate, and nitrate on thyroid function in workers exposed to perchlorate long-term. J Clin Endocrinol Metab. 2005;90:700–706. doi: 10.1210/jc.2004-1821. [DOI] [PubMed] [Google Scholar]
- Braverman LE, Pearce EN, He X, Pino S, Seeley M, Beck B, et al. Effects of six months of daily low-dose perchlorate exposure on thyroid function in healthy volunteers. J Clin Endocrinol Metab. 2006;91:2721–2724. doi: 10.1210/jc.2006-0184. [DOI] [PubMed] [Google Scholar]
- Brechner RJ, Parkhurst GD, Humble WO, Brown MB, Herman WH. Ammonium perchlorate contamination of Colorado River drinking water is associated with abnormal thyroid function in newborns in Arizona. J Occup Environ Med. 2000;42:777–782. doi: 10.1097/00043764-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Buratti M, Xaiz D, Caravelliand G, Colombi A. Validation of urinary thiocyanate as a biomarker of tobacco smoking. Biomarkers. 1997;2:81–85. doi: 10.1080/135475097231797. [DOI] [PubMed] [Google Scholar]
- Burstein PJ, Draznin B, Johnson CJ, Schalch DS. The effect of hypothyroidism on growth, serum growth hormone, the growth hormone-dependent somatomedin, insulin-like growth factor, and its carrier protein in rats. Endocrinology. 1979;104:1107–1111. doi: 10.1210/endo-104-4-1107. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Atlanta, GA: CDC, Department of Health and Human Services; 2008. National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population 1999-2002. [Google Scholar]
- Chang S, Crothers C, Lai S, Lamm S. Pediatric neurobehavioral diseases in Nevada counties with respect to perchlorate in drinking water: an ecological inquiry. Birth Defects Res A Clin Mol Teratol. 2003;67:886–892. doi: 10.1002/bdra.10089. [DOI] [PubMed] [Google Scholar]
- Chomard P, Vernhes G, Autissier N, Debry G. Serum concentrations of total T4, T3, reverse T3 and free T4, T3 in moderately obese patients. Hum Nutr Clin Nutr. 1985;39:371–378. [PubMed] [Google Scholar]
- Crump C, Michaud P, Téllez R, Reyes C, Gonzalez G, Montgomery EL, et al. Does perchlorate in drinking water affect thyroid function in newborns or school-age children? J Occup Environ Med. 2000;42:603–612. doi: 10.1097/00043764-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Czernichow S, Kengne AP, Huxley RR, Batty GD, de Galan B, Grobbee D, et al. Comparison of waist-to-hip ratio and other obesity indices as predictors of cardiovascular disease risk in people with type-2 diabetes: a prospective cohort study from ADVANCE. Eur J Cardiovasc Prev Rehabil. 2011a;18:312–319. doi: 10.1097/HJR.0b013e32833c1aa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011b;12:680–687. doi: 10.1111/j.1467-789X.2011.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groef B, Decallonne BR, Van der Geyten S, Darras VM, Bouillon R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects. Eur J Endocrinol. 2006;155:17–25. doi: 10.1530/eje.1.02190. [DOI] [PubMed] [Google Scholar]
- Deming J. Application of the Gompertz curve to the observed pattern of growth in length of 48 individual boys and girls during the adolescent cycle of growth. Hum Biol. 1957;29:83–122. [PubMed] [Google Scholar]
- Du ST, Zhang YS, Lin XY. Accumulation of nitrate in vegetables and its possible implications to human health. Agric Sci China. 2007;6:1246–1255. [Google Scholar]
- Freedman DS, Kahn HS, Mei Z, Grummer-Strawn LM, Dietz WH, Srinivasan SR, et al. Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr. 2007;86:33–40. doi: 10.1093/ajcn/86.1.33. [DOI] [PubMed] [Google Scholar]
- Gatseva PD, Argirova MD. High-nitrate levels in drinking water may be a risk factor for thyroid dysfunction in children and pregnant women living in rural Bulgarian areas. Int J Hyg Environ Health. 2008;211:555–559. doi: 10.1016/j.ijheh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Greer MA, Goodman G, Pleus RC, Greer SE. Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ Health Perspect. 2002;110:927–937. doi: 10.1289/ehp.02110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer MA, Stott AK, Milne KA. Effects of thiocyanate, perchlorate and other anions on thyroidal iodine metabolism. Endocrinology. 1966;79:237–247. doi: 10.1210/endo-79-2-237. [DOI] [PubMed] [Google Scholar]
- Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591–2599. doi: 10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. 2008. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health 7 27; doi: 10.1186/1476-069X-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. 2004. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 112 1734 1740; doi: 10.1289/ehp.7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- Laitinen J, Power C, Järvelin MR. Family social class, maternal body mass index, childhood body mass, and age at menarche as predictors of adult obesity. Am J Clin Nutr. 2001;74:287–294. doi: 10.1093/ajcn/74.3.287. [DOI] [PubMed] [Google Scholar]
- Lamm SH. Perchlorate exposure does not explain differences in neonatal thyroid function between Yuma and Flagstaff. J Occup Environ Med. 2003;45:1131–1132. doi: 10.1097/01.jom.0000094991.31330.d3. [DOI] [PubMed] [Google Scholar]
- Lamm SH, Braverman LE, Li FX, Richman K, Pino S, Howearth G. Thyroid health status of ammonium perchlorate workers: a cross-sectional occupational health study. J Occup Environ Med. 1999;41:248–260. doi: 10.1097/00043764-199904000-00006. [DOI] [PubMed] [Google Scholar]
- Lau FK, deCastro BR, Mills-Herring L, Tao L, Valentin-Blasini L, Alwis KU, et al. Urinary perchlorate as a measure of dietary and drinking water exposure in a representative sample of the United States population 2001-2008. J Expo Sci Environ Epidemiol. 2013;23:207–214. doi: 10.1038/jes.2012.108. [DOI] [PubMed] [Google Scholar]
- Laurberg P, Andersen S, Knudsen N, Ovesen L, Nøhr SB, Bülow Pedersen I. Thiocyanate in food and iodine in milk: from domestic animal feeding to improved understanding of cretinism. Thyroid. 2002;12:897–902. doi: 10.1089/105072502761016520. [DOI] [PubMed] [Google Scholar]
- Li FX, Squartsoff L, Lamm SH. Prevalence of thyroid diseases in Nevada counties with respect to perchlorate in drinking water. J Occup Environ Med. 2001;43:630–634. doi: 10.1097/00043764-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Li Z, Li FX, Byrd D, Deyhle GM, Sesser DE, Skeels MR, et al. Neonatal thyroxine level and perchlorate in drinking water. J Occup Environ Med. 2000;42:200–205. doi: 10.1097/00043764-200002000-00020. [DOI] [PubMed] [Google Scholar]
- Matzen LE, Kvetny J, Pedersen KK. TSH, thyroid hormones and nuclear-binding of T3 in mononuclear blood cells from obese and non-obese women. Scand J Clin Lab Invest. 1989;49:249–253. [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. 2007. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect 115 1029 1034; doi: 10.1289/ehp.9852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. 2010;44(4):1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiratta SK, Dotson RL, Brooker RT. In: Kirk-Othmer Encyclopedia of Chemical Technology, Paper to Pigment Dispersions (Vol. 18) (Kroschwitz JI, Howe-Grant M, eds). 4th ed. New York: John Wiley & Sons Inc.; 1996. Perchloric acid and perchlorates. pp. 157–170. [Google Scholar]
- Mervish N, Blount B, Valentin-Blasini L, Brenner B, Galvez MP, Wolff MS, et al. Temporal variability in urinary concentrations of perchlorate, nitrate, thiocyanate and iodide among children. J Expo Sci Environ Epidemiol. 2012;22:212–218. doi: 10.1038/jes.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michajlovski N, Langer P. Studies on relations between thiocyanate formation and goitrogenic properties of foods. I. Preformed thiocyanate content of some foods [in German]. Hoppe Seylers Z Physiol Chem. 1958;312:26–30. [PubMed] [Google Scholar]
- Miell JP, Taylor AM, Zini M, Maheshwari HG, Ross RJ, Valcavi R. Effects of hypothyroidism and hyperthyroidism on insulin-like growth factors (IGFs) and growth hormone- and IGF-binding proteins. J Clin Endocrinol Metab. 1993;76:950–955. doi: 10.1210/jcem.76.4.7682563. [DOI] [PubMed] [Google Scholar]
- Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration’s Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol. 2008;18:571–580. doi: 10.1038/sj.jes.7500648. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int J Androl. 2008;31:201–208. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council, Committee to Assess the Health Implications of Perchlorate Ingestion) Washington, DC: National Academies Press; 2005. Health Implications of Perchlorate Ingestion. [Google Scholar]
- Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–171. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Reinehr T, Andler W. Thyroid hormones before and after weight loss in obesity. Arch Dis Child. 2002;87:320–323. doi: 10.1136/adc.87.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Pacific Grove, CA: Duxbury; 2000. Fundamentals of Biostatistics. 5th ed. [Google Scholar]
- Rubin BS, Soto AM. Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol. 2009;304:55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell LM, Gallo MV, Denham M, Ravenscroft J, DeCaprio AP, Carpenter DO. 2008. Relationship of thyroid hormone levels to levels of polychlorinated biphenyls, lead, p,p’-DDE, and other toxicants in Akwesasne Mohawk youth. Environ Health Perspect 116 806 813; doi: 10.1289/ehp.10490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalitin S, Yackobovitch-Gavan M, Phillip M. Prevalence of thyroid dysfunction in obese children and adolescents before and after weight reduction and its relation to other metabolic parameters. Horm Res. 2009;71:155–161. doi: 10.1159/000197872. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. 2007. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect 115 876 882; doi: 10.1289/ehp.9882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Miller MD, Cushing L, Blount BC, Smith AH. 2013. Combined effects of perchlorate, thiocyanate, and iodine on thyroid function in the National Health and Nutrition Examination Survey 2007–08. Environ Res 123 17 24; doi: 10.1016/j.envres.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Miller MD, Howd R. 2007. Impact of smoking and thiocyanate on perchlorate and thyroid hormone associations in the 2001–2002 National Health and Nutrition Examination Survey. Environ Health Perspect 115 1333 1338; doi: 10.1289/ehp.10300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajtáková M, Semanová Z, Tomková Z, Szökeová E, Majoros J, Rádiková Z, et al. Increased thyroid volume and frequency of thyroid disorders signs in schoolchildren from nitrate polluted area. Chemosphere. 2006;62:559–564. doi: 10.1016/j.chemosphere.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Téllez Téllez R, Michaud Chacón P, Reyes Abarca C, Blount BC, Van Landingham CB, Crump KS, et al. Long-term environmental exposure to perchlorate through drinking water and thyroid function during pregnancy and the neonatal period. Thyroid. 2005;15:963–975. doi: 10.1089/thy.2005.15.963. [DOI] [PubMed] [Google Scholar]
- Terry MB, Wei Y, Esserman D. Maternal, birth, and early-life influences on adult body size in women. Am J Epidemiol. 2007;166:5–13. doi: 10.1093/aje/kwm094. [DOI] [PubMed] [Google Scholar]
- Thurlow RA, Winichagoon P, Pongcharoen T, Gowachirapant S, Boonpraderm A, Manger MS, et al. 2006. Risk of zinc, iodine and other micronutrient deficiencies among school age children. Eur J Clin Nutr 60: 623 632 [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14:1012–1019. doi: 10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- Valentin-Blasini L, Blount BC, Delinsky A. Quantification of iodide and sodium-iodide symporter inhibitors in human urine using ion chromatography tandem mass spectrometry. J Chromatogr A. 2007;1155:40–46. doi: 10.1016/j.chroma.2007.04.014. [DOI] [PubMed] [Google Scholar]
- van Maanen JM, van Dijk A, Mulder K, de Baets MH, Menheere PC, van der Heide D, et al. Consumption of drinking water with high nitrate levels causes hypertrophy of the thyroid. Toxicol Lett. 1994;72:365–374. doi: 10.1016/0378-4274(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Werner SC, Ingbar SH, Braverman LE, Utiger RD, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. [Google Scholar]
- Willett W. New York: Oxford University Press; 1998. Nutritional Epidemiology. 2nd ed. [Google Scholar]
- Xi B, Mi J, Zhao M, Zhang T, Jia C, Li J, et al. Trends in abdominal obesity among US children and adolescents. Pediatrics. 2014;134:e334–e339. doi: 10.1542/peds.2014-0970. [DOI] [PubMed] [Google Scholar]
- Youso SL, Rockwood GA, Logue BA. The analysis of protein-bound thiocyanate in plasma of smokers and non-smokers as a marker of cyanide exposure. J Anal Toxicol. 2012;36:265–269. doi: 10.1093/jat/bks017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MB. The adverse effects of mild-to-moderate iodine deficiency during pregnancy and childhood: a review. Thyroid. 2007;17:829–835. doi: 10.1089/thy.2007.0108. [DOI] [PubMed] [Google Scholar]
- Zoeller RT. Environmental chemicals impacting the thyroid: targets and consequences. Thyroid. 2007;17:811–817. doi: 10.1089/thy.2007.0107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


