Abstract
Background:
Few reports have described endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) for biliary lesions. In addition, adverse events were not completely examined in previous reports, due to the inclusion of cases in which biliary stents had already been placed. The present study aimed to investigate the diagnostic yield and adverse events of EUS-FNA for biliary lesions as the first-line diagnostic modality for consecutive prospectively registered patients.
Methods:
Inclusion criteria were as follows: (1) patients with suspected cholangiocarcinoma (CCA) based on computed tomography or other imaging modalities; (2) patients who had not previously undergone endoscopic retrograde cholangiopancreatography or EUS-FNA; (3) absence of surgically altered anatomy, such as Roux-en-Y anastomosis or duodenal obstruction caused by tumor invasion, through which an endoscope could not pass; and (4) provision of written informed consent to all procedures associated with the study.
Results:
A total of 47 consecutive patients with suspected CCA were registered to this study. Sensitivity and accuracy were 89% and 87%, respectively. On multivariate analysis, puncture site was the only factor associated with reduced diagnostic yield (hazard ration, 6.879; 95% confidence interval, 1.172–40.374; P = 0.033). Remarkably, no adverse events such as bleeding or bile leakage were associated with EUS-FNA in any of the 47 patients.
Conclusions:
Our results suggest that EUS-FNA can be safely performed for biliary disease without biliary stenting. Furthermore, this procedure may warrant use as the first-line diagnostic method, although our results need to be validated in future prospective studies.
Keywords: biliary cancer, endoscopic ultrasound, endoscopic ultrasound-guided fine needle aspiration, endoscopic retrograde cholangiopancreatography
Introduction
Although cholangiocarcinoma (CCA) is a relatively rare cancer of the biliary tree, its incidence is increasing [Gores, 2003, Shaib and El-Serag, 2004]. Most cases of this disease are diagnosed in an advanced stage, so the mortality rate is also increasing worldwide [Khan et al. 2003]. On the other hand, the cumulative 5-year survival rate of patients who undergo surgical resection at the T1 stage is extremely high [Mizumoto et al. 1993]. The only treatment that offers long-term survival for CCA is thus surgical resection. However, in cases of suspected hepatic hilar CCA, after surgical resection around 13–24% of patients show benign disease, such as primary sclerosing cholangitis, Mirizzi’s syndrome, or other inflammatory lesions [Clayton et al. 2003; Gerhards et al. 2001; Nakayama et al. 1999]. Differentiating CCA from benign conditions is thus critical, because treatment strategies and prognoses differ markedly. In addition, among patients with advanced-stage disease, rapid diagnosis of CCA based on cytohistological evidence impacts potential targeted chemotherapies.
However, despite advances in imaging modalities, differentiating CCA from begin lesions remains challenging. To obtain histological evidence, the gold-standard method is forceps biopsy or bile cytology under endoscopic retrograde cholangiopancreatography (ERCP). Bile juice cytology under ERCP has been reported to offer variable accuracy, ranging from 33% to 80% [Mansfield et al. 1997]. The accuracy of biliary tract biopsy under ERCP has likewise been reported to range from 41% to 92% [Tamada et al. 2002; Domagk et al. 2002]. These results may not be sufficient for CCA patients with poor prognosis. In addition, ERCP is associated with critical adverse events such as acute pancreatitis [Freeman et al. 1996; Loperfido et al. 1998; Wang et al. 2009; Cheng et al. 2006; Testoni et al. 2010; Meister et al. 2011]. Improvements in the accuracy and safety of diagnostic modality are thus still required.
Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) has been the established diagnostic modality to obtain submucosal tissue specimens from diverse types of lesions, particularly pancreatic tumors [Yamao et al. 2005; Hasan and Hawes, 2012]. Compared with ERCP, EUS-FNA offers high diagnostic sensitivity and accuracy for pancreatic tumors, as well as a low risk of adverse events. However, only a few reports have described EUS-FNA for biliary lesions [Frischer-Ravens et al. 2000, 2004; Rösch et al. 2004; Lee et al. 2004; Eloubeidi et al. 2004; Byrne et al. 2004; Meara et al. 2006; DeWitt et al. 2006; Mohamadnejad et al. 2011; Ohshima et al. 2011; Nayar et al. 2011; Weilert et al. 2014]. In addition, most of those reports examined results of EUS-FNA in cases that could not be diagnosed using ERCP. Adverse events such as bile leakage or cholangitis were thus not completely examined because of the inclusion of cases in which biliary stents had already been placed.
The present study examined the diagnostic yield and adverse events of EUS-FNA for biliary lesions as a registration study with consecutive patients.
