Abstract
Background
Small studies have reported women to have worse outcomes and more adverse events after implantation of mechanical circulatory support device compared to men. To further evaluate sex differences in outcome we utilized the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS).
Method and Results
There were 401 women (pulsatile devices=78) and 1535 men (pulsatile devices=402) from 89 institutions who were prospectively entered into the INTERMACS database for primary implantation of a left ventricular assist device (LVAD) between June 23, 2006 until March 31, 2010. Extensive pre-implant data and outcome data was collected on all patients. With a mean follow-up of 7 months, 67 females (17%) died and 250 males (16%) died. There was no statistically significant sex difference in mortality for either pulsatile (p=0.82) or continuous-flow devices (p=0.95) in adjusted and unadjusted models. There were also no statistically significant sex differences with time to first infection, bleeding or device malfunction. However, female gender was associated with an increase hazard of first neurologic event (adjusted hazard ratio 1.44 [1.05-1.96], p=0.020).
Conclusions
There were no significant sex differences in mortality, time to first infection, bleeding or device malfunction with either pulsatile or continuous-flow LVADs. However, women had an increased risk of first neurologic event. For urgent/emergent mechanical support, the benefit of LVAD support likely outweighs the risk but it remains less clear for women undergoing elective LVAD implantation.
Keywords: Heart failure, heart-assist device, sex, prognosis
Sex specific mechanical circulatory support outcome data remains limited. Several studies have suggested an increase mortality and/or higher likelihood of bleeding and neurologic events in women compared with men after implantation of mechanical circulatory support devices (MCSD)1-6. However, these studies often had few women participants, focused mainly on use of pulsatile-flow devices, and provided limited information regarding severity of illness prior to mechanical circulatory support. In one single center study in which women had a worse prognosis, survival post mechanical circulatory support correlated best with the degree of medical severity prior to ventricular assist device implantation and not sex6.
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) provides a unique opportunity to evaluate sex differences in outcome and adverse events after implantation of mechanical support. Since the first annual report in 20085, the number of women in the database has increased from 83 to 401 and the number of men has increased from 304 to 1535. There is also sufficient data to compare pulsatile with continuous-flow devices. Our objective was to determine if there are any sex differences in outcome and adverse events after primary implantation of left ventricular assist device (LVADs).
Methods
INTERMACS Registry
The INTERMACS is a national registry for patients implanted with a Food and Drug Administration (FDA) approved mechanical circulatory support device designed to support patients for long periods of time. It is an audited registry and adverse events are reviewed by a Medical Events Committee for reasonableness and internal consistency. Registry participation is mandatory for all Centers for Medicare and Medicaid Services (CMS) approved “destination” MCSD implantation centers. The registry was created and has been maintained by the University of Alabama INTERMACS Data Coordinating Center since June 2005, and is supported by the National Heart, Lung and Blood Institute, FDA and CMS. Participating centers are required to obtain Institutional Review Board approval before initiating data collection and data is transmitted from sites using a Web-based system to a secure server provided by the United Network for Organ Sharing (UNOS).
Patient Population
From June 23, 2006 until March 31, 2010, 1936 adult patients (21% female) from 89 institutions were prospectively entered into the INTERMACS database for primary implantation of an intracorporeal LVAD. Patients receiving primary right ventricular assist devices (n=0), biventricular assist device (n=121), total artificial hearts (n=73), and extracorporeal LVADS (n=86) were excluded.
Outcomes and Adverse Events
The primary outcome was all-cause mortality with data censored at time of transplantation or device removal after recovery of myocardial function. Secondary outcomes were time to first major bleed, device malfunction, major infection, or neurologic dysfunction. These adverse events were prospectively defined and contained in the operations manual. Briefly, suspected internal or external major bleeding was defined as bleeding that resulted in death, re-operation, hospitalization, or blood transfusion. Device malfunction was defined as failure of one of the parts of the mechanical circulatory support device. Major infection was defined clinically with symptoms and/or signs warranting the use of antibiotics for treatment. Neurologic dysfunction was defined as a transient ischemic attack or cerebrovascular accident (hemorrhagic or ischemic). Participating registry centers were required to update the database within 30 days of an event except for device malfunction, which was to be entered within 72 hours of occurrence (www.INTERMACS.org).
Statistical Analysis
Sex specific baseline characteristics were reported for adult patients who underwent primary implantation of an intracorporeal LVAD. Continuous variables were expressed as means except for skewed variables which were expressed as medians. Categorical variables were expressed as frequencies. Discrete variables were compared using the chi-square test of significance unless the frequencies were quite small in which case Fisher’s exact test was used. Median values were compared with a nonparametric test (median two sample test). Otherwise continuous variables were compared using the t-test. For variables with ≤12% data omitted, we used mean value imputation to fill in the missing data. We excluded the following variables which were missing >12% data: college education, total cholesterol, pre-albumin, cardiac output, cardiac index, right atrial pressure, pulmonary capillary wedge pressure, pulmonary artery pressures, pulmonary vascular resistance, left ventricular ejection fraction <20%, severe right ventricular dysfunction, and left ventricular end diastolic diameter. Sex specific survival analysis was performed for pulsatile and continuous-flow LVADs using the Kaplan-Meier method with censoring for heart transplantation or cardiac recovery. Cox proportional hazard models were created to assess the association between sex and outcome including mortality, bleeding, infection, device malfunction, and neurological event. Two models were created for each outcome: Model 1 adjusted for sex and Model 2 adjusted for sex, age, bilirubin, ascites, INTERMACS level, INR, creatinine, platelets, AST, hemoglobin, BUN, albumin, inotropes, and type of LVAD support. Variables included in regression models were selected for the known significance based on prior literature7-10. We evaluated the proportional hazards assumption and found no obvious violations. A p ≤ 0.05 was considered statistically significant. All analyses were performed using SAS 8.2 software (SAS Institute, Cary, NC). Competing outcome figures were calculated with an internally written SAS macro that implements the Nelson method for estimating the distribution of cumulative, repeating events11.
