Abstract
Mounting evidence supports that fine particulate matter adversely impacts cardio-metabolic diseases particularly in susceptible individuals; however, health effects induced by the extreme concentrations within megacities in Asia is not well described. We enrolled 65 nonsmoking adults with metabolic syndrome and insulin resistance in the Beijing metropolitan area into a panel study of four repeated visits across four seasons since 2012. Daily ambient fine particulate matter (PM2.5) and personal black carbon (BC) levels ranged from 9.0 to 552.5 μg/m3 and 0.2 to 24.5 μg/m3, respectively, with extreme levels observed during January 2013. Cumulative PM2.5 exposure windows across the prior 1-7 days were significantly associated with systolic blood pressure (BP) elevations ranging from 2.0 (95% confidence interval 0.3-3.7) to 2.7 (0.6-4.8) mmHg per standard deviation increase [67.2 μg/m3]), while cumulative BC exposure during the previous 2-5 days were significantly associated with ranges in elevations in diastolic BP from 1.3 (0.0-2.5) to 1.7 (0.3-3.2) mmHg per standard deviation increase [3.6 μg/m3]). Both BC and PM2.5 were significantly associated with worsening insulin resistance (0.18 (0.01-0.36) and 0.22 (0.04-0.39) unit increase per standard deviation increase of personal-level BC, and 0.18 (0.02-0.34) and 0.22 (0.08-0.36) unit increase per standard deviation increase of ambient PM2.5 on lag days 4 and 5). These results provide important global public health warnings that air pollution may pose a risk to cardio-metabolic health even at the extremely high concentrations faced by billions of people in the developing world today.
Keywords: Air pollution, Hypertension, Diabetes Mellitus, Insulin Resistance, Metabolis
INTRODUCTION
Fine particulate matter (PM) air pollution < 2.5 μm in aerodynamic diameter (PM2.5) ranks among the leading risk factors for global morbidity and mortality [1]. Numerous studies demonstrate that short-term exposures over a few days can trigger acute cardiovascular events (e.g., myocardial infarctions [2, 3], strokes [4]). Perhaps even more important from a public health standpoint, is that living in highly-polluted regions increases cardiovascular morbidity and mortality several fold more than the risk conveyed by brief exposures [5, 6]. One plausible explanation may be that long-term exposures to PM2.5 can potentiate the development of chronic disease states, thereby synergistically heightening future cardiovascular risk [7]. In this regard, we and others have shown that the inhalation of air pollutants may promote the development of cardio-metabolic disorders, including hypertension and type 2 diabetes mellitus (DM) [8-14]. Indeed, a growing number of animal and human experiments add support for the mechanistic plausibility of these associations [15-19].
Recent estimates suggest that more than three-quarters of the world’s population live in regions exceeding the World Health Organization (WHO) annual air quality standards for PM2.5 (>10 μg/m3) [1, 20]. Coincident with burgeoning air pollution levels, rapid urbanization in these societies has led to a co-epidemic of the cardio-metabolic syndrome. In fact, the majority of cardiovascular deaths, as well as the largest portion of the world’s population suffering from hypertension and/or DM, resides among these same highly polluted countries, posing a threat to global health [1, 21-23].
Most of the studies on exposure response relationships of air pollution and cardiovascular morbidity and mortality or surrogates have mostly come from the western hemisphere, where the levels are significantly lower [24]. Given the fact that the shape of the concentration response relationship suggests attenuation of effects at high doses, it is important to investigate the impact of air pollution at high levels. Our main goal in the Air Pollution and Cardiometabolic Disease (AIRCMD-China) study, was to investigate if the extraordinary high levels of particulate pollutants encountered within a typical Chinese global megacity (i.e., population > 10 million residents) adversely impacts blood pressure (BP) and insulin sensitivity [14, 25]. In our initial report, we found that brief personal-level black carbon (BC) exposures, a metric of combustion-related PM, were associated with rapid elevations (within 6-10 hours) in both BP and heart rate [14]. Here, we present the main study results regarding the associations of sub-acute (prior 1-7 days) BC and PM2.5 exposures measured at the personal- and ambient-level with changes in hemodynamics and insulin sensitivity among at-risk individuals with the metabolic syndrome.
METHODS
Full details regarding the protocol have been previously published [14, 25]. Briefly, AIRCMD-China was a prospective study conducted from February 14, 2012 until July 4, 2013 investigating the associations between prior 1-7 days exposures to particulate air pollutants with cardio-metabolic responses among participants with the metabolic syndrome defined by International Diabetes Federation criteria specific for Asians [25]. The primary outcomes were changes in resting BP levels and metabolic insulin sensitivity determined by the homeostasis model assessment of insulin resistance (HOMA-IR). The study population was comprised of nonsmoking adults residing in the Beijing-area recruited from clinics affiliated with Peking Union Medical College (PUMC) Hospital and followed-up to complete four study visit periods during separate seasons within a one year time frame. The institutional review board at PUMC Hospital approved the protocol and all subjects gave written informed consent.