Materials and methods
Patients
Between April 2012 and October 2013, consecutive patients with suspected CCA seen at our hospital were registered to this study. Inclusion criteria were as follows: (1) patients with suspected CCA based on computed tomography or other imaging modalities; (2) patients who had not previously undergone ERCP or EUS-FNA; (3) absence of surgically altered anatomy, such as Roux-en-Y anastomosis or duodenal obstruction caused by tumor invasion, through which an endoscope could not pass; and (4) provision of written informed consent to all procedures associated with the study.
Final diagnosis was based on the pathological examination of specimens obtained by surgical resection or bile cytology or forceps biopsy using ERCP or EUS-FNA. If signs of malignancy were absent at the end of follow up (disease regression or lack of evidence of disease progression), CCA was ruled out. The final diagnosis was thus benign disorder if the clinical course of the patient was consistent after follow up for at least 1 year.
Diagnostic protocol
All patients underwent EUS, and checked for potential tumor sites. We also measured tumor size in this modality. To obtain histological evidence, EUS-FNA was first performed for rapid diagnosis, and results were evaluated as malignancy or no malignancy by a cytopathologist or cytotechnologist. Next, if the result of EUS-FNA suggested malignancy, we placed a stent after several days. A metallic stent was used in unresectable patients, and a plastic stent or endoscopic nasal biliary drainage tube was used in resectable patients, if necessary. If on-site results of EUS-FNA were benign, suspicious of malignancy, or showing atypical cells, we performed bile cytology or forceps biopsy under ERCP after several days. We prospectively registered these results, including adverse events, clinical data such as obstructive jaundice, cholangitis and final diagnosis in our database.
EUS-FNA technique
We performed EUS at 7.5 MHz using a convex linear-array echoendoscope (UCT260; Olympus Optical, Tokyo, Japan) connected to an ultrasound device (SSD5500; Aloka, Tokyo, Japan) and a 22-gauge FNA needle (Sono Tip Pro Control 19G; Medi-Globe, Rosenheim, Germany; Medico’s Hirata, Osaka, Japan).
First, we visualized tumors of the common bile duct (CBD) or hepatic hilum from the duodenal bulb, and punctured the tumors while using color Doppler ultrasonography to avoid any intervening vessels. Stroke was carefully performed approximately 20 times within the tumor or thick-walled CBD to avoid bile leakage (Figures 1 and 2). Aspirated material was divided up for rapid cytopathological evaluation and histological preparation. The material aspirated was immediately evaluated (Diff-Quik staining) by a cytopathologist or cytotechnologist for rapid on-site evaluation (ROSE), as described. The aspirated material was directly fixed in 10% formalin in a standard specimen bottle, centrifuged, and embedded in paraffin for histological analysis. Sections were visualized using hematoxylin and eosin (HE), as well as immunohistochemical staining, if necessary.
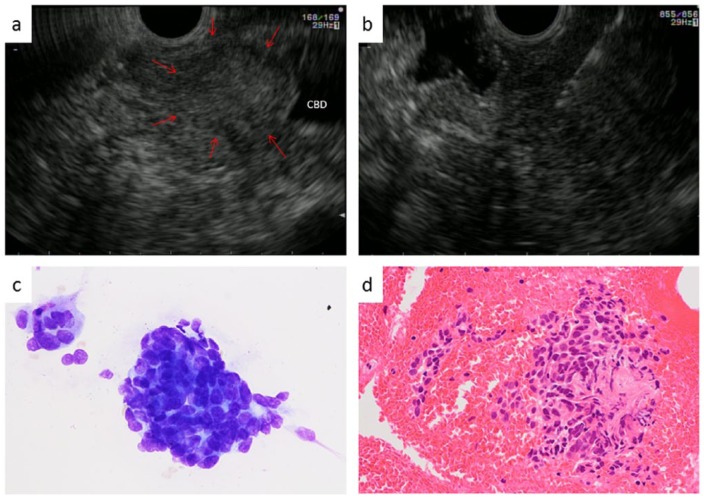
Figure 1.
Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) for the lower bile duct. (a) Mass lesion was seen in the lower bile duct (arrow). (b) Using a 22-guage FNA needle, we puncture the tumor from the duodenum. (c) The cytological result is adenocarcinoma. (d) Histologically, this patient was also diagnosed with cholangiocarcinoma.
