Abstract
Background:
High radon exposure is a risk factor for squamous cell carcinoma, a major lung cancer histology observed in former uranium miners. Radon exposure can cause oxidative stress, leading to pulmonary inflammation. Interleukin-6 (IL-6) is a pro-carcinogenic inflammatory cytokine that plays a pivotal role in lung cancer development.
Objectives:
We assessed whether single nucleotide polymorphisms (SNPs) in the IL6 promoter are associated with lung cancer in former uranium miners with high occupational exposure to radon gas.
Methods:
Genetic associations were assessed in a case–control study of former uranium miners (242 cases and 336 controls). A replication study was performed using data from the Gene Environment Association Studies (GENEVA) Genome Wide Association Study (GWAS) of Lung Cancer and Smoking. Functional relevance of the SNPs was characterized using in vitro approaches.
Results:
We found that rs1800797 was associated with squamous cell carcinoma in miners and with a shorter time between the midpoint of the period of substantial exposure and diagnosis among the cases. Furthermore, rs1800797 was also associated with lung cancer among never smokers in the GENEVA dataset. Functional studies identified that the risk allele was associated with increased basal IL-6 mRNA level and greater promoter activity. Furthermore, fibroblasts with the risk allele showed greater induction of IL-6 secretion by hydrogen peroxide or benzo[a]pyrene diolepoxide treatments.
Conclusions:
An IL6 promoter variant was associated with lung cancer in uranium miners and never smokers in two external study populations. The associations are strongly supported by the functional relevance that the IL6 promoter SNP affects basal expression and carcinogen-induced IL-6 secretion.
Citation:
Leng S, Thomas CL, Snider AM, Picchi MA, Chen W, Willis DG, Carr TG, Krzeminski J, Desai D, Shantu A, Lin Y, Jacobson MR, Belinsky SA. 2016. Radon exposure, IL-6 promoter variants, and lung squamous cell carcinoma in former uranium miners. Environ Health Perspect 124:445–451; http://dx.doi.org/10.1289/ehp.1409437
Introduction
Radon is an inert gas released during the decay of radium-226. Radon gas is ubiquitous in indoor and outdoor air and contaminates many underground mines (Sethi et al. 2012). Cohort studies of underground miners have established a strong association between high levels of radon exposure and increased risk for lung cancer (Archer 1988; Archer et al. 2004; Gilliland et al. 2000). Moreover, combined exposure to radon and tobacco smoke through uranium mining may further increase lung cancer risk (Archer 1988). Lung squamous cell carcinoma is the predominant histological type of lung cancer observed in former uranium miners, and it is likely driven by the localization of radon within the upper airways owing to its binding to silica and diesel particles inhaled by the miners (Saccomanno et al. 1996; Samet 1989; Sethi et al. 2012). An association between residential radon exposure and lung cancer risk was also identified in seven North American case–control studies (Krewski et al. 2006).
The high–linear energy transfer alpha particles emitted by radon and radon daughters can directly attack genomic DNA and cause mainly double-strand breaks in DNA (Prise et al. 2001; Sethi et al. 2012). In addition, overproduction of reactive oxygen species in the lungs caused by persistent radon exposure may cause oxidative stress, leading to pulmonary inflammation, tissue damage, and eventually to chronic lung diseases such as chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, and lung cancer (Archer et al. 1998; Iyer et al. 2000; Mapel et al. 1997; Narayanan et al. 1997; Rosanna and Salvatore 2012; Schubauer-Berigan et al. 2009). Strong associations between pulmonary inflammation as manifested in COPD and/or chronic mucous hypersecretion and risk for subsequent lung cancer incidence support the premise that persistent inflammation is involved in the etiology of lung cancer (Brenner et al. 2012; Wilson et al. 2008). Among the cytokines and chemokines produced by persistent pulmonary inflammation, interleukin-6 (IL-6) plays a pivotal role in promoting cancer development as shown in studies using in vitro and in vivo models of lung carcinogenesis (Chen et al. 2012; Dougan et al. 2011; Gao et al. 2007; Ochoa et al. 2011; Qi et al. 2014).
IL6 has a promoter with several single nucleotide polymorphisms (SNPs) that show large differences in minor allele frequency (MAF) across major ethnic populations. Increased induction of IL6 promoter activity by norepinephrine, lipopolysaccharide, and IL-1 in an in vitro plasmid construct carrying the G allele of rs1800795 has been identified (Cole et al. 2010; Fishman et al. 1998). Gel shift assays confirmed the exclusive binding of GATA1 to the sequence containing the rs1800795 G allele following norepinephrine induction (Cole et al. 2010). However, results from population studies assessing the association between rs1800795 and plasma levels of IL-6 as an indicator for systematic inflammation were inconsistent (Fishman et al. 1998; He et al. 2009; Ljungman et al. 2009a, 2009b; Sousa et al. 2012; Zakharyan et al. 2012).
The present study assessed the association between IL6 promoter SNPs and squamous cell carcinoma in uranium miners. Generalization to populations with residential radon exposure was examined using the Gene Environment Association Studies (GENEVA) Genome Wide Association Study (GWAS) of Lung Cancer and Smoking. The functional relevance of significant variants was also assessed in vitro using multiple cell types.
Methods
Former uranium miners. A cumulative incidence case–control study was conducted in the Saccomanno Uranium Miner cohort of male former uranium miners (n = 17,000) who worked underground at the Colorado plateau and participated in sputum cytology screening for lung cancer detection between 1957 and 2002 (Saccomanno et al. 1996). A pool of confirmed deceased former uranium miners (360 squamous cell carcinoma cases and 810 lifetime lung cancer–free miners) with available data for essential variables including age and smoking history at sputum collection, working level month (WLM), age at death, age at lung cancer diagnosis (for cases), and survival after lung cancer diagnosis (for cases) were used in the present study. Squamous cell carcinoma cases were identified from the St. Mary’s Hospital Cancer Registry and the St. Mary’s Saccomanno Research Institute Cancer Research Database. Because 94% of the miners (n = 1,100) were Caucasian, the study was restricted to this ethnic group to minimize bias owing to ancestry differences. The sputum specimens proximal to cancer diagnosis (cases) or the last follow-up examination (controls) were used for DNA isolation. Study participants for whom DNA samples could not be recovered from sputum cytology slides based on amplification of a 180-bp DNA fragment in the human KRAS gene were excluded from the present study. A total of 267 cases and 383 controls were included for the genotyping study. For the miners selected as the controls, cumulative radon exposure at work, expressed as WLM, and age at death were similar to those reported for other lifetime lung cancer–free uranium miners who were born during 1904–1933 [WLM, 0.8 vs. 0.7 kWLMs; age at death, 68.7 vs. 68.5 years (Leng S., unpublished data)]. No personal identifiers accompanied the transfer of material from the St. Mary’s Saccomanno Research Institute to Lovelace Respiratory Research Institute. The present study was conducted under an Institutional Review Board–approved protocol that was exempt from informed consent requirements based on the Department of Health and Human Services regulations under 45CFR46.102(f), which defines a human subject as a living individual.