Results
Patient Characteristics
There were 401 women (78 pulsatile devices) and 1535 men (402 pulsatile devices) who underwent primary implantation of an intracorporeal LVAD. Baseline characteristics are shown in Table 1. Women, as compared to men, were younger, had smaller body surface area (BSA), more history of cancer, less alcohol abuse, less coronary artery bypass graft surgery, and lower serum BUN, creatinine, hemoglobin, and total bilirubin. There were no clinically significant sex differences in the following baseline characteristics: diabetes mellitus, chronic obstructive lung disease, peripheral vascular disease, current tobacco usage, implantable cardioverter defibrillators, usage of LVAD as a bridge to transplantation, systolic blood pressure, serum sodium, and liver function tests. More women were listed in critical cardiogenic shock with no sex differences in use of intra-aortic balloon pumps, inotrope dependency, and ventilator support. There were also no sex differences in concurrent surgery during implantation of LVAD.
Table 1.
Gender Differences: Baseline Characteristics (adult primary intracorporeal LVADs, n = 1936)
| Pre-Implant | |||
|---|---|---|---|
| Demographics | Male N= 1535 |
Female N= 401 |
p-value |
| White, n (%) | 71% | 59% | <0.0001 |
| Hispanic, n (%) | 7% | 6% | 0.57 |
| Age (yrs), mean (std dev) | 54.1 (12.2) | 50.9 (12.8) | <0.0001 |
| Married, n (%) | 67% | 47% | <0.0001 |
| BMI* (kg per meter2), mean (std dev) | 28.7 (6.6) | 29.0 (7.4) | 0.46 |
| BSA† (m2), mean (std dev) | 2.14 (0.30) | 1.91 (0.28) | <0.0001 |
| Clinical | |||
| Diabetes, n (%) | 39% | 35% | 0.14 |
| Inotropes, n (%) | 83% | 85% | 0.30 |
| Pre-COPD, n (%) | 13% | 11% | 0.37 |
| INTERMACS Patient Profile Level 1: Critical Cardiogenic Shock, n (%) |
19% | 26% | 0.01 |
| INTERMACS Patient Profile Level 2: Progressive Decline, n (%) |
45% | 43% | 0.45 |
| Bridge to Transplant: Listed, n (%) | 43% | 48% | 0.09 |
| Bridge to Transplant: Likely to be listed, n (%) |
30% | 27% | 0.36 |
| Bridge to Transplant: Moderately likely to belisted, n (%) |
10% | 9% | 0.48 |
| Bridge to Transplant: Unlikely to be listed, n (%) |
4% | 5% | 0.22 |
| Destination Therapy, n (%) | 11% | 7% | 0.01 |
| NYHA‡ = 4, n (%) | 80% | 82% | 0.29 |
| Diagnosis CAD§, n (%) | 10% | 8% | 0.24 |
| CVA§, n (%) | 7% | 8% | 0.68 |