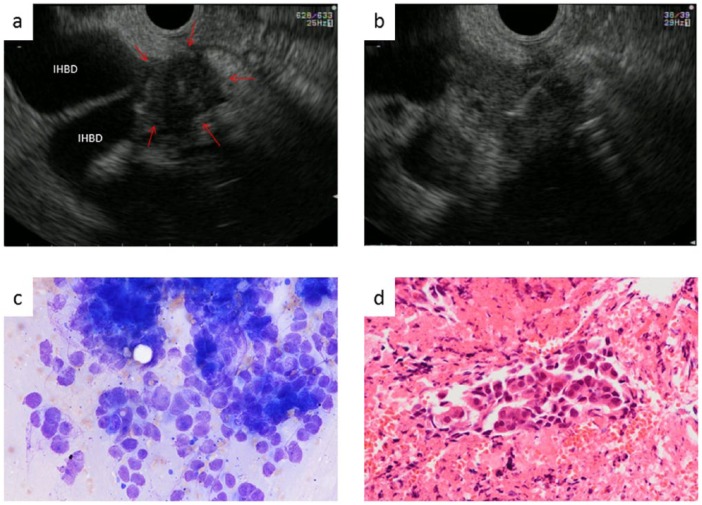
Figure 2.
Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) for the hepatic hilum. (a) Mass lesion in the hepatic hilum (arrow). (b) Using a 22-gauge FNA needle, we puncture the tumor from the duodenal bulb. (c) The cytological result is adenocarcinoma. (d) Histologically, this patient was also diagnosed with cholangiocarcinoma.
Forceps biopsy and bile cytology technique under ERCP
The ERCP cannula was inserted into the CBD, then a 0.025-inch guidewire (Revowave; Piolax Medical Devices, Kanagawa, Japan) was placed (Figure 3). Next, without performing endoscopic sphincterotomy, we inserted the forceps into the CBD, and advanced them to the obstructed site. We then performed forceps biopsy to obtain 2–4 tissue samples. After forceps biopsy, we aspirated bile juice, and performed brushing cytology, and wash cytology using 20 ml of normal saline. In addition, if a 5-Fr pigtail endoscopic nasobiliary drainage (ENBD) tube (COOK Medical, Bloomington, IN) was placed, we directly aspirated bile juice tube 3–6 times via the ENBD.
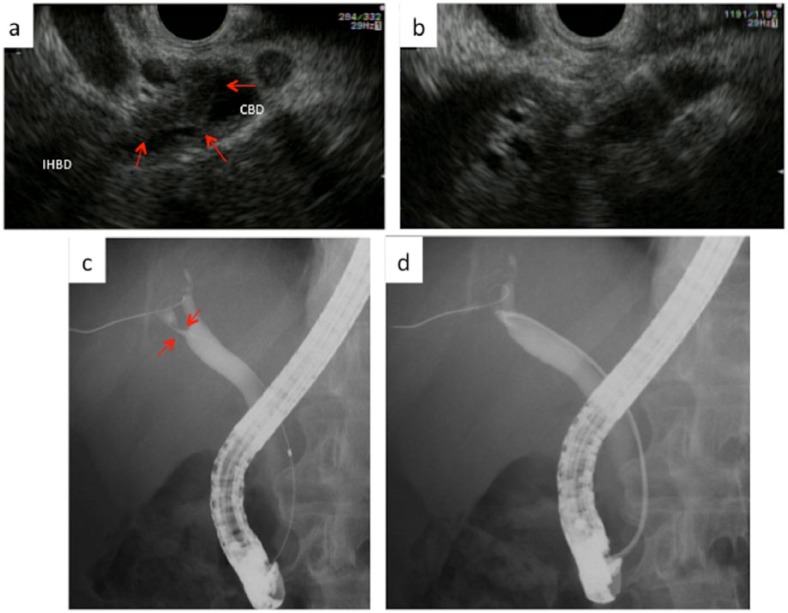
Figure 3.
Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) for the common bile duct (CBD).
(a) A thick-walled CBD is seen (arrow). (b) Using a 22-G FNA needle, we carefully puncture the thick-walled CBD from the duodenal bulb. However, EUS-FNA showed no malignancy. (c) Bile duct stenosis is seen (arrow). (d) Forceps biopsy is performed under ERCP guidance without sphincterotomy. This patient was finally diagnosed with benign stricture.
Diagnostic criteria for material obtained by EUS-FNA
EUS-FNA results were categorized as ‘malignancy’, ‘suspicious of malignancy’, atypical cells or no malignancy. In this study, ‘malignancy’ was considered as malignancy, while all other lesions (‘suspicious of malignancy’, ‘atypical cells’, or ‘no malignancy’) were considered as benign.
Statistical analysis
All data were analyzed using SPSS version 11.0 statistical software (SPSS, Chicago, IL). Continuous variables are expressed as median values. Logistic regression was used to estimate the hazard ratio (HR) and the 95% confidence interval (CI) of various factors associated with reducing diagnostic yield. Differences with values of P<0.05 were considered statistically significant.
