Abstract
Primed neutrophils that are capable of releasing matrix metalloproteinases (MMPs) into the circulation are thought to play a significant role in the pathophysiology of acute respiratory distress syndrome (ARDS). We hypothesized that direct measurement of plasma MMP-9 activity may be a predictor of incipient tissue damage and subsequent lung injury, which was investigated in both an animal model of ARDS and a small cohort of 38 critically ill human patients. In a mouse model of ARDS involving instillation of intratracheal LPS to induce lung inflammation, we measured neutrophil-mediated inflammation, along with MMP-9 activity in the airways and lung tissue and MMP-9 expression in the plasma. Neutrophil recruitment, inflammation, and MMP-9 activity in the airways and lung tissue increased throughout the 72 hours after LPS instillation, while plasma MMP-9 expression was greatest at 12–24 hours after LPS instillation. The results suggest that the peak in plasma MMP-9 activity may precede the peak of neutrophil inflammation in the airways and lung tissue in the setting of ARDS. Based on this animal study, a retrospective observational cohort study involving 38 patients admitted to a surgical intensive care unit (SICU) at a tertiary care university hospital with acute respiratory failure requiring intubation and mechanical ventilation was conducted. Plasma samples were collected daily, and MMP-9 activity was compared with lung function as determined by the PaO2/FiO2 ratio. In patients that developed ARDS, a notable increase in plasma MMP-9 activity on a particular day correlated with a decrease in the PaO2/FiO2 ratio on the following day (r = −0.503, p < 0.006). Taken together, these results suggest that plasma MMP-9 activity changes as a surrogate for primed neutrophils may have predictive value for the development of ARDS in a selected subset of critically ill patients.
Keywords: Matrix metalloproteinases, acute lung injury, acute respiratory distress syndrome, neutrophil, neutrophil priming
Introduction
Acute Respiratory Distress Syndrome (ARDS), inclusive of the former entity Acute Lung Injury (ALI), is the most common type of organ failure in patients with sepsis and has mortality rates as high as 45% (1). In May 2000, the ARDS Network trial demonstrated that mortality rates in patients with ARDS could be decreased 22% by employing a lower tidal volume ventilation strategy that was shown to reduce ventilator-associated lung injury, and a recent meta-analysis by Serpa Neto et al found similar results (2, 3). However, since the publication of the landmark trial more than ten years ago the management of ARDS remains largely supportive with few effective new treatments (4).
While there are several biomarkers that have been studied for the diagnosis of ARDS, there are currently no molecular tools available to the clinician that can be used at the bedside to predict which patients are at highest risk for development of ARDS, and it was only recently that the Lung Injury Prediction Score, a risk prediction model for ARDS, became available (5). Development of ARDS in the setting of severe sepsis or trauma results from a large-scale inflammatory response by the patient’s innate immune system. This involves cytokine-mediated recruitment and priming of neutrophils and macrophages, release of proteases and cytotoxins including reactive oxygen species (ROS), and subsequent damage to pulmonary capillary endothelial cells leading to capillary leak and lung destruction (6–8). In addition, cross-talk between neutrophils, the coagulation system and coagulopathy in the development of ARDS has been alluded to by several studies and may play a significant role in its pathogenesis (9, 10). Profiling circulating cytokines can assist in predicting the severity of sepsis, but to date has not been particularly accurate in predicting which patients will subsequently develop ARDS (11, 12). Advance knowledge prior to the onset of ARDS might allow clinicians to facilitate timely therapeutic interventions, such as APRV, that has been shown to significantly increase P/F ratios, improve cardiac function, reduce vasopressor use, and reduce length of intubation and ICU stay in human polytrauma patients (ISS>40) (13), and in pig studies has been shown to decrease the incidence of ARDS, preserve epithelial integrity, and reduce histopathologic stigmata of ARDS following sepsis and injury (14).
In the past two decades, a growing body of research involving both animal and human studies has suggested that the increased expression of matrix metalloproteinases (MMPs) within the airways plays an important role in the pathogenesis of ARDS (c.f. references (15–19)). MMPs are a family of zinc-dependent endoproteases that can degrade extracellular matrix proteins and process a variety of bioactive molecules. Most of the previous studies have focused on the increased expression of the gelatinases MMP-9 and MMP-2 within the lung and bronchoalveaolar fluid in the setting of ARDS. Early in the development of the disease, there is marked accumulation of neutrophils in the microvasculature of the lung. Following sequestration in the pulmonary vasculature, neutrophils adhere to the surface of the pulmonary endothelium and subsequently migrate through the endothelial cell layer and the underlying basement membrane to infiltrate the lung tissue and alveolar spaces (20). The release of MMP-9 by neutrophils is thought to play a significant role in facilitating lung inflammation by degrading the basement membrane and enabling neutrophil migration into the lung tissue and airways (21). Interestingly, Chakrabarti and Patel reported that IL-8 engagement of the CXCR-2 receptor on suspension phase neutrophils was sufficient to induce their secretion of MMP-9 from tertiary granules (22), while Pugin et al. showed that LPS binding to CD14 on solution phase neutrophils similarly induced pro-MMP9 secretion (23). We therefore hypothesized that direct measurements of increased MMP-9 activity in the circulation, as an indicator for neutrophil priming, might signify incipient tissue damage and increased risk for subsequent development of lung injury. The initial aim of this study was to determine if measurements of a time course of MMP-9 activity in the circulation utilizing a mouse model of lung injury could provide additional insight into the timing and role of plasma MMP-9 expression in the pathogenesis of ARDS. Following the animal study, our subsequent aim was to investigate if the results generated from the mouse model could be translated to humans. This was examined by performing a limited retrospective observational cohort study looking specifically at whether increased plasma MMP-9 activity might precede and potentially correlate with evidence for the subsequent development of lung injury and deterioration of lung function in critically ill ICU patients at risk for ARDS.
Materials and Methods
Neutrophil Purification
Purification of neutrophils from whole blood was performed as described previously (24). In brief, blood was obtained by venipuncture from healthy volunteers into blood collection tubes containing EDTA (Becton Dickinson, Franklin Lakes, NJ). Neutrophils were separated from PBMCs by Ficoll gradient centrifugation (GE Healthcare, Piscataway, NJ), and then from RBCs by Dextran sedimentation (Dulbecco’s PBS without Ca2+ and Mg2+ containing 2% dextran (GE Healthcare)), and hypotonic lysis respectively. The purified neutrophils were more than 97% viable by trypan blue staining (Sigma-Aldrich, St. Louis, MO).