| TIA∥, n (%) | 4% | 3% | 0.29 |
| Cancer, n (%) | 5% | 12% | <0.0001 |
| Current Smoker, n (%) | 13% | 13% | 0.90 |
| Current Drug Abuse, n (%) | 3% | 1% | 0.15 |
| Alcohol Abuse, n (%) | 19% | 8% | <0.0001 |
| Blood Type O, n (%) | 50% | 51% | 0.74 |
| Rheumatologic Disease, n (%) | 3% | 5% | 0.16 |
| Hepatitis B, n (%) | 2% | 2% | 0.75 |
| Hepatitis C, n (%) | 2% | 2% | 0.45 |
| Dialysis, n (%) | 3% | 2% | 0.42 |
| History of CABG**, n (%) | 22% | 11% | <0.0001 |
| History of Valve Surgery, n (%) | 6% | 9% | 0.10 |
| ICD††, n (%) | 78% | 76% | 0.37 |
| IABP‡‡, n (%) | 34% | 35% | 0.90 |
| Ventilator, n (%) | 11% | 12% | 0.33 |
| Peripheral vascular disease, n (%) | 7% | 4% | 0.11 |
| Carotid artery disease, n (%) | 10% | 9% | 0.36 |
| B-Blockers, n (%) | 76% | 70% | 0.03 |
| ACE Inhibitors§§, n (%) | 54% | 52% | 0.34 |
| Laboratory | |||
| Sodium (mmol/L), mean (std dev) | 134.2 (5.2) | 134.8 (5.0) | 0.04 |
| Creatinine (mg/dL), mean (std dev) | 1.55 (0.87) | 1.26 (0.67) | <0.0001 |
| BUN ∥ ∥ (mg/dL), mean (std dev) | 32.2 (20.6) | 25.7 (15.3) | <0.0001 |
| Total bilirubin (mg/dL), mean (std dev) | 1.55 (1.66) | 1.28 (1.31) | 0.005 |
| Cholesterol (mg/dL), mean (std dev) | 124 (42.5) | 131.3 (41.6) | 0.05 |
| INR ## (international units), mean (std dev) | 1.38 (0.5) | 1.3 (0.4) | 0.01 |
| Hemoglobin (mg/dL), mean (std dev) | 11.4 (2.0) | 10.5 (1.7) | <0.0001 |
| Platelet (K/uL), mean (std dev) | 202 (97) | 217 (97) | 0.002 |
| Protein C (%), mean (std dev) | 89 (34) | 84 (39) | 0.64 |
| Protein S (%), mean (std dev) | 84 (35) | 81 (29) | 0.77 |
| CRP*** (mg/L), median (IQR†††) | 6.0 (1.4-18.1) | 4.7 (2.0-17.6) | 0.47 |
| BNP ‡‡‡ (pg/ml), mean (std dev) | 1244 (1093) | 1311 (1186) | 0.48 |
| SGOT/AST (u/L), median (IQR) | 32 (23-53) | 31 (21-46) | 0.13 |
| SGPT/ALT (u/L), median (IQR) | 34 (21-63) | 28 (18-45) | <0.0001 |
| WBC §§§ (K/uL), mean (std dev) | 9.06 (3.8) | 8.87 (3.93) | 0.39 |
| Hemodynamics | |||
| Systolic blood pressure (mmHg), mean (std dev) |
101 (16) | 100 (17) | 0.33 |
| Diastolic blood pressure (mmHg), mean (std dev) |
62 (12) | 61 (12) | 0.06 |
| Mitral Regurg (Moderate/Severe), n (%) | 58% | 63% | 0.17 |
| Tricuspid Regurg (Moderate/Severe), n (%) |
44% | 54% | 0.004 |
| Aortic Regurg (Moderate/Severe), n (%) | 6% | 3% | 0.05 |
| Operative | |||