Results
Patient characteristics
A total of 47 consecutive patients (34 men, 13 women; mean age, 69.1 ± 12.5 years) with suspected CCA were registered to this study (Table 1). Serum bilirubin level was 6.63 ± 6.24 mg/dl, and serum white blood cell count was 6654 ± 2566 μl. A total of 29 patients (61.7%) had obstructive jaundice, and 5 (10.6%) had cholangitis. On EUS, mean tumor size was 15.3 ± 7.5 mm. Mean number of needle passes was 2.0 ± 1.1 (range 1–5).
Table 1.
Patient’s characteristics.
| Age (range) | 69.1 ± 12.5 (36–89) |
| Gender male:female | 34:13 |
| Total serum bilirubin (mg/dl) | 6.63 ± 6.24 |
| Total white blood cell (μl) | 6654 ± 2566 |
| Obstructive jaundice (n) | 29 |
| Cholangitis (n) | 5 |
| Number of puncture passes (range) | 2.0 ± 1.1 (1–5) |
| Puncture site of tumor | |
| Malignant | |
| Hepatic hilum | 23 |
| Upper, middle bile duct | 15 |
| Lower bile duct | 2 |
| Benign | |
| Hepatic hilum | 4 |
| Upper, middle bile duct | 2 |
| Lower bile duct | 1 |
| Size of tumor or wall (mm, mean ± SD) | 15.3 ± 7.5 |
| Final diagnosis | |
| Hepatic hilum carcinoma | 12 |
| Common bile duct carcinoma | 25 |
| Hepatocellular carcinoma | 3 |
| Begin biliary stenosis | 7 |
Puncture sites were as follows: hepatic hilum, n = 27 (57.4%; malignant, n = 23; benign, n = 4); upper and middle bile duct, n = 17 (36.2%; malignant, n = 15; benign, n = 2); and lower bile duct, n = 3 (6.4%; malignant, n = 2; benign, n = 1).
Final diagnosis was as follows: CCA, n = 37 (78.7%; hepatic hilum, n = 12; CBD, n = 25); hepatocellular carcinoma (HCC), n = 3 (6.4%); and benign biliary stenosis, n = 7 (14.9%). The final diagnosis was based on surgical specimens (n = 8, 21.6%) or clinical follow up (n = 39, 78.4%). Disease based on surgical specimens was CCA in seven cases and HCC in one case.
Diagnostic flow
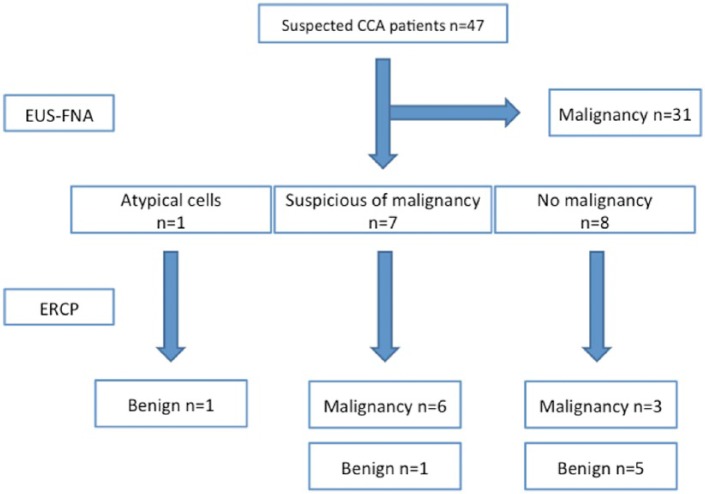
Among the 47 patients with suspected CCA, 31 were diagnosed with malignancy by EUS-FNA (Figure 4). A total of 16 patients were diagnosed as follows: no malignancy, n = 8; suspicious of malignancy, n = 7; and atypical cells, n = 1. Among these 16 patients, 9 were diagnosed with malignancy by ERCP, and 7 as benign. Final diagnosis was obtained from surgical results in 8 cases, and clinical follow up in 39. These results were confirmed from preoperative or biopsy results.
Figure 4.
Diagnostic flow.
Diagnostic yield of EUS-FNA for biliary tumors
Adequate material was obtained from all 47 patients who underwent EUS-FNA (Table 2). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the CBD were 89%, 100%, 100%, 60% and 90%, respectively (Table 2). On the other hand, diagnostic yields of the hepatic hilum were 68%, 100%, 100%, 36% and 73%, respectively. Overall, these values from the two analyses combined were 84%, 100%, 100%, 63% and 87%, respectively. If ‘suspicious of malignancy’ was instead considered as malignancy, the overall sensitivity and accuracy of EUS-FNA were 93% and 91%, respectively.
Table 2.
Diagnostic yield of endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) for biliary disease.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| Biliary tract | 89% (16/18) | 100% (3/3) | 100% (16/16) | 60% (3/5) | 90% (19/21) |
| Hepatic hilum | 68% (15/22) | 100% (10/10) | 100% (15/15) | 36% (4/11) | 73% (19/26) |
| Total | 84% (31/37) | 100% (10/10) | 100% (31/31) | 63% (10/16) | 87% (41/47) |
PPV, positive predictive value; NPV, negative predictive value.