Cumulative radon exposure. WLM, a time-integrated measure of radon progeny exposure from mine air, was calculated for each individual as the product of the exposure time in working months (1 month = 170 hr) and average working level to estimate the cumulative radon exposure for each miner. Working level was defined as the measure of the energy released by airborne radon progeny that was measured based on counting the emissions of alpha particles in a representative volume of air [National Research Council (NRC) 1994]. One working level equals any combination of radon progeny in 1 L of air that results in the ultimate emission of 130,000 MeV of energy from alpha particles. The information used for WLM assessment was obtained from the mining history for each miner and from the accumulated mine measurement data collected by the National Institute for Occupational Safety and Health (NIOSH) (Archer et al. 2004; Lundin et al. 1971). WLM was calculated up to the time of cancer diagnosis for squamous cell carcinoma cases and for the entire uranium mining occupation for the controls.
GENEVA GWAS of Lung Cancer and Smoking. Lung cancer cases (n = 2,522) and controls (n = 2,725) drawn from the Environment and Genetics in Lung Cancer Etiology (EAGLE) study (1,816 cases and 1,984 controls) and the Prostate, Lung, Colon and Ovarian (PLCO) cancer screening trial (706 cases and 741 controls) comprised the GENEVA GWAS of Lung Cancer and Smoking (Landi et al. 2009), with genotype data obtained using Illumina HumanHap550v3.0 chips (dbGaP Study Accession number: phs000093.v2.p2; see Supplemental Material, Table S1). The EAGLE and PLCO studies enrolled participants from general populations in the Lombardy region of Italy and in 10 locations throughout the United States, respectively (see Supplemental Material, “Introduction for GENEVA GWAS of Lung Cancer and Smoking,” for a detailed introduction of these two studies); thus, participants in these studies would not be expected to have occupational exposure to radon or other radiation. Allele dosage for rs1800797 was extracted from imputation data that were obtained using European populations from the 1000 Genomes Project as the reference (1000 Genomes Project Consortium et al. 2012). This study was selected to assess whether the association with lung cancer that was observed for rs1800797 in miners can be generalized to populations with residential radon exposure because of the large sample size and available smoking history data. Stratification analysis by smoking status or tumor histology was conducted because a combined analysis of seven large-scale case–control studies conducted in North America identified a significant association between residential radon exposure and lung cancer risk in adenocarcinoma (Krewski et al. 2006). In addition, the role of residential radon exposure in the etiology of lung cancer may be more prominent in never smokers (Samet et al. 2009). The informed consent document signed by the PLCO study participants allows use of these data by investigators for discovery and hypothesis generation in the investigation of the genetic contributions to cancer and other adult diseases as well as for development of novel analytical approaches for GWASs. Use of the EAGLE data set is limited to scientific genetic research related to the etiology, molecular basis, and outcome of lung disease and smoking. Thus, IRB approval was not required for the use of these deidentified data for genetic association analysis in the present study.
SNP selection and genotyping. All common SNPs (minor allele frequency > 0.05) within the IL6 locus (chr7: 22721293–22776691, genome build 36; see Supplemental Material, Figure S1) were predicted for their functional potential using SNP Web Info (Xu and Taylor 2009). Four SNPs (rs12700386, rs2069827, rs1800797, and rs2069840) in promoters not in high linkage disequilibrium (LD) (r 2 < 0.22) were suggested to affect the binding of transcription factors as indicated by the fact that MATCHTM (Kel et al. 2003) predicted a transcriptional factor binding site with one allele but not with the other or the difference in the matrix similarity scores or core similarity scores between the two alleles was ≥ 0.2, and thus were selected for genotyping using the TaqMan genotyping assay (Life Technologies). The rs1800797 A allele is in almost perfect LD with the rs1800795 C allele (r 2 = 0.97). Participants with missing genotype data for more than two SNPs (5 squamous cell carcinoma cases and 23 controls) were excluded from the present analysis. Thus, 242 cases and 336 controls were eventually included in the genetic association analysis.
Real-time polymerase chain reaction. RNA was isolated from low-passage (≤ 2) primary human bronchial epithelial cell (BEC) cultures (n = 85) and human skin fibroblast lines (n = 6) that were established from cells obtained by bronchoscopy from current or former smokers examined at pulmonary clinics (Leng et al. 2012) or were obtained from infant foreskin at University of New Mexico Hospital, respectively. BEC cultures established from unaffected sites were used for this experiment for patients with subsequent lung cancer diagnosis. TaqMan real-time polymerase chain reaction (qPCR) was performed to quantify IL6 gene expression in cDNA using the delta threshold cycle method with β-actin as the endogenous control.
Resequencing of the IL-6 promoter. Twenty-one alleles of the IL6 promoter (690 bps, chr7:22732707–22733396) that contained 9 rs1800797 A alleles and 12 rs1800797 G alleles were successfully amplified using primers listed in Supplemental Material, Table S2, from 13 human BEC cultures that were heterozygous for rs1800797 and were directionally cloned into the pGL2-basic luciferase reporter vector (Promega) upstream of the luciferase coding sequence using MluI and BglII cloning sites according to the manufacturer’s instructions. Bidirectional Sanger sequencing was performed to confirm the haplotype alleles that contained four SNPs: rs1800797, rs1800796, rs36215814, and rs1800795.