Neutrophil Priming for MMP Production
Priming of human neutrophils was performed following our previously published protocol (24). Human recombinant TNF-α was obtained from R&D Systems (Minneapolis, MN). Human recombinant GM-CSF was obtained from Genzyme (Cambridge, MA). Neutrophils purified from healthy volunteers were primed by adding 5 uL of freshly diluted priming agent or its vehicle Dulbecco’s PBS with Ca2+ and Mg2+ (Invitrogen) to 500 uL aliquots of 5 × 106 neutrophils in Dulbecco’s PBS with Ca2+ and Mg2+ and incubating the neutrophils for 30 or 60 minutes at 37°C. TNF-α was used at a concentration of 20 ng/mL and GM-CSF was used at a concentration of 24 ng/mL. To prepare conditioned media, neutrophils were centrifuged for 1 minute at 17,720 × g in 4°C after incubation with the priming agents, and 10 uL of the “neutrophil supernatant” were used for gelatin zymography.
ROS Production Assay
ROS production was assayed using luminol-dependent chemilumenescence with a Centro LB 960 Microplate Luminometer (Berthold Technologies, Oak Ridge, TN) (24). This device measures neutrophil respiratory burst activity by detecting and recording the number of photons emitted when luminol is oxidized by the ROS produced by activated neutrophils. To prime neutrophils for ROS production, 5 uL of freshly diluted priming agent or its vehicle Dulbecco’s PBS with Ca2+ and Mg2+ were added to 500 uL aliquots of 2 × 106 neutrophils in Dulbecco’s PBS with Ca2+ and Mg2 and neutrophils were incubated for 30 minutes at 37°C. TNF-α was used at a concentration of 20 ng/mL and GM-CSF was used at a concentration of 24 ng/mL. After the incubation period, 500 uL of Dulbecco’s PBS with Ca2+ and Mg2+ containing 400 uM luminol (Sigma-Aldrich) were added to the neutrophils and 150 uL of the primed neutrophils with luminol were transferred to microplate wells. To trigger the respiratory burst, 50 uL of the freshly diluted activating agent N-formyl-methionyl-leucyl-phenylalanine (fMLP) (Sigma-Aldrich) or its vehicle Dulbecco’s PBS with Ca2+ and Mg2+ were added to the neutrophils. fMLP was used at a concentration of 100 nM. The respiratory burst was measured as number of chemiluminescence events per 0.1 seconds.
Mouse Bone Marrow Preparation
Bone marrow was flushed from long bones of two Balb/c mice (Charles River Laboratories, Wilmington, MA) with Hank’s balanced salt solution without Ca2+ and Mg2+ (Sigma-Aldrich), 0.25% fatty acid-free bovine serum albumin, 15 mM HEPES, pH 7.4, at room temperature. Bone marrow was dispersed and debris was removed. Following pelleting by centrifugation, cells were resuspended in 20 mL Dulbecco’s PBS with Ca2+ and Mg2+. 5 uL of the resuspended mouse bone marrow cells were used for the positive control in gelatin zymography assays.
Mouse Model Protocol
A previously published mouse model using intratracheal LPS instillation to cause lung inflammation and injury was utilized (25). Male Balb/c mice, 8–12 weeks of age and weighing 20–25 g, were obtained from Charles River Laboratories (Wilmington, MA). The protocol was approved by the Massachusetts Institute of Technology Committee on Animal Care. Mice were randomly assigned to control (n = 15) and experimental (n = 25) groups. After anesthesia by intraperitoneal injection of 300–450 ug/g Avertin (Sigma-Aldrich), control mice were treated with a 50 uL intratracheal instillation of PBS, followed by a 30 uL/g bolus of air. Experimental mice were treated with a 50 uL intratracheal instillation of PBS containing a 2 ug/g dose of E. coli derived LPS (Sigma-Aldrich) followed by a 30 uL/g bolus of air. After treatment, mice were placed on a heating pad for recovery from anesthesia. At 4, 12, 24, 48, and 72 hours after treatment, three control and five experimental mice were selected randomly for euthanasia by carbon dioxide asphyxiation. Blood was collected by cardiac puncture and centrifuged at 1000 × g for 10 minutes at 4°C. The plasma was separated into aliquots and frozen at −80°C until further use.
Bronchoalveolar Lavage (BAL)
Following mouse euthanasia at 4, 12, 24, 48, and 72 hours after intratracheal PBS or LPS instillation, the thorax was opened to expose the trachea. The trachea was intubated with 0.965 mm diameter polyethylene tubing (Becton Dickinson). A 750 uL aliquot of sterile PBS was instilled with a syringe through the tubing and withdrawn slowly. This was performed four times to obtain an adequate BAL specimen. The BAL fluids were then centrifuged at 1000 × g for 10 minutes at 4°C. The supernatant was separated into aliquots and frozen at −80°C until use for gelatin zymography. The cell pellet was resuspended in 400 uL PBS consisting of 2.5% cetyltrimethylammonium bromide (Sigma-Aldrich), 50 mM KPO4, pH 6.0, sonicated, and centrifuged at 17,720 × g for 30 minutes at 4°C. The supernatant was separated into aliquots and frozen at −80°C until use for the myeloperoxidase activity (MPO) assay.
Lung Sample Preparation
To prepare lung samples, lungs were harvested after BAL was performed. Lungs from each mouse were homogenized in 500 uL of PBS, 50 mM KPO4, pH 7.4 and centrifuged at 17,720 × g for 30 minutes at 4°C. The supernatant was separated into aliquots and frozen at −80°C until use for gelatin zymography. The lung pellet was resuspended in 400 uL PBS consisting of 2.5% cetyltrimethylammonium bromide, 50 mM KPO4, pH 6.0, sonicated, and centrifuged at 17,720 × g for 30 minutes at 4°C. The supernatant was separated into aliquots and frozen at −80°C until use for the MPO assay.
Myeloperoxidase Activity Assay
35 uL aliquots of BAL and lung samples were added to 665 uL of reaction buffer containing 50 mM KPO4, 60 uM o-dianisidine dihydrochloride (Sigma-Aldrich), and 200 uM hydrogen peroxide (Sigma-Aldrich) at room temperature. After 10 minutes, the reaction was stopped by adding10 uL of 20% NaN3. Absorbance at 490 nm was measured using a Beckman Coulter DU640 spectrophotometer (Brea, CA).