| Concommitant surgery, n (%) | 37% | 34% | 0.38 |
| ∥ ∥ ∥ LV Continuous Flow device, n (%) | 74% | 81% | 0.01 |
| Failure to wean, n (%) | 1% | 2% | 0.30 |
| Implant after cardiac surgery, n (%) | 2% | 3% | 0.56 |
BMI=body mass index;
BSA=body surface area;
NYHA=New York Heart Association;
CAD=coronary artery disease;
CVA=cerebral vascular accident;
TIA=transient ischemic attack;
CABG= coronary artery bypass grafts;
ICD=implantable cardioverter defibrillator;
IABP=intra-aortic balloon pump;
ACE inhibitors=angiotensin converting enzyme inhibitors;
BUN=blood urea nitrogen;
INR=international normalized ratio;
CRP=c-reactive protein;
IQR=interquartile range;
BNP= brain natriuretic peptide;
WBC=white blood cell count;
LV=left ventricular
Outcomes
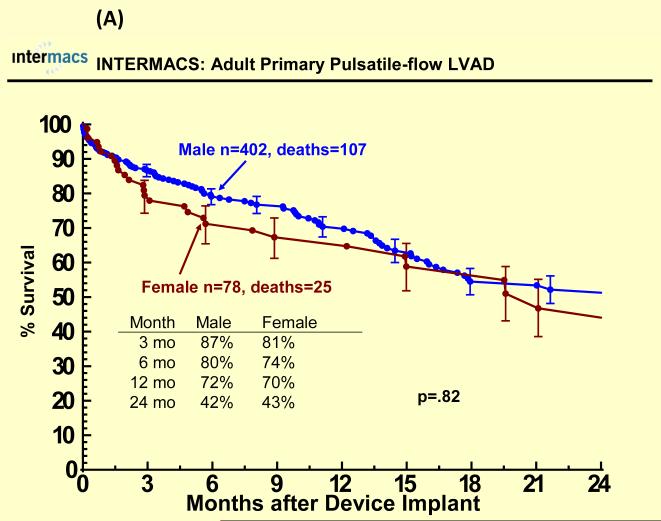
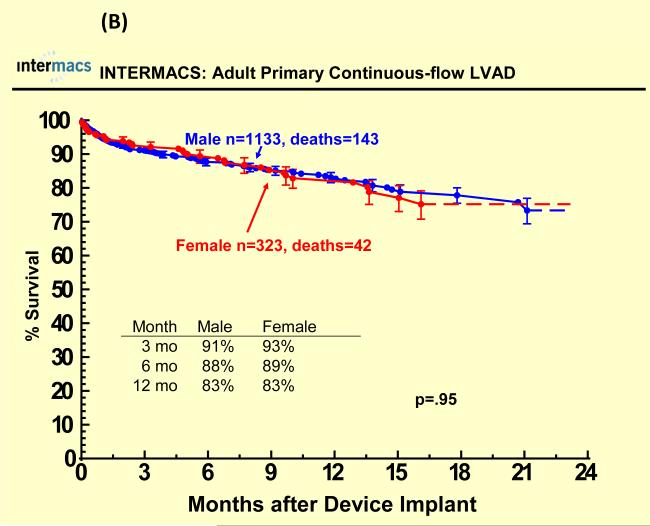
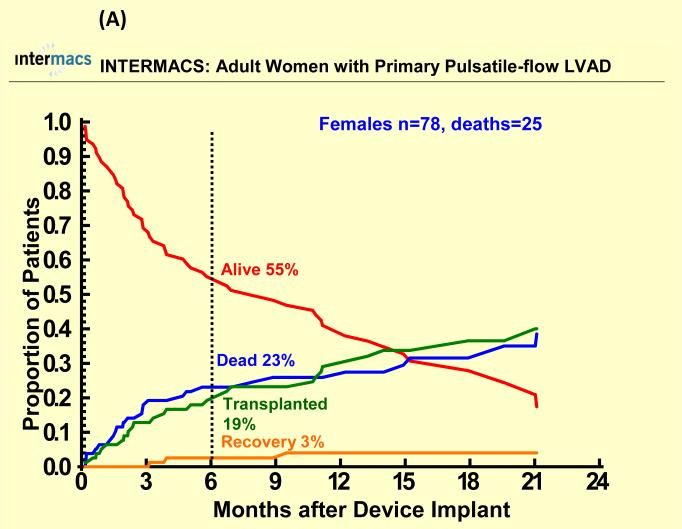
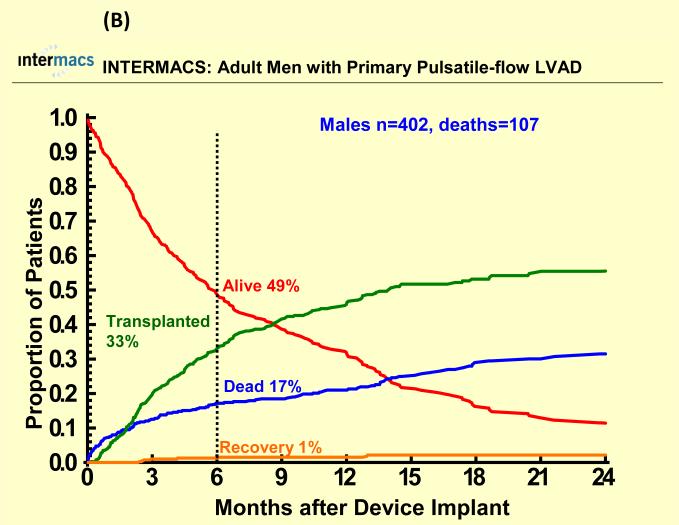
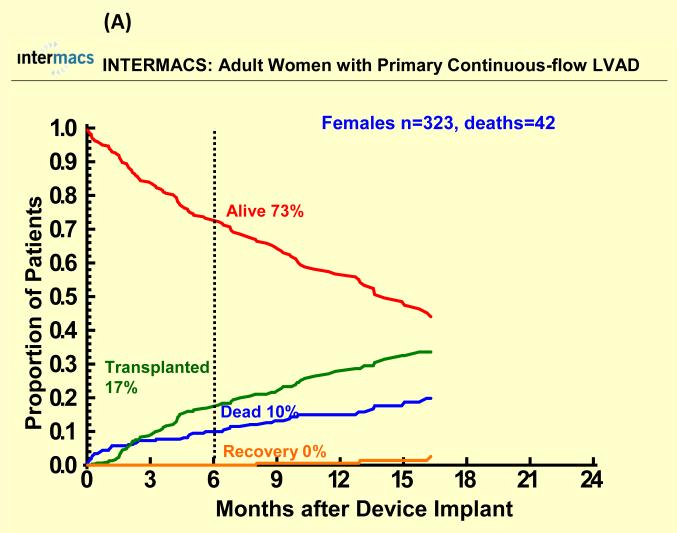
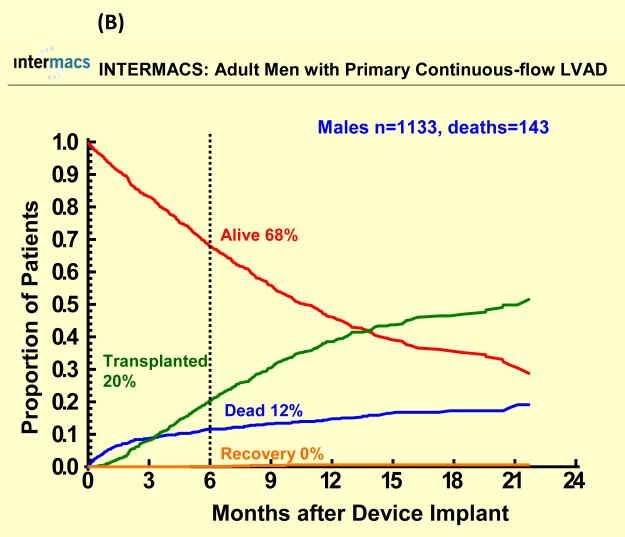
With a mean follow-up of 7 months, 67 (17%) females died (25 with pulsatile-flow devices) and 250 (16%) males died (107 with pulsatile-flow devices). Cause of death was similar among groups except for a higher percentage of cardiovascular deaths and a lower percentage of neurologic deaths among men compared to women for both pulsatile and continuous flow devices (Table 2). There were 622 patients who underwent heart transplantation (95 women, 527 men). Few patients recovered and had the LVAD removed (< 2%). One year survival after primary LVAD implantation was 70% for women and 72% for men with pulsatile-flow devices and 83% for women and men with continuous flow devices There was no statistically significant sex difference in all-cause mortality for pulsatile or continuous-flow devices in both unadjusted (Figure 1 A and B) and unadjusted analyses (Table 2). However, survival was better for continuous-flow devices compared with pulsatile-flow devices for both women and men. Competing outcome data for pulsatile-flow intracorporeal LVADs (see Figure 2A and B) was notable for women and men with similar 6 months survival. However men with a pulsatile-flow LVAD underwent heart transplantation more frequently than women. For continuous-flow intracorporeal LVADs women and men had similar competing outcome data as shown in Figure 3A and B.
Table 2.