Health outcomes
Demographic characteristics, medical history, anthropometrics, and self-reported dietary intake and physical activity were collected at the screening visit. During each of the 4 study visit periods, participants performed personal-level BC exposure monitoring for 5 consecutive days prior to the measurement of health responses. Study outcomes were performed fasting in the morning of each visit day and have been described in detail previously[14, 25]. HRV was performed using the SpaceLabs EVO digital Holter system (SpaceLabs Healthcare, WA, USA). Aortic augmentation and carotid-femoral PWV determined by SphygmoCor CP device (AtCor Medical, Austrlia) and reactive hyperemial index (RHI) determined using peripheral arterial tonometry (EndoPAT2000, Itamar Medical, Israel). Supine BP was measured using the Omron HEM 907XL device. Fasting venous blood samples for plasma glucose, plasma insulin, lipoproteins, glycated hemoglobin, adiponectin and leptin was measured at PUMC. HOMA-IR was calculated using fasting insulin and glucose measures. Concomitant with day 4 of personal BC, 24-hour ambulatory BP levels was determined and reported previously[14, 25].
Exposure Measurements and Meteorological Variables
Ambient PM2.5 levels were obtained from 3 locations in Beijing: US Embassy in Beijing situated in the Northeast 3rd Ring Road with PM2.5 readings posted on a Twitter feed; the Institute of Atmospheric Physics (IAP) near the North 2nd Ring Road using a tapered element oscillating microbalance (TEOM) sampler until April 30, 2013; and Tsinghua University at northwest Beijing (between the 4th and 5th Ring Roads) with PM2.5 measured from TEOM sampler since June 15, 2012. The data collected at the US Embassy were the most complete and were thus used for evaluating the associations with study outcomes.
To assess personal exposures to BC, we used a microaethalometer (AethLabs MicroAeth AE51). Subjects carried the AE51 with them throughout their daily activities or placed them nearby when indoors (e.g., when sleeping) to measure personal mass concentration of BC every 5 minutes throughout the five-day period prior to the clinic visit. To characterize the component of BC exposure related to the overall ambient conditions in the city, five-minute city center ambient BC concentrations were measured from a second story window located at the Peking Union Medical College Hospital using a dual wavelength aethalometer (Aethalometer AE20; Magee Scientific, CA). This instrument started operation in May 28, 2012, and thus these data were not available for the first season of subject visits. Meteorological information on daily temperature and humidity at a fixed monitoring station close to PUMC Hospital were obtained from the Weather Underground website.
Statistical Analysis
Descriptive statistics of demographic characteristics were computed for all subjects. Comparisons of functional end-points across four repeated visits were performed by repeated measures analysis of variance, with degrees of freedom 3 and a compound symmetry covariance structure for random effect.
The mean concentrations of ambient exposure during the preceding 1-7 days before the measurement of health responses at each visit were computed because most associations with study outcomes were observed during this time period. Similarly, personal level BC concentrations were averaged at the preceding 1 to 5 days for each subject. Levels of exposure variables 1-day prior to each study visit are reported, while distributions of longer lagging days are not shown because of similar patterns. Transformation of variables was not applied because normal distributions was observed. To examine associations between exposure variables and outcomes across four visits, we used linear mixed models with a random intercept for each subject to account for within-subject correlations due to repeated measurements. In our models, the outcomes of interest include resting BP, HOMA-IR, HRV, PWV and PWA, endothelial function index, and blood biomarkers. We investigated exposure windows during the preceding 1-7 days (1-7-day lags) before the measurement of health responses for BC and PM2.5. Effects of cumulative exposure ranging from 1-7 days prior to the clinic visit (1-7-day averages) on health outcomes were also assessed. All models were adjusted for age, gender, BMI, hypertension (yes/no), temperature, relative humidity, and visits (categorical); which were selected based upon prior knowledge and bivariate association analyses with the outcome. Linear terms of temperature and relative humidity were found to be adequate by the Akaike Information Criterion. All models were evaluated for outliers and influential observations by residual diagnostics. Estimated effects on health outcomes were presented with each standard deviation increase in 1-day lag of personal BC or ambient PM2.5. All analyses were performed with statistical package R version 3.1.2 (www.r-project.org). Statistical significance was assessed using a two-sided Wald test at the significance level of 0.05.
RESULTS
The majority of patients were overweight (mean body mass index of 26.0), with a history of hypertension (83%). Mean age of the population was 61±9 years with females predominating. Forty eight percent of the participants had previously lived with a smoker for at least 6 months. The baseline hemoglobin A1c in the participants was 6.09 (0.92) suggested pre-diabetic state in the participants with no trend towards deterioration in glycemic control over 12 months. Table 1 shows the results for all health outcomes measured at each of the 4 study visits. Four participants dropped out (2 after their first visits, 2 after their third visits), and 3 subjects intermittently missed one or two visits, leaving a total of 248 measurements for each study endpoint. Mean ambulatory and clinic BP levels were within the pre-hypertensive range.
Table 1.