Gelatin Zymography
10 uL diluted sample aliquots were mixed with non-reducing 2X sodium dodecyl sulfate (SDS) sample buffer containing 0.125 M Tris pH 6.8, 20% glycerol, 4% SDS, and 0.05% bromophenol blue and loaded onto a 7.5% polyacrylamide gel containing 0.2% copolymerized porcine gelatin (Sigma-Aldrich). After electrophoresis, gels were washed with 2.5% Triton X-100, rinsed with water, and incubated for 15 hours in digestion buffer containing 50 mM Tris pH 7.4, 0.2 M NaCl, 5 mM CaCl2, 1 mM ZnCl2, 0.02% Brij 35, and 0.002% NaN3. The gels were stained with Coomassie Brilliant Blue and de-stained in a solution of 50% methanol and 10% acetic acid. Molecular weight standards were run in each gel. MMP-9 activity was detected as a clear band on a blue background. Identity of MMP-9 was confirmed with western blotting. The gels were scanned as a black-and-white image and inverted. MMP-9 activity was determined by measuring the density of the bands of gelatin hydrolysis using the Imagequant® software (Molecular Dynamics, Sunnyvale, CA). An internal standard allowed for comparison between gels.
Western Blotting
10 uL diluted sample aliquots were loaded onto a 7.5% polyacrylamide gel for SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% milk (Carnation) in Tris-buffered saline containing 0.2% Tween 20 (TBST) for 1 hour at room temperature and probed overnight at 4°C with an anti-mouse MMP-9 antibody (Abcam, Cambridge, MA) diluted 1:5000. Membranes were washed with TBST and probed with a horseradish peroxidase (HRP)-conjugated secondary antibody (Bio-Rad, Hercules, CA) for 1 hour. The membranes were washed with TBST, chemiluminescence substrate (PerkinElmer, Waltham, MA) was applied, and the membranes were exposed to film. The films were scanned as a black-and-white image and MMP-9 expression was determined by measuring the density of MMP-9 bands using the Imagequant® software. An internal standard was used to allow comparison between gels.
Human Subjects
The retrospective observational cohort study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center. A total of 38 patients with various diagnoses admitted to the surgical intensive care unit (SICU) with acute respiratory failure requiring intubation and mechanical ventilation between 2005 and 2008 were randomly selected for inclusion into the study. Patients were defined as having Acute Lung Injury (ALI) and/or ARDS according to criteria from the 1994 American-European Consensus Conference on ARDS at the time of data collection, but for the purposes of this manuscript ALI has been reported as ARDS in lieu of the updated Berlin Definition of ARDS (1). Patients were diagnosed with ARDS when they presented with an acute onset of respiratory failure, bilateral pulmonary infiltrates on chest radiograph, PaO2/FiO2 ratio < 300, and a pulmonary artery wedge pressure < 18 mmHg or absence of clinical evidence of left ventricular heart failure. Arterial and venous anti-coagulated blood samples were collected from patients for each day mechanical ventilation was required. The blood samples were centrifuged within one hour of collection and the plasma was stored at −80°C until further use. Daily PaO2 values were determined from arterial blood gas results. Severity of illness at the time of admission was assessed using the APACHE II score. Patients were considered to have sepsis when they met the criteria defined by the American College of Chest Physicians and Society of Critical Care Medicine Consensus Conference of 1992. Age, gender, diagnosis, and outcome were also recorded.
Statistics
Results are expressed as mean values ± SEM. Statistical analysis was performed using the method on calculating correlation coefficients with repeated observations as described by Bland and Altman (26, 27). The statistical software was SAS 9.2 (SAS Institute, Cary, NC). A p-value ≤ 0.05 was considered significant.
Results
Priming of human neutrophils results in extracellular release of MMP-9 in the absence of ROS production
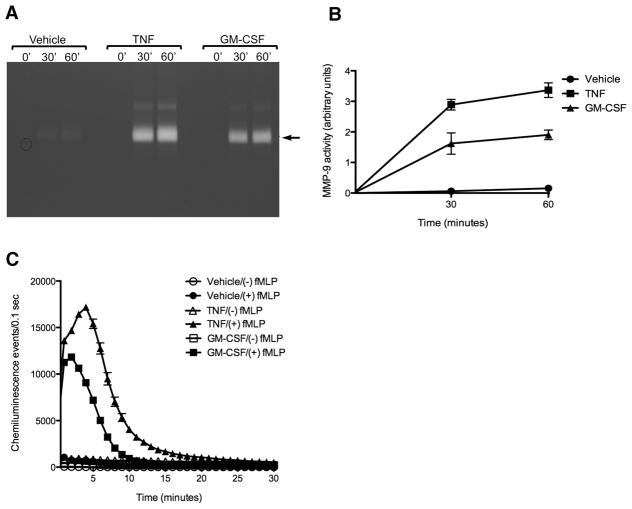
When resting human neutrophils purified from healthy volunteers were incubated with the priming agent TNF-α (20 ng/mL) or GM-CSF (24 ng/mL), an increase in the 92KDa MMP-9 activity, compared to unprimed controls, was detected in the culture media by gelatin zymography (Fig. 1A). This increase in gelatinase activity was evident within 30 minutes, and increased further by 60 minutes after priming (Fig. 1B). In contrast to MMP-9, no MMP-2 activity was detectable by gelatin zymography in the conditioned media from these primed neutrophils. Furthermore, neutrophil priming with either TNF-α or GM-CSF did not generate significant ROS production and release by neutrophils as assayed by luminol chemiluminescence, but did significantly enhance subsequent ROS production after addition of the activating agent fMLP, compared with fMLP treatment alone, (Fig. 1C). Taken together, these results suggest that release of enzymatically active 92 kDa MMP-9 might be a useful functional in-vivo marker for neutrophil priming.
Figure 1. Human neutrophils are induced by priming agents to release MMP-9.
A, Representative gelatin zymogram demonstrating increased MMP-9 activity in supernatants from human neutrophils purified from a healthy volunteer after treatment with either TNF α (20 ng/mL) or GM-CSF (24 ng/mL) for 30 or 60 minutes. B, Quantification of MMP-9 activity determined by zymography in supernatants of human neutrophils treated with priming agents compared to control. Data is expressed as mean ± SEM (n=3). C, Measurement of ROS production by human neutrophils determined by luminometry demonstrating that neutrophils treated only with TNF α (20 ng/mL) or GM-CSF (24 ng/mL) are not activated to produce ROS. Primed neutrophils are activated to produce ROS after treatment with the agonist fMLP (100 nM). Experiment performed in duplicate. Data is expressed as mean ± half range.