Gender Differences in Cause of Death
| Pulsatile (N=132) | Continuous (N=185) | |||
|---|---|---|---|---|
| Primary Cause of Death |
Male (N=107) | Female (N=25) | Male (N=143) | Female (N=42) |
| Arterial Embolism | 1 (1 %) | 0 (0 %) | 2 (1 %) | 0 (0 %) |
| Cancer | 1 (1 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) |
| Cardiovascular | 11 (10 %) | 1 (4 %) | 15 (10 %) | 2 (5 %) |
| Device Malfunction | 6 (6 %) | 1 (4 %) | 1 (1 %) | 0 (0 %) |
| Infection | 9 (8%) | 3 (12 %) | 19 (13 %) | 3 (7 %) |
| Liver Failure | 2 (2 %) | 0 (0 %) | 4 (3 %) | 0 (0 %) |
| Other Chronic Illness | 2 (2 %) | 0 (0 %) | 1 (1 %) | 1 (2 %) |
| Pancreatitis | 1 (1 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) |
| Renal Failure | 2 (2 %) | 0 (0 %) | 2 (1%) | 3 (7 %) |
| RV* Failure | 8 (7 %) | 1 (4 %) | 6 (4 %) | 5 (12 %) |
| Sudden Death | 2 (2 %) | 0 (0 %) | 5 (3 %) | 2 (5 %) |
| VT†/VF ‡ | 3 (3%) | 0 (0 %) | 1 (1 %) | 0 (0 %) |
| CNS § | 7 (7 %) | 3 (12 %) | 12 (8 %) | 6 (14 %) |
| Fluid/Electrolyte Disorder | 0 (0 %) | 0 (0 %) | 0 (0 %) | 1 (2 %) |
| Hemorrhage | 10 (9 %) | 1 (4 %) | 12 (8 %) | 3 (7 %) |
| Hematologic | 0 (0 %) | 0 (0 %) | 1 (1 %) | 0 (0 %) |
| Trauma/Accident | 0 (0 %) | 0 (0 %) | 0 (0 %) | 2 (5 %) |
| Pulmonary | 6 (6 %) | 1 (4 %) | 9 (6 %) | 0 (0 %) |
| Suicide | 0 (0 %) | 0 (0 %) | 1 (1 %) | 0 (0 %) |
| Unknown | 7 (7 %) | 3 (12 %) | 15 (10 %) | 5 (12 %) |
| Other | 29 (27 %) | 11 (44 %) | 37 (26 %) | 9 (21 %) |
RV=right ventricular ;
VT=ventricular tachycardia,
VF=ventricular fibrillation,
CNS=central nervous system
Figure 1.
Sex specific Kaplan-Meier plots for (A) pulsatile and (B) continuous-flow intracorporeal left ventricular assist devices. Panel A shows the sex specific Kaplan-Meier analysis of survival for the 480 adult patients who underwent implantation of a primary pulsatile-flow intracorporeal LVAD from June 23, 2006 until March 31, 2010, with data censored for heart transplantation and recovery of ventricular function allowing device explant. Panel B shows the sex specific Kaplan-Meier analysis of survival for the 1456 adult patients who underwent implantation of a primary continuous-flow intracorporeal LVAD from June 23, 2006 until March 31, 2010, with data censored for heart transplantation and recovery of ventricular function.
Figure 2.
Competing outcomes are shown for (A) women and (B) men with primary pulsatile-flow intracorporeal left ventricular assist devices. Panel A shows all outcomes over time for the 78 women who received a primary pulsatile-flow intracorporeal LVAD from June 23, 2006 until March 31, 2010. Panel B shows all outcomes over time for the 402 men who received a primary pulsatile-flow intracorporeal LVAD from June 23, 2006 until March 31, 2010.
Figure 3.
Competing outcomes are shown for (A) women and (B) men with primary continuous-flow intracorporeal left ventricular assist devices. Panel A shows all outcomes over time for the 323 women who received a primary continuous-flow intracorporeal LVAD from June 23, 2006 until March 31, 2010. Panel B shows all outcomes over time for the 1133 men who received a primary continuous-flow intracorporeal LVAD from June 23, 2006 until March 31, 2010.