Luciferase reporter assay. pGL2 constructs generated using the method described above that contained two common haplotype alleles (A-G-A8T12-C and G-G-A10T11-G) of the IL6 promoter were sequence verified for transfection experiments. Transfection was performed in a human embryonic kidney cell line and a human lung fibroblast line (HEK293 and HFL1, respectively; both obtained directly from the American Type Culture Collection) using TransIT-2020 transfection reagent (Mirus Bio LLC) and in a normal human bronchial epithelial cell line (HBEC2) immortalized by insertion of the telomerase catalytic subunit and cyclin-dependent kinase 4 (obtained from Shay and Minna, Southwestern Medical Center, Dallas, TX) using a Neon electroporation system (Life Technologies). Cells were harvested 48 hr after transfection, and reporter activity was measured using the Dual Luciferase Assay System (Promega). Control experiments were conducted in the same manner as described above, except the IL6 promoter constructs were replaced with promoterless or SV40 promoter constructs. The constructs carrying two major haplotype alleles (A-G-A8T12-C and G-G-A10T11-G) in the IL6 promoter generated 5–30 times higher reporter activity relative to the promoterless construct in the three cell lines tested (not shown), suggesting that IL6 has a robust promoter.
Benzo[a]pyrene diolepoxide– and hydrogen peroxide–induced IL-6 secretion in fibroblast lines. Lung fibroblasts appear to be a major source of IL-6 in the microenvironment of normal lungs based on the findings of greater basal and tobacco carcinogen–induced secretion of IL-6 in human lung fibroblasts than in lung epithelial cells (Chen et al. 2012). A dose-dependent induction of IL-6 was observed in lung fibroblasts treated with benzo[a]pyrene diolepoxide (BPDE) (Chen et al. 2012), and this response was replicated in skin fibroblasts (Figure 1). Thus, owing to the lack of lung fibroblasts with varied genotypes, the effect of IL6 variants on IL-6 induction by lung carcinogen treatment was assessed in skin fibroblasts that were wild homozygous (GG, n = 3) and heterozygous (AG, n = 3) for rs1800797. Low-passage skin fibroblast lines (n = 6) were maintained in Dulbecco’s modified essential medium (DMEM) with 10% fetal bovine serum (FBS). Prior to treatment with BPDE or hydrogen peroxide (H2O2), cells were washed twice with phosphate-buffered saline (PBS) to remove any residual FBS in the medium. Cells were then cultured in DMEM without FBS and treated with BPDE or H2O2 at varied concentrations for 1 hr. The medium with carcinogens was then replaced with fresh medium without FBS. The level of IL-6 in the medium was measured 24 hr later using an enzyme-linked immunosorbent assay (ELISA) kit (eBioscience).
Figure 1.
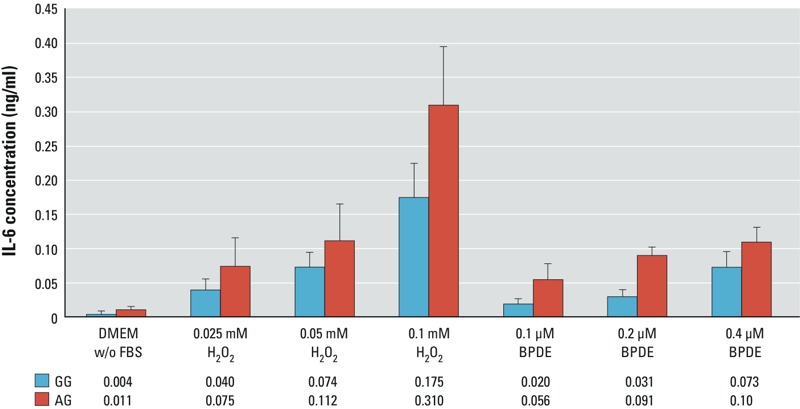
Promoter single nucleotide polymorphisms (SNPs) affect IL-6 secretion in fibroblast cells treated with H2O2 and benzo[a]pyrene diolepoxide (BPDE). Abbreviations: DMEM, Dulbecco’s modified essential medium; FBS, fetal bovine serum. Six fibroblast cell lines, three wild homozygotes (GG), and three heterozygotes (AG) for rs1800797 were treated with H2O2 and BPDE. The height of the bar is the average concentration of IL-6 in culture medium that is also expressed as the number under the figure. The error bar is the standard deviation from three independent experiments. A generalized linear model was used to assess the effects of H2O2 or BPDE treatment and rs1800797 genotype on IL-6 concentration detected in the medium. A strong IL-6 induction was identified for both carcinogens (p-values < 0.0001). The slope for the induction of IL-6 secretion by H2O2 or BPDE treatment is 74% and 39% greater in AG lines than GG lines (p-values = 0.017 for H2O2 and 0.13 for BPDE).
Statistical analysis. Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between case–control status and each SNP using an additive inheritance model that evaluated the contribution of each allele to cancer risk. Models were adjusted for cumulative radon exposure (WLM), which was modeled using one indicator variable for ≥ 0.895 kWLM and one indicator variable for missing WLM (n = 27), with < 0.895 kWLM as the common reference exposure, and for smoking pack-years (≥ 35 vs. < 35) and age at sputum collection. The missing indicator variable for WLM was included to maximize the number of observations included in the analysis.
The complete occupational histories for underground uranium mining were available for 162 miners with squamous cell carcinoma diagnosis, which allowed calculation of the mid-induction latency (MIL), defined as the time from the midpoint of the period of substantial exposure (an annual accumulative dose of ≥ 50.6 mSv) to squamous cell carcinoma diagnosis (Archer et al. 2004). All 162 miner cases achieved this level of exposure before the mid-1960s, when effective radiation control began to be implemented in underground uranium mines. The least-square means of MIL for miners with GG, GA, and AA genotypes of rs1800797 were calculated using a generalized linear model with adjustment for underground mining history and smoking history surveyed at cancer diagnosis. Cox proportional hazard models were used for 162 cases to estimate associations between IL6 variants and the time to cancer diagnosis. Underground mining history (age at start of underground uranium mining, average WLM per month, and total years of underground mining) and smoking history (age at start of cigarette smoking, average number of cigarettes smoked per day, total years of cigarette smoking, and number of years after quitting smoking) surveyed at squamous cell carcinoma diagnosis were included in the model for covariate adjustment.