Lung tissue and airway neutrophil inflammation is increased in a mouse model of lung injury
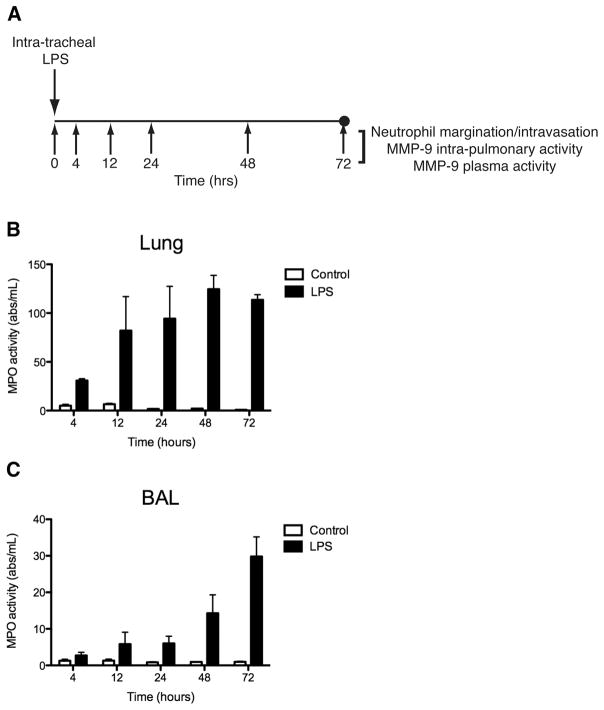
To explore if plasma MMP-9 could be used as an indicator of neutrophil priming and activation in the setting of lung inflammation and injury, we next used a murine model of lung injury involving the intra-tracheal instillation of LPS. Myeloperoxidase (MPO) activity was used to assay neutrophil infiltration into both lung parenchyma and conducting airways at 4, 12, 24, 48, and 72 hours after intratracheal LPS administration (Fig. 2A). Lung tissue MPO activity was increased in experimental mice and appeared to peak at 48 hours to approximately 60-fold greater compared to control (Fig. 2B). Neutrophil infiltration in the airways was assayed by measuring the MPO activity in the BAL samples. BAL MPO activity was increased in experimental mice compared to control and appeared to increase steadily throughout the 72-hour time period after LPS administration (Fig. 2C). At 72 hours after LPS instillation, BAL MPO activity in experimental mice was increased to approximately 30-fold greater than control mice.
Figure 2. MPO activity is increased in lung tissue and airways after intratracheal LPS instillation in mouse model of lung injury/ARDS.
A, Schematic of mouse model of lung injury/ARDS. Balb/c mice are administered a 2 mg/kg dose of LPS by the intratracheal route. Neutrophilic inflammation and MMP activity were measured in the lung tissue and airways at 4, 12, 24, 48, and 72 hours after LPS treatment. B, Mouse lung MPO activity. C, Mouse BAL MPO activity. Data is expressed as mean ± SEM (n = 3–5).
Lung tissue and airway MMP-9 activity is increased in a mouse model of lung injury
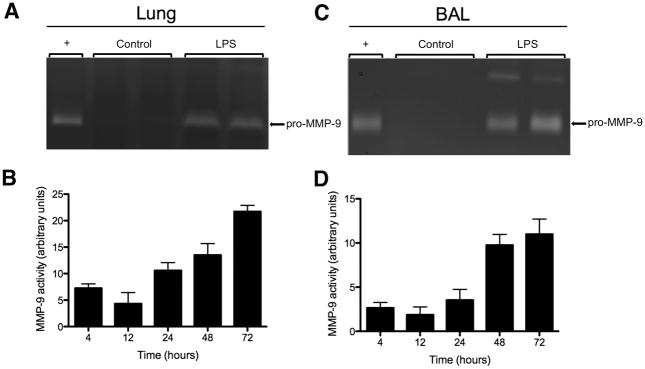
In experimental mice, 92 kDa MMP-9 activity was detected at all time points in lung tissues after LPS administration (Fig. 3). In control mice, lung MMP-9 activity was not detectable at any time point. Fig. 3A shows a representative gelatin zymogram demonstrating MMP-9 activity in lung samples from an experimental mouse. When the zones of gelatin hydrolysis were quantified, lung MMP-9 activity of experimental mice appeared to be elevated as early as 4 hours after LPS treatment and increased steadily throughout the measured time points (Fig. 3B). MMP-9 activity in the airways was assayed using BAL samples collected from mice. Similar to lung tissues, MMP-9 activity from the 92 kDa form was detected at all time points in BAL samples after intratracheal LPS administration, but was not detectable in the control mice at any time point. Fig. 3C shows a representative gelatin zymogram demonstrating MMP-9 activity in BAL samples from an experimental mouse. MMP-9 activity in the airways also appeared to increase steadily throughout the 72-hour time period after LPS administration (Fig. 3D). Surprisingly, gelatin zymography was unable to detect MMP-9 activity in the plasma from either control or intratracheal LPS-treated mice, and no MMP-2 activity was detected by zymography in BAL and lung samples or plasma from either the LPS-treated or control mice.
Figure 3. MMP-9 activity is increased in lung tissue and airways after intratracheal LPS instillation in mouse model of lung injury/ARDS.
A, Representative gelatin zymogram demonstrating increased MMP-9 activity in lung samples at 72 hours after intratracheal LPS instillation. ‘+’ represents the positive control and is MMP-9 activity from bone marrow cells of untreated Balb/c mice. B, Quantification of MMP-9 activity determined by zymography in lung samples at 4, 12, 24, 48, and 72 hours after intratracheal LPS instillation. C, Representative gelatin zymogram demonstrating increased MMP-9 activity in BAL samples at 72 hours after intratracheal LPS instillation. D, Quantification of MMP-9 activity determined by zymography in BAL samples at 4, 12, 24, 48, and 72 hours after intratracheal LPS instillation. Data is expressed as mean ± SEM (n = 3–5).