Descriptive statistics of the study outcomes
| Outcome | Visit 1 (n=65) |
Visit 2 (n=61) |
Visit 3 (n=61) |
Visit 4 (n=61) |
Overall | p† |
|---|---|---|---|---|---|---|
| Ambulatory Blood Pressure | ||||||
| 24-hour mean SBP (mmHg) |
124.4 (11.9) | 123.3 (10.4) | 124.7 (10.7) | 123.0 (8.3) | 123.8 (10.4) | 0.26 |
| 24-hour mean DBP (mmHg) |
75.8 (8.0) | 75.0 (7.5) | 75.4 (7.7) | 74.1 (6.9) | 75.1 (7.5) | 0.03 |
| 24-hour mean HR (beats/min) |
70.1 (9.2) | 68.4 (9.3) | 70.1 (9.1) | 69.2 (8.3) | 69.5 (9.0) | 0.07 |
| Clinic Blood Pressures | ||||||
| SBP (mmHg) | 130.4 (16.0) | 125.0 (15.3) | 126.6 (14.9) | 124.9 (14.2) | 126.8 (15.2) | 0.001 |
| DBP (mmHg) | 81.1 (9.8) | 77.7 (10.7) | 78.7 (10.5) | 77.2 (9.6) | 78.7 (10.2) | <.0001 |
| HR (beats/min) | 62.8 (8.9) | 62.5 (8.1) | 64.0 (10.1) | 62.6 (8.3) | 63.0 (8.9) | 0.21 |
| Heart Rate Variability * | ||||||
| 5-min LF (msec2) | 5.21 (0.98) | 5.41 (1.04) | 5.06 (0.98) | 5.14 (1.08) | 5.20 (1.02) | 0.12 |
| 5-min HF (msec2) | 5.29 (1.09) | 5.26 (1.19) | 5.14 (1.04) | 5.22 (1.07) | 5.23 (1.09) | 0.69 |
| 5-min LF/HF | −0.08 (1.11) | 0.15 (0.99) | −0.08 (1.09) | −0.08 (1.04) | −0.03 (1.06) | 0.34 |
| 5-min SDNN (msec) | 3.86 (0.45) | 4.11 (0.44) | 3.71 (0.41) | 3.75 (0.48) | 3.86 (0.47) | <.0001 |
| 5-min rMSSD (msec) | 3.43 (0.44) | 3.47 (0.48) | 3.35 (0.48) | 3.38 (0.49) | 3.41 (0.47) | 0.37 |
| 5-min pNN50 (%) | 1.61 (1.16) | 1.45 (1.17) | 1.15 (1.21) | 1.30 (1.27) | 1.38 (1.21) | 0.04 |
| Blood Metabolic Biomarkers | ||||||
| Fasting glucose (mmol/L) | 5.39 (0.74) | 5.42 (0.67) | 5.69 (0.86) | 5.72 (1.00) | 5.55 (0.83) | <.0001 |
| Fasting insulin (μU/mL) | 10.3 (5.4) | 11.4 (5.8) | 12.5 (7.1) | 11.5 (6.4) | 11.4 (6.2) | 0.007 |
| HOMA-IR | 2.51 (1.33) | 2.74 (1.44) | 3.22 (1.93) | 2.97 (1.88) | 2.86 (1.68) | <.0001 |
| Low-density lipoprotein (mmol/L) |
2.71 (0.87) | 2.66 (0.79) | 2.63 (0.89) | 2.69 (0.78) | 2.67 (0.83) | 0.58 |
| High-density lipoprotein (mmol/L) |
1.26 (0.29) | 1.21 (0.26) | 1.22 (0.25) | 1.22 (0.24) | 1.23 (0.26) | 0.05 |
| Triglyceride (mmol/L) | 1.60 (0.73) | 1.60 (0.79) | 1.50 (0.71) | 1.57 (0.70) | 1.56 (0.73) | 0.78 |
| Total cholesterol (mmol/L) |
4.70 (1.18) | 4.65 (1.14) | 4.55 (1.06) | 4.58 (0.97) | 4.62 (1.08) | 0.53 |
| Glycated hemoglobin (%) | 6.09 (0.92) | 6.23 (1.01) | 6.21 (0.78) | 6.15 (0.81) | 6.17 (0.88) | 0.61 |
| Adiponectin (μg/mL) | 2.32 (1.21) | 2.08 (1.11) | 2.14 (1.11) | 2.13 (0.99) | 2.17 (1.11) | 0.37 |
| Leptin (μg/L) | 5.77 (4.88) | 4.95 (4.09) | 5.24 (5.87) | 4.86 (4.57) | 5.22 (4.89) | 0.08 |
| Vascular Outcomes | ||||||
| Augmentation index at heart rate of 75 beats/min |
24.6 (8.6) | 26.9 (7.5) | 25.7 (8.8) | 25.4 (9.9) | 25.6 (8.7) | 0.24 |
| Pulse wave velocity | 7.52 (1.74) | 7.73 (1.34) | 7.17 (1.51) | 7.77 (1.61) | 7.56 (1.57) | 0.02 |
| Reactive Hyperemia Index |
2.20 (0.56) | 2.10 (0.53) | 2.00 (0.52) | 1.95 (0.60) | 2.07 (0.56) | 0.01 |
Results are presented as mean (SD). SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LF, low frequency peak; HF, high frequency peak; SDNN, standard deviation of N-N intervals; rMSSD, root mean square of successive differences between N-N intervals; pNN50, proportion of N-N intervals greater than 50 milliseconds; HOMA-IR, homeostasis model assessment of insulin resistance.
p-value shows significance of differences between four visits using repeated measures analysis of variance, with 3 degrees of freedom and a compound symmetry covariance structure for random effect.