Univariate and multivariate analysis using logistic regression
We also tried to evaluate factors reducing diagnostic yield using various factors (age, sex, jaundice, puncture site, cholangitis, disease, and tumor size); see Table 3.
Table 3.
Logistic analysis of reducing factors for diagnostic yield.
| Variables (n) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | ||||
| ≧70 (27) | 0.667 | 0.547 | ||
| <70 (20) | (0.178 to 2.491) | |||
| Gender | ||||
| Male (34) | 2.291 | 0.333 | ||
| Female (13) | (0.428 to 12.26) | |||
| Jaundice | ||||
| Presence (29) | 1.209 | 0.781 | 1.493 | 0.629 |
| Absence (13) | (0.318 to 4.602) | (0.294 to 7.587) | ||
| Puncture site | ||||
| Hilum (27) | 5.937 | 0.035 | 6.879 | 0.033 |
| CBD (20) | (1.132 to 31.15) | (1.172 to 40.374) | ||
| Cholangitis | ||||
| Presence (5) | 0.750 | 0.168 | 0.736 | 0.736 |
| Absence (42) | (0.160 to 3.523) | (0.111 to 4.887) | ||
| Disease | ||||
| Malignancy (40) | 2.583 | 0.266 | 0.944 | 0.944 |
| Benign (7) | (0.486 to 13.734) | (9.132E-2 to 13.079) | ||
| Tumor size | ||||
| ≧10 mm (34) | 2.857 | 0.146 | 2.894 | 0.294 |
| <10 mm (13) | (0.694 to 11.764) | (0.398 to 21.039) | ||
HR, hazard ratio; CI, confidence interval.
On univariate analysis using logistic regression, age >70 years [hazard ratio (HR) 0.667; 95% CI 0.178–2.491, p = 0.547], male sex (HR 2.291; 95% CI 0.428–12.26, p = 0.333), presence of jaundice (HR 1.209; 95% CI 0.318–4.602; p = 0.781), presence of cholangitis (HR 0.750; 95% CI 0.160–3.523; p = 0.168), malignant disease (HR 2.583; 95% CI 0.486–13.734; p = 0.266), and tumor size >10 mm (HR 2.857; 95% CI 0.694–11.764; p = 0.146) were not associated with the reduced diagnostic yield of EUS-FNA. On the other hand, only puncture site at the hepatic hilum was associated with reduced diagnostic yield (HR 5.937; 95% CI 1.132–31.147; p = 0.035). In addition, on multivariate analysis, puncture site was the only factor associated with reduced diagnostic yield (HR 6.879; 95% CI 1.172–40.374; p = 0.033).
Adverse events
Remarkably, although all patients underwent EUS-FNA without biliary stenting, no adverse events such as bleeding or bile leakage were associated with EUS-FNA in any of the 47 patients. On the other hand, post-ERCP pancreatitis was seen in 6.3% (1/16, mild pancreatitis). In addition, tumor seeding was not seen in any patients during follow up.
Discussion
Despite improvements in diagnostic modalities such as three-dimensional multidetector row computed tomography, magnetic resonance imaging [including magnetic resonance cholangiopancreatography (MRCP) and diffusion-weighted imaging] and per-oral video cholangioscopy with narrow-band imaging, differentiation of CCA from other inflammatory lesions remains challenging.
The next step in the diagnosis of biliary disease may be histological assessment. To obtain histological tissues, ERCP or percutaneous transhepatic cholangiodrainage (PTCD) can be selected. AS PTCD has carries a risk of tumor seeding [Chapman et al. 1989], ERCP is usually selected. Although ERCP is the gold-standard method of the diagnosis for biliary lesions, post-ERCP pancreatitis remains an important and sometimes fatal (2–15%) problem [Freeman et al. 1996; Loperfido et al. 1998; Wang et al. 2009; Cheng et al. 2006; Testoni et al. 2010; Meister et al. 2011]. On the other hand, EUS-FNA can be performed for pancreatic masses with high sensitivity and safety compared with ERCP [Yamao et al. 2005; Hasan and Hawes, 2012]. However, only 12 reports have described EUS-FNA for biliary disease. Table 4 shows an overview of EUS-FNA for the biliary tract [Frischer-Ravens et al. 2000, 2004; Rösch et al. 2004, Lee et al. 2004; Eloubeidi et al. 2004; Byrne et al. 2004; Meara et al. 2006; DeWitt et al. 2006; Mohamadnejad et al. 2011; Ohshima et al. 2011; Nayar et al. 2011; Weilert et al. 2014]. According to those reports, sensitivity and accuracy have been widely reported as 43–100% and 54–100%, respectively. Our study showed 84% sensitivity and 87% accuracy. The present results may therefore be reliable, because the diagnostic yield was similar to that in previous reports. In previous reports, the sensitivity of EUS-FNA was higher for distal malignant biliary strictures than for proximal strictures. Mohamadnejad and colleagues reported that the sensitivity of EUS-FNA was higher for distal stricture (81%) than for proximal stricture (59%, p = 0.04). In addition to this report, the sensitivity of EUS-FNA in our study was higher for distal stricture (89%) than for proximal stricture (68%), and proximal site was the only factor reducing diagnostic yield.