Plasma MMP-9 expression is increased in mouse model of lung injury
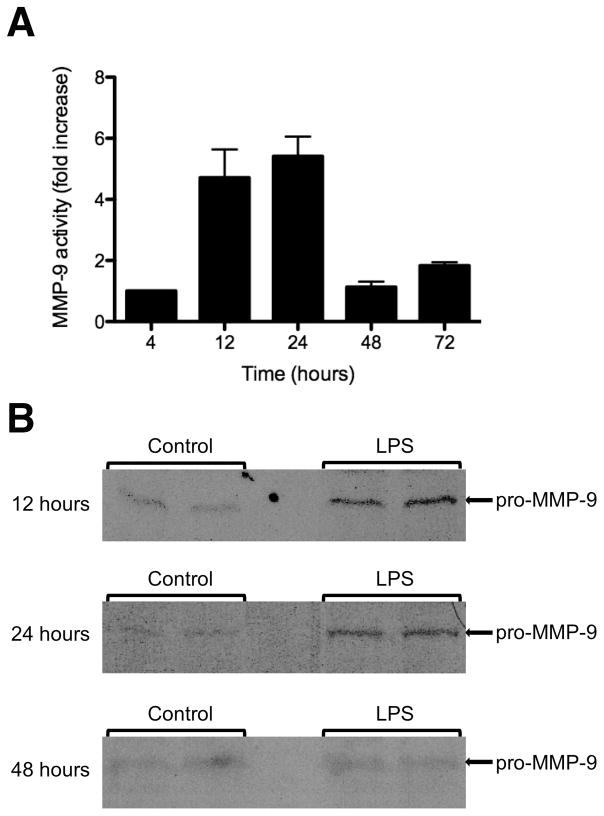
Western blotting was then used to examine the expression of MMP-9 in mouse plasma due to its increased sensitivity over gelatin zymography in detecting the presence of MMP-9. Mouse MMP-9 was detected as a band at 92 kDa (Fig 4A). The enzymatically cleaved version of MMP-9 was not detected by western blotting in either experimental or control mice. When quantified, an approximately five-fold increase in plasma MMP-9 expression was seen in experimental mice compared to controls at 12 and 24 hours after intratracheal instillation of LPS (Fig 4B). By 48 hours after intratracheal LPS administration, plasma MMP-9 expression had returned to baseline levels.
Figure 4. MMP-9 expression is increased in mouse plasma 12–24 hours after intratracheal LPS treatment.
A, Western blots demonstrating increased expression of the pro-MMP-9 form (92 kDa) in mouse plasma 12 and 24 hours after intratracheal LPS instillation when compared to mouse plasma at 48 hours after LPS treatment. B, Quantification of plasma MMP-9 expression demonstrating fold increase of plasma pro-MMP-9 expression in intratracheal LPS treatment groups compared to controls. Data is expressed as mean ± SEM (n = 3–5).
Patient and clinical data
The study population consisted of 38 patients with various primary diagnoses admitted to the SICU for acute respiratory failure from 2005 through 2008. Table 1 outlines the clinical data of the study population. Sepsis and trauma accounted for 61% of the SICU admissions. 26 patients developed ARDS and primary pulmonary pathology accounted for 14 (54%) of those cases. The mortality rate of patients in the study was 26%.
Table 1.
Clinical data of study population. Data is expressed as mean ± SD.
| No. of patients in study | 38 |
|---|---|
| Patients with ALI/ARDS | 26 |
| Age | 62 ± 18 |
| Sex, M/F | 15/23 |
| Apache II score | 14 ± 7 |
| Main pathology | |
| Sepsis | 9 |
| Trauma | 14 |
| Other (liver failure, kidney failure, GI bleed, limb ischemia, abdominal aortic aneurysm, intracranial hemorrhage) | 15 |
| Cause of ARDS (pulmonary/systemic) | 14/12 |
| ICU day for onset of ALI/ARDS | 2 ± 1.83 |
| Outcome (dead/alive) | 8/30 |
Plasma MMP-9 activity is observed in septic patients with equal activity in the arterial and venous circulation
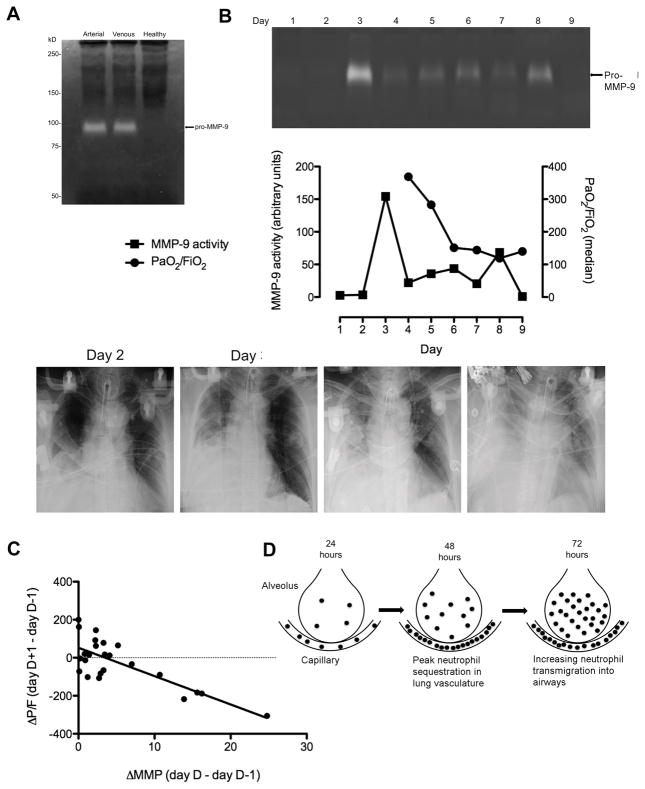
In initial experiments, MMP-9 activity in arterial and venous blood from septic patients was measured using gelatin zymography to determine whether there were observable differences in utilizing arterial or venous blood for measuring MMP-9 activity due to washout or sequestration of MMP9 from the lung vasculature (Fig. 5A). We observed no difference in MMP-9 activity in arterial blood compared to venous blood in the course of our measurements, suggesting that both sources of access were equivalent.
Figure 5. An increase in plasma MMP-9 activity correlates with a subsequent decline in PaO2/FiO2 in patients that developed ARDS.
A, Representative gelatin zymogram demonstrating equivalent increased arterial and venous MMP-9 activity in a septic patient compared to a healthy volunteer. B, Example of gelatin zymogram demonstrating time course of plasma MMP-9 activity in an ICU patient that developed ARDS. Lanes represent MMP-9 activity in plasma samples obtained over a number of days during which the patient required mechanical ventilation. Graph is shown demonstrating the time course of plasma MMP-9 activity quantified from the gelatin zymogram with the daily median PaO2/FiO2 values for the patient. Chest x-rays for the patient are shown. The chest x-rays obtained on day 2 and 3 of the study shows a moderately sized right pleural effusion with atelectasis. The lung fields are otherwise clear. Plasma MMP-9 activity has peaked on day 3 and lung function begins to deteriorate in the next 48 hours. On day 6, when the patient clinically appears to have moderate ARDS with a PaO2/FiO2 < 200, the chest x-ray shows the presence of bilateral infiltrates and an interval enlargement in the size of the right pleural effusion. The bilateral infiltrates are seen more clearly in the chest CT obtained on this day. The last chest x-ray shows worsening of the bilateral pulmonary infiltrates and further increase in size of the right pleural effusion. C, Correlation between an increase in plasma MMP-9 activity and change in lung function the following day in the 23/38 SICU patients that developed ARDS who had detectable plasma MMP-9 activity, r = −0.687, p = 0.028. Only those days in which there was a detectable increase in plasma MMP-9 activity are included. Of these 23 patients, 14 developed ARDS due to a primary pulmonary source while 9 developed ARDS from a non-pulmonary source. MMP-9 activity was determined by gelatin zymography and normalized according to an internal standard. Lung function was determined daily by the median PaO2/FiO2 ratio. D, Neutrophil sequestration in lung vasculature and transmigration into airways. Lung MPO activity in experimental mice is greatest at 48 hours after intratracheal LPS treatment indicating peak neutrophil sequestration in the lung vasculature. Over the following 24 hours, MPO activity in BAL fluid continues to increase indicating increasing neutrophil-mediated inflammation and transmigration into the alveoli.