All heart rate variability indices are summarized on the log scale.
Air pollution levels during the study are presented in Table 2. Both personal and stationary ambient measures of BC exposures were high. The correlation between personal BC and ambient BC had a correlation coefficient of 0.88 (Supplemental Table S1). Correlation of PM2.5 between sites was high (r>0.90, p<0.0001). Personal BC correlated significantly with PM2.5 measurements at all sites but particularly with the US Embassy site (r<0.80, p<0.0001). The temporal correlations among measurements in different locations indicate that a large component of day-to-day variations in exposure among study subjects are common and influenced by variation in large scale emission patterns and atmospheric conditions (e.g., meteorology).
Table 2.
Descriptive statistics of air pollutants and meteorological parameters collected during the preceding 24 hours before the measurement of health responses.
| Visit 1 (n=65) |
Visit 2 (n=61) |
Visit 3 (n=61) |
Visit 4 (n=61) |
Overall | p§ | |
|---|---|---|---|---|---|---|
| Personal BC, μg/m 3 | ||||||
| Mean ± SD | 4.5 ± 2.5 | 6.7 ± 3.1 | 7.7 ± 5.0 | 4.7 ± 2.0 | 5.9 ± 3.6 | <.0001 |
| Min | 0.3 | 0.9 | 0.4 | 0.7 | 0.3 | |
| Max | 9.7 | 15.3 | 16.5 | 10.5 | 16.5 | |
| IQR | 4.5 | 4.8 | 8.6 | 3.1 | 4.5 | |
| Ambient BC at PUMC, μg/m3 * | ||||||
| Mean ± SD | 5.0 ± 1.5 | 6.1 ± 2.4 | 8.4 ± 5.3 | 5.2 ± 1.8 | 6.5 ± 3.7 | <.0001 |
| Min | 2.6 | 1.2 | 1.1 | 1.1 | 1.1 | |
| Max | 6.5 | 9.9 | 17.5 | 8.3 | 17.5 | |
| IQR | 2.8 | 5.2 | 9.2 | 3.0 | 4.3 | |
| Ambient PM2.5 at US Embassy, μg/m3 † | ||||||
| Mean ± SD | 75.2 ± 39.9 | 104.7 ± 61.0 | 145.5 ± 97.3 | 80.5 ± 36.7 | 99.5 ± 67.2 | <.0001 |
| Min | 18.0 | 18.0 | 18.2 | 18.2 | 18.0 | |
| Max | 135.5 | 204.9 | 298.0 | 138.8 | 298.0 | |
| IQR | 68.7 | 119.3 | 155.8 | 72.0 | 81.8 | |
| Temperature, °C | ||||||
| Mean ± SD | 18.8 ± 6.0 | 24 ± 3.5 | 6.3 ± 9.1 | 15.6 ± 8.0 | 16.2 ± 9.4 | <.0001 |
| Min | −3.3 | 15.6 | −7.2 | −7.2 | −7.2 | |
| Max | 26.7 | 28.9 | 21.7 | 28.9 | 28.9 | |
| IQR | 6.7 | 5.0 | 15.0 | 13.3 | 12.2 | |
| Relative humidity, % | ||||||
| Mean ± SD | 52.3 ± 20.9 | 72.8 ± 14.9 | 60.6 ± 20.1 | 51.4 ± 17.4 | 59.2 ± 20.3 | <.0001 |
| Min | 18 | 22 | 22 | 27 | 18 | |
| Max | 91 | 91 | 91 | 89 | 91 | |
| IQR | 36 | 17 | 23 | 17 | 30 | |
BC indicates black carbon; IQR, interquartile range; PM, particulate matter; and PUMC, Peking Union Medical College.
P value shows significance of differences between 4 study visits using repeated measures ANOVA.
Ambient BC at PUMC was collected starting from May 28, 2012.
PM 2.5 measured at the US Embassy was used for evaluating the associations of ambient PM 2.5 levels with the study health outcomes.