A generalized linear model was used to assess the association between rs1800797 and IL6 expression in 85 primary human BECs and between subsequent lung cancer diagnosis and IL6 expression in a subset of primary human BECs with adjustment for rs1800797 genotype coded as 0, 1, and 2 for GG, GA, and AA, respectively (n = 78). A likehood ratio test implemented in Merlin (Abecasis et al. 2002) was applied to test the association between rs1800797 and steady-state IL6 expression (GI_10834983-S) in 79 Epstein-Barr virus (EBV)–transformed lymphoblastoid cell lines from CEU families (Utah residents with ancestry from northern and western Europe) in the HapMap project (Abecasis et al. 2002; Holm et al. 2010). The effects of rs1800797 and dose of carcinogen exposure on IL-6 secretion in human skin fibroblast lines (n = 6) were assessed using generalized linear models. The comparison of slopes for the dose–response curves by rs1800797 genotype was conducted using the likelihood ratio test by including an interaction term for genotype and carcinogen treatment in the generalized linear models. A two-sided p-value of 0.05 was used to define a significant association. Statistical analyses were conducted using SAS 9.2 (SAS Institute Inc.).
Results
Demographics of the miners. A total of 242 cases and 336 controls were eventually included in the genetic association analysis (Table 1). Cases were older and had more pack-years than controls at the time of the latest sputum collection. Half of the cases died within 6 months of diagnosis. Cases were an average of 6 years younger at death than controls. All study participants were male and of non-Hispanic white (NHW) ethnicity.
Table 1.
Characteristics of squamous cell carcinoma cases and controls.
| Variable | Case | Control | p-Value |
|---|---|---|---|
| n | 242 | 336 | |
| Age at lung cancer diagnosis (years, mean ± SD) | 60.3 ± 10.4 | — | |
| Survival after diagnosis [years, median (Q1–Q3)] | 0.6 (0.1–2.3) | — | |
| Age at death (years, mean ± SD) | 62.6 ± 10.6 | 69.2 ± 12.5 | 2.8 × 10–10a |
| Sex (male percent) | 100 | 100 | |
| Ethnicity (non-Hispanic white %) | 100 | 100 | |
| WLM [kWLMs,b median (Q1–Q3)] | 1.0 (0.5–2.1) | 0.8 (0.4–1.5) | 0.001c |
| < 0.895 (%) | 41.1 | 54.0 | 0.055d |
| ≥ 0.895 (%) | 48.6 | 45.4 | |
| Missing (%) | 10.4 | 0.6 | |
| Mid-induction latency [years, median (Q1–Q3)]e | 19 (12.0–26.5) | ||
| WLM, working level month. aStudent’s t-test. bTwenty-seven miners had missing cumulative WLM estimates. WLM, a time-integrated measure, was calculated as the product of time in working months (1 month = 170 hr) and working levels to estimate the cumulative exposure to radon daughter radiation for each miner. cWilcoxon rank-sum test. dChi-square test for differences between cases and controls. The missing group was not included in this test. eSquamous cell carcinoma cases (n = 162) with complete information for latency and genotypes. Mid-induction latency defined as time from midpoint of the period of substantial exposure (an annual accumulative dose of ≥ 50.6 mSv) to squamous cell carcinoma diagnosis (Archer et al. 2004). | |||
Association between IL6 promoter SNPs and squamous cell carcinoma in miners. Miners with cumulative radon exposure levels ≥ 0.895 kWLM had a statistically significant greater risk for squamous cell carcinoma than miners with < 0.895 kWLM (OR = 1.51, 95% CI: 1.05, 2.18, p = 0.026 with adjustment for rs1800797 genotype, pack-years, and age at sputum collection), consistent with radon progeny exposure as a risk factor for squamous cell carcinoma in miners. More cases had missing WLM estimates than controls (OR = 18.6, 95% CI: 4.2, 82.8, p = 0.00012). The variant allele A of rs1800797 was a statistically significant risk factor for squamous cell carcinoma in former uranium miners (OR = 1.36, 95% CI: 1.05, 1.75, p = 0.018; Table 2). A sensitivity analysis of miners with nonmissing WLM estimates did not change the genetic associations observed for IL6 promoter SNPs (OR = 1.34, 95% CI: 1.04, 1.73, p = 0.023).
Table 2.
Association between IL-6 promoter single nucleotide polymorphisms (SNPs) and squamous cell carcinoma in miners (odds ratios based on adjusted logistic regression models of 242 cases and 336 controls) and hazard ratios for the time from the onset of high exposure to diagnosis among 162 cases.
| SNP | Allelea | Case–control study of squamous cell carcinomab | Mid-induction latencyc | |||
|---|---|---|---|---|---|---|
| Cased | Controld | OR (95% CI)e | Average (years)f | Hazard ratioe | ||
| rs12700386 | C/G | 0.66/0.29/0.05 | 0.65/0.31/0.04 | 0.94 (0.69, 1.30) | 19.3/20.1/18.4 | 0.81 (0.59, 1.11) |
| rs2069827 | G/T | 0.84/0.15/0.01 | 0.83/0.14/0.04 | 0.84 (0.56, 1.26) | 19.5/19.9/13.8 | 1.30 (0.86, 1.95) |
| rs1800797 | G/A | 0.30/0.50/0.21 | 0.39/0.45/0.16 | 1.36 (1.05, 1.75) | 20.7/19.1/18.8 | 1.57 (1.22, 2.01) |
| rs2069840 | C/G | 0.45/0.45/0.10 | 0.44/0.37/0.19 | 0.77 (0.60, 1.01) | 20.1/19.3/17.5 | 0.95 (0.71, 1.28) |
| Abbreviations: CI, confidence interval; OR, odds ratio. aAll forward strand RefSNP alleles. Alleles underlined were minor allele and test allele in the logistic regression. bAdjustment for cumulative working level month (WLM) estimate, pack-years and age at sputum collection was included in logistic regression models. Analysis was conducted in 242 lung squamous cell carcinoma cases and 336 controls. cAdjustment for underground mining history and smoking history surveyed at squamous cell carcinoma diagnosis was included in Cox regression models. Analysis was conducted in 162 cases owing to unavailability of the complete record of the occupational history for underground uranium mining. dForward slashes separate the frequencies of wild homozygotes, heterozygotes, and variant homozygotes for each individual SNP. eOR or hazard ratio was calculated for the association between genotype of each IL6 variant and risk for squamous cell carcinoma or latency, respectively. Each variant was coded as 0, 1, or 2 for wild homozygote, heterozygote, or variant homozygote. fForward slashes separate the average mid-induction latency for participants carrying wild homozygotes, heterozygotes, and variant homozygotes for each individual SNP. Mid-induction latency was defined as time from midpoint of the period of substantial exposure (an annual accumulative dose of ≥ 50.6 mSv) to squamous cell carcinoma diagnosis (Archer et al. 2004). | ||||||
Association between IL6 promoter SNPs and latency in miners with squamous cell carcinoma. The median of latency (19 years) in the 162 miners with squamous cell carcinoma was comparable to that seen for a large group of underground uranium miners (18.9 years, n = 505) from the United States who were either current smokers or had quit smoking < 10 years when diagnosed with lung cancer (Archer et al. 2004). The least-square means of MIL calculated using a generalized linear model with adjustment for underground mining history and smoking history surveyed at cancer diagnosis were 20.7 (standard error, 0.68), 19.5 (0.50), and 18.0 (0.73) for miners with GG, GA, and AA genotypes of rs1800797, respectively (p = 0.0075). Consistent with being a risk allele for squamous cell carcinoma, each copy of the A allele of rs1800797 was associated with a hazard ratio (HR) of 1.57 (95% CI: 1.22, 2.01, p = 0.00037) for latency (Table 2).