Adverse Events
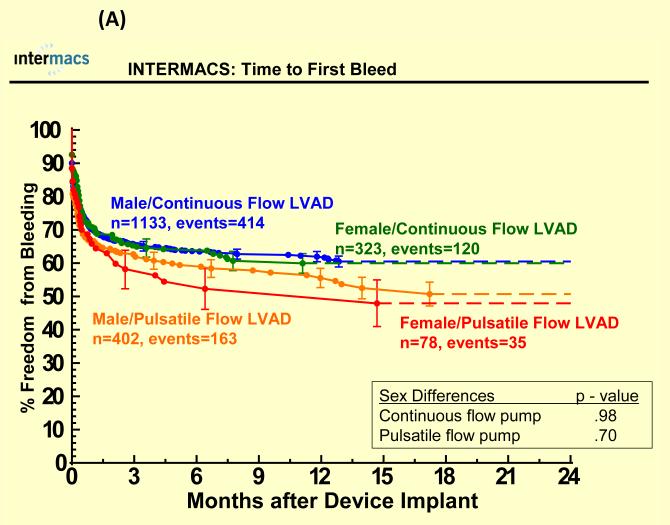
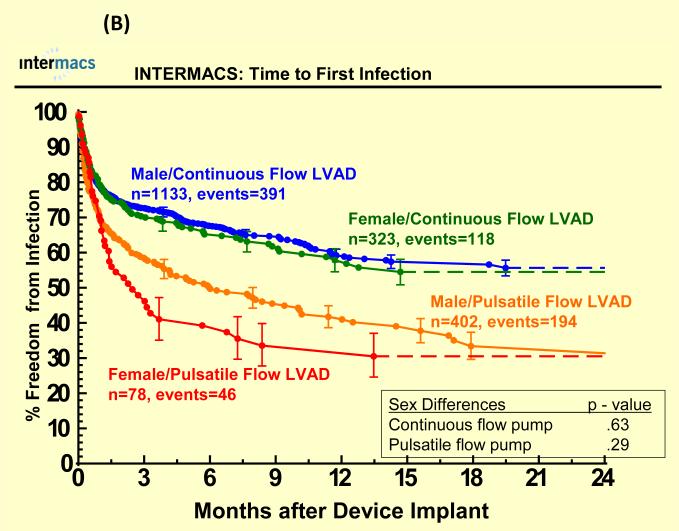
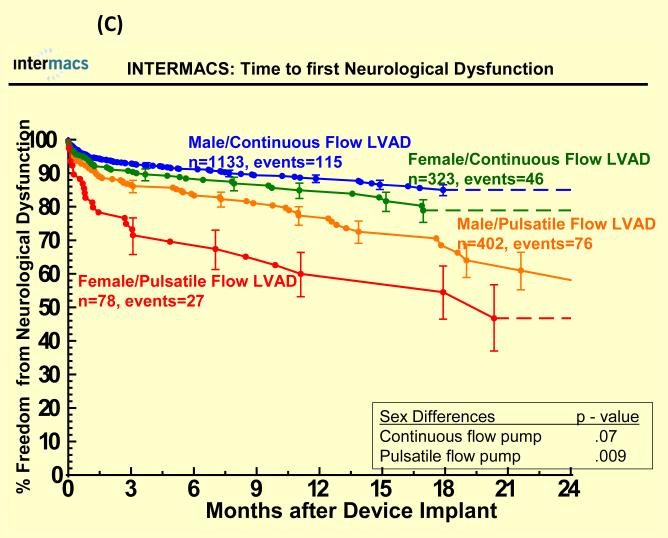
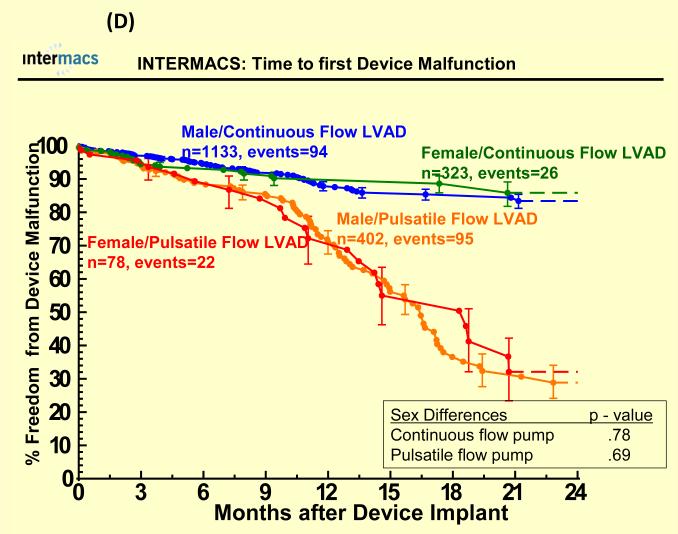
Adverse events were more common in pulsatile-flow LVADs than with continuous-flow LVADs (see Figure 4). There were no statistically significant sex differences in time to first infection, bleeding or device malfunction with use of continuous or pulsatile-flow LVADs (see Figure 4 A, B, D) even after multivariate analyses (Table 3). However, women compared to men had shorter time to first neurologic event with pulsatile-flow LVADs and a trend toward shorter time to first neurologic event with continuous-flow LVADs (see Figure 4 C). Multivariate analysis adjusting for many risk factors including type of device, prior CVA/TIA, carotid disease, and BSA revealed female gender to be associated with an increase hazard of neurologic events (Table 3). There was no interaction between gender and device type for any of the adverse events, suggesting that the gender differences in neurologic event after multivariate analysis were not dependent on type of LVAD implanted.
Figure 4.
Time to first (A) major bleed, (B) infection, (C) neurologic dysfunction, and (D) device malfunction for women and men with primary intracorporeal left ventricular assist devices. Panel A shows time to first major bleed in the 1936 patients who received a primary intracorporeal LVAD from June 23, 2006 until March 31, 2010. Panel B shows time to first major infection in the 1936 patients who received a primary intracorporeal LVAD from June 23, 2006 until March 31, 2010. Panel C shows time to first neurologic event in the 1936 patients who received a primary intracorporeal LVAD from June 23, 2006 until March 31, 2010. Panel D shows time to first device malfunction in the 1936 patients who received a primary intracorporeal LVAD from June 23, 2006 until March 31, 2010.
Table 3.