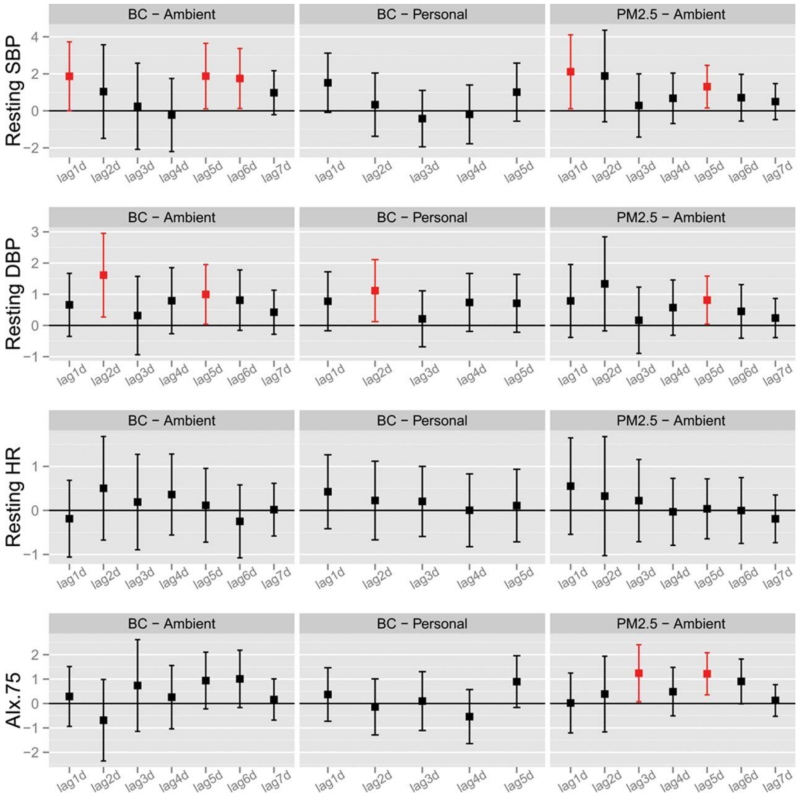
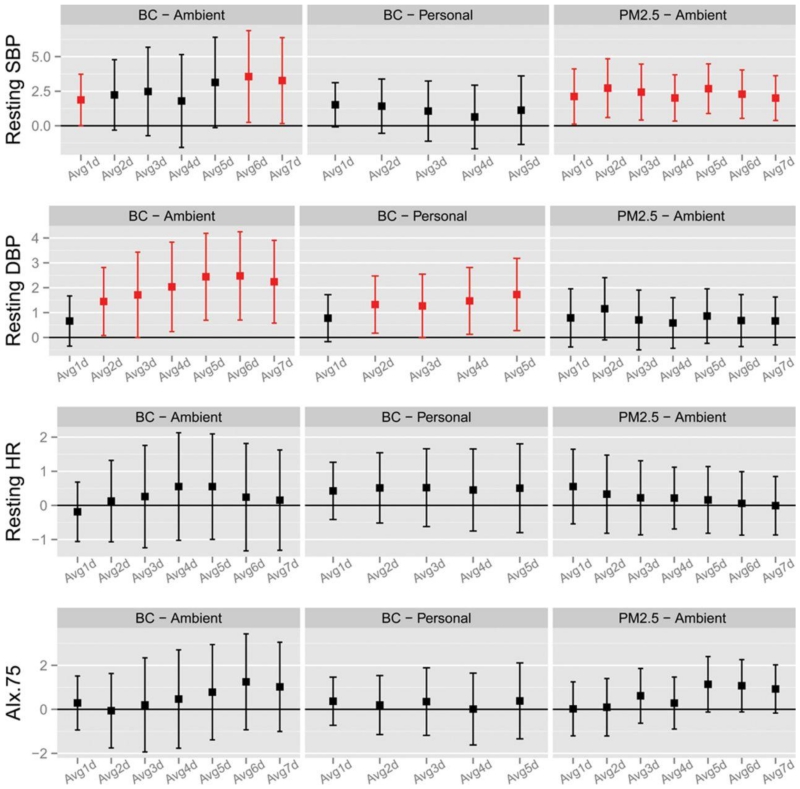
The effects of air pollution exposures on BP are shown in Figures 1 and 2. Significant association with ambient BC and elevated systolic and diastolic BP, personal-level BC and diastolic BP and ambient PM2.5 with elevated systolic and diastolic BP and AIx@75 were observed at several different single lag day periods of exposure (Figure 1). The most consistent associations were for cumulative PM2.5 exposure windows across the prior 1-7 days with resting systolic BP. The increase ranged from 2.0 (95% CI 0.3-3.7) to 2.7 mm Hg (95% CI 0.6-4.8) per SD rise in PM2.5 (67.2 μg/m3) (Figure 2). Cumulative personal BC exposures during the previous 2-5 days corresponded with elevation in diastolic BP, ranging from 1.3 (95% CI 0.0-2.5) to 1.7 mm Hg (95% CI 0.3-3.2) per SD increase [3.6 μg/m3]). No PM2.5 or BC metrics were associated with change in heart rate (Figures 1 and 2), RHI or PWV (data not shown).
Figure 1. Changes in Seated Clinic Blood Pressure Levels Associated with Previous Single Day Lag Concentrations of PM2.5 and Black Carbon.
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; AI.75, augmentation index at a heart rate of 75 beats/min.
Data points and error bars represent the estimated changes and 95% confidence intervals associated with a 3.61 μg/m3 (standard deviation of 1-day lag of personal BC) increase in 1-day to 7-day single day lags of personal and ambient BC concentration, as well as a 67.2 μg/m3 (standard deviation of 1-day lag of ambient PM2.5) increase in 1-day to 7-day single day lags of ambient PM2.5 concentration using linear mixed models. Significant associations (p-value≤0.05) were shown in red. All models controlled for age, sex, body mass index, temperature, relative humidity, hypertension, diabetes mellitus, and study visit.
Figure 2. Changes in Seated Clinic Blood Pressure Levels Associated with Previous 1 to 7 day Long Cumulative PM2.5 and Black Carbon Concentrations.
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; AI.75, augmentation index at a heart rate of 75 beats/min.
Data points and error bars represent the estimated changes of the health endpoints and 95% confidence intervals associated with a 3.61 μg/m3 increase in 1-day to 7-day long cumulative personal and ambient BC concentrations, as well as a 67.2 μg/m3 increase in 1-day to 7-day long cumulative ambient PM2.5 concentrations using linear mixed models. Significant associations (p-value ≤ 0.05) were shown in red. All models controlled for age, sex, body mass index, temperature, relative humidity, hypertension, diabetes mellitus, and study visit.