Association between rs1800797 and risk for lung cancer in the GENEVA dataset. The GWAS of 2,522 lung cancer cases and 2,725 controls identified an increased risk for lung cancer associated with the rs1800797 A allele (OR = 1.10, 95% CI: 1.01, 1.20, p = 0.04; Table 3). Associations between rs1800797 and lung cancer varied significantly by smoking status, with OR = 1.41 (95% CI: 1.05, 1.91) for each A allele in never smokers (interaction p-value = 0.05 for the difference from current smokers); OR = 1.08 (95% CI: 0.95, 1.24) for each A allele in former smokers (interaction p-value = 0.03 for the difference from current smokers); and OR 1.06 (95% CI: 0.92, 1.22) in current smokers (Table 3). Associations with each rs1800797 A allele also varied by histologic subtype, with a significant positive association with adenocarcinoma (OR = 1.16; 95% CI:1.04, 1.31; p = 0.009) compared with squamous cell carcinoma (OR = 1.08; 95% CI: 0.93, 1.25; p = 0.3) (Table 3).
Table 3.
Association between rs1800797 and risk for lung cancer in the GENEVA dataset.
| Variable | Control (n)a | Case (n)a | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Overall | 0.67 ± 0.67 (2,725) | 0.70 ± 0.67 (2,522) | 1.10 (1.01, 1.20) | 0.045b |
| Smoking statusc | ||||
| Never smokers | 0.60 ± 0.63 (633) | 0.70 ± 0.64 (138) | 1.41 (1.05, 1.91) | 0.024d |
| Former smokers | 0.67 ± 0.68 (1,125) | 0.72 ± 0.69 (1,204) | 1.08 (0.95, 1.24) | 0.24d |
| Current smokers | 0.70 ± 0.68 (967) | 0.67 ± 0.66 (1,180) | 1.06 (0.92, 1.22) | 0.43d |
| Histology | ||||
| Adenocarcinoma | 0.67 ± 0.67 (2,725) | 0.72 ± 0.69 (986) | 1.16 (1.04, 1.31) | 0.0091d |
| Squamous cell carcinoma | 0.67 ± 0.67 (2,725) | 0.69 ± 0.67 (582) | 1.08 (0.93, 1.25) | 0.33d |
| Small cell | 0.67 ± 0.67 (2,725) | 0.62 ± 0.65 (256) | 0.90 (0.73, 1.11) | 0.32d |
| Others | 0.67 ± 0.67 (2,725) | 0.70 ± 0.67 (698) | 1.04 (0.91, 1.19) | 0.59d |
| Abbreviations: CI, confidence interval; GENEVA, Gene Environment Association Studies; OR, odds ratio. aA allele dosage for rs1800797 presented as mean ± standard deviation. bAdjustment for age, sex, smoking status, pack-years, and cohort was included in logistic regression models. cThe interaction terms between never versus current smokers and rs1800797 and between former versus current smokers and rs1800797 were included together in the logistic regression with statistical significance identified for both interaction terms (p-values = 0.050 and 0.029, respectively). dAdjustment for age, sex, pack-years, and cohort was included in logistic regression models. | ||||
Association between rs1800797 and IL6 expression. The risk allele of rs1800797 was associated with increased IL6 expression in an allelic dose-dependent manner assessed in 85 primary human BECs from current and former smokers (p = 0.009) and 79 lymphoblastic cell lines from the HapMap CEU population (p = 0.034) (Table 4). Interestingly, human BECs collected from patients with a subsequent lung cancer diagnosis (n = 56) had significantly higher IL6 expression than those without (n = 22) (0.0029 ± 0.0034 vs. 0.0013 ± 0.0014, p = 0.027 with adjustment for the rs1800797 genotype).
Table 4.
Association between rs1800797 and IL6 expression in primary human bronchial epithelial cells and lymphoblastic cell lines.
| Tissuea | rs1800797 | n | IL6 expression (mean ± SD) | p-Value |
|---|---|---|---|---|
| Human bronchial epithelial cells | 0.009b | |||
| GG | 42 | 0.0016 ± 0.0019 | ||
| GA | 32 | 0.0031 ± 0.0036 | ||
| AA | 11 | 0.0041 ± 0.0038 | ||
| Lymphoblastic cell lines | 0.034c | |||
| GG | 18 | 6.18 ± 0.33 | ||
| GA | 43 | 6.19 ± 0.33 | ||
| AA | 18 | 6.36 ± 0.37 | ||
| aPrimary human bronchial epithelial cell (BEC) cultures (n = 85) were established from cells obtained by bronchoscopy from current or former smokers examined at the pulmonary clinic of University of New Mexico Hospital. BEC cultures established from unaffected sites were used for this experiment for patients with subsequent lung cancer diagnosis. Epstein-Barr virus–transformed lymphoblastoid cell lines (n = 79) from CEU families (Utah residents with ancestry from northern/western Europe) were established by the HapMap project. bGeneralized linear model with adjustment for lung cancer diagnosis status. rs1800797 genotype was coded as 0, 1, or 2 for GG, GA, or AA genotype, respectively. IL6 expression was expressed as relative quantification with β-actin as the endogenous control. cLikelihood ratio test implemented in MERLIN was used to evaluate the association between rs1800797 and IL6 expression (GI_10834983-S). The family structure and sex were included in the models for covariate adjustment. | ||||
Haplotype alleles in the IL6 promoter. AGC and GGG are two major haplotype alleles in the IL6 promoter that contain rs1800797, rs1800796, and rs1800795 and have cumulative allele frequency > 0.95 in European populations from the 1000 Genomes Project (1000 Genomes Project Consortium et al. 2012). However, the phasing status is unclear between rs36215814 as an AnTn polymorphism located between rs1800796 and rs1800795 and the other three SNPs. Cloned sequencing of 21 IL6 promoter alleles amplified from 13 self-identified non-Hispanic white study participants who were heterozygous for rs1800797 identified eight different compositions of the AnTn polymorphism with A8T12 enriched in the AGC allele and A10T11 enriched in the GGG allele (see Supplemental Material, Table S2). According to the allele frequencies for AGC (0.5) and GGG (0.45) in the European populations (1000 Genomes Project Consortium et al. 2012), the estimated frequencies for haplotype alleles A-G-A8T12-C and G-G-A10T11-G are 0.5 and 0.15, respectively.