Plasma MMP-9 activity appears to show time-dependent changes in SICU patients that developed ARDS
Plasma MMP-9 activity in patients was determined by gelatin zymography for each day that mechanical ventilation was required. In patients with ARDS, plasma MMP-9 activity was detectable by zymography in 88% of cases. 100% of patients with ARDS attributable to primary pulmonary pathology had plasma MMP-9 activity that was detectable by zymography. Among SICU patients without ARDS, plasma MMP-9 activity was detectable by zymography in 83% of cases. Fig. 5B shows a representative gelatin zymogram demonstrating a time course of plasma MMP-9 activity for a patient who developed ARDS followed by a graph demonstrating the plasma MMP-9 activity time course with the daily median PaO2/FiO2 values for the patient. Chest x-rays taken of patient during the course of the study are also shown. The chest x-ray obtained on day 2 shows a moderate sized right pleural effusion with atelectasis. The lung fields are otherwise clear. Plasma MMP-9 activity for the patient appears to peak on day 3 and lung function begins to deteriorate within the next 48 hours. On day 6, when the patient clinically appears to have moderate ARDS with PaO2/FiO2 < 200, the chest x-ray only shows an interval enlargement in the size of the right pleural effusion. The last chest x-ray obtained four days later shows bilateral pulmonary infiltrates indicative of ARDS. This patient represents an example where the radiographic evidence of ARDS lags behind the clinical presentation.
A marked increase in plasma MMP-9 activity, but not the absolute level of activity, correlates with a subsequent deterioration in lung function
Because a number of patients had missing clinical data after the sixth day of SICU admission, correlation of plasma MMP-9 activity with PaO2/FiO2 ratio was determined by analyzing data gathered from only the first six days of SICU admission. For patients that developed ARDS, statistical analysis did not reveal a significant correlation between the absolute value of plasma MMP-9 activity and PaO2/FiO2 ratio on a particular day. However, there was a statistically significant rise in plasma MMP-9 activity on a particular day relative to the day before,(i.e. ΔMMP from D-1 to D) that was negatively correlated with a subsequent change in the PaO2/FiO2 ratio on the following day for the 23/38 patients that developed ARDS who had detectable plasma MMP-9 activity by gel zymography (r = −0.503, p < 0.006). When evaluating only those days for which there was an increase in plasma MMP-9 activity, the negative correlation between rising MMP-9 activity on D-1 and falling PaO2/FiO2 ratio on the following day was stronger (r = −0.687 by the method of Bland and Altman (26, 27). p = 0.028) (Fig. 5C). No statistically significant correlation was observed when the absolute plasma MMP-9 activity was compared with mortality rate or APACHE II score.
Discussion
Few animal or human studies have been performed that examine the utility of plasma MMP-9 activity as a predictive tool for ARDS. Initially, MMP-9 is released in its 92 kDa pro-form and further enzyme activation subsequently occurs through either limited proteolysis or through conformational changes that expose the active site (28). Our data, and previously published studies have shown that agents that prime for the neutrophil oxidative burst induce neutrophil release of MMP-9, indicating that MMP-9 release may be indicative of systemic neutrophil priming, with additional MMP-9 release seen upon later activation (7, 22, 23). Neutrophil priming occurs in both the circulating and marginated neutrophil populations and refers to a state in which neutrophils are not actively producing and releasing ROS, but upon exposure to a second stimulus exhibit a potentiated respiratory burst. The primed state has been implicated to play a role in the inappropriate or excessive neutrophil activation responsible for the inflammatory tissue injury seen in clinical diseases such as ARDS, rheumatoid arthritis, and ischemia- reperfusion injury (7, 29). While several studies have evaluated MMP-9 levels and/or activity in BAL fluid for mechanistic and pathophysiologic involvement in ARDS in both human patients and animal models (15–19, 30), and others have intermittently reported an association between elevated plasma MMP-9 protein levels (31) or leukocyte MMP-9 mRNA levels (32) and severe sepsis, to our knowledge this is the first study that specifically aimed to evaluate a possible predictive capability of plasma MMP-9 activity to identify patients at risk for future development of ARDS.
In ARDS, the pathophysiology of lung injury involves the sequestration of primed neutrophils in the pulmonary vasculature, followed by adhesion and transmigration into the alveolar spaces, and finally activation with potentiated release of reactive oxygen species and proteolytic enzymes that become effectors of tissue injury (6–8, 29). We hypothesized that if MMP-9 release in the plasma is indicative of systemic neutrophil priming before or during sequestration in the lung vasculature, then plasma MMP-9 release and activity might be predictive of subsequent neutrophil activation and development of ARDS. In our ex vivo experiments we found rapid MMP-9 release occurred following neutrophil priming by using the priming agents TNF-α and GM-CSF, consistent with prior studies (22, 23). In addition, neutrophil activation and production of reactive oxygen species were not induced by priming agents alone, but required the subsequent addition of a neutrophil agonist such as fMLP, confirming the primed state (24).
After obtaining results suggesting that MMP-9 release may be indicative of neutrophil priming, we utilized a previously published mouse model of lung injury using intratracheal LPS instillation to cause lung inflammation and injury to study plasma MMP-9 activity in the direct setting of ARDS (25). The purpose of using intratracheal LPS to cause lung injury in this model was to mimic the inflammatory response seen in pneumonia caused by gram negative bacteria, a common cause of ARDS in patients (4). In our mouse model, we were able to demonstrate that intratracheal instillation of LPS causes an increase in neutrophil-mediated inflammation in both lung tissue and airways, as indicated by the increased lung and BAL MPO activity in experimental mice compared to controls. Neutrophil-mediated inflammation in lung tissue appears to peak at 48 hours after intratracheal LPS treatment, and the level of elevation remains stable for the following 24 hours. With regards to neutrophil-mediated inflammation in the airways, MPO activity in the BAL fluid appears to increase as early as 4 hours after LPS instillation and increases steadily throughout the 72-hour study duration. This suggests that in our mouse model the recruitment and sequestration of neutrophils in the lung vasculature is upregulated during the first 48 hours and remains stable for at least an additional 24 hours. In the BAL fluid, the steadily increasing MPO activity in response to LPS instillation is indicative of increasing numbers of neutrophils continuing to migrate out of the lung vasculature and infiltrate the alveolar spaces in the 72 hours following LPS instillation (Fig. 5D).