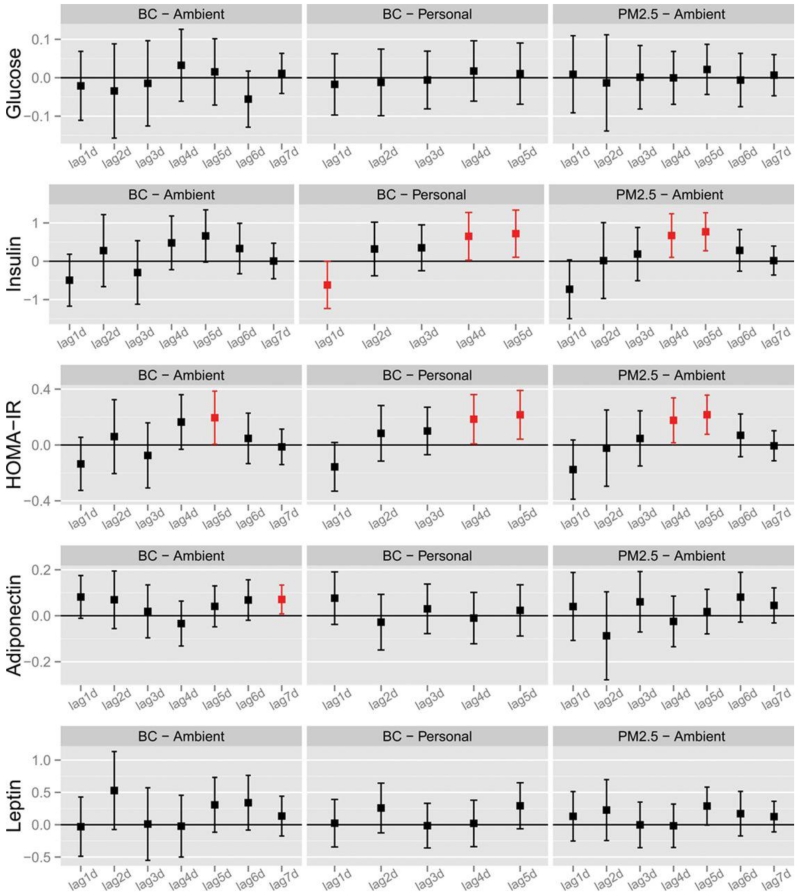
The effects of air pollution exposure on metabolic biomarkers are shown in Figure 3 and the online supplement (Figure S2). Air pollutants worsened insulin sensitivity, albeit in a somewhat delayed fashion. Both personal-level BC and ambient PM2.5 were significantly associated with increases in HOMA-IR (0.18 (95% CI 0.01-0.36) and 0.22 unit (95% CI 0.04-0.39) per SD increase of personal-level BC and 0.18 (95% CI 0.02-0.34) and 0.22 unit (95% CI 0.08-0.36) per SD increase of ambient PM2.5 on individual lag-days 4 and 5) (Figure 3). Due to the null association during early individual lag days, cumulative exposure windows were not related to HOMA-IR (Figure S2). Finally, changes in leptin and adiponectin (Figure 3 and S2) and multiple HRV metrics (Figures S3 and S4) were not consistently associated with air pollution metrics.
Figure 3. Changes in Metabolic Biomarkers Associated with Previous Single Day Lag Concentrations of PM2.5 and Black Carbon.
HOMA-IR, homeostasis model assessment of insulin resistance.
Data points and error bars represent the estimated changes of the health endpoints and 95% confidence intervals associated with a 3.61 μg/m3 increase in 1-day to 7-day single day lags of personal and ambient BC concentration, as well as a 67.2 μg/m3 increase in 1-day to 7-day single day lags of ambient PM2.5 concentration using linear mixed models. Significant associations (p-value ≤ 0.05) were shown in red. All models controlled for age, sex, body mass index, temperature, relative humidity, hypertension, diabetes mellitus, and study visit.
By chance the study period encompassed a highly publicized air pollution excursion that occurred in January-February 2013 in eastern China [26]. This period of extreme air pollution (e.g., maximum of 552.2 μg/m3 in Beijing and strongly elevated levels of BC (Supplemental Figure S1)) were captured when 15 subjects were participating in their third observation period and thus we additionally analyzed their responses. Supplemental Table S2 presents the changes in LF and SDNN and demonstrates a decrease in SDNN and LF in these subjects during this period.
DISCUSSION
Our findings demonstrate an impact of short-term variation in very high levels of ambient air pollution metrics on health outcomes in Beijing China [27]. We show that both PM2.5 and BC are linked to increases in BP and insulin resistance, even among at-risk individuals who have been living for long periods in these conditions. This observation supports our hypothesis that the rising twin epidemics of cardio-metabolic diseases [21, 22] and poor air quality [20] among developing regions may be more than a simple coincidence arising from the common soil of urban lifestyles [8, 10-13, 28].