Female Gender and Outcome After Primary LVAD Implantation: Cox Proportional Hazards Analyses.
| Model | Hazard Ratio (95%CI) |
P |
|---|---|---|
| Death | ||
| Unadjusted | 0.97 (0.74-1.27) | 0.810 |
| Multivariable adjusted | 1.17 (0.88-1.55) | 0.270 |
| First Bleeding | ||
| Unadjusted | 1.01 (0.84-1.20) | 0.960 |
| Multivariable adjusted | 1.03 (0.85-1.25) | 0.740 |
| First Infection | ||
| Unadjusted | 1.06 (0.89-1.27) | 0.480 |
| Multivariable adjusted | 1.03 (0.85-1.24) | 0.770 |
| First Device Malfunction | ||
| Unadjusted | 0.90 (0.65-1.24) | 0.490 |
| Multivariable adjusted | 0.87 (0.62-1.22) | 0.410 |
| First Neurological Event | ||
| Unadjusted | 1.43 (1.08-1.88) | 0.010 |
| Multivariable adjusted | 1.43 (1.08-1.88) | 0.020 |
CVA=cerebral vascular accident,
TIA=transient ischemic attack,
BSA=body surface area.
Multivariable analysis was adjusted for age, bilirubin, ascites, INTERMACS level, INR, creatinine, platelets, AST, hemoglobin, BUN, albumin, inotropes, and type of LVAD support
Discussion
There was no significant sex difference in mortality with either a pulsatile or continuous-flow LVAD for the 1936 patients (21% female) in the INTERMACS registry and no significant sex differences in time to first bleed, infection or device malfunction. However, women compared to men had shorter time to first neurologic event that was statistically significant in unadjusted and adjusted models. Women and men with continuous-flow LVADs had similar competing outcomes but women compared to men with pulsatile-flow LVADs were less likely to undergo heart transplantation. Few patients recovered left ventricular systolic function after VAD implantation and survival was best for both women and men after implantation of continuous-flow LVADs compared to pulsatile-flow devices.
Our findings regarding the benefit and risks of LVADs in women and men is the largest published analysis to date assessing sex differences. The lack of sex differences in all-cause mortality after LVAD implantation is reassuring and the excellent survival rate for continuous-flow devices is similar to recently published studies12, 13. One year survival after implantation of continuous-flow LVADs was 83% which is better than smaller studies12-15 and comparable to the one year expected survival after heart transplantation in the United States16 making it reasonable as a bridge to transplant for heart failure patients who have failed medical therapy. Adverse events were similar between women and men except for first neurologic events which were more likely in women even after adjusting for type of LVAD, history of carotid disease, CVA/ TIA, BSA, and age. A higher rate of neurologic events in women has been previously reported in smaller studies for the HeartMate II 2, 12. In preliminary studies with the HeartMate II continuous-flow devices, patients (n=10) with a smaller BSA (1.2 m2 −1.5 m2) had a higher incidence of neurologic events than those patients (n=126) with larger BSAs. The cohort with smaller BSA were all women raising concern that the finding may be related to sex since in the aggregate HeartMate II study with 194 patients there were more neurologic events in women (N=44) compared to men (N=150)2. With over 1900 patients in the INTERMACS registry including 401 women, the higher risk for first neurologic event in women after LVAD implantation remains a concern even after adjusting for many variables including type of LVAD and BSA. The cause for sex differences remains unknown. Information regarding INR, von Willebrand factor and anticoagulation therapy at time of neurologic event was not available in our registry but in a retrospective HeartMate II LVAD analysis that showed women to be at higher risk for stroke, there was no sex difference in mean serum INR, partial thromboplastin time, platelet count, or average systolic blood pressure at time of neurologic event12. Further research is necessary to determine whether sex differences in neurologic events are due to sex differences in the pharmcokinetics and pharmacodynamics of antiplatelet and anticoagulation therapy or due to sex differences in thrombotic risk after implantation of a LVAD. Given the the higher risk of thromboembolism in women with atrial fibrillation compared to men17 and the sex differences in the pharmacokinetics and pharmacodynamics of many cardiovascular drugs18-21, both hypotheses are plausible.
Overall, there was better survival and fewer adverse events for patients with primary implantation of continuous-flow devices compared with pulsatile-flow devices. This is very important especially for women in light of FDA criticism that the HeartMate II was approved with preliminary data showing a higher rate of stroke in women compared to men and a trend to a higher rate of infection and bleeding22. Although women have a higher risk of first neurologic events after LVAD implantation, there were fewer adverse events and better survival with continuous-flow devices compared with pulsatile-flow devices.
Competing outcomes were similar for women and men who underwent implantation of continuous-flow LVAD. However, women who had pulsatile-flow devices were less likely than men to undergo heart transplantation despite similar pre-implant device strategy (listed for bridge to transplant 43% male, 48% female). The reason for this remains unclear. The INTERMACS registry did not track cause for inactivation (UNOS status 7), date of reactivation, or important determinants of transplantation such as degree of sensitization (i.e. high Panel of Reactive Antibodies). Given the higher percentage of male heart donors in the United States16, sex differences in rate of heart transplantation may simply reflect the higher likelihood that a male donor heart would be best suited, based on size, to fit in a male heart failure recipient and the lower likelihood that a male recipient would be sensitized.
Limitations of this study include the inability of our registry to confirm death/heart transplantation with external sources like the Social Security Death Index and UNOS database since patient identification was not collected in order to maintain patient confidentiality. Compliance with data entry was excellent but certain data such as central hemodynamics, total cholesterol, estimates of severe right ventricular dysfunction, and left ventricular end diastolic diameter were missing in >40% of the patients and therefore not utilized in this study. Other information not obtained included data regarding inactivation on the heart transplant wait list, date of reactivation, amount of anticoagulation/antiplatelet therapy, quantification of “reactive antibodies,” and von Willebrand factor-dependent ristocetin-induced platelet aggregation which may be in the future collected and help us better understand other sex differences. Our data was also limited to 89 centers, which is not all inconclusive but does represent the majority of U.S. cardiac surgical centers implanting mechanical circulatory support. Registries are also subject to human error with respect to data entry and for quality control, however INTERMACS did have an operational auditing program.