By measuring a time course of MMP-9 expression/activity in mouse plasma, BAL, and lung tissue, we were able to gain further insight into the role of MMP-9 in ARDS. The main finding of our mouse model was an observed increase in the expression of plasma MMP-9 at 12 and 24 hours after intratracheal LPS instillation using western blotting. By 48 and 72 hours after LPS administration, plasma MMP-9 expression had returned to baseline levels. When examining MMP-9 activity in the BAL fluid and lung tissue, we observed that MMP-9 activity continued to increase throughout the 72-hour time period, indicative of an upregulation in MMP-9 secretion by primed neutrophils sequestered in the lung vasculature and activated neutrophils that have transmigrated into the alveolar spaces. The increase in mouse plasma MMP-9 expression in the first 24 hours after LPS instillation suggests the early presence of primed neutrophils in the circulation that release MMP-9 into the plasma prior to being sequestered in the lung vasculature. Because plasma MMP-9 expression does not mirror the increase in MMP-9 activity in BAL and lung samples, the results also suggest that the MMP-9 that is spilled over into the circulation from degranulation during neutrophil sequestration and transmigration is a nominal amount. Our findings are therefore consistent with a model in which acute lung injury induces a generalized neutrophil priming phenomenon, with secretion of MMP-9 by primed neutrophils in the pulmonary vasculature promoting their subsequent migration into the alveolar space (21) with development of lung injury/ARDS. More significantly, an increase in plasma MMP-9 expression may be a specific indicator of early systemic inflammation that portends subsequent lung inflammation, injury, and ARDS.
Though we had difficulty using gelatin zymography to detect MMP-9 activity in mouse plasma after LPS instillation, we were still able to observe evidence of increased plasma MMP-9 expression at early time points by immunoblotting. Based on these results, we measured a time course of plasma MMP-9 activity in a series of SICU patients admitted with acute respiratory failure to determine if there might be some predictive value for MMP-9 measurements in a clinical setting. In contrast to our mouse model in which we were not able to detect plasma MMP-9 activity in experimental mice, we were able to detect MMP-9 activity in 88% of patients with ARDS using gelatin zymography. In addition, we were able to detect plasma MMP-9 activity in 100% of patients with ARDS attributable to primary pulmonary pathology. This was likely due to the presence of a much higher concentration of MMP-9 in the plasma of human subjects with ARDS compared to the experimental mice in our animal study. We first compared plasma MMP-9 activity in arterial and venous blood from ICU patients and determined that there was no significant difference in MMP-9 activity from the two components of the circulation. This result suggests that MMP-9 washout into arterial or venous blood from sequestered neutrophils in the lung vasculature is either a similar or negligible amount, and taken together with our animal model data suggests that MMP-9 measurements may reflect levels of circulating primed neutrophils as opposed to washout from inflamed lungs. In addition, this indicates that arterial and venous sources of blood are likely equivalent for MMP-9 measurements in a clinical setting.
After measuring plasma MMP-9 activity time courses in our series of SICU patients, we observed a statistically significant inverse correlation between a change in plasma MMP-9 activity on a particular day and a change in the PaO2/FiO2 ratio the following day in patients that developed ARDS (r = −0.503, p < 0.006). These results suggest that an increased plasma MMP-9 activity might be indicative of a subsequent decline in lung function the following day, and conversely that a decrease in plasma MMP-9 activity might signify an improvement in lung function the following day.
Based on this observed inverse correlation between changing MMP-9 activity and changing PaO2/FiO2 ratios, we asked whether some absolute elevated value of plasma MMP-9 activity might be indicative of subsequent deterioration in lung function. However, we were unable to observe any statistically significant correlation between the absolute value of plasma MMP-9 activity on a specific day and the PaO2/FiO2 ratio measured the following day in patients that developed ARDS. Instead, it was the change in MMP-9 activity levels relative to the day before that correlated with a subsequent change in lung function. Whether the lack of an absolute cut-off value for MMP-9 activity as a predictive marker results from patient to patient variability in the extent of neutrophil priming and MMP-9 secretion as a consequence of other organ injuries or a generalized septic-like state, or from patient-specific differences in clearance of circulating MMP-9 is not known.
Our data suggest that time-dependent measurements of plasma MMP-9 activity in critically ill ICU patients may be useful for a subset at risk for worsening lung function. Importantly, however, our study is entirely retrospective, small in size, and limited due to the infrequency with which ARDS presents in our patient population. Our data also indicates that elevated plasma MMP-9 activity is not specific for lung injury, as 10 of the 12 patients without ARDS had detectable plasma MMP-9 activity by zymography, likely reflective of the general pro-inflammatory state of critical illness. It should also be noted that 4 of those patients presented with associated brain injury and there is existing evidence in the literature indicating an upregulation in MMP-9 expression in the setting of brain injury (33). Two other patients presented with septic shock and associated acute respiratory failure, however one of them expired early during SICU admission and the other did not meet criteria for diagnosis of ARDS. Trauma and mesenteric ischemia were among the diagnoses for the remaining 4 patients with detectable plasma MMP-9 activity that did not develop ARDS. This result is not unexpected given the broad function MMPs serve in host defense, tissue injury, inflammation, and wound repair in addition to their role in other pathological disorders including arthritis, cancer, lung disease, and cardiovascular disease (34). Additional studies involving ICU patients presenting with trauma would help to address this issue. Other modulators of MMP-9 activity such as TIMP-1 and NGAL might also be useful for prognostication in combination with measurements of MMP-9 activity. Paradoxically, high ratios of TIMP-1/MMP-9 protein levels in serum, which inhibits MMP-9 activity in vitro, were correlated with increased mortality in sepsis (35), while higher ratios within BAL fluid were observed in patients during prolonged courses of ARDS (18), suggesting that the functional connection between TIMP-1 and MMP-9 in vivo may be complex. Other products released by primed neutrophils, such as IL-8, may also play a role.