We recently demonstrated that personal-level exposure to BC in Beijing (a marker of traffic-related pollutants) increases BP and heart rate within a few hours [14]. These main results from the AIRCMD-China study confirm and extend our previous observations by showing that city-wide “ambient” levels of PM2.5 and BC (as well as BC measured at the personal-level) over the prior 1-7 days are linked to higher BP levels and insulin resistance. While air pollutants have also been associated with a risk for developing diabetes [8, 10, 11], very few studies have evaluated effects on glucose metabolism or insulin sensitivity in humans [28-33]. This current study is the first to be performed at such high levels among individuals with the metabolic syndrome over multiple seasons. It is important to note that the observed effects (i.e., increased BP and insulin resistance) occurred among at-risk individuals within the context of acute elevation in air pollution levels on top of continuing chronic exposures and still remain capable of provoking biological responses linked to risk of developing hypertension and diabetes. Under conditions of extremely high levels of air pollution, there is the potential of alteration of parasympathetic tone that may also be important as demonstrated in a subset of 15 patients who underwent biologic measurements during a period of extremely high levels of air pollution.
Particulate air pollutants have been postulated to trigger elevation in BP and worsen insulin resistance via several pathways [7, 8, 16, 18, 28]. Despite a sound mechanistic basis that air pollutants could provoke adverse cardio-metabolic responses by mechanisms such as increase in circulating cytokines and inflammation neither PM2.5 nor BC were related to these outcomes in this study. Microvascular endothelial dysfunction (i.e., lower RHI) and impaired arterial compliance (i.e., higher PWV) are also unlikely explanations for BP elevation given their lack of association. It is possible that chronic high degrees of exposure as was the case with this study, may have slightly different underlying mechanisms [16, 18].
PM2.5, hypertension, and DM are each independent major risk factors for global morbidity and mortality [1]. Even low-levels of PM2.5 are linked to excess cardiovascular events, suggesting that there are no known “safe” lower thresholds at the population-level [5, 7]. Similarly, small elevations in BP and plasma glucose (e.g., pre-hypertension and pre-diabetes) over the long-term are both causally-related to adverse cardiovascular health outcomes [34, 35]. Therefore, even modest perturbations in BP and metabolic control, could be linked to significant health burden given the billions of people affected by air pollution globally and continually.
The strengths of the study include repeated assessments over 12 months in the same individual and performance of the study at a time of unprecedented levels of air pollution levels. Further the performance in an “at risk” patient population renders the findings of our study all the more important. We acknowledge multiple limitations, including lack of specific PM characterization that may have allowed better delineation of constituents driving the relationships in this study. We also do not have detailed characterization of insulin responses assessed using clamp measures. Given the nature of the design (4 seasons) this may have been hard to accomplish. Finally it is possible that other environmental determinants that co-segregate with air pollution may have contributed to these effects and it is impossible to definitively rule out the contribution of these factors.
PERSPECTIVES
Worldwide data support that most cardiovascular events, as well as the highest prevalence of cardiometabolic conditions, are among developing nations [21, 22]. Our findings provide evidence for an influential role for air pollution even at extremely high concentrations faced by billions of people in the developing world today. We recognize that many other factors must also be important (e.g., obesity, diet, exercise) as hypertension and diabetes rates are increasing in North America despite falling PM2.5 levels [20-22]. Nonetheless, even very low levels of air pollution have been associated with an excess of cardio-metabolic disorders [10-12]. As such, we believe more studies are urgently needed to confirm this hypothesis of potential worldwide public health importance.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
We have evaluated the effects of air pollutants on cardio-metabolic outcomes in subjects with metabolic syndrome living in a highly-polluted megacity in Asia.
Both personal and city-wide ambient levels of BC (as well as PM2.5 measured at ambient level) over the prior 1-5 days are linked to higher BP levels and insulin resistance.
In our study, a subset of subjects underwent measurements during the severe air pollution haze event over Eastern China in January 2013 (PM2.5 >500 mg/m3).
What Is Relevant?
The association between personal-level exposure to BC and elevation in BP within a few hours was found in our previous observation. The longitudinal analysis on the sub-acute exposure confirmed and extended the relationships between air pollutants and cardio-metabolic outcomes. The consistency of our findings across seasons, a range of doses, and study designs render our work much more relevant.
Summary
Our results suggest sub-acute exposures to particulate air pollutants are associated with adverse changes in BP and insulin sensitivity among at-risk individuals living in a region chronically suffering from very high ambient levels.
ACKNOWLEDGMENTS
We would like to thank the staff of PUMC medical college for their help in this study.
SOURCES OF FUNDING
This study was supported by the National Institute of Environmental Health Sciences grants R01ES017290, R01ES015146 and R01ES019616, Ministry of Science and Technology of the People’s Republic of China grant 2012AA02A616, and Beijing Municipal Science and Technology Commission grant Z131107002213176.
Footnotes
CONFLICTS OF INTEREST / DISCLOSURES
No other conflicts of interest have been disclosed.