Conclusion
In summary there were no significant sex differences in mortality with either a pulsatile or continuous-flow device, but women had a shorter time to first neurologic event in both unadjusted and adjusted analyses. Further research is needed to better understand the mechanisms underlying these sex differences.
Acknowledgments
Funding Sources: Supported by American Heart Association Scientist Development Grant 0730307N(EH), National Heart Lung and Blood Institute Contract HHSN268201100025C (DCN, SLM, KLU, JBY), and the Cleveland Clinic Kaufman Center for Heart Failure (EMH, DS, JBY)
Kathleen Grady receives NHLBI funding for the REVIVE-IT trial.
Footnotes
Conflict of Interest Disclosures: David Naftel is a consultant to Berlin Hearts, Inc. and Syncardia, Inc.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [Last accessed on February 15, 2011];Intermacs: Adverse event rates in patients receiving pulsatile vad’s: Incidence and composition change dramatically 3 months after implant. Available at http://www.Uab.Edu/ctsresearch/intermacs/document%20library/global%20ae%20rates%20after%20mcsd%20implant%20very%20%20final%20versionishlt2009.Ppt.
- 2. [Last accessed February 15, 2011];Summary of safety and effectiveness data. Pma 060040. Thoratec heartmate ii left ventricular assist system (lvad) 2008 Apr; Available at http://www.Accessdata.Fda.Gov/cdrh_docs/pdf6/p060040b.Pdf.
- 3. [Last accessed February 15, 2011];Intermacs: Early neurological adverse events (nae) after pulsatile vad implantation in 455 patients: Incidence, severity, and outcome. Available at http://www.Uab.Edu/ctsresearch/intermacs/document%20library/ae%20neurological%20final%2 0presentation.Ppt.
- 4.Hernandez AF, Grab JD, Gammie JS, O’Brien SM, Hammill BG, Rogers JG, Camacho MT, Dullum MK, Ferguson TB, Peterson ED. A decade of short-term outcomes in post cardiac surgery ventricular assist device implantation: Data from the society of thoracic surgeons’ national cardiac database. Circulation. 2007;116:606–612. doi: 10.1161/CIRCULATIONAHA.106.666289. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Stevenson LW, Kormos RL, Pagani FD, Miller MA, Ulisney K, Young JB. Intermacs database for durable devices for circulatory support: First annual report. J Heart Lung Transplant. 2008;27:1065–1072. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Morgan JA, Weinberg AD, Hollingsworth KW, Flannery MR, Oz MC, Naka Y. Effect of gender on bridging to transplantation and posttransplantation survival in patients with left ventricular assist devices. J Thorac Cardiovasc Surg. 2004;127:1193–1195. doi: 10.1016/s0022-5223(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 7.Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Usefulness of the intermacs scale to predict outcomes after mechanical assist device implantation. J Heart Lung Transplant. 2009;28:827–833. doi: 10.1016/j.healun.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Holman WL, Kormos RL, Naftel DC, Miller MA, Pagani FD, Blume E, Cleeton T, Koenig SC, Edwards L, Kirklin JK. Predictors of death and transplant in patients with a mechanical circulatory support device: A multi-institutional study. J Heart Lung Transplant. 2009;28:44–50. doi: 10.1016/j.healun.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Hunt SA. Mechanical circulatory support: New data, old problems. Circulation. 2007;116:461–462. doi: 10.1161/CIRCULATIONAHA.107.715300. [DOI] [PubMed] [Google Scholar]
- 10.Matthews JC, Pagani FD, Haft JW, Koelling TM, Naftel DC, Aaronson KD. Model for end-stage liver disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation. 2010;121:214–220. doi: 10.1161/CIRCULATIONAHA.108.838656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson W. Graphical analysis of system repair data. J Qual Technol. 1988;20:24–35. [Google Scholar]
- 12.Bogaev RC, Pamboukian SV, Moore SA, Chen L, John R, Boyle AJ, Sundareswaran KS, Farrar DJ, Frazier OH. Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant. 2011;30:515–522. doi: 10.1016/j.healun.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 14.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 15.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, Conte JV, Bogaev RC, MacGillivray TE, Naka Y, Mancini D, Massey HT, Chen L, Klodell CT, Aranda JM, Moazami N, Ewald GA, Farrar DJ, Frazier OH. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 16. [Last accessed february 15, 2011];Organ procurement and transplantation network. Last accessed on november 19, 2010. Available at http://optn.Transplant.Hrsa.Gov/latestdata/step2.Asp?
- 17.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 18.Jochmann N, Stangl K, Garbe E, Baumann G, Stangl V. Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases. Eur Heart J. 2005;26:1585–1595. doi: 10.1093/eurheartj/ehi397. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser J. Gender in the pharmacy: Does it matter? Science. 2005;308:1572. doi: 10.1126/science.308.5728.1572. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther. 2007;82:87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- 21.Stangier J, Stahle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47:47–59. doi: 10.2165/00003088-200847010-00005. [DOI] [PubMed] [Google Scholar]
- 22.Dhruva SS, Redberg RF. The need for sex-specific data prior to food and drug administration approval. J Am Coll Cardiol. 2010;55:261. doi: 10.1016/j.jacc.2009.08.053. author reply 261-262. [DOI] [PubMed] [Google Scholar]