IL6 promoter activity by haplotype alleles. The reporter assay showed that haplotype allele A-G-A8T12-C carrying the risk allele of rs1800797 had 45–92% increased promoter activity compared with G-G-A10T11-G in HFL1, HEK293, and HBEC2 cell lines (p-values < 0.00062; Figure 2).
Figure 2.
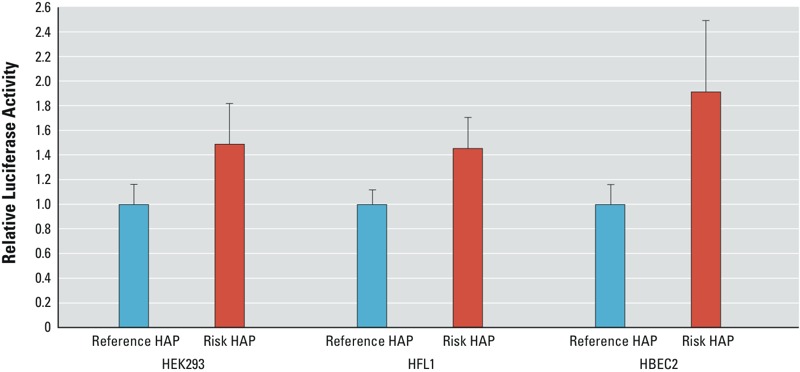
IL6 promoter activity by haplotype alleles. Luciferase reporter construct containing haplotype allele A-G-A8T12-C (Risk HAP) that carried variant alleles for rs1800797 (A) and rs1800795 (C) had significantly higher reporter activity than was observed for G-G-A10T11-G (Reference HAP) in HEK293 (p-value = 3.42 × 10–6), HFL1 (p-value = 1.86 × 10–5), and HBEC2 (p-value = 6.2 × 10–4). The height of the bar is the average luciferase activity standardized by the levels observed for G-G-A10T11-G set to one. The error bar is the standard deviation.
IL6 promoter SNPs and the effects of H2O2 and BPDE on IL-6 secretion in human fibroblasts. Six skin fibroblast lines (three GGs and three AGs for rs1800797 as described in “Methods”) were used to assess whether the rs1800797 genotype could modify IL-6 secretion as the response to DNA damage indicative of radon or tobacco carcinogen exposure. IL6 mRNA expression was 10 times higher in AG lines than in GG lines (0.0021 ± 0.0011 vs. 0.00020 ± 0.00009, respectively; p = 0.061, data not shown). Treating cells with H2O2 and BPDE creates oxidative damage and BPDE-DNA adducts that mimic the damage induced by radon and by the tobacco carcinogen benzo[a]pyrene, respectively (Narayanan et al. 1997; Tellez et al. 2011). The effects of the rs1800797 genotype (coded as 0 for GG and 1 for AG) and the dose of carcinogen (concentration in medium) on IL-6 secretion in human skin fibroblast lines (n = 6) were assessed using generalized linear models. A dose-dependent induction of IL-6 secretion was seen in skin fibroblasts treated with H2O2 and BPDE (p-values < 0.0001; Figure 1). AG lines had consistently higher levels of IL-6 secretion than GG lines (p-values < 0.015) with both treatments. Moreover, the slopes for the induction of IL-6 secretion by increasing concentrations of H2O2 and BPDE were 74% and 39% greater, respectively, in AG lines than in GG lines [2.97 (0.44) vs. 1.71 (0.20), p = 0.017 for H2O2 and 0.24 (0.04) vs. 0.17 (0.02), p = 0.13 for BPDE], indicating a stronger induction kinetic for IL-6 secretion in AG lines. Furthermore, the strong correlation between IL6 mRNA expression and protein secretion (Pearson correlation coefficient = 0.78) suggested that the IL-6 secretion induced by carcinogen treatment stems from gene transcription.
Discussion
In this study, we comprehensively evaluated the association between four IL6 promoter variants predicted by in silico analyses to affect binding of transcription factors and lung squamous cell carcinoma in former uranium miners with high levels of radon exposure. We found that rs1800797 was significantly associated with increased odds for squamous cell carcinoma and shortened latency for development of squamous cell carcinoma. In particular, rs1800797 was associated with increased basal expression and induced secretion of IL-6 by fibroblasts in response to DNA damage in vitro, supporting a role for IL-6 in the association between rs1800797 and squamous cell carcinoma in the former uranium miners. The association between rs1800797 and squamous cell carcinoma in the former uranium miners was somewhat consistent with the association between rs1800797 and all lung cancers among never smokers in the GENEVA GWAS of Lung Cancer and Smoking study population, although the association was stronger for adenocarcinoma than for squamous cell carcinoma when evaluated by case subtype. Radon exposures were not assessed in GENEVA participants, but radon is a potential cause of lung cancer among never smokers. This finding reinforces the importance of considering the levels of environmental exposures when studying the association between IL6 promoter variants and environmental disease phenotype (Cole et al. 2010; Ljungman et al. 2009b). In addition, a stratified analysis by tumor histology identified a significant association between rs1800797 and lung adenocarcinoma, a result consistent with the finding that > 73% of lung cancer patients who were never smokers were diagnosed with adenocarcinoma in the GENEVA dataset.