REFERENCES
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Perier MC, Marijon E, Vernerey D, Empana JP, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. Jama. 2012;307(7):713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 3.Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, Newby DE, Mills NL. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382(9897):1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu XB, Su JW, Li XY, Chen G. Short-term effects of particulate matter on stroke attack: meta-regression and meta-analyses. PloS one. 2014;9(5):e95682. doi: 10.1371/journal.pone.0095682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, Khan S, Atari DO, Jerrett M, Pope CA, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect. 2012;120(5):708–714. doi: 10.1289/ehp.1104049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health. 2013;12(1):43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep. 2010;12(5):291–300. doi: 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- 8.Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61(12):3037–3045. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens. 2009;3(5):332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Brook JR, et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect. 2013;121(7):804–810. doi: 10.1289/ehp.1205958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. European journal of endocrinology / European Federation of Endocrine Societies. 2014;171(5):R173–182. doi: 10.1530/EJE-14-0365. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Kopp A, et al. Spatial association between ambient fine particulate matter and incident hypertension. Circulation. 2014;129(5):562–569. doi: 10.1161/CIRCULATIONAHA.113.003532. [DOI] [PubMed] [Google Scholar]
- 13.Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of exposure to PM2.5 on blood pressure: a systematic review and meta-analysis. Journal of hypertension. 2014;32(11):2130–2140. doi: 10.1097/HJH.0000000000000342. discussion 2141. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X, Sun Z, Ruan Y, Yan J, Mukherjee B, Yang F, Duan F, Sun L, Liang R, Lian H, et al. Personal black carbon exposure influences ambulatory blood pressure: air pollution and cardiometabolic disease (AIRCMD-China) study. Hypertension. 2014;63(4):871–877. doi: 10.1161/HYPERTENSIONAHA.113.02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Bai Y, Xu X, Sun L, Wang A, Wang TY, Maurya SK, Periasamy M, Morishita M, Harkema J, et al. Exaggerated effects of particulate matter air pollution in genetic type II diabetes mellitus. Particle and fibre toxicology. 2014;11:27. doi: 10.1186/1743-8977-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Fonken LK, Wang A, Maiseyeu A, Bai Y, Wang TY, Maurya S, Ko YA, Periasamy M, Dvonch T, et al. Central IKKbeta inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part Fibre Toxicol. 2014;11:53. doi: 10.1186/s12989-014-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Xu X, Bai Y, Wang TY, Rao X, Wang A, Sun L, Ying Z, Gushchina L, Maiseyeu A, et al. Air pollution-mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environmental health perspectives. 2014;122(1):17–26. doi: 10.1289/ehp.1306841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, Zhao J, Liu D, Morishita M, Sun Q, et al. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect. 2014;122(1):79–86. doi: 10.1289/ehp.1307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Z, Xu X, Zhang X, Wang A, Zhang C, Huttemann M, Grossman LI, Chen LC, Rajagopalan S, Sun Q, et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol. 2013;58(1):148–154. doi: 10.1016/j.jhep.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Donkelaar A, Martin RV, Brauer M, Boys BL. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ Health Perspect. 2015;123(2):135–143. doi: 10.1289/ehp.1408646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310(9):959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 22.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Burnett RT, Pope CA, 3rd, Ezzati M, Olives C, Lim SS, Mehta S, Shin HH, Singh G, Hubbell B, Brauer M, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014;122(4):397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pope CA, 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ, Pope CA. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Mukherjee B, Brook RD, Gatts GA, Yang F, Sun Q, Brook JR, Fan Z, Rajagopalan S. Air-Pollution and Cardiometabolic Diseases (AIRCMD): a prospective study investigating the impact of air pollution exposure and propensity for type II diabetes. Sci Total Environ. 2013;448:72–78. doi: 10.1016/j.scitotenv.2012.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beijing air pollution off the charts— NYTimes.com. NYTimes.com http://www.nytimes.com/2013/01/13/science/earth/beijing-air-pollution-off-the-charts.html?_r<0.
- 27.Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR, et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA. 2012;307(19):2068–2078. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brook RD, Xu X, Bard RL, Dvonch JT, Morishita M, Kaciroti N, Sun Q, Harkema J, Rajagopalan S. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ. 2013;448:66–71. doi: 10.1016/j.scitotenv.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JH, Hong YC. GSTM1, GSTT1, and GSTP1 polymorphisms and associations between air pollutants and markers of insulin resistance in elderly Koreans. Environ Health Perspect. 2012;120(10):1378–1384. doi: 10.1289/ehp.1104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203(1):311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Tamayo T, Rathmann W, Kramer U, Sugiri D, Grabert M, Holl RW. Is particle pollution in outdoor air associated with metabolic control in type 2 diabetes? PLoS One. 2014;9(3):e91639. doi: 10.1371/journal.pone.0091639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiering E, Cyrys J, Kratzsch J, Meisinger C, Hoffmann B, Berdel D, von Berg A, Koletzko S, Bauer CP, Heinrich J. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia. 2013;56(8):1696–1704. doi: 10.1007/s00125-013-2925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleisch AF, Gold DR, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, Coull BA, Zanobetti A, Gillman MW, et al. Air pollution exposure and abnormal glucose tolerance during pregnancy: the project Viva cohort. Environ Health Perspect. 2014;122(4):378–383. doi: 10.1289/ehp.1307065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Su L, Cai X, Mai W, Wang S, Hu Y, Wu Y, Tang H, Xu D. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. Am Heart J. 2014;167(2):160–168. e161. doi: 10.1016/j.ahj.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59(7):635–643. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.