Table 4.
Previous reports of diagnostic yield of endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) for biliary tract.
| Study | N | Disease | Puncture site (n) |
Sensitivity | Specificity | Accuracy | Adverse events | |
|---|---|---|---|---|---|---|---|---|
| Hepatic hilum | CBD | |||||||
| Fritscher-Ravens et al. [2000] | 10 | Cholangio Ca (8), HCC (1), Signet ring cell ca (1) | 10 | 0 | 89% | N/D | 89% | None |
| Fritscher-Ravens et al. [2004] | 44 | Cholangio Ca (30), Sclerosing cholangitis (4), Primary sclerosing cholangitis (4), Sarcoid-like lesion (1), Other malignancy (5) | 44 | 0 | 89% | 100% | 91% | None |
| Rösch et al. [2004] | 50 | Pancreatic tumor (16), Biliary tumor (12), Chronic pancreatitis (6), CBD stricture (16) | 16 | 34 | 43% | 100% | 70% | N/D |
| Lee et al. [2004] | 40 | Pancreatic or biliary carcinoma (23), Metastatic carcinoma (1), Primary sclerosing cholangitis (3), Chronic pancreatitis (2), Neuroma (1), Stricture secondary to cholangitis (5), Idiopathic benign stricture (5) | 1 | 39 | 47% | 100% | 63% | N/D |
| Eloubeidi et al. [2004] | 28 | N/D | 15 | 13 | 86% | 100% | 88% | N/D |
| Byrne et al. [2004] | 35 | Cholangio Ca (12), Metastatic Ca (4), Benign (19) | 3 | 32 | 86% | N/D | N/D | N/D |
| Meara et al. [2006] | 46 | N/D | N/D | N/D | 87% | 100% | N/D | None |
| DeWitt et al. [2006] | 24 | Cholangio Ca (19), Gallbladder Ca (1), Lymphoma (2), Benign stricture (2) | 24 | 0 | 77% | 100% | 79% | None |
| Mohamadnejad et al. [2011] | 81 | Cholangio Ca (81) | 30 | 51 | 73% | N/D | N/D | Hemobilia (1) |
| Ohshima et al. [2011] | 22 | Cholangio Ca (10), Gallbladder Ca (5), Metastasis (1), Benign (6) | 0 | 14 | 100% | 100% | 100% | None |
| Nayar et al. [2011] | 32 | Cholangio Ca (24), Benign (8) | 0 | 32 | 68% | 100% | 54% | None |
| Weilert et al. [2014] | 51 | Pancreatic Ca (34), Cholangio Ca (13), Gallbladder Ca (1), Autoimmune pancreatitis (1), Chronic pancreatitis (1), Autoimmune cholangiopathy (1) | 7 | 44 | 94% | 100% | 94% | None |
Ca, carcinoma, HCC, hepatocellular carcinoma; CBD, common bile duct; N/D, not discussed.
Interestingly, almost no adverse events have been reported (only one adverse event, as hemobilia), as in our results. However, previously reports included pancreatic disease and patients who had previously undergone biliary stenting under ERCP, and thus did not truly indicate that EUS-FNA for biliary disease offers a high diagnostic yield and safety without adverse events such as bile leakage. Our study included only biliary disease, and no patients under conditions such as biliary stent placement were included. Our study thus truly reflected the diagnostic yield and adverse events associated with EUS-FNA for biliary disease. According to our results, EUS-FNA for biliary disease may safely offer a high diagnostic yield. However, the risk of tumor seeding needs to be evaluated by long-term follow up and a larger patient group. In addition, puncturing masses while avoiding puncture of the biliary tract lumen is not always possible, and EUS-FNA for the biliary tract should thus be performed when the biliary tract can be easily and safely accessed.