Despite the limitations of this study, the data from the mouse model of lung injury/ARDS and the observational cohort study involving SICU patients suggests that there may be a potential role for using the kinetics of plasma MMP-9 activity measurements as an indicator of neutrophil priming and predictor of risk for subsequent lung injury and lung function deterioration in critically ill patients. If these results can be substantiated in a larger prospective clinical trial, then they may indicate an optimal period in which institution of appropriate therapy in critically ill patients could reduce the development of ARDS or multiple organ failure and improve survival.
Acknowledgments
This work was funded by NIH grants R01-GM59281, P50-GM68762, and UM1-HL120877. AH was supported by the National Institutes of Health Ruth L. Kirschstein National Research Service Award.
We gratefully acknowledge technical support from members of the Yaffe laboratory, and support and encouragement from the entire staff of the Trauma and Neuro Surgical ICUs at the Beth Israel Deaconess Medical Center.
References
- 1.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, de Pasqualucci MO, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308(16):1651–9. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369(9572):1553–64. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 5.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, 3rd, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J, Malinchoc M U. S. C. Illness and I. Injury Trials Group. Lung Injury Prevention Study: Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–70. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):337–49. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 7.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. Subpopulation of hyperresponsive polymorphonuclear neutrophils in patients with adult respiratory distress syndrome. Role of cytokine production. Am Rev Respir Dis. 1992;146(4):990–6. doi: 10.1164/ajrccm/146.4.990. [DOI] [PubMed] [Google Scholar]
- 8.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17(3–4):293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gando S, Kameue T, Nanzaki S, Hayakawa T, Nakanishi Y. Increased neutrophil elastase, persistent intravascular coagulation, and decreased fibrinolytic activity in patients with posttraumatic acute respiratory distress syndrome. J Trauma. 1997;42(6):1068–72. doi: 10.1097/00005373-199706000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD L. National Heart and N. Blood Institute Acute Respiratory Distress Syndrome Clinical Trials. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35(8):1821–8. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberholzer A, Souza SM, Tschoeke SK, Oberholzer C, Abouhamze A, Pribble JP, Moldawer LL. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock. 2005;23(6):488–93. [PubMed] [Google Scholar]
- 12.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(2):R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putensen C, Zech S, Wrigge H, Zinserling J, Stuber F, Von Spiegel T, Mutz N. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–9. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 14.Roy S, Habashi N, Sadowitz B, Andrews P, Ge L, Wang G, Roy P, Ghosh A, Kuhn M, Satalin J, Gatto LA, Lin X, Dean DA, Vodovotz Y, Nieman G. Early airway pressure release ventilation prevents ARDS-a novel preventive approach to lung injury. Shock. 2013;39(1):28–38. doi: 10.1097/SHK.0b013e31827b47bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer JM. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154(2 Pt 1):346–52. doi: 10.1164/ajrccm.154.2.8756805. [DOI] [PubMed] [Google Scholar]
- 16.Torii K, Iida K, Miyazaki Y, Saga S, Kondoh Y, Taniguchi H, Taki F, Takagi K, Matsuyama M, Suzuki R. Higher concentrations of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155(1):43–6. doi: 10.1164/ajrccm.155.1.9001287. [DOI] [PubMed] [Google Scholar]
- 17.Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27(2):304–12. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 18.Lanchou J, Corbel M, Tanguy M, Germain N, Boichot E, Theret N, Clement B, Lagente V, Malledant Y. Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit Care Med. 2003;31(2):536–42. doi: 10.1097/01.CCM.0000048626.02184.F8. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg J, Halter J, Schiller HJ, Dasilva M, Landas S, Gatto LA, Maisi P, Sorsa T, Rajamaki M, Lee HM, Nieman GF. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J Surg Res. 2003;111(2):185–95. doi: 10.1016/s0022-4804(03)00089-1. [DOI] [PubMed] [Google Scholar]
- 20.Aldridge AJ. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg. 2002;168(4):204–14. doi: 10.1080/11024150260102807. [DOI] [PubMed] [Google Scholar]
- 21.Delclaux C, Delacourt C, D’Ortho MP, Boyer V, Lafuma C, Harf A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol. 1996;14(3):288–95. doi: 10.1165/ajrcmb.14.3.8845180. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol. 2005;78(1):279–88. doi: 10.1189/jlb.1004612. [DOI] [PubMed] [Google Scholar]
- 23.Pugin J, Widmer MC, Kossodo S, Liang CM, Preas HLn, Suffredini AF. Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol. 1999;20(3):458–64. doi: 10.1165/ajrcmb.20.3.3311. [DOI] [PubMed] [Google Scholar]
- 24.Brown GE, Stewart MQ, Bissonnette SA, Elia AE, Wilker E, Yaffe MB. Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase. The Journal of biological chemistry. 2004;279(26):27059–68. doi: 10.1074/jbc.M314258200. [DOI] [PubMed] [Google Scholar]
- 25.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1--Correlation within subjects. BMJ. 1995;310(6977):446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 2--Correlation between subjects. BMJ. 1995;310(6980):633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vempati P, Karagiannis ED, Popel AS. A biochemical model of matrix metalloproteinase 9 activation and inhibition. J Biol Chem. 2007;282(52):37585–96. doi: 10.1074/jbc.M611500200. [DOI] [PubMed] [Google Scholar]
- 29.Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 1998;94(5):461–71. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 30.Fligiel SE, Standiford T, Fligiel HM, Tashkin D, Strieter RM, Warner RL, Johnson KJ, Varani J. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol. 2006;37(4):422–30. doi: 10.1016/j.humpath.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Yazdan-Ashoori P, Liaw P, Toltl L, Webb B, Kilmer G, Carter DE, Fraser DD. Elevated plasma matrix metalloproteinases and their tissue inhibitors in patients with severe sepsis. J Crit Care. 2011;26(6):556–65. doi: 10.1016/j.jcrc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Kalkoff M, Cursons RT, Sleigh JW, Jacobson GM. The use of real time rtPCR to quantify inflammatory mediator expression in leukocytes from patients with severe sepsis. Anaesth Intensive Care. 2004;32(6):746–55. doi: 10.1177/0310057X0403200603. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg GA. Matrix metalloproteinases in brain injury. J Neurotrauma. 1995;12(5):833–42. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- 34.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19(1):34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorente L, Martin MM, Sole-Violan J, Blanquer J, Labarta L, Diaz C, Borreguero-Leon JM, Orbe J, Rodriguez JA, Jimenez A, Paramo JA. Association of sepsis-related mortality with early increase of TIMP-1/MMP-9 ratio. PLoS One. 2014;9(4):e94318. doi: 10.1371/journal.pone.0094318. [DOI] [PMC free article] [PubMed] [Google Scholar]