The association between the IL6 promoter variants and increased risk for lung cancer was strongly supported by functional studies. We found that rs1800797 was significantly associated with increased IL6 mRNA expression in primary HBECs, lymphoblastic cells, and fibroblasts, which may at least partially stem from the increased gene transcription assessed using the luciferase reporter assay. Our cloning strategy recapitulated the physical linear allelic combination for the four common SNPs at this 690-bp promoter region for the two common haplotype alleles studied (A-G-A8T12-C and G-G-A10T11-G) and allows for the detection of potential combined function of the four individual SNPs on gene transcription, a key factor in detecting the difference in basal expression of IL6 (Terry et al. 2000). Other studies that compared luciferase activities for two promoter constructs that differed by only one base (rs1800795, G/C) with one allele not naturally existing did not see an increase in basal transcription associated with the rs1800795 C allele (Cole et al. 2010; Fishman et al. 1998; Kristiansen et al. 2003).
Compelling evidence suggests that IL-6 signaling in the tumor microenvironment is an essential factor promoting tumor cell proliferation, survival, and metastasis (Fisher et al. 2014). Consistent with this supposition, our previous studies support a paracrine-dominant mechanism for IL-6 signaling mediated by lung fibroblasts that increases the risk of malignant transformation in human bronchial epithelial cells (Chen et al. 2012). In contrast, inhibition of IL-6 secretion in lung fibroblasts greatly reduced transformation of bronchial epithelial cells exposed chronically to tobacco carcinogens (Chen et al. 2012). In the present study, we observed a strong induction of IL-6 secretion in fibroblasts with exposure to H2O2 and BPDE. Because treatment of cells with H2O2 and BPDE creates oxidative damage and BPDE-DNA adducts that mimic the damage induced by radon and the tobacco carcinogen benzo[a]pyrene (Narayanan et al. 1997; Tellez et al. 2011), respectively, these findings further implicate the involvement of IL-6 in promoting the development of lung squamous cell carcinoma in uranium miners. Of great importance, assessment of the effects of the rs1800797 genotype on the slopes for IL-6 induction by H2O2 or BPDE treatments identified greater induction of IL-6 secretion in rs1800797 AG lines than in GG lines. Thus, the elevated levels of basal and carcinogen-induced IL-6 secretion observed in fibroblasts carrying IL-6 variants could lead to a microenvironment that may favor clonal expansion and progression of lung premalignant field defects. This microenvironment would in turn contribute to an increased risk for lung cancer in populations with exposure to radon and/or to cigarette smoke.
Controls were selected from uranium miners who had no lung cancer diagnosis during their entire lives; thus, our study design was optimal for a genetic association study because no misclassification for a control becoming a case was possible. However, caution should be taken in the interpretation of the associations between radon exposure, smoking history, and risk for lung cancer. WLM was calculated up to cancer diagnosis for cancer cases and for the entire uranium-mining occupation for the controls. Smoking history was obtained at the time of sputum collection with the assumption that heavy smokers (≥ 35 pack-years) would maintain a heavy smoking status for their entire lifetime. It is unknown whether smoking behavior changed after sputum was collected. Thus, radon and cigarette smoke exposures were not estimated for controls at the point when controls had person-time contributions proportional to those of cases at cancer diagnosis. A nested case–control study design may be more appropriate to address the temporal relationship between radon exposure, smoking history, and risk for lung cancer.
Conclusions
Our findings suggest that sequence variants in the IL6 promoter modulate gene transcription and responses to environmental carcinogens by lung fibroblasts. In particular, in vitro evidence that the rs1800797 variant modulated IL6 expression supports a role for IL-6 in pathogenic mechanisms associated with squamous cell lung cancer in uranium miners and with lung cancer in never smokers.
Supplemental Material
Acknowledgments
We thank Y. Liu and G. Wu for the statistical assistance related to the latency analysis and for SNP–IL-6 expression analysis using Merlin, respectively; D.M. Klinge for sequencing and cloning the IL-6 promoter; and T.J. Gagliano for the scientific editing of the figures.
Footnotes
This study was supported by the National Institute of Environmental Health Sciences, National Institutes of Health (to S.A.B.) (NIEHS/NIH; R01 ES015262), the U.S. Department of Energy (to S.A.B.)(DE-FG02-09ER64783), and the NIH/National Cancer Institute (NCI) (P30 CA118100). W.C. was supported by an NCI award (R03CA167727).
The funding organizations played no role in the design and conduct of the study; in collection, management, analysis, and interpretation of the data; or in the presentation, review, or approval of the manuscript.
The authors declare they have no actual or potential competing financial interests.