Only one report, by Weilert and colleagues [Weilert et al. 2014], has described EUS-FNA safety for suspected malignant biliary obstruction before ERCP, and the diagnostic yield of EUS-FNA was compared with that of ERCP. In that study, EUS-FNA was superior to ERCP tissue sampling for pancreatic masses (sensitivity, 100% versus 38%; p < 0.001). However, no significant differences were seen between biliary mass or stricture (79% each) and indeterminate stricture (80% each). According to both that report and our own findings, EUS-FNA for biliary disease may be safely performed without placement of a biliary stent. Because there is no possibility of acute pancreatitis, EUS-FNA for biliary disease may offer a first-line diagnostic modality that is preferable to forceps biopsy or bile cytology under ERCP, although this needs to be confirmed in a prospective randomized study of a larger number of patients. Another advantage of EUS-FNA for biliary disease, if performed with ROSE, may be as follows: if the results of EUS-FNA show malignancy, a metallic stent can be placed during the initial ERCP for cases of unresectable CCA. This may lead to a reduced risk of post-ERCP pancreatitis. If forceps biopsy or bile cytology under ERCP is performed, a plastic stent or endoscopic nasal biliary drainage may be selected until results are available. Re-ERCP must then be performed to place the metallic stent, which may increase the risk of post-ERCP pancreatitis. A favorable cost benefit ration may thus be obtained with EUS-FNA, but this too must be confirmed in a prospective randomized study of a larger number of patients.
Univariate and multivariate analyses for factors reducing diagnostic yield were also performed in this study. Only puncture site was independently associated with reduced yield in multivariate analysis (HR 6.879; 95% CI 1.172–40.374, p = 0.033). This may be because, compared with CBD, visualization of the hepatic hilum is technically difficult. EUS-FNA for the hepatic hilum may thus be difficult compared with EUS-FNA for CBD. Improvements to FNA needles and echoendoscopes are still required to overcome this problem. Our study also showed limitations such as small sample size and patient selection bias. In addition, although none of the patients developed malignant disease during clinical follow up, patients who underwent clinical follow up were included in this study, representing a limitation to the study design.
In conclusion, we analyzed factors reducing diagnostic yield on EUS-FNA for biliary disease alone. In addition, we demonstrated that EUS-FNA for biliary disease could be safely performed without biliary stenting. However, EUS-FNA and ERCP were complementary in the diagnostic workup. Our results need to be validated in future prospective studies.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Saori Onda, Second Department of Internal Medicine, Osaka Medical College, Japan.
Takeshi Ogura, Second Department of Internal Medicine, Osaka Medical College, 1-1 Daigakuchou, Takatsukishi, Osaka 464-8681, Japan.
Yoshitaka Kurisu, Departments of Pathology, Osaka Medical College, Japan.
Daisuke Masuda, Second Department of Internal Medicine, Osaka Medical College, Japan.
Tatsushi Sano, Second Department of Internal Medicine, Osaka Medical College, Japan.
Wataru Takagi, Second Department of Internal Medicine, Osaka Medical College, Japan.
Shinya Fukunishi, Second Department of Internal Medicine, Osaka Medical College, Japan.
Kazuhide Higuchi, Second Department of Internal Medicine, Osaka Medical College, Japan.
References
- Byrne M., Gerke H., Mitchell R., Stiffler H., McGrath K., Branch M., et al. (2004) Yield of endoscopic ultrasound-guided fine-needle aspiration of bile duct lesions. Endoscopy 36: 715–719. [DOI] [PubMed] [Google Scholar]
- Chapman W., Sharp K., Weaver F., Sawyers J., et al. (1989) Tumor seeding from percutaneous biliary catheters. Ann Surg 209: 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Shermen S., Watkins J., Barnett J., Freeman M., Geenen J., et al. (2006) Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 101: 139–147. [DOI] [PubMed] [Google Scholar]
- Clayton R., Clarke D., Currie E., Madhavan K., Parks R., Garden O. (2003) Incidence of benign pathology in patients undergoing hepatic resection for suspected malignancy. Surgeon 1: 32–38. [DOI] [PubMed] [Google Scholar]
- DeWitt J., Misra V., Leblanc J., McHenry L., Sherman S., et al. (2006) EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc 64: 325–333. [DOI] [PubMed] [Google Scholar]
- Domagk D., Poremba C., Dietl K., Senninger N., Heinecke A., Domschke W., et al. (2002) Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study. Gut 51: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloubeidi M., Chen V., Jhala N., Eltoum I., Jhala D., Chhieng D., et al. (2004) Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol 2: 209–213. [DOI] [PubMed] [Google Scholar]
- Freeman M., Nelson D., Sherman S., Haber G., Herman M., Dorsher P., et al. (1996) Complications of endoscopic sphincterotomy. N Engl J Med 335: 909–918. [DOI] [PubMed] [Google Scholar]
- Frischer-Ravens A., Broering D., Knoefel W., Rogiers X., Swain P., Thonke F., et al. (2004) EUS-guided fine-needle aspiration biopsy of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol 99: 45–51. [DOI] [PubMed] [Google Scholar]
- Frischer-Ravens A., Broering D., Sriram P., Topalidis T., Jaeckle S., Thonke F., et al. (2000) EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc 52: 534–540. [DOI] [PubMed] [Google Scholar]
- Gerhards M., Vos P., van Gulik T., Rauws E. A., Bosma A., Gouma D., et al. (2001) Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg 88: 48–51. [DOI] [PubMed] [Google Scholar]
- Gores G. (2003) Cholangiocarcinoma: current concepts and insights. Hepatology 37: 961–969. [DOI] [PubMed] [Google Scholar]
- Hasan M., Hawes R. (2012) EUS-guided FNA of solid pancreas tumors. Gastrointest Endosc Clin N Am 22: 155–167. [DOI] [PubMed] [Google Scholar]
- Khan S., Taylor-Robinson S., Toledano M., Beck A., Elliott P., Thomas H. (2002) Changing international trends in mortality rates for liver, biliary and pancreatic tumors. J Hepatol 37: 806–813. [DOI] [PubMed] [Google Scholar]
- Lee J., Salem R., Aslanian H., Chacho M., Topazian M., et al. (2004) Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol 99: 1069–1073. [DOI] [PubMed] [Google Scholar]
- Loperfido S., Angelini G., Benedetti G., Chilovi F., Costan F., De Berardinis F., et al. (1998) Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc 48: 1–10. [DOI] [PubMed] [Google Scholar]
- Mansfield J., Griffin S., Wadehra V., Matthewson K., et al. (1997) A prospective evaluation of cytology from biliary strictures. Gut 40: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meara R., Jhala D., Eloubeidi M., Eltoum I., Chhieng D., Crowe D., et al. (2006) Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: analysis of 53 cases. Cytopathology 17: 42–49. [DOI] [PubMed] [Google Scholar]
- Meister T., Heinzow H., Heinecke A., Hoehr R., Domschke W., Domagk D., et al. (2011) Post-ERCP pancreatitis in 2364 ERCP procedures: is intraductal ultrasonography another risk factor? Endoscopy 43: 331–336. [DOI] [PubMed] [Google Scholar]
- Mizumoto R., Ogura Y., Kusuda T. (1993) Definition and diagnosis of early cancer of biliary tract. Hepatogastroenterology 40: 69–77. [PubMed] [Google Scholar]
- Mohamadnejad M., DeWitt J., Sherman S., LeBlanc J., Pitt H., House M., et al. (2011) Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc 73: 71–78. [DOI] [PubMed] [Google Scholar]
- Nakayama A., Imamura H., Shimada E., Miyagawa S., Makuuchi M., Kawasaki S., et al. (1999) Proximal bile duct stricture disguised as malignant neoplasm. Surgery 125: 514–521. [PubMed] [Google Scholar]
- Nayar M., Manas D., Wadehra V., Oppong K. (2011) Role of EUS/EUS-guided FNA in the management of proximal biliary strictures. Hepatogastroenterology 58: 1862–1865. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Yasuda I., Kawakami H., Kuwatani M., Mukai T., Iwashita T., et al. (2011) EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J Gastroenterol 46: 921–928. [DOI] [PubMed] [Google Scholar]
- Rösch T., Hofrichter K., Frimberger E., Meining A., Born P., Weigert N., et al. (2004) ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc 60: 390–396. [DOI] [PubMed] [Google Scholar]
- Shaib Y., El-Serag H. (2004) The epidemiology of cholangiocarcinoma. Semin Liver Dis 24: 115–125. [DOI] [PubMed] [Google Scholar]
- Tamada K., Tomiyama T., Wada S., Ohashi A., Satoh Y., Ido K., et al. (2002) Endoscopic transpapillary bile duct biopsy with the combination of intraductal ultrasonography in the diagnosis of biliary strictures. Gut 50: 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testoni P., Mariani A., Giussani A., Vailati C., Masci E., Macarri G., et al. (2010) Risk factors for post-ERCP pancreatitis in high-and low-volume centers and among expert and non-expert operators: a prospective multicenter study. Am J Gastroenterol 105: 1753–1761. [DOI] [PubMed] [Google Scholar]
- Wang P., Li Z., Liu F., Ren X., Lu N., Fan Z., et al. (2009) Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol 104: 31–40. [DOI] [PubMed] [Google Scholar]
- Weilert F., Bhat Y., Binmoeller K., Kane S., Jaffee I., Shaw R., et al. (2014) EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. PII: S0016–5107(13)02714–4. DOI: 10.1016/j.gie.2013.12.031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Yamao K., Sawaki A., Mizuno M., Shimizu Y., Yatabe Y., Koshikawa T., et al. (2005) Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol 40: 251–254. [DOI] [PubMed] [Google Scholar]