References
- 1000 Genomes Project Consortium, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Archer VE. Lung cancer risks of underground miners: cohort and case-control studies. Yale J Biol Med. 1988;61:183–193. [PMC free article] [PubMed] [Google Scholar]
- Archer VE, Coons T, Saccomanno G, Hong DY. Latency and the lung cancer epidemic among United States uranium miners. Health Phys. 2004;87:480–489. doi: 10.1097/01.hp.0000133216.72557.ab. [DOI] [PubMed] [Google Scholar]
- Archer VE, Renzetti AD, Doggett RS, Jarvis JQ, Colby TV. Chronic diffuse interstitial fibrosis of the lung in uranium miners. J Occup Environ Med. 1998;40:460–474. doi: 10.1097/00043764-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Brenner DR, Boffetta P, Duell EJ, Bickeböller H, Rosenberger A, McCormack V, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol. 2012;176:573–585. doi: 10.1093/aje/kws151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Xu X, Bai L, Padilla MT, Gott KM, Leng S, et al. Low-dose gamma-irradiation inhibits IL-6 secretion from human lung fibroblasts that promotes bronchial epithelial cell transformation by cigarette-smoke carcinogen. Carcinogenesis. 2012;33:1368–1374. doi: 10.1093/carcin/bgs159. [DOI] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, et al. Computational identification of gene–social environment interaction at the human IL6 locus. Proc Natl Acad Sci USA. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, Li D, Neuberg D, Mihm M, Googe P, Wong KK, et al. A dual role for the immune response in a mouse model of inflammation-associated lung cancer. J Clin Invest. 2011;121:2436–2446. doi: 10.1172/JCI44796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol. 2014;26:38–47. doi: 10.1016/j.smim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland FD, Hunt WC, Archer VE, Saccomanno G. Radon progeny exposure and lung cancer risk among non-smoking uranium miners. Health Phys. 2000;79:365–372. doi: 10.1097/00004032-200010000-00004. [DOI] [PubMed] [Google Scholar]
- He JQ, Foreman MG, Shumansky K, Zhang X, Akhabir L, Sin DD, et al. Associations of IL6 polymorphisms with lung function decline and COPD. Thorax. 2009;64:698–704. doi: 10.1136/thx.2008.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm K, Melum E, Franke A, Karlsen TH. 2010. SNPexp—a web tool for calculating and visualizing correlation between HapMap genotypes and gene expression levels. BMC Bioinformatics 11 600, doi: 10.1186/1471-2105-11-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Lehnert BE, Svensson R. Factors underlying the cell growth-related bystander responses to α particles. Can Res. 2000;60:1290–1298. [PubMed] [Google Scholar]
- Kel AE, Gossling E, Reuter I, Cheremushkin E, Kel-Margoulis OV, Wingender E. MATCH™: a tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res. 2003;31:3576–3579. doi: 10.1093/nar/gkg585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A. 2006;69:533–597. doi: 10.1080/15287390500260945. [DOI] [PubMed] [Google Scholar]
- Kristiansen OP, Nolsøe RL, Larsen L, Gjesing AM, Johannesen J, Larsen ZM, et al. Association of a functional 17β-estradiol sensitive IL6-174G/C promoter polymorphism with early-onset type 1 diabetes in females. Hum Mol Genet. 2003;12:1101–1110. doi: 10.1093/hmg/ddg132. [DOI] [PubMed] [Google Scholar]
- Landi MT, Chatterjee N, Yu K, Goldin LR, Goldstein AM, Rotunno M, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009;85:679–691. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng S, Stidley CA, Liu Y, Edlund CK, Willink RP, Han Y, et al. Genetic determinants for promoter hypermethylation in the lungs of smokers: a candidate gene-based study. Cancer Res. 2012;72:707–715. doi: 10.1158/0008-5472.CAN-11-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P, Bellander T, Nyberg F, Lampa E, Jacquemin B, Kolz M, et al. DNA variants, plasma levels and variability of interleukin-6 in myocardial infarction survivors: results from the AIRGENE study. Thromb Res. 2009a;124:57–64. doi: 10.1016/j.thromres.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Ljungman P, Bellander T, Schneider A, Breitner S, Forastiere F, Hampel R, et al. 2009b. Modification of the interleukin-6 response to air pollution by interleukin-6 and fibrinogen polymorphisms. Environ Health Perspect 117 1373 1379, doi: 10.1289/ehp.0800370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin FE, Wagoner JK, Archer VE. Washington, DC: U.S. Public Health Service; 1971. Radon Daughter Exposure and Respiratory Cancer Quantitative and Temporal Aspects: Report from the Epidemiological Study of United States Uranium Miners. [Google Scholar]
- Mapel DW, Coultas DB, James DS, Hunt WC, Stidley CA, Gilliland FD. Ethnic differences in the prevalence of nonmalignant respiratory disease among uranium miners. Am J Public Health. 1997;87:833–838. doi: 10.2105/ajph.87.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- NRC (National Research Council, Committee on Health Effects of Exposure to Radon) 1994. Health Effects of Exposure to Radon: Time for Reassessment? Washington, DC:National Academy Press. [Google Scholar]
- Ochoa CE, Mirabolfathinejad SG, Ruiz VA, Evans SE, Gagea M, Evans CM, et al. Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev Res (Phila) 2011;4:51–64. doi: 10.1158/1940-6207.CAPR-10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prise KM, Pinto M, Newman HC, Michael BD. A review of studies of ionizing radiation-induced double-strand break clustering. Radiat Res. 2001;156(5 pt 2):572–576. doi: 10.1667/0033-7587(2001)156[0572:arosoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Promega Corporation. pGL2 Luciferase Reporter Vectors. TM003. 2015 Available: https://www.promega.com/~/media/files/resources/protocols/technical%20manuals/0/pgl2%20luciferase%20reporter%20vectors%20protocol.pdf [accessed 27 August 2015]
- Qi Y, Zhang M, Li H, Frank JA, Dai L, Liu H, et al. Autophagy inhibition by sustained overproduction of IL6 contributes to arsenic carcinogenesis. Cancer Res. 2014;74:3740–3752. doi: 10.1158/0008-5472.CAN-13-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanna DP, Salvatore C. Reactive oxygen species, inflammation, and lung diseases. Curr Pharm Des. 2012;18:3889–3900. doi: 10.2174/138161212802083716. [DOI] [PubMed] [Google Scholar]
- Saccomanno G, Auerbach O, Kuschner M, Harley NH, Michels RY, Anderson MW, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer. 1996;77:1278–1283. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1278::AID-CNCR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Samet JM. Radon and lung cancer. J Natl Cancer Inst. 1989;81:745–757. doi: 10.1093/jnci/81.10.745. [DOI] [PubMed] [Google Scholar]
- Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15:5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubauer-Berigan MK, Daniels RD, Pinkerton LE. Radon exposure and mortality among white and American Indian uranium miners: an update of the Colorado Plateau cohort. Am J Epidemiol. 2009;169:718–730. doi: 10.1093/aje/kwn406. [DOI] [PubMed] [Google Scholar]
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol. 2012;10:157–164. [PubMed] [Google Scholar]
- Sousa AL, Fava VM, Sampaio LH, Martelli CM, Costa MB, Mira MT, et al. Genetic and immunological evidence implicates interleukin 6 as a susceptibility gene for leprosy type 2 reaction. J Infect Dis. 2012;205:1417–1424. doi: 10.1093/infdis/jis208. [DOI] [PubMed] [Google Scholar]
- Tellez CS, Juri DE, Do K, Bernauer AM, Thomas CL, Damiani LA, et al. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res. 2011;71:3087–3097. doi: 10.1158/0008-5472.CAN-10-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Taylor JA. 2009 SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res 37(Web Server issue):W600–W605.
- Zakharyan R, Petrek M, Arakelyan A, Mrazek F, Atshemyan S, Boyajyan A. Interleukin-6 promoter polymorphism and plasma levels in patients with schizophrenia. Tissue Antigens. 2012;80:136–142. doi: 10.1111/j.1399-0039.2012.01